CHAPTER 41 Primary Tumors
INTRODUCTION
Primary tumors of the spine are uncommon, accounting for 5–10% of all primary skeletal tumors. At the Rizzoli Institute, 323 patients (adults and children) with primary spine neoplasms were seen over the past 50 years; 27% were malignant and 73% were benign.1 The most commonly encountered benign tumors of the mobile spine (the cervical, thoracic, and lumbar segments) include osteoblastomas, osteochondromas, giant cell tumors, hemangiomas, osteoid osteomas, and aneurysmal bone cysts. Common malignant primary tumors of the mobile spine include chordomas, chondrosarcomas, osteosarcomas, and Ewing’s sarcoma (Table 41.1). Less common malignant lesions of the mobile spine include solitary myeloma, solitary lymphoma, fibrosarcoma, and hemangioendothelioma.2 Chordomas and Ewing’s sarcomas are much more common in the sacrum. In fact, the incidences and clinical characteristics of many sacral tumors differ from those found in the mobile spine. Therefore, statistics and characteristics regarding sacral lesions are included in the discussion of these particular tumor types that are commonly found in the sacrum.
| Common Benign Primary | Common Malignant Primary |
|---|---|
| Tumors of the Mobile Spine2,5 (%) | Tumors of the Mobile Spine2 (%) |
| Osteoblastoma (20–23) | Chordoma (33) |
| Osteochondroma (13–23) | Chondrosarcoma (25) |
| Giant cell tumors (16–22) | Osteosarcoma (19) |
| Hemangioma (10–20) | Ewing’s (8) |
| Osteoid osteoma (7–21) | |
| Aneurysmal bone cyst (13) |
There is a strong correlation between patient age and likelihood of malignancy in primary tumors of the spine.3,4,5 In children, nearly 70% of primary tumors of the spine are benign,6 and 70% of primary spine tumors in adults (>21 years old) are malignant.7 As in many tumors, certain tumors of the spine have a predilection for certain age groups. In patients younger than 10 years, neuroblastoma, eosinophilic granuloma, and Ewing’s sarcoma dominate. Aneurysmal bone cysts, giant cell tumors, osteoid osteomas, osteoblastomas, and eosinophilic granulomas are the most frequent primary spine tumors found in adults less than 30 years of age. Patients between 30 and 50 years of age more often have chondrosarcoma, chordoma, lymphoma, and hemangioma as well as metastatic lesions. Those over 50 years of age mostly likely have metastatic disease, but may present with solitary myeloma or chondrosarcoma.8
The spine can be divided into anterior elements and posterior elements (Fig. 42.1). Anterior elements consist of the vertebral bodies, and the posterior elements include the remainder of the vertebra (pedicles, transverse processes, laminae, and the spinous process). Most malignant tumors, both primary and metastatic, occur in the anterior elements. Primary spine tumors located in the anterior elements have a 76% probability of being malignant. Those tumors located in the posterior elements are more likely benign (64%).6 Metastatic lesions of the spine are found in the anterior elements approximately 95% of the time. Common primary spine tumors involving the posterior elements are aneurysmal bone cysts, osteoblastomas, and osteoid osteomas. Primary spine tumors that have a predilection for the vertebral body include osteosarcomas, chordomas, solitary lymphomas, eosinophilic granulomas, giant cell tumors, and hemangiomas.8
CLINICAL PRESENTATION
As mentioned previously, early diagnosis is critical in primary spinal tumors. Early detection can lead to optimal local control and prevention of distant spread. Therefore, a high index of clinical suspicion and an awareness of symptoms is vital. A careful history describing the characteristics and timeline of symptoms is important in diagnosis and may direct the work-up and treatment. A proper history includes a summary of prior treatment and provides a baseline to evaluate the course of the disease and the effect of therapy.
Pain
Back pain is the most common presenting symptom in both primary (84%)5 and secondary tumors (>90%)9,10 of the spine. Tumor pain is typically unrelenting, progressive, and often present during the night, although many types of pain can present. These include local, axial, radicular, and myelopathic pain, which are discussed in detail in the following chapter. Pain at night is particularly suggestive of tumors such as osteoid osteoma and sometimes more aggressive lesions. Tumor pain may be localized to a specific spinal segment or reproduced by pressure/percussion over the involved area. Tumor expansion may erode the cortical margins leading to pathologic fractures. Both pathologic fractures and tumor growth may involve the spinal canal and neural foramina, with compression of the cord and/or nerve roots resulting in neurologic deficits as well as pain. Ongoing destruction may also lead to development of spinal instability.
A history of persistent back pain should be taken seriously and usually warrants further investigation. Patients with a history of irradiation or Paget’s disease of the spine warrant added vigilance due to their risk of secondary osteosarcoma.11 In general, pain from tumors commonly mimics the pain produced by nontumorous disorders. Thus, it is necessary for the clinician to have a high index of suspicion when dealing with back pain, even if the patient does not present with the characteristic types of pain associated with spinal tumors.
Neurologic impairments
Neurological presentation entirely depends on the level of the lesion and the degree of nerve or cord compression. Neurological manifestations, including radiculopathy and myelopathy, may present some time after the onset of pain. Forty-one percent of patients with primary spine tumors complained of a subjective sense of weakness on initial presentation.5 Malignant tumors are associated with a higher incidence of neurologic deficit than are benign lesions.12 Benign tumors of the spine can often present with a history of symptoms of long duration or may remain asymptomatic for some time.
Other symptoms
Systemic or constitutional symptoms tend to be more common with malignant or metastatic disease than in benign lesions. As such, constitutional symptoms such as fatigue, fever, and unexpected weight loss must be included in a careful review of symptoms when a malignant or metastatic lesion is suspected. Red flags that suggest malignancy of the spine are presented in Table 41.2.
Table 41.2 Elements of the Presentation that are Worrisome for Spinal Malignancy
| Red Flags |
|---|
| History of prior malignancy |
| Back pain worse at night/pain and wakes patient from sleep |
| Consistent progression of pain |
| Pain unchanged during rest or activity |
| Acute neurologic deterioration |
| Presence of a mass |
| Presence of constitutional symptoms |
PHYSICAL EXAM
Musculoskeletal inspection of the spine
The patient should be inspected for any obvious deformities of the spine and abnormal posturing. A bony prominence, kyphotic deformity, or acute angular scoliosis can be observed after vertebral collapse.13 Scoliosis is often associated with osteoid osteoma and osteoblastoma located on the concave side of the apical portion of the deformity. Spine tumors are rarely palpable due to the amount of tissue and muscle layers superficial to the spine. However, the spinous processes of the spine should be palpated, while paying special attention to any tenderness, masses, vertebral defects, and spastic paraspinal musculature. Range of motion testing in flexion, extension, rotation, and lateral bending should be carefully performed.
Neurologic examination
In evaluating neurologic function, motor, sensory, and reflex function must be assessed and recorded. Although weakness is rarely the first symptom of a spinal column tumor, it can be objectively identified in 55% of patients with malignant lesions and in 35% of patients with benign lesions.5 Sensory findings are less common than motor findings in patients with either radiculopathy or cord compression. However, testing with pinprick and light touch, particularly in the sacral dermatomes should be performed. Saddle sensory loss may be associated with tumors in the area of the cauda equina. Compression above the cauda equina often spares the sensation to these sacral dermatomes.14
WORK-UP
Laboratory studies
The laboratory work-up in a patient with a suspected tumor of the spine can be involved. A complete blood count (CBC) with a differential is important when working up any suspected malignancy. Elevated erythrocyte sedimentation rates (ESR) and C-reactive protein (CRP) levels signal that an inflammatory process is involved, but cannot consistently differentiate an infectious process from a malignancy. Lactate dehydrogenase (LDH) levels can be elevated in sarcomas, and LDH isoenzymes 2 and 3 can suggest a diagnosis of lymphoma.15 To evaluate for liver cancer, alpha fetoprotein (AFP) levels are often obtained in patients with hepatitis C or those who are heavy drinkers. Carcinoembryonic antigen (CEA) is a marker of adenocarcinomas such as colonic, rectal, pancreatic, gastric, and breast.16 Prostate specific antigen (PSA) levels can help diagnose prostate cancer. A thyroid panel can help eliminate the suspicion of a rare thyroid primary, and parathyroid hormone (PTH) can be ordered to look for hyperparathyroidism. An elevated PTH level may lead to diagnosis of a brown tumor of the spine. The diagnosis of myeloma can be confirmed by the identification of monoclonal proteins in the serum or urine via serum protein electrophoresis (SPEP) or urine protein electrophoresis (UPEP);17 however, monoclonal proteins are more often absent or undetectable in solitary myeloma compared to multiply myeloma.18 A chemistry panel can be used to assess kidney function and allows calcium and phosphate levels to be followed to detect and avoid the development of malignant hypercalcemia. An elevated alkaline phosphatase level can also suggest a neoplastic bone disease.
Imaging techniques
Bone scan
Bone scanning (skeletal scintigraphy) utilizes a disphosphonate compound, tagged with technetium 99m, which becomes incorporated into bone by osteoblastic activity after intravenous injection. Bone scans may be instrumental in detecting tumors of the spine, and images of the entire body can be obtained in a fairly short period of time. One weakness is low specificity. Bone scans are known to be highly sensitive in localizing osteoid osteomas.19 This provides an earlier diagnosis and accurate localization of the tumor. Bone scans are also useful in identifying multifocal lesions and for evaluating metastatic disease.
Computed tomography
Computed tomography provides the best images of bone architecture and readily detects small areas of bone destruction or blastic change, although magnetic resonance imaging is more effective in detecting lesions before changes in bone structure can be demonstrated. In the past, CT was not considered a good screening tool for lesions in the spine, but with multidetector scanners, the entire spine can be scanned in great detail in under 5 minutes. Images can be reconstructed into any plane for the evaluation of bone alignment and extent of compression in a compression fracture. CT imaging can also provide the spine surgeon with an image of remaining bone in an abnormal vertebra, a factor in the feasibility of fixation. CT imaging is also valuable for planning and guiding percutaneous biopsies of vertebral lesions. CT imaging of the spine is especially useful in those patients who cannot undergo MRI (claustrophobic, cannot lie flat for long periods of time, or have implanted, metallic devices).
Myelography (conventional and CT-myelography)
To perform a myelogram, iodinated contrast material is instilled into the dural sac in order to detect external compression of the sac or space-occupying lesions within the spinal cord. Therefore, it is an invasive procedure with inherent risks. Before MRI, conventional myelography was the gold standard for detection of cord compression and intrinsic cord lesions, but it has been largely replaced by MR scanning, and by CT-myelography when MRI is contraindicated. Myelography may fail to reveal secondary sites of epidural spinal cord compression and has been shown to be less sensitive in diagnosing spinal tumors than MRI.20 CT-myelography, like conventional myelography, involves the instillation of contrast into the dural sac, but the amount of contrast used is much less due to the enhanced ability of CT to depict subtle contrast differences. By employing various window settings for the images, details of the paraspinal structures, bone, and dural sac contents are well demonstrated. Both conventional and CT-myelography may be used when metallic fixation devices have been placed in and around the spine and MRI is unable to provide adequate images. This, however, is becoming less frequent with the increased use of titanium spinal hardware.
Positron emission tomography
The most common radiotracer used in clinical positron emission tomography (PET) imaging is fluorine-18-fluoro-2-D-deoxyglucose (18F-FDG), which accumulates in areas of high glycolysis and membrane transport of glucose, both known to be increased in malignant tissue. Unlike the agent used in bone scanning, 18F-FDG may detect bone marrow-occupying lesions before cortical involvement occurs, thus detecting bone metastases before they can be found on bone scans. Sclerotic metastases, however, as found in some breast and prostate cancers, are less likely to be detected by PET as these lesions have lower glycolytic rates and are less cellular than lytic metastases.21 18F-FDG is not specific for tumors and may accumulate at sites of infection but is less likely to be detected at sites of degenerative change than technetium 99m, the agent used in bone scans. Therefore, it is somewhat more specific for tumors. PET also demonstrates metastases in soft tissue throughout the body, resulting in additional diagnostic value.
In addition to detecting spine tumors, PET may also be useful in distinguishing malignant lesions from benign. One study of 29 patients with cancer and spine abnormalities showed that two nuclear medicine physicians were in agreement in calling abnormalities benign, equivocal, or metastasis in 90%.22 In addition, 100% of abnormalities interpreted as benign or malignant were correctly identified. The only discrepancies were in three abnormalities that were interpreted as equivocal and which turned out to be metastatic. CT and/or MRI were important in arriving at the final diagnosis in equivocal cases.
The ability of PET to evaluate the response of bone tumors to chemotherapy has also been studied. In one study of patients who underwent preoperative chemotherapy for osteosarcoma, changes in tumor 18F-FDG uptake were correlated with percentage tumor necrosis on histopathology. Tumor necrosis was accurately predicted on PET scan in 15 out of 16 patients by visual assessment and in 14 out of 15 patients by final tumor to background ratio (TBR).23
Biopsy
Types of biopsy
There are two types of biopsy commonly used for biopsy of spinal lesions: percutaneous, guided and open, surgical biopsy. Both fluoroscopic-guided and CT-guided percutaneous biopsies can be utilized, and both are effective. The tip accuracy of CT makes it superior when dealing with small, deep-seated lesions especially in the cervical and thoracic regions.24 CT better allows selection of the optimal location to sample tissue. For lesions visible via fluoroscopic monitoring, fluoroscopic-guided biopsy offers real-time positioning of the needle. Open biopsy maximizes tissue retrieval and providing the highest diagnostic success rate; however, it is typically reserved for failed percutaneous biopsies due to the increased morbidity of the open procedure and greater risk of wound contamination with tumor. Regardless of which method is used, the goal is to obtain an adequate amount of tissue while minimizing complications.
Biopsy success rate
Accurate diagnosis of tumorous and nontumorous lesions using CT-guided biopsy is achieved more than 90% of the time.24–26 In lesions with central necrosis, the ability to obtain the correct diagnosis may be enhanced by obtaining tissue from the periphery of the lesion. In paucicellular aspirates, a cell block can be prepared or additional tissue, such as a core biopsy, can be obtained. If histology yields only peripheral blood in an obviously destructive mass, biopsy can be repeated, by directing the needle/device at a slightly different area of the lesion.26 If indicated, corticosteroids should ideally be administered after biopsy due to their lytic effect on certain tumors, including leukemia; this lytic effect can lead to a nondiagnostic biopsy.
Percutaneous biopsy of solitary lesions
The approach to the percutaneous biopsy of a solitary spinal lesion is fairly straightforward. Usually, the approach involves the shortest path to the lesion that does not place vital structures at risk. For biopsies of the spine, this typically involves a posterior approach; however, in the cervical spine anterolateral approaches are often used. A posterior transpedicular approach is often used to biopsy lesions in the vertebral body. The transpedicular approach helps to avoid vital structures while minimizing the amount of tissue susceptible to tumor contamination of the needle tract. Virtually any lesion within the vertebral body of cervical, thoracic, or lumbar vertebrae can by accessed via this approach.34 Lesions located in the posterior elements are typically easy to biopsy with a direct approach. Occasionally, primary lesions of the spine can locally metastasize to other vertebrae. In this instance, the decision of which lesion to biopsy is important and should be based on several factors including the size of the lesion, radiologic morphology, location along the spinal column, and location within the vertebra. These factors are discussed further in the next chapter.
Complications of biopsy
Biopsies of potentially tumorous lesions should be well planned. It is well known that inadequate or inappropriate biopsies adversely affect outcome. Complications arising in these unsound biopsies include disability due to more complex resection, loss of function, local recurrence, and death.28 The surgeon that will be performing the definitive surgical procedure, if further surgery becomes necessary, should always perform the open biopsy. This ensures that the subsequent surgery can be performed using the optimal incision and approach, while excising the biopsy incision and tract. This can also help eliminate unnecessary and improperly performed open biopsies.
Complications of percutaneous needle biopsy include bleeding, infection, neurologic compromise, fracture, biopsy tract contamination, and death, although serious complications are rare. Due to the risk of tumor contamination of the biopsy tract,29 the needle tract should be excised if a subsequent surgery is indicated, although this is somewhat controversial. Whenever possible, guided biopsies should be done at the same institution where definitive surgical treatment will occur. Typically, pathologists at the larger referral centers will be more experienced with uncommon primary and secondary malignant tissues obtained from the spine and will typically review specimens despite previous histologic diagnosis from outside institutions. Also, a team approach between the interventional radiologist and the treating surgeon is more likely to produce a favorable result.
BENIGN TUMORS OF THE SPINE
Eosinophilic granuloma
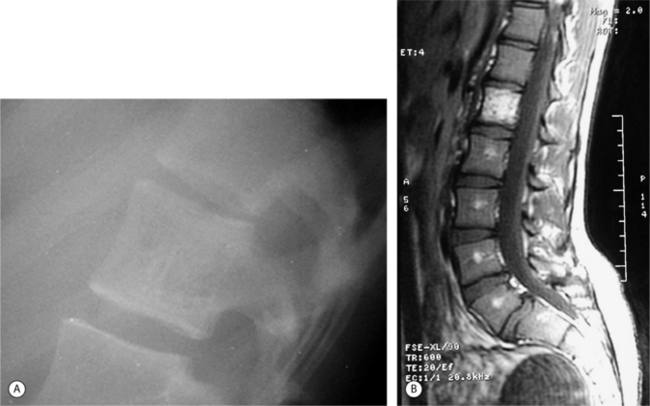
Also known as Langerhans cell histiocytosis (LCH), eosinophilic granuloma is a benign and self-limiting process that can lead to focal destruction of bone. It is most prevalent in children, with half of patients under the age of 10 years. The etiology is unknown and the lesion is comprised of lipid-containing histiocytes from the reticuloendothelial system and eosinophils. Lesions are most common in the skull although virtually any bone may be affected, with vertebral involvement occurring in approximately 10–15% of cases. The most common appearance is a well-circumscribed, punched-out lesion with no periosteal reaction. Less common is the moth-eaten pattern with periosteal reaction. Both are demonstrated in Figure 41.2. Vertebral destruction with complete collapse of the vertebral body can occur and is classically referred to as ‘vertebra plana.’ Multiple vertebrae may occasionally be involved. Collapse can produce pain and spasm of the paraspinal muscles. Deformities in the form of gibbus or kyphus may develop in some cases. Diagnosis is confirmed by needle or open biopsy.
Most lesions of eosinophilic granuloma regress without any treatment or with bracing alone. Local steroid injections have also been used.30,31 Chemotherapy is recommended in cases of LCH with disseminated lesions. When neurologic symptoms are present, with or without vertebral collapse, irradiation and immobilization is recommended.32 Neurologic recovery is usually excellent and partial reconstitution of vertebral height is often seen in young patients. In rare cases of cord compression, surgical decompression and stabilization are indicated.
Osteochondroma
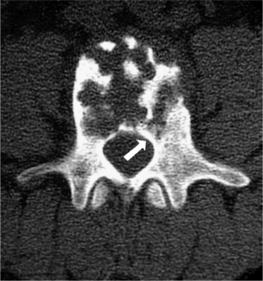
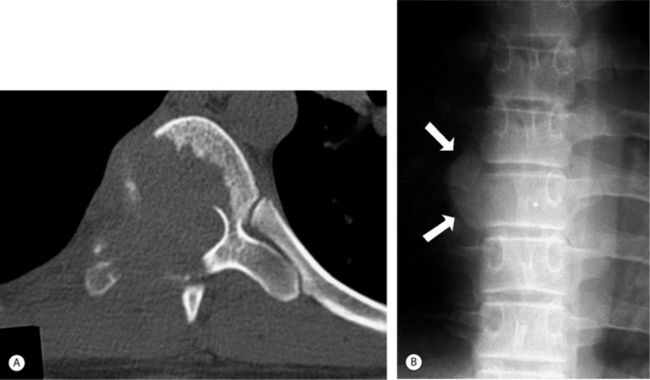
Osteochondromas, also known as exostoses, are cartilage capped, and the cortex of the lesion is continuous with the cortex of the involved bone. An osteochondroma of the cervical vertebra is shown in Figure 41.3. Osteochondromas accounted for 35% of all benign bone tumors in one series.2 They can occur in any bone in which enchondral ossification occurs, but are usually found in the metaphyseal region of long bones in the limbs. These lesions involve the spine in 3–7% of cases2,33 and make up approximately 2% of all solitary spine tumors and 13–23% of all benign tumors of the spine.2 Osteochondromas typically occur in patients aged 20–30 years. They are often painless, and neurologic deficits with this type of spinal lesion are rare. The cartilage cap of osteochondromas should be less than 2 cm in adults. Lesions with a cap greater than 2 cm should be suspected of malignant transformation to chondrosarcoma. Solitary osteochondromas are reported to have a 3% chance of malignant transformation, which increases to 27% if the lesion is one of the many found in multiple hereditary exostoses (MHE).34 Patients with MHE typically develop lesions earlier in life and more frequently experience neurologic deficits. Neurologic deficit due to canal or foraminal encroachment and pain are the main indications for surgical intervention. Because these lesions progress slowly, surgical excision often results in neurologic recovery. No adjuvant treatment is usually required.
Osteoid osteoma/osteoblastoma
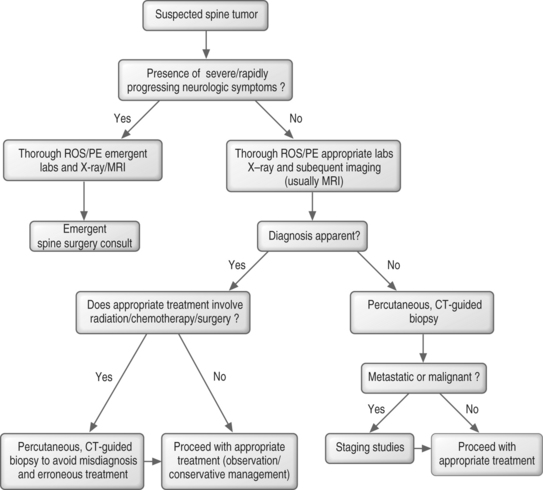
Histologically, osteoid osteomas and osteoblastomas are alike; however, they have different clinical characteristics. Osteoid osteomas are smaller, by definition less than 2 cm in diameter, and tend to have thick sclerotic margins. Approximately 10% of osteoid osteomas are located within the spine. Osteoid osteomas have a distinct predilection for the posterior elements in the spine and typically present in patients 10–20 years of age. Scoliosis is often found in both osteoid osteoma and osteoblastoma, with 50–63% of patients with either condition having significant scoliosis.35,36 Osteoid osteoma lesions secrete prostaglandins, and patients often present with the classic history of night pain and pain relief with NSAIDs. In the spine, an osteoid osteoma can be difficult to identify on plain radiographs as the diameter of the lesion is less than 2 cm and is often obscured by overlapping shadows of the vertebral column. MRI may demonstrate surrounding bone and soft tissue edema creating the false impression of an aggressive lesion. The bone scan is the most sensitive test for locating osteoid osteomas, and the central nidus shows markedly increased uptake. Fine-cut (1 mm) CT scans show the lesion clearly and best differentiate it from other lesions. An osteoid osteoma of the spine is shown in Figure 41.4. The treatment of choice for spinal osteoid osteomas is surgical excision. The entire tumor nidus should be removed. Reliable pain relief is achieved, and the secondary spinal deformity resolves in most cases. Percutaneous CT-guided resection has also been shown to be useful.37,38 Radiofrequency (RF) ablation has also been utilized in the spine, but is associated with higher risks. Some authors feel that RF ablation may have a limited role in osteoid osteomas of the spine.39 Occasionally, long-term treatment with NSAIDSs may be a viable option especially when the lesion is in a difficult location, as spontaneous regression is known to occur over a period of time and malignant transformations are rare.
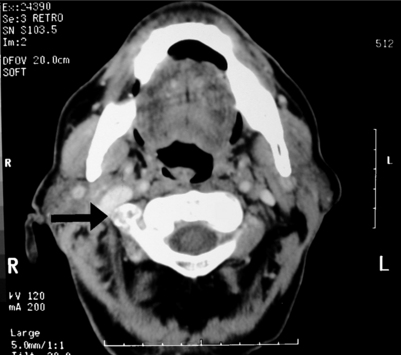
In comparison to osteoid osteomas, osteoblastomas are larger than 2 cm, and antiinflammatory medications provide little or no symptom relief, as osteoblastomas are not known to secrete prostaglandin. The spinal column and sacrum are involved in approximately 42% of all lesions.2,35 Like osteoid osteomas, osteoblastomas tend to involve the posterior elements. Rarely is osteoblastoma confined to the vertebral body. Similar to osteoid osteomas, the population affected is young, 10–30 years of age. Osteoblastoma is a rare tumor with occasional malignant transformation and should not be labeled as just a large osteoid osteoma. Radiographically, they are usually characterized by a lytic lesion with expansile behavior and varying lesional ossification (Fig. 41.5). It is important to remember that an osteoblastoma is a benign, but often locally aggressive tumor, and must be treated as such. Marked radionucleotide uptake is exhibited on bone scans. MRI is non-specific, but is used to assess the effect of the tumor on surrounding tissues and the spinal cord if cord involvement is suspected. These lesions are radioresistant. Marginal resection is usually the best surgical option. When complete excision of the osteoblastoma is not feasible, curettage and bone grafting may provide an acceptable long-term result.35,40 Aggressive osteoblastomas have a recurrence rate of approximately 50% if negative margins are not obtained.
Hemangioma
Hemangiomas of the spine are common, occurring in approximately 10% of all adults and are typically in the anterior elements. These lesions are rarely symptomatic and are typically found incidentally, although they may occasionally exhibit extraosseous extension and neural compromise. Hemangiomas typically contain trabecular condensations surrounded by abnormal vascular channels which are more lucent on plain films and CT, giving the vertebral body vertical striations on plain films, which is popularly referred to as the ‘jailhouse’ vertebra (Fig. 41.6A). The appearance of these lesions on axial CT images resembles polka-dots.2 They may exhibit sclerotic margins. On MRI, hemangiomas are hyperintense on T1 (Fig. 41.6B), which may make them undistinguishable from normal fatty marrow, and are hyperintense on T2-weighted images.2,41 The trabecular condensations may appear on MRI as dark dots against a more hyperintense background, giving a ‘salt and pepper’ appearance. Hemangiomas demonstrate contrast enhancement on MRI.
Asymptomatic hemangiomas rarely develop into symptomatic lesions. Symptomatic hemangiomas usually respond well to conservative surgical procedures. Selective arterial embolization may be a safer and more effective treatment than radiation. Hemangiomas respond to low-dose radiation, but radiation may not be an ideal treatment due to the risk of radiation-induced secondary sarcomas. Vertebroplasty has been used in Europe to treat these lesions and reinforce the vertebral body percutaneously.13 Anterior resection and fusion are reserved for pathologic collapse and neural compromise or refractory cases.
Aneurysmal bone cyst
Aneurysmal bone cysts (ABCs) are sometimes excluded from discussions of neoplasms because they have been known to regress after incomplete removal. The cause of these lesions is unknown, but approximately 28% of the time, they arise secondary to other lesions such as giant cell tumor, chondroblastoma, and osteosarcoma.42 The region around the knee, including distal femur and proximal tibia, are the most common locations for this lesion. ABCs involving the spine are not common, representing approximately 13% of benign spine tumors. They are most commonly found in the lumbar region. Presenting patients are most often younger than 20 years of age. Both the anterior and posterior elements may be involved (Fig. 41.7A) with the posterior elements being involved most of the time. These lesions can also involve adjacent vertebrae, sometimes spanning more than three vertebrae. Radiographs usually demonstrate an expansive osteolytic cavity (Fig. 41.7B) with a ‘soap-bubble’ appearance within the lesion. The cortex is often expanded and thinned. MRI is especially helpful in the evaluation of patients with suspected ABC of the spine. MRI characteristically shows a multiloculated expansile lesion with multiple characteristic (but not pathognomonic) fluid-fluid levels. The lesions are often extremely vascular. Selective arterial embolization has become a useful adjunct to surgical excision of spinal lesions to reduce intraoperative bleeding potential. Curettage usually eradicates the lesion, and recurrences, which do not tend to invade vital structures, may be successfully treated by repeated curettage or excision.33
Giant cell tumor
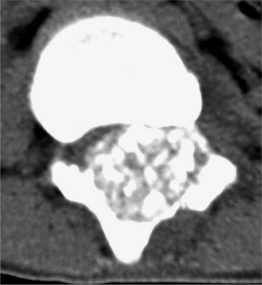
Giant cell tumors (GCTs) of the spine are most commonly found in the sacrum. These are usually benign, but locally aggressive, and may not present until the third or fourth decade of life. Cord compression has been noted in about 20% of cases. Even though the tumor is classified as benign, it metastasizes in approximately 3% of the cases.2 There is a 2% risk of lung metastasis associated with GCT and is unrelated to the extent of radiographic aggressiveness. GCTs usually involve the vertebral body, but may extend to the surrounding bone and soft tissues as the tumor enlarges (Fig. 41.8A, B). Plain films show an area of focal destruction of the vertebral body and lesions are lucent with marginal sclerosis.
Giant cell tumors can be highly vascular. In the authors’ experience, embolization of sacral lesions has been a successful primary approach for management.43 It is reasonably noninvasive, has documented excellent functional outcomes and is not associated with neurologic complications. Marginal and, when appropriate, wide excision with adjuvant therapy may be indicated in some cases (especially in those resistant to embolization). Because of the tendency of these lesions to recur, CT and MRI are important in planning an operation that will provide wide margins. In cases not amenable to surgical excision, low-dose radiation therapy (less than 30 Gy) with contemporary techniques carries a small rate of secondary malignancy. Bisphosphonates have been shown to induce apoptosis in giant cell tumors.44,45 Treatment of spine GCTs with bisphosphonates and radiotherapy has been reported to produce favorable results in three patients.46
The distinction between a benign vertebral body tumor such as a giant cell tumor and an aneurysmal bone cyst sometimes can be difficult. The distinction, nevertheless, may be important because of a giant cell tumor’s potentially more aggressive nature that demands more complete excision than an aneurysmal bone cyst. An aneurysmal bone cyst, while commonly involving the vertebral body, virtually always involves a pedicle or other posterior elements. Therefore, a lesion that only involves the vertebral body is more likely to be a giant cell tumor than an aneurysmal bone cyst, although metastatic lesions and myeloma are also in the differential of a lytic, expansile lesion in the vertebral body.
MALIGNANT TUMORS OF THE SPINE
Solitary myeloma (solitary plasmacytoma)
Solitary myeloma is one of many B-cell lymphoproliferative diseases, which include multiple myeloma. Multiple myeloma of the spine, which is discussed in the next chapter, is typically due to metastatic spread to the spine. True solitary myeloma is relatively rare, constituting only 3% of all plasma cell neoplasms.47 Of all solitary plasmacytomas, approximately 55% involve the vertebral column.48 Solitary plasmacytoma of the spine usually affects the vertebral body. As mentioned previously, monoclonal proteins are more often absent or undetectable in solitary myeloma compared to multiply myeloma.18
Solitary myeloma is an isolated lesion and treatment may result in cure. Treatment of choice in solitary plasmacytoma and multiple myeloma is radiation. The prognosis for patients with solitary plasmacytoma is much better than that for multiple myeloma. Disease-free survival for patients with solitary plasmacytoma of the spine is 60% at 5 years with a median survival of 92 months.49 Surgical intervention is typically reserved for decompression of neural structures and stabilization in cases of severe destruction. Postoperative adjuvant radiation is recommended. Surgery is the treatment of choice for recurrent lesions or those that do not respond to radiotherapy in which en bloc resection and prophylactic reconstruction with repeat radiotherapy may provide extended disease-free survival. Dissemination of myeloma can occur after years of disease-free survival, and patients should undergo regularly scheduled follow-up. MRI provides the earliest indication of local recurrence, and serum protein electrophoresis (SPEP) has proven to be the best indicator of dissemination. The treatment for disseminated myeloma is systemic chemotherapy.
Osteosarcoma
Approximately 3% of all primary osteosarcomas arise in the spine,2 and this percentage increases with age.50 Spinal osteosarcoma arises in the vertebral body in approximately 95% of cases. Plain films will often reveal cortical disruption, soft tissue calcification, periosteal reactions, and in advanced cases, vertebral collapse. Paraspinal soft tissue involvement can involve vascular, neural, and other contiguous structures.
Median survival of these patients typically ranges from 6 to 18 months.51–53 However, extensive anterior and posterior resection and adjuvant chemotherapy have improved local tumor control, neurologic function, and survival.53 When local control can be obtained with an appropriate surgical margin, survival is comparable to a similar lesion in an extremity. However, complete resection can be difficult and is not possible in the majority of spinal osteosarcomas.13,48
Secondary osteosarcomas
Within osteosarcomas, there is a subgroup of secondary osteosarcomas which arise secondarily in Pagetoid or irradiated bone. This subgroup accounts for approximately 4% of all intramedullary osteosarcomas.50 However, of all osteosarcomas within the spine, secondary osteosarcomas represent almost 30%.51,52 The majority of patients with secondary osteosarcoma are over 60 years of age.50 Lesions that develop in pagetoid bone progress rapidly and produce extensive destruction of bone. Prognosis in these patients is poor, with less than 5% achieving long-term survival. The majority of patients with osteosarcoma secondary to radiation have received 50 Gy or more. Most patients were exposed to radiation for nonosseous disease including Hodgkin’s lymphoma, breast cancer, and carcinoma of the cervix. The 5-year disease-free survival rate is approximately 17%, slightly better results than in those with pagetoid osteosarcoma. Both forms of secondary osteosarcoma can present long after exposure. One patient presented with osteosarcoma of the spine 31 years after being irradiated for Hodgkin’s lymphoma.54
Ewing’s sarcoma
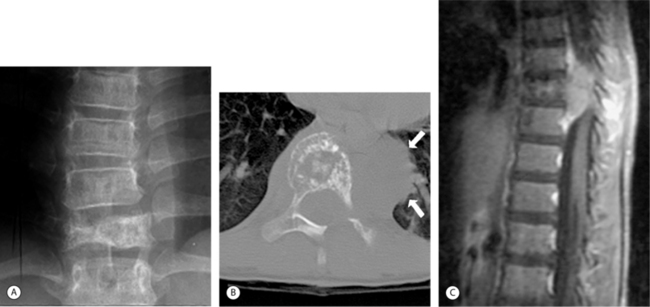
Ewing’s sarcoma presents as a primary, and rarely a secondary, tumor of the spine. Approximately 3% of all Ewing’s tumors arise in the mobile spine, and 6.4% are located in the sacrum.2 Most patients (88%) with primary Ewing’s of the spine are 20 years or younger. Sometimes difficult to detect on plain radiographs, these tumors have a permeative, destructive pattern. The first radiographic findings are usually vertebral collapse and vertebra plana (Fig. 41.9A), which may make it difficult to differentiate from eosinophilic granuloma. Frequently, due to the aggressive nature of this tumor, extraosseous involvement is present at initial diagnosis (Fig. 41.9B, C). Often neurologic symptoms due to intraspinal extension occur prior to radiographic detection, making MRI the imaging procedure of choice for evaluation of cord and nerve root involvement.
Chordoma
Chordoma is a low-grade, relatively common malignancy of the spine typically found in patients in their fourth to sixth decade. It accounts for approximately 6% of all primary malignant bone tumors.2 An even higher percentage (8.4%) was found in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) study. Chordomas, always localized to the midline, arise from primitive notochord remnants and are primarily found in the sacrococcygeal area (47%) and spheno-occipital region (38%) in the base of the skull.2 The rest of the time they are found in the cervical, thoracic, and lumbar areas. Approximately 5% of patients develop metastases; sites include the liver, lungs, lymph nodes, peritoneum, skin, and heart. Although metastasis is relatively rare, nearly 70% of patients die from these lesions, illustrating the severity of local tumor extension.55 Chordomas grow slowly and progressively, reaching considerable size before metastasizing, sometimes causing nerve root compression, constipation, and urinary frequency. Occasionally, an advanced, very large chordoma can be palpated posteriorly on rectal examination. Initial symptoms are typically mild in nature and progress as the tumor slowly enlarges. Chordomas on T1-weighted MRI are typically hypointense to isointense compared to the surrounding musculature (Fig. 41.10A). In T2-weighted images, they invariably show high signal and an inhomogeneous texture (Fig. 41.10B). Surgical resection with wide margins is the only curative procedure. Intralesional resection is associated with a high rate of recurrence and mortality.56 Appropriate staging studies should be done prior to biopsy. Biopsy should be performed through a direct posterior approach, and the biopsy incision should be excised en bloc with the tumor at the time of resection.11
Chondrosarcoma
Chondrosarcoma is another low-grade, slow-growing malignancy. Chondrosarcomas are primarily a tumor of adulthood and old age. Approximately 8% of chondrosarcomas are primary tumors of the spine.2 Advanced chondrosarcoma usually has a characteristic radiologic appearance. Lesions often appear with a large area of destruction containing flocculent calcifications and have an associated soft tissue mass. If there is no soft tissue mass, the vertebral lesion may be primarily lytic with sclerotic margins and without calcification. CT is useful in evaluating the extent of the lesion and amount of spinal canal compromise. On MRI, the signal intensity of chondrosarcoma is heterogeneous because of the mixture of soft tissue cartilage, calcification, and hemorrhage. Associated soft tissue masses are defined well by MRI (Fig. 41.11A). High signal intensity on T2-weighted and STIR images (Fig. 41.11B) is typical. Areas of mineralization show low intensity in both sequences. These tumors are relatively resistant to radiation and chemotherapy. Like chordomas, chondrosarcomas tend to recur locally and require complete surgical resection with a wide margin of uninvolved tissue to achieve cure. They also have a poor prognosis. In cases where a clear margin is not obtained, adjuvant radiotherapy may improve local control.
Solitary lymphoma
Lymphoma may present as an isolated tumor within bone, referred to in the past as reticulum cell sarcoma, or as a systemic disease. Primary lymphomas of the bone accounted for 3% of all malignant bone tumors in one review.44,45 Whether considered a primary or secondary lesion, lymphomas do account for a significant number of spinal tumors. In cases of solitary lymphoma, patients typically have none of the general constitutional complaints so commonly associated with systemic lymphoma, even when lesions are extensive. However, those lesions that involve the spine frequently have neurologic symptoms. Surgical intervention is typically reserved for decompression of neural structures and stabilization in cases of severe destruction and is used as an adjuvant to radiation and chemotherapy. Systemic lymphoma involving the spine is discussed in the next chapter as a secondary tumor.
MANAGEMENT
General medical treatment
Bisphosphonates
Bisphosphonates are drugs that inhibit osteoclastic activity, suppressing bone resorption. The use of bisphosphonates in metastatic disease has been studied much more than primary tumors of bone. Studies have shown that bisphosphonates induce apoptosis in giant cell tumors,46 and giant cell tumors of the spine have been treated with radiotherapy and bisphosphonates alone with favorable results.57 There are orthopedic surgeons who are currently using bisphosphonate-soaked cancellous allograft when treating aneurysmal bone cysts, although this has not been published. Bisphosphonates have also been studied as a potential treatment for osteosarcoma. Bisphosphonates have been shown to induce apoptosis in osteosarcoma cells58 and inhibit canine osteosarcoma tumor growth.59 Further investigation into the efficacy of bisphosphonate treatment in these primary bone tumors is needed.
Corticosteroids
Corticosteroids, by reducing the vasogenic edema of acute spinal cord compression, stabilize or improve neurologic status and relieve pain in some patients. Due to the low mineralocorticoid activity, low cost, and use in clinical trials, dexamethasone is commonly used. The optimal dose used to treat acute spinal cord compression is controversial. One randomized, control trial showed that an initial 100 mg i.v. bolus of dexamethasone and subsequent doses of 96 mg/day (split into four daily doses) in patients with epidural spinal cord compression provided a significantly higher percentage of patients that were still ambulatory at long-term follow-up.60 One retrospective study comparing 16 and 96 mg/day doses demonstrated a significantly higher incidence of both serious and nonserious side effects with the higher dose.59 This study also showed no difference in efficacy between the two doses; therefore, the recommended dose for symptomatic patients is a 10 mg i.v. bolus followed by 16 mg/day, administered four times daily. The larger dose of 96 mg/day should only be administered to patients with rapidly progressing neurologic deficits.61
Steroids are recommended for neurologic compromise of acute onset. However, caution must be taken in a patient with an undiagnosed spinal mass with regards to corticosteroids treatment. One must not deliver steroids prior to biopsy because of the oncolytic effect for certain tumors, such as lymphoma.60 Other complications of corticosteroid treatment include metabolic abnormalities, GI bleeding/perforation, steroid withdrawal, osteoporosis, osteonecrosis, and psychosis. Postoperative infection and wound breakdown are also increased with corticosteroid use.
Tumor-specific medical treatment
Chemotherapy
The use of chemotherapy in the treatment of spine tumors depends on the chemosensitivity of the tumor in question. Chemotherapeutics have been useful in Ewing’s and osteosarcoma. Also, patients with myelomas and lymphomas that become disseminated can benefit from chemotherapy. Possible complications of chemotherapy vary depending on the chemotherapeutic agent used, but typically include immunosuppression, delayed wound healing, and perioperative wound infection. Interestingly, chemotherapy for bone sarcomas does not negatively affect fertility rates or childbirth; the authors showed that 15 of the 36 patients attempted conceptions, and all were successful.62
Minimally invasive procedures
Radiofrequency ablation
Radiofrequency (RF) ablation has been used more commonly in metastatic spine lesions.63–65 However, one study of 263 patients that underwent RF ablation for osteoid osteoma included 3 patients with osteoid osteoma of the spine.39 These authors suggest that the electrode should be at least 1 cm away from major nerves; therefore, most spinal osteoid osteomas should not be treated with this method.
Embolization
Selective arterial embolization is not a curative treatment for spinal tumors, but it can reduce operative blood loss and reduce the size of tumors. Preoperative embolization of hypervascular vertebral tumors can make a previously unresectable tumor resectable. Embolization has become a useful preoperative adjunct to surgical excision of spinal ABCs, and it may be a safer and more effective option than radiation for the treatment of hemangiomas. As mentioned previously, the authors have found embolization of sacral lesions to be a successful approach for primary management.43 It is reasonably noninvasive, has documented excellent functional outcomes, and has not been associated with neurologic complications.
Vertebroplasty/Kyphoplasty
Percutaneous vertebroplasty using polymethyl methacrylate (PMMA) has been used in the treatment of benign compression fractures since the late 1980s. Some authors have reported the use of vertebroplasty in treating local and axial pain due to vertebral metastases,66–68 although treatment of primary spine tumors has not been well described. Over the past decade vertebroplasty has been used in Europe to treat hemangiomas, reinforcing the vertebral body.13
Surgical treatment
Surgery plays an important role in the management of primary tumors of the spine. For benign disease, surgery can be curative. For malignant disease, the outcome of adjuvant therapy may depend on the ability of surgery to achieve adequate removal of tumor, preferentially with negative margins. In one report, patients with malignant lesions that were completely excised had a 75% 5-year survival, while those with incompletely resected lesions had a 19% 5-year survival.6 The evolution of instrumentation and surgical techniques has provided the surgeon with a wide armamentarium with which to adequately excise or debulk tumors, decompress the neural structures, and stabilize the spine.
Indications
The indications for surgery in patients with primary tumors include impingement of neural structures causing myelopathy or intractable pain, structural instability, presence of tumor type that is radioresistant, tumor recurrence in a patient who cannot receive further medical or radiotherapy, fractures or impending fractures, and the need for a diagnostic biopsy in a patient with an unknown primary (approximately 9% of patients).69 The objective of surgery should be to achieve a cure, extend life expectancy, or provide palliation but the decision should be carefully discussed with the oncology management team and the patient and family. Once the objective is established, desirable goals are early patient mobilization, relief of pain, spinal alignment, decompression to relieve neurologic deficits to permit return of useful ambulation and bowel function, and stabilization of the involved motion segments. Surgery is contraindicated in patients with quadriplegia and no reasonable chance to restore neurologic function, diffuse spinal involvement of the operative segment, and a life expectancy of less than 4 months.70–72 The decision to operate within this range should weigh the patient’s remaining quality of life against the pain, life disruption, hospitalization, and recovery necessary after an operation.
Surgical approaches
Sacrum
Primary tumors of the sacrum such as Ewing’s sarcoma, giant cell tumor, chondrosarcoma, aneurysmal bone cysts, osteoblastoma, synovial sarcoma, and chordomas are often large at presentation. Benign tumors can be cured with intralesional curettage and adjuvant therapy in contrast to radio- and chemoresistant malignancies that require en bloc wide resection.73 It is often difficult to adequately resect malignant tumors without neurologic deficits and diminished bowel and bladder function. In order to achieve en bloc resection, a circumferential approach is needed and begins with an anterior transperitoneal approach. The L5–S1 disc is removed and the internal iliac vessels are ligated. A rectus abdominus pedicle flap may be placed in the pelvis and used to fill the defect that remains after a staged posterior sacrectomy and en bloc removal of the tumor. The thecal sac is ligated, which often requires sacrificing many of the sacral roots, and the L5 nerve roots are preserved during the posterior dissection. Bowel function can be assisted with laxatives and diet control, and self-catherization is needed for bladder control.74 Stabilization is achieved between the lumbar vertebras and the ilium.75
Operative stabilization
Orthopedic spine surgeons are increasingly trained to apply recent, advanced techniques of instrumentation using pedicle and lateral mass screws, rods, plates, and vertebral body replacement devices such as titanium mesh cages. With these newer stabilization techniques and devices, orthopedic surgeons are more aggressively removing tumors and restoring adequate stability. Stabilization after corpectomy or vertebrectomies can be achieved with either humeral or fibula strut grafts, but metallic cages are more durable in cases of recurrence and are preferred if postoperative radiation will be used since there is a 50% nonunion rate with bone graft in the face of radiation.76 Polymethyl methacrylate (PMMA) can be used to fill vertebral body defects without risk of cord damage from the heat generated from polymerization.77 Postoperative radiation should be postponed for 1 month to maximize wound healing.
ALGORITHMIC APPROACH TO TREATMENT
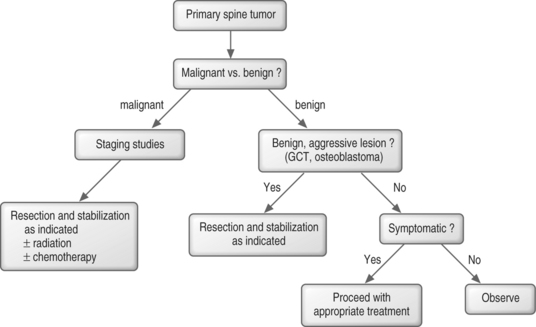
In benign spine tumors, the question becomes one of aggressiveness. Typical benign, aggressive lesions are giant cell tumors and osteoblastomas. These are treated with marginal resection, and stabilization when need. If the benign tumor is not an aggressive type, it can often be observed or treated, depending on how symptomatic it is (Fig. 41.12).
1 Campanacci M, Boriani S, Savini R. Staging, biopsy, surgical planning of primary spinal tumors. Chir Organi Mov. 1990;751(Suppl):99-103.
2 Unni KK. Dahlin’s Bone tumors. New York: Lippincott, 1996.
3 Sachs BL, Carter JR, Thompson GH. Primary osseous neoplasms of the thoracic and lumbar spine. Orthop Trans. 1984;83:422-423.
4 Sim FH, Stauffer RN, Laws ER. Primary bone tumors simulating lumbar disc syndrome. Spine. 1977;22:65-74.
5 Weinstein J, McLain RF. Primary tumors of the spine. Spine. 1987;129:843-851.
6 Weinstein JN. Surgical approach to spine tumors. Orthopedics. 1989;126:897-905.
7 Bohlman HH, Sachs BL, Carter JR, et al. Primary neoplasms of the cervical spine. Diagnosis and treatment of twenty-three patients. J Bone Joint Surg [Am]. 1986;684:483-494.
8 Parks PF. Spine tumors – patient evaluation. Semin Spine Surg. 1990:2154.
9 Nazarian S. Place de la chirurgie dans le traitement des metastases du rachis. Rev Chi Orthop. 1997;83(Suppl. III):109-174.
10 Nazzaro JM. Metastatic spinal lesions. In: Benzel E, editor. Spine surgery: techniques, complication avoidance, and management. New York: Churchill Livingstone; 1999:679-695.
11 Weinstein J, McLain RF. Tumors of the spine. In: Rothman R, Simeone FA, editors. The spine. Philadelphia: WB Saunders; 1992:1279-1318.
12 Bell GR. Surgical treatment of spinal tumors. Clin Orthop. 1997;335:54-63.
13 Cohen D. Tumors of the spine. In: Koval K, editor. Orthopaedic knowledge update. Rosemont, IL: AAOS; 2002:673-687.
14 Schiff D. Clinical features and diagnosis of epidural spinal cord compression, including cauda equina syndrome. UpToDate Online, 2003.
15 Dumontet C, et al. Profiles and prognostic values of LDH isoenzymes in patients with non-Hodgkin’s lymphoma. Leukemia. 1999;135:811-817.
16 Sarobe P, Huarte E, Lasarte JJ, et al. Carcinoembryonic antigen as a target to induce anti-tumor immune responses. Curr Cancer Drug Targets. 2004;45:443-454.
17 Durie BG, Young LA, Salmon SE. Human myeloma in vitro colony growth: interrelationships between drug sensitivity, cell kinetics, and patient survival duration. Blood. 1983;615:929-934.
18 Bataille R, Sany J. Solitary myeloma: clinical and prognostic features of a review of 114 cases. Cancer. 1981;483:845-851.
19 Israeli A, Zwas ST, Horoszowski H, et al. Use of radionuclide method in preoperative and intraoperative diagnosis of osteoid osteoma of the spine. Case report. Clin Orthop. 1983;175:194-196.
20 Carmody RF, Yang PJ, Seeley GW, et al. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;1731:225-229.
21 Hiraga T, Mundy GR, Yoneda T. Bone metastases – morphology. In: Rubins R, Mundy GR, editors. Cancer and the skeleton. London: Martin Danitz; 2000:65-74.
22 Bohdiewicz PJ, Wong CY, Kondas D, et al. High predictive value of F-18 FDG PET patterns of the spine for metastases or benign lesions with good agreement between readers. Clin Nucl Med. 2003;2812:966-970.
23 Nair N, Ali A, Green AA, et al. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging. 2000;32:79-83.
24 Babu NV, Titus VT, Chittaranjan S, et al. Computed tomographically guided biopsy of the spine. Spine. 1994;1921:2436-2442.
25 Minart D, Vallee JN, Cormier E, et al. Percutaneous coaxial transpedicular biopsy of vertebral body lesions during vertebroplasty. Neuroradiology. 2001;435:409-412.
26 Ozsarlak O, De Schepper AM, Wang X, et al. CT-guided percutaneous needle biopsy in spine lesions. JBR-BTR. 2003;865:294-296.
27 Hadjipavlou AG, Kontakis GM, Gaitanis JN, et al. Effectiveness and pitfalls of percutaneous transpedicle biopsy of the spine. Clin Orthop. 2003;411:54-60.
28 Mankin HJ, Mankin J, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg [Am]. 1996;785:656-663.
29 Schwartz HS, Spengler DM. Needle tract recurrences after closed biopsy for sarcoma: three cases and review of the literature. Ann Surg Oncol. 1997;43:228-236.
30 Egeler RM, Thompson RCJr, Voute PA, et al. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;126:811-814.
31 Scaglietti O, Marchetti PG, Bartolozzi P. Final results obtained in the treatment of bone cysts with methylprednisolone acetate (depo-medrol) and a discussion of results achieved in other bone lesions. Clin Orthop. 1982;165:33-42.
32 Green NE, Robertson WWJr, Kilroy AW. Eosinophilic granuloma of the spine with associated neural deficit. Report of three cases. J Bone Joint Surg [Am]. 1980;627:1198-1202.
33 Chapman M. Chapman’s orthopaedic surgery. Philadelphia: Lippincott Williams & Wilkins, 2000.
34 Garrison RC, Unni KK, McLeod RA, et al. Chondrosarcoma arising in osteochondroma. Cancer. 1982;499:1890-1897.
35 Marsh BW, Bonfiglio M, Brady LP, et al. Benign osteoblastoma: range of manifestations. J Bone Joint Surg [Am]. 1975;571:1-9.
36 Pettine KA, Klassen RA. Osteoid-osteoma and osteoblastoma of the spine. J Bone Joint Surg [Am]. 1986;683:354-361.
37 Roger B, Bellin MF, Wioland M, et al. Osteoid osteoma: CT-guided percutaneous excision confirmed with immediate follow-up scintigraphy in 16 outpatients. Radiology. 1996;2011:239-242.
38 Towbin R, Kaye R, Meza MP, et al. Osteoid osteoma: percutaneous excision using a CT-guided coaxial technique. Am J Roentgenol. 1995;1644:945-949.
39 Rosenthal DI, Hornicek FJ, Torriani M, et al. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;2291:171-175.
40 Griffin JB. Benign osteoblastoma of the thoracic spine. Case report with fifteen-year follow-up. J Bone Joint Surg [Am]. 1978;606:833-835.
41 Ross JS, Masaryk TJ, Modic T, et al. Vertebral hemangiomas: MR imaging. Radiology. 1987;1651:165-169.
42 Martinez V, Sissons HA. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;6111:2291-2304.
43 Lackman RD, Khoury LD, Esmail A, et al. The treatment of sacral giant-cell tumours by serial arterial embolisation. J Bone Joint Surg [Br]. 2002;846:873-877.
44 Chang SS, Suratwala SJ, Jung KM, et al. Bisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosis. Clin Orthop. 2004;426:103-109.
45 Cheng YY, Huang L, Kumta SM, et al. Cytochemical and ultrastructural changes in the osteoclast-like giant cells of giant cell tumor of bone following bisphosphonate administration. Ultrastruct Pathol. 2003;276:385-391.
46 Fujimoto N, Nakagawa K, Seichi A, et al. A new bisphosphonate treatment option for giant cell tumors. Oncol Rep. 2001;83:643-647.
47 Corwin J. Solitary plasmacytoma of bone vs. extramedullary plasmacytoma and their relationship to multiple myeloma. Cancer. 1979;43:1007-1013.
48 Bielack SS, Wulff B, Delling G, et al. Osteosarcoma of the trunk treated by multimodal therapy: experience of the Cooperative Osteosarcoma Study Group (COSS). Med Pediatr Oncol. 1995;241:6-12.
49 McLain RF, Weinstein JN. Solitary plasmacytomas of the spine: a review of 84 cases. J Spinal Disord. 1989;22:69-74.
50 Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;577:1442-1449.
51 Barwick KW, Huvos AG, Smith J. Primary osteogenic sarcoma of the vertebral column: a clinicopathologic correlation of ten patients. Cancer. 1980;463:595-604.
52 Shives TC, Dahlin DC, Sim FH, et al. Osteosarcoma of the spine. J Bone Joint Surg [Am]. 1986;685:660-668.
53 Sundaresan N, Rosen G, Huvos AG, et al. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988;236:714-719.
54 Sundaresan N, Huvos AG, Rosen G, et al. Postradiation osteosarcoma of the spine following treatment of Hodgkin’s disease. Spine. 1986;111:90-92.
55 Bjornsson J, Wold LE, Ebersold MJ, et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;713:735-740.
56 Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996;2113:1569-1577.
57 Mackie PS, Fisher JL, Zhou H, et al. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;847:951-958.
58 Farese JP, Ashton J, Milner R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim. 2004;403–404:113-117.
59 Heimdal K, Hirschberg H, Slettebo H, et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;122:141-144.
60 Sorensen S, Helweg-Larsen S, Mouridsen H, et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A1:22-27.
61 Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(Suppl 2):S202-S213.
62 Hosalkar HS, Weiss A. Chemotherapy for bone sarcoma does not affect fertility rates or childbirth. Clin Orthop. 2004;429:352.
63 Callstrom MR, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;2241:87-97.
64 Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;157:707-712.
65 Poggi G, et al. Percutaneous ultrasound-guided radiofrequency thermal ablation of malignant osteolyses. Anticancer Res. 2003;236D:4977-4983.
66 Barr JD, Barr MS, Lemley TJ, et al. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;258:923-928.
67 Deramond H, Depriester C, Toussaint P, et al. Percutaneous vertebroplasty. Semin Musculoskelet Radiol. 1997;12:285-296.
68 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg Spine. 2003;981:21-30.
69 Ratanatharathorn V, Powers WE. Epidural spinal cord compression from metastatic tumor: diagnosis and guidelines for management. Cancer Treat Rev. 1991;181:55-71.
70 Bilsky MH, Lis E, Raizer J, et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459-469.
71 Boriani S, De Lure F. Bone tumors of the spine and epidural cord compression: treatment options. Semin Spine Surg. 1995;7:317-322.
72 Vieweg U, Meyer B, Schramm J. Tumour surgery of the upper cervical spine – a retrospective study of 13 cases. Acta Neurochir (Wien). 2001;1433:217-225.
73 Sar C, Eralp L. Surgical treatment of primary tumors of the sacrum. Arch Orthop Trauma Surg. 2002;1223:148-155.
74 Gokaslan ZL, Romsdahl MM, Kroll SS, et al. Total sacrectomy and Galveston L-rod reconstruction for malignant neoplasms. Technical note. J Neurosurg. 1997;875:781-787.
75 Doita M, Harada T, Iguchi T, et al. Total sacrectomy and reconstruction for sacral tumors. Spine. 2003;2815:E296-E301.
76 Rao S, Badani K, Schildhauer T, et al. Metastatic malignancy of the cervical spine. A nonoperative history. Spine. 1992;1710(Suppl):S407-S412.
77 Wang GJ, Reger SI, McLaughlin RE, et al. The safety of cement fixation in the cervical spine. Studies of a rabbit model. Clin Orthop. 1979;139:276-282.