Primary malignant tumours of the liver
Hepatocellular carcinoma
HCC accounts for 90% of all primary liver malignancy and its incidence continues to increase. It is the sixth most common neoplasm, accounting for more than 5% of all cancers, and is the third most common cause of cancer-related death. The International Agency for Research on Cancer has estimated in 2008 through its GLOBOCAN series that primary liver cancer caused more than 690 000 deaths worldwide, similar to colon or rectal cancer.1
Control of HCC nodules may be achieved successfully by surgical resection and by percutaneous treatment. The indications for these therapies depend on the morphological features of the tumour and the functional status of the non-tumorous liver. Unfortunately, these treatments are associated with a high incidence of tumour recurrence due to the persistence of the underlying cirrhosis, which represents a preneoplastic condition. Liver transplantation may seem a logical alternative treatment but has its own limitations, including tumour recurrence, the limited availability of grafts, and cost. The most exciting areas of progress are the control of hepatitis B virus (HBV) or hepatitis C virus (HCV), the prevention of carcinogenesis in patients with chronic liver disease, early radiological screening and the development of medical therapies. In the setting of liver surgery, better liver function assessment and understanding of the segmental liver anatomy with more accurate imaging evaluation are the most important factors that have led to a decrease in postoperative mortality. Active follow-up and treatment of recurrence have also contributed to increased 5-year survival rates as high as 70%.2
Incidence of HCC
The world age-adjusted incidence of HCC in men is 14.9 per 100 000, but has geographical variation related to the prevalence of HBV and HCV infections, which are the two main risk factors worldwide and account for more than three-quarters of all cases (Table 5.1). The incidence may be as high as 99 cases per 100 000 in Mongolian men; other high-rate areas include Senegal, Gambia, South Korea, Hong Kong and Japan. By contrast, North and South America, Northern Europe and Oceania are areas with low incidences (less than 5 cases per 100 000); in these areas, HCV is the main risk factor, together with alcohol abuse, non-alcoholic fatty liver disease and obesity. Southern European countries have intermediate rates.
Risk factors for HCC
HBV infection
The risk of HBV-associated HCC increases with the severity of the underlying hepatitis, age at infection and duration of infection, as well as level of viral replication. An Asian patient with HBV-related cirrhosis has a 17% cumulative risk of developing HCC over a 5-year period. In the West, this cumulative risk is 10%. This may be explained by the earlier acquisition of HBV in Asia through vertical transmission (rather than horizontal transmission in the West through sexual or parenteral routes), longer duration of disease, or additional exposure to environmental factors. Ongoing HBV replication or hepatitis B e-antigen (HBeAg) infection accelerates the progression to cirrhosis and also to HCC. A study conducted in Taiwanese men reported that the risk of HCC increased 10-fold when HBsAg was present and 60-fold when HBeAg was present. Similarly, HBV-DNA levels greater than 104 or 106 copies/mL are associated with a 2.3 and 6.1 hazard risk, respectively, compared to patients with lower levels of replication.3 Additional cofactors that increase the risk of HCC are male gender (three- to sixfold), age > 40 years, concurrent HCV infection (twofold), HDV co-infection (threefold), heavy alcohol consumption (two- to threefold) and, in endemic regions, aflatoxin ingestion.
Human immunodeficiency virus (HIV) infection
The incidence of HCC is expected to rise in HIV-positive persons predominantly because of the higher prevalence of associated well-known risk factors: not only co-infection with HCV and HBV, but also alcohol abuse, non-alcoholic steatohepatitis (NASH) and diabetes. HIV-positive patients who are co-infected with HBV or HCV may have more rapidly progressive liver disease, and when they develop cirrhosis they also have an increased risk of HCC. The Mortavic study indicated that HCC caused 25% of all liver-related deaths among HIV patients.5
Cirrhosis and HCC occur 15–20 years earlier in HIV–HCV co-infected patients than in patients infected by HCV alone. The course of the disease is also considered more aggressive.6 Screening for HCC should, however, be the same as in HIV-negative patients.
Non-alcoholic fatty liver disease (NAFLD)
An association between NAFLD and HCC was first identified in 2002 by several studies focusing on HCC patients with chronic liver disease in the absence of HBV/HCV infection or alcohol abuse. In this population, there was a much higher prevalence of obesity, diabetes, hypertriglyceridaemia and pathological features of NAFLD. At the same time, evidence was accumulating linking common features of the metabolic syndrome/NASH with HCC. In particular, obesity was noted to increase the mortality from liver cancer far more than for any other cancer.7 Similarly, diabetes was found to increase the risk of HCC with and without acute or chronic liver disease.8
Precise figures on the incidence of HCC in patients with NAFLD are still lacking. It increases with male gender, increasing age, sinusoidal iron deposition and severity of underlying liver disease. In surgical series, overt cirrhosis is present in only one-third of patients while the others have less severe liver damage.9 In addition, there is also evidence that NAFLD may act synergistically with other risk factors, such as chronic HCV or alcoholic consumption, in the development of HCC.
Hereditary haemochromatosis
Hereditary haemochromatosis (HH) is an autosomal recessive disorder associated with homozygosity for the C282Y mutation in the haemochromatosis gene and characterised by excessive gastrointestinal absorption of iron. HH is a long-known risk factor for HCC, and the risk increases in patients with cirrhosis. Other risk factors include male gender and diabetes. Several additional risk factors such as HBV infection (4.9-fold), age greater than 55 years (13.3-fold) and alcohol abuse (2.3-fold) may act synergistically with iron overload to increase the risk of HCC in patients with cirrhosis caused by hereditary haemochromatosis. In a recent meta-analysis of nine studies including 1102 HCC cases, mainly from European populations, it has been reported that C282Y mutation was associated with increased risk of HCC (fourfold) in patients with alcoholic liver cirrhosis, but not in those with viral liver cirrhosis.10 Interestingly, pathological conditions other than haemochromatosis that are associated with iron overload, such as homozygous β-thalassaemia or the so-called African overload syndrome, are also associated with an increased risk of HCC. Similarly, there is evidence of a link between iron deposits within the liver and HCC in patients with and without cirrhosis.
Cirrhosis of other aetiologies
Primary biliary cirrhosis (PBC) has been considered as a low-risk factor for HCC, not only because of its rare incidence but also because it predominantly affects women (with a sex ratio of 9:1). A recent meta-analysis of 12 studies has reported that PBC is significantly associated with an increased risk of HCC (18.8-fold) compared to the general population.11 However, there were several confounding factors in this meta-analysis, such as advanced histological stage of PBC, history of blood transfusion, and smoking or drinking habits that might be associated with an increased probability for HCC development in PBC patients, or may be directly associated with PBC development. In contrast, HCC development in patients with secondary biliary cirrhosis is exceptional if it even exists.
Autoimmune hepatitis has a low risk of HCC development. Potential reasons are the female predominance and the delayed development of cirrhosis through corticosteroid therapy. HCV infection needs to be ruled out as it may induce autoantibodies. Recent data reported that cirrhosis at presentation is an important prognostic risk factor for HCC. In a prospective multicentre cohort study evaluating 193 Japanese patients with autoimmune hepatitis, seven (3.6%) developed HCC during an 8-year period, all of whom had underlying cirrhosis.12
Adenoma, contraceptives and androgens
Like adenoma in other locations, hepatocellular adenomas (HCAs) have a risk of malignant transformation and hepatocyte dysplasia is the intermediate step between HCAs and HCC. A recent systematic review estimated the risk to be 4.2%.13 This risk and the treatment strategy to prevent it may, however, be refined.
HCAs are most classical in women of child-bearing age and are associated with the prolonged use of contraceptives and oestrogen treatments. Discontinuation of oral contraceptives does not completely avoid the risk of malignant transformation. Malignancy within HCAs measuring less than 4 cm in diameter is exceptional. There is also recent evidence that HCAs may develop in men, especially if there is a background of a metabolic syndrome. The risk of malignant transformation in men is 50% (10 times higher than in women) and malignancy can occur in HCAs as small as 1 cm.14 Therefore, whereas resection of HCAs larger than 4 cm is recommended in women, all HCAs irrespective of size should be resected (or ablated) in men.
The number of HCAs does not appear to increase the risk of malignant transformation and, in particular, patients with polyadenomatosis are not at increased risk.14–16
Malignant transformation of HCAs has also been linked to the genotype and phenotype of HCAs. It is more prevalent in telangiectatic or atypical HCAs than in steatotic HCAs. Most importantly, the presence of a β-catenin mutation (observed in approximately 10–15% of HCAs) confers a particularly high risk of malignancy.17
Pathology of HCC and nodular lesions in chronic liver disease
Microscopically, HCCs exhibit variable degrees of differentiation that are usually stratified into four different histological grades, known as Edmondson grades 1–4, which correspond to well-differentiated, moderately differentiated, poorly differentiated and undifferentiated types. The degree of differentiation typically decreases as the tumour increases in diameter. Very-well-differentiated HCCs can resemble normal hepatocytes and the trabecular structure may reproduce a near normal lobar architecture so that histological diagnosis by biopsy or following resection may be difficult. A number of immunomarkers have been described to selectively identify the malignant nature of these HCCs, not only in resected specimens but also in liver biopsies: glypican 3 (GPC3), heat shock protein 70 (HSP70) and glutamine synthetase (GS). Positive immunomarker staining for any two markers can detect early and well-differentiated HCC in 50–73% of cases, with 100% specificity when the analysis is performed on resected specimens.18
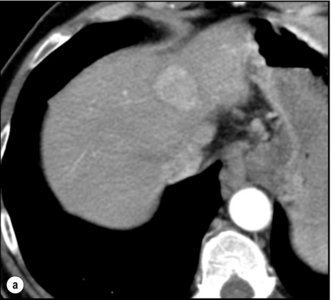
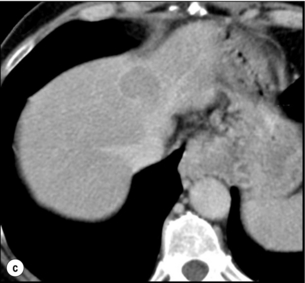
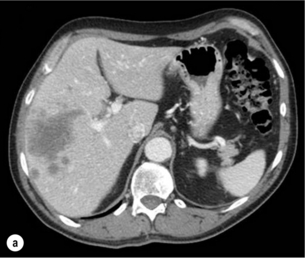
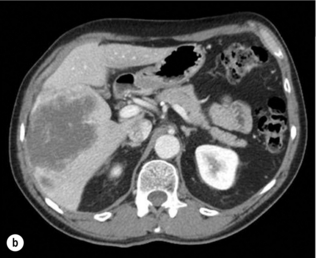
HCC has a great tendency to spread locally and to invade blood vessels. The rate of portal invasion is higher in the expansive type, in poorly differentiated HCCs and in large tumours. Characteristically, microscopic vascular invasion is seen in 20% of tumours measuring 2 cm in diameter, in 30–60% of cases with nodules measuring 2–5 cm and in up to 60–90% when nodules are more than 5 cm in size. The presence of portal invasion is the most important predictive factor associated with recurrence. The tumour thrombus has its own arterial supply, mainly from the site of the original venous invasion. Once HCC invades the portal vein, tumour thrombi grow rapidly in both directions, and in particular towards the main portal vein. As a consequence, tumour fragments spread throughout the liver as the thrombus crosses segmental branches. Once the tumour thrombus has extended into the main portal vein, there is a high risk of complete thrombosis and increased portal hypertension. This accounts for the frequent presentation with fatal rupture of oesophageal varices, or liver decompensation including ascites (Fig. 5.1), jaundice and encephalopathy. Invasion of hepatic veins is possible, although less frequent. The thrombus eventually extends into the suprahepatic vena cava or the right atrium and is associated with a high risk of lung metastases. Rarely, HCC may invade the biliary tract and give rise to jaundice or haemobilia. Mechanisms of HCC-induced biliary obstruction include:
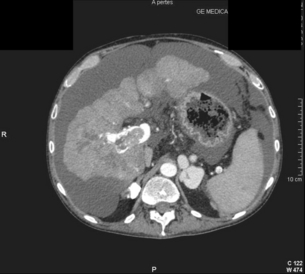
Figure 5.1 CT scan of a patient with a tumour thrombus originating from an HCC located in the right liver. The thrombus extends in to the main portal vein. Ascites is present.
Clinical presentation
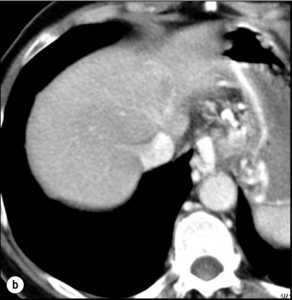
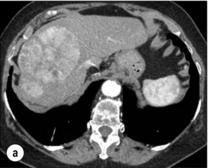
Spontaneous rupture occurs in 5–15% of patients and is observed particularly in patients with superficial or protruding tumours. The diagnosis should be suspected in patients with known HCC or cirrhosis presenting with acute epigastric pain, as well as in Asian or African men who develop an acute abdomen (Fig. 5.2). Minor rupture manifests as abdominal pain or haemorrhagic ascites, and hypovolaemic shock is only present in about half of the patients. Portal vein invasion may manifest as upper gastrointestinal bleeding or acute ascites, and invasion of hepatic veins or the inferior vena cava may result in pulmonary embolism or sudden death.
Liver function tests and tumour markers
Serum tumour markers
α-Fetoprotein: Serum α-fetoprotein (AFP) is the most widely recognised serum marker of HCC. It is secreted during foetal life but residual levels are very low in adults (0–20 ng/mL). It may increase in patients with an HCC, and serum levels greater than 400 ng/mL can be considered as diagnostic of HCC with 95% confidence. Levels may exceed 10 000 ng/mL in 5–10% of patients with HCC. Very high levels usually correlate with poor differentiation, tumour aggressiveness and vascular invasion. An AFP > 20 ng/mL has a sensitivity of 60% and therefore a surveillance programme using this cut-off value would miss 40% of tumours. If a value of > 200 ng/mL is used, 22% of tumours would be missed. Only 10% of small tumours are associated with raised AFP levels, whereas 30% of patients with chronic active hepatitis without an HCC have a moderately increased AFP. This usually correlates with the degree of histological activity and raised levels of transaminase, and it may therefore fluctuate. Tumours other than HCC can also be associated with increased AFP levels, but these are rare (non-seminal germinal tumours, hepatoid gastric tumours, neuroendocrine tumours).
Others serum tumour markers: Alternative serum markers for HCC, such as des-γ-carboxy prothrombin (DCP) or prothrombin induced by vitamin K absence (PIVKA-II; > 40 mAU/mL) and AFP-L3 (> 15%) have not come into common practice except in Japan, where they are covered under the national health insurance. A prospective study of at-risk patients comparing the accuracy of AFP and DCP in the early detection of HCC showed that the combination of both markers increased the sensitivity from 61% and 74%, for each marker alone, to 91% for both markers combined.19
Radiological studies
Computed tomography
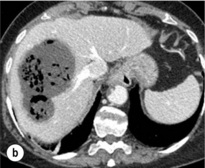
CT is more accurate than US in identifying HCCs and their lobar or segmental distribution, particularly with the development of helical and multislice spiral scanners. Spiral CT is undertaken without contrast and during arterial (25–50 s), portal (60–65 s) and equilibrium (130–180 s) phases after contrast administration. In addition, it is useful for identifying features of underlying cirrhosis, accurately measuring liver and tumour volumes, and assessing extrahepatic tumour spread. HCCs are usually hypodense and spontaneous hyperdensity is usually associated with iron overload or fatty infiltration, which is seen in 2–20% of patients. Specific features are early uptake of contrast and a mosaic shape pattern. During the portal phase, the density diminishes sharply and results in washout (tumour is hypodense compared to adjacent parenchyma) during the late phase (Fig. 5.3). HCCs may show variable vascularity depending on tumour grade and some are poorly vascularised. The capsule, when present, is best seen during the portal or late phase as an enhanced thickening at the periphery (delayed vascular enhancement is characteristic of fibrosis). Vascular invasion of segmental branches may also be identified. Intratumoral arterioportal fistula may develop and present as early enhancement of portal branches or as a triangular area distal to the tumour with contrast enhancement different from the adjacent parenchyma. Nonetheless, such fistulas are seen frequently in cirrhotic patients without HCC as infracentimetric hypervascular subcapsular lesions.
Contrast-enhanced ultrasound
Contrast-enhanced US (CEUS) is the most recent technique to assess vascularisation of tumours. A contrast agent (stabilised microbubbles) is administered intravenously via a bolus injection followed by saline flush. Enhancement patterns are typically described during the arterial (10–20 s postinjection), portal venous (30–80 s) and late phase (120–360 s). Whereas US microbubbles are confined to the vascular spaces, contrast agents for CT and MRI are rapidly cleared from the blood into the extracellular space. The sensitivity of CEUS to detect arterial enhancement is greater than that of CT or MRI because of the continuous monitoring of the images. Washout is slower for well-differentiated than for poorly differentiated tumours. However, it is subject to the same limitations as other US modes: if the baseline scan is unsatisfactory, the CEUS study will also be unsatisfactory. The advent of the second-generation US contrast agent Sonazoid, approved exclusively in Japan in 2007, has made Sonazoid-CEUS more effective for screening and staging than CEUS using other vascular agents such as SonoVue. Sonazoid contrast agent is taken up by Kupffer cells in the postvascular phase or Kupffer phase (starting 10 min postinjection) and provides extremely stable Kupffer images suitable for repeated scanning from 10 to about 120 min after injection.20
Other imaging
Angiography: Although the diagnostic usefulness of angiography has been considerably reduced, it is still widely used as part of arterial chemoembolisation. Arteriography shows early vascular uptake (blush) and, if used, lipiodol injection is retained selectively for a prolonged period by the tumour. On subsequent CT, the retained radiodense lipiodol reveals the tumour as a high-density area. Uptake within the liver is not specific for HCC, since all hypervascular liver tumours, including focal nodular hyperplasia, adenoma, angioma and metastases, will retain Lipiodol. False-negative results may be observed with an avascular, necrotic or fibrotic HCC.
Positron emission tomography: The contribution of fluorodeoxyglucose (FDG)-positron emission tomography (PET) in the diagnosis of HCC remains limited because of low sensitivity (60%). FDG-PET can detect poorly differentiated but not-well differentiated HCC due to similarities between the metabolism of FDG in normal hepatocytes and hepatocellular cells. Recently, [18 F]fluorocholine (FCH), a PET tracer of lipid metabolism, has been shown to be significantly more sensitive than [18 F]FDG at detecting HCC, particularly in well-differentiated tumours.21 Interestingly, in metastatic HCC, FDG-PET has a high sensitivity and is more suitable for the detection of bone metastases than CT or bone scintigraphy.22
Diagnosis of HCC
The first attempt to standardise the diagnostic criteria was in 2000 by the European Association for the Study of Liver Disease (EASLD). Since the last publication of the AASLD practice guidelines for the management of HCC in 2005, several studies have reported that AFP determination lacks adequate sensitivity and specificity for effective surveillance and diagnosis.19 The advocated strategy in the 2011 updated version of the diagnostic criteria23 was based on imaging techniques and/or biopsy as follows:
• For nodules less than 1 cm found on US, it was considered that other imaging techniques would be unlikely to reliably confirm the diagnosis. Since the accuracy of liver biopsy for such small lesions and the likelihood of HCC are low, it was felt reasonable to repeat an US at 3- to 6-month intervals until the lesion disappeared, enlarged or displayed characteristics of HCC. If there has been no growth over a period of up to 2 years, routine surveillance can be resumed.
• For nodules larger than 1 cm found on US screening of a cirrhotic liver, diagnosis of HCC can be established by one contrast-enhanced imaging technique (multidetector CT or dynamic contrast-enhanced MRI). The specific imaging pattern of HCC is defined by intense contrast uptake during the arterial phase followed by contrast washout during the venous or delayed phases. The value of these non-invasive criteria for HCC in cirrhosis has been confirmed prospectively.24–26 These typical imaging features have a specificity and predictive positive value of approximately 100% and sensitivity of 71%.
• If the findings are not characteristic or the vascular features are not typical, and in other clinical settings (e.g. absence of cirrhosis), a diagnostic biopsy was recommended, although it was acknowledged that a negative biopsy did not exclude the diagnosis.
Natural history of HCC and staging systems
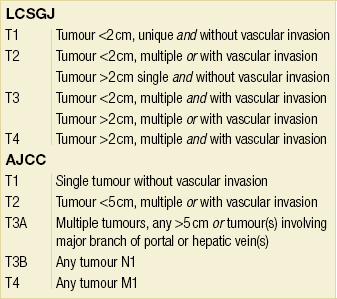
• Although staging was assessed initially by the TNM classification, the pathological staging of HCC has evolved in Eastern (Liver Cancer Study Group of Japan) and Western (American Joint Committee on Cancer, International Union Against Cancer) countries. These take into account the number of tumours, vascular invasion and tumour size (Table 5.2). A limitation is that they are based on pathological findings and can only be applied accurately (retrospectively) in operated patients.
Table 5.2
Comparison of the tumour (T) staging in the Liver Cancer Study Group of Japan (LCSGJ) and American Joint Committee on Cancer (AJCC) staging systems
• Cancer-related symptoms have a detrimental impact on outcome that is assessed by the WHO performance status or the Karnofsky index. The presence of pain is a poor indicator of outcome.
• Liver damage induced by underlying liver disease has traditionally been assessed by the Child–Pugh score. This was, however, designed to assess the functional reserve of cirrhotic patients undergoing portocaval shunt surgery and is not entirely appropriate for HCC patients in whom therapeutic options may include liver transplantation and liver resection.
Several groups have attempted to combine these features within integrated staging systems. There are currently six such systems, designated as the CLIP (from Italy), GRETCH (from France), BCLC (from Spain), CUPI (from China), JSS and JIS (from Japan) scores. It is beyond the scope of this chapter to detail all of them (Table 5.3). It should be appreciated that these scores have been computed retrospectively by multivariate analysis of a specific patient population and not all have been externally validated.
Table 5.3
Main variables retained in prognostic models
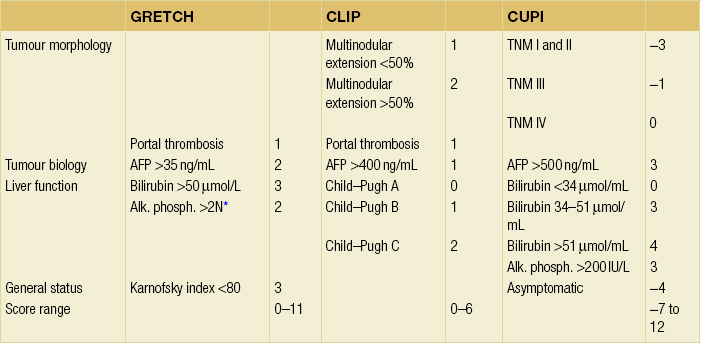
The numbers refer to the score given to each variable. A total score is obtained by adding each individual score. In the CLIP score, the median survivals according to the score in the initial30 and prospective validations32 were: score 0, 36–42 months; score 1, 22–32 months; score 2, 8–16 months; score 3, 4–7 months, score 4 or above, 1–3 months.
Screening for HCC
No clear evidence is available to determine the optimal interval for periodic screening. Tumour doubling times vary widely, with an average of 200 days. It has been estimated that the time taken for an undetectable lesion to grow to 2 cm is about 4–12 months, and that it takes 5 months for the most rapidly growing HCC to reach 3 cm. Because treatments are most effective for tumours < 3 cm, screening programmes are usually performed at 6-monthly intervals. The efficacy of screening to improve the prognosis of HCC has mainly been demonstrated in China on HBV carriers.27 These results need to be validated in other geographical areas. Until then, most rely on a 6-month interval (3–4 months in Japan) in high-risk patients. It has been reported that surveillance is cost-effective if the expected HCC risks exceeds 1.5% per year in patients with HCV and 0.2% per year in patients with HBV.28
Treatment options
HCC in normal livers
The treatment of choice in patients with no or minimal coexisting fibrosis is partial liver resection. The non-tumorous liver has a high regenerating capacity, allowing even major hepatectomies to be performed. Perioperative mortality and morbidity are less than 1% and 15%, respectively. Five-year survival is greater than 50%.29 These results may, however, vary according to the population studied. Patients with a metabolic syndrome in particular are at increased risk of postoperative mortality. Lymphadenectomy is recommended as the prevalence of lymph node metastases is approximately 15%, compared to less than 5% in cirrhotic patients. Adjuvant chemotherapy is not recommended. Regular follow-up with throraco-abdominal CT at 6-monthly intervals is recommended, as early detection and treatment of recurrence may improve survival.
There is very little place for other invasive treatments. Percutaneous ablation, as a rule, has no role due to the usually large tumour size at diagnosis. Liver transplantation is associated with a perioperative mortality of 10%, a need for long-term immunosuppression and long-term results not significantly different from those of resection. In a recent multicentre study based on a collaboration of 38 European transplant centres, only 105 patients transplanted for an HCC occurring in a normal liver were identified.30 Transplantation had been performed as the primary treatment because partial liver resection was precluded by anatomical factors or the need to preserve a sufficient volume of liver remnant, or as rescue treatment for intrahepatic tumour recurrence not amenable to repeat resection. The 5-year survival rate was 59% in patients without macrovascular or lymph node invasion, irrespective of tumour size and differentiation.
Liver resection of HCC in cirrhotic patients
Main limitations: Resection of HCC has four limitations in cirrhotic patients: (i) the tumour is multifocal in 20–60% of patients at the time of diagnosis and liver resection can normally only be considered in patients with unifocal tumours; (ii) cirrhosis is an important risk factor for the development of postoperative complications; (iii) oncological resections dictate wide margins whereas the diseased underlying liver usually requires parenchymal sparing; (iv) recurrence is invariable as cirrhosis persists.
Risk of surgery and patient selection: The risk of hepatectomy is increased in cirrhotic patients due to coagulation defects, portal hypertension, liver failure and impaired regeneration. In-hospital death was 10% in the 1990s (even higher in some subgroups) but has decreased since then as a result of improved patient selection, operative technique and perioperative management. Although some very large series report no mortality, the average mortality rates in national surveys or registries are 4–6% and are therefore higher than in non-cirrhotic patients or after resection of other malignancies.
Additional selection criteria for surgery have therefore been proposed for Child–Pugh A patients. In Japan, the indocyanine green (ICG) test is usually used. After injection of 0.5 mg ICG/kg body weight, retention of ICG is measured in peripheral blood, in particular 15 min after the injection (ICG-R15). Normal values of ICG-R15 are 10%. In cirrhotic patients, minor resections can be performed when it is 22% or less, but major resections only if it less than 14–17%. In contrast, in Europe and the USA, selection mainly relies on the absence of significant portal hypertension or cytolysis. This requires that patients have no evidence of oesophageal varices, splenomegaly, portosystemic shunts (including a patent umbilical vein) or ascites (even on imaging studies), and that they have a platelet count greater than 100 × 109/L. Some groups even advocate that invasive measurement of the hepatic vein–portal vein gradient should be less than 10 mmHg. Several studies have shown that a normal serum bilirubin and the absence of clinically significant portal hypertension are the best predictors of good outcomes after resection.31 Recently, the MELD score and thrombocytopenia, irrespective of Child–Pugh grade and tumour features, have been shown to be associated not only with postoperative mortality and morbidity but also with long-term survival.32,33
There has been considerable interest during the past 5 years in the optimal management of the remnant liver. This includes: (i) more selective use of inflow occlusion; (ii) avoiding excessive mobilisation of the liver; and (iii) measuring the future remnant liver volume (RLV) using CT reconstruction. In patients with chronic liver disease, an RLV of approximately 40% of the total liver volume is required before a major hepatectomy is performed. When this is not the case, preoperative portal vein embolisation (PVE) is indicated as a means of increasing the RLV and, perhaps more importantly, preoperatively testing the ability of the liver to regenerate. When right hepatectomy is contemplated (the most frequent circumstance when there is a risk of small RLV), the right portal vein is percutaneously injected, under ultrasound guidance, with glue or ethanol. This should induce atrophy of the right liver within 2–6 weeks and a compensatory hypertrophy of the left RLV. Due to its efficacy, PVE (alone or in association with transarterial chemoembolisation) has become almost routine before a right hepatectomy in cirrhotic patients.34 The absence of hypertrophy of the left liver following a successful right PVE means that the liver is unable to regenerate and that hepatectomy is contraindicated. There is also increasing evidence that parenchymal size alone does not necessarily reflect function and there is therefore interest in the functional evaluation of the remnant liver volume.
Technique: There is increasing evidence that both anatomical resections (as opposed to tumorectomies) and wide (as opposed to limited) margins may improve long-term survival without increasing the perioperative risk. The rationale is tumour spread through microvascular invasion, the incidence and extent of which is related to tumour diameter and degree of tumour differentiation. Several retrospective studies have reported an approximately 20% improvement in overall and disease-free survival following anatomical compared to limited resections.35 The impact of the margin width has been evaluated in a prospective controlled trial.36 A 2-cm margin was associated with a 75% 5-year survival as compared to 49% for 1-cm margins. Both concepts are not exclusive and should be taken into account, especially in tumours with diameters between 2 and 5 cm.
There is increasing interest in laparoscopic resections for HCC.37 Although not formally proven yet, it may have the advantage of less intraoperative bleeding, postoperative complications, postoperative analgesic drug consumption and a shorter hospitalisation time. More specifically in the context of cirrhotic patients, it may also reduce the risk of postoperative ascites and its consequences, as well as facilitate subsequent liver transplantation if required, because of fewer adhesions.38
Outcome after resection: The largest series from the Liver Cancer Study Group in Japan has reported 1-, 3-, 5- and 10-year survival rates of 87%, 66%, 48% and 21%, respectively in 11 631 cirrhotic patients treated by hepatic resection between 1992 and 2003. Comparable results have been reported by other groups worldwide, with no differences between Western and Asian studies. Independent predictors of survival are age, degree of liver damage, AFP level, tumour diameter, number of nodules, vascular invasion and surgical margins. Survival rates as high as 68% at 5 years have been reported in Child grade A patients with well-encapsulated tumours of 2 cm diameter or less. These figures continue to improve, even when patients with larger tumours are included. Active treatment of recurrences has been a major reason for this improvement.
Treatment of recurrence: Tumour recurrence is the major cause of death following resection of HCC in the cirrhotic patient. Its incidence is 40% within the first year, 60% at 3 years and approximately 80% at 5 years. However, it is invariable if follow-up is extended beyond 10 years as the precursor condition (cirrhosis) persists after surgery. It is frequently difficult to differentiate true recurrence from de novo tumours. The former tend to occur within the first 2 years and their main risk factors are vascular invasion, poor histological differentiation, presence of satellites and number of nodules. De novo recurrent tumours occur later and the main risk factors are the same as those of a primary HCC. Molecular analysis suggests that their respective proportions are 60–70% and 30–40%. Recurrence within the liver is multifocal in 50% of patients and is associated with distant metastasis in 15%, especially in the lungs, adrenal gland or bones. Extrahepatic recurrence without simultaneous intrahepatic recurrence is infrequent in cirrhotic livers. Anatomical resection and a tumour-free margin of 2 cm are associated with improved survival.
Evidence that neoadjuvant or adjuvant treatments reduce the risk of recurrence is currently lacking and these treatments are therefore not recommended. This applies to preoperative chemoembolisation, neoadjuvant or adjuvant chemotherapy, internal radiation with 131I-labelled Lipiodol, adoptive immunotherapy, retinoic acid or interferon, although some of these strategies initially showed promising results. An updated follow-up of the only randomised trial of adjuvant 131I-labelled Lipiodol has shown that the improved overall and disease-free survivals in the treatment group persisted until the seventh postoperative year.39 This study was characterised by a very high proportion of HBV-related HCC (88%). Three recent meta-analyses of published studies40–42 favour the use of interferon to reduce the risk of HCC recurrence; however, the quality of the studies was low due to heterogeneity of the patient populations, interferon used, duration of the treatment regimen and whether results were independent of the effect of viral suppression. No study has confirmed the potential efficacy of retinoic acid. Anti-angiogenic treatments are currently being evaluated.
Rationale: HCC is the only tumour for which transplantation plays a significant role, and this is the most attractive therapeutic option because it removes both detectable and undetectable tumour nodules together with all the preneoplastic lesions that are present in the cirrhotic liver. In addition, it simultaneously treats the underlying cirrhosis and prevents the development of postoperative or distant complications associated with portal hypertension and liver failure.
Patient selection: LT is not readily available in most high endemic areas of HCC. Even when available, there is donor shortage; LT can therefore only be performed in a fraction (less than 5% in most Western countries) of HCC patients. HCC patients are considered potential candidates for LT if their anticipated survival is approximately the same as those patients transplanted for other indications. This may be achieved if strict selection criteria are applied; otherwise, HCC patients are at high risk of death from tumour recurrence. These include an HCC: (i) confined to the liver (i.e. no extrahepatic disease, including lymph nodes); (ii) without vascular extension; and (iii) with limited tumour burden.
Tumour burden was initially defined as a single tumour less than 5 cm in diameter or the presence of two or three tumours less than 3 cm in dameter (the so-called Milan criteria).43 With the adoption of these criteria, the 5-year survival after LT ranges between 60% and 75%. There have subsequently been concerns that these criteria were too restrictive, which led to the proposal of expanded criteria. The best known and validated of these are the UCSF criteria – a single tumour less than 6.5 cm in diameter, or three or fewer tumours, the largest of which is less than 4.5 cm with the sum of the tumour diameters being less than 8 cm.44 Others take into account poor tumour differentiation or a high (or rapidly increasing) AFP serum concentration. An international consensus conference held in 2012 recommended a limited expansion of the listing criteria beyond the standard Milan criteria.45 Predicting tumour biology through molecular profiling rather than tumour morphology is the aim of current research in this field.
Treatment on the waiting list: The average time from listing to transplantation in Europe and the USA is usually greater than 12 months. Up to 25% of patients may be excluded from the waiting list due to disease progression. Three approaches have been developed to avoid these drop-outs:
• Living-donor liver transplantation (LDLT) is an alternative source of grafts but has its own drawbacks, including the inherent risk for the donor, the risk of small-for-size grafts and the fact that only 25–30% of transplant candidates have a potential donor. It has the advantage of being performed rapidly, so avoiding drop-out on the waiting lists. The number of LDLT in Western countries has, however, decreased recently and the trend is to favour cadaveric transplantation through changes in allocation policies.
• New rules of graft allocation have been implemented, initially in the USA and subsequently in Europe. In the USA, the Model of End-stage Liver Disease (MELD) organ allocation policy implemented in 2002 has given priority to candidates with HCC within the Milan criteria. Waiting times have shortened, obviating the need for LDLT. Similar policies have been applied in other countries such as France and the UK.
• Treatment of the tumour by resection, ablation or chemoembolisation is widely used while the patient is on the waiting list to avoid tumour progression beyond the oncological criteria. There is some evidence that these treatments may reduce drop-out rates on the waiting lists, but it is not clear if the outcome is the same for patients within or beyond the Milan criteria. The impact of these treatments on downstaging and post-transplantation survival is similarly uncertain. A specific advantage of resection over ablation or chemoembolisation is that it provides pathological details of the tumours. However, it is still unclear if the presence of poor prognostic factors should encourage or discourage transplantation.
Transarterial chemoembolisation (TACE):
Technique: HCC, in contrast to the liver parenchyma, receives almost 100% of its blood supply from the artery. When the feeding artery is obstructed, the tumour experiences an ischaemic insult that results in extensive necrosis. With the development of more supraselective embolisations, greater attention is paid to accessory arteries that may contribute to tumour vascularity, such as the diaphragmatic or mammary arteries, that should also be embolised to achieve adequate control. Injection of iodised oil has been combined to improve the efficacy of embolisation. Iodised oil (Lipiodol), which is hyperdense on CT, is cleared from the normal hepatic parenchyma but retained in malignant tumours for periods ranging from several weeks to over a year. This accumulation, which is not associated with significant adverse effects, may be used for targeting cytotoxic drugs and increasing their concentration in tumour cells. Recently drug-eluting beads (DC-Beads) loaded with doxorubicin have been developed. This technique is much more expensive than conventional TACE, but preliminary results show superior treatment response and delayed tumour progression.46 Combination of TACE and anti-angiogenic treatments is under evaluation.47
Contraindications: TACE should not be performed in patients with liver decompensation, biliary obstruction, bilioenteric anastomosis and impaired kidney function. Portal vein thrombosis is also a contraindication unless it is limited to a liver section only and TACE can be performed in a highly selective manner on a limited tumour volume.
Morbidity and mortality: Mortality is less than 1% if these contraindications are applied. Overall, more than 75% of patients develop a postembolisation syndrome characterised by fever, abdominal pain, nausea and raised serum transaminase levels. These symptoms, which are not prevented by antibiotics or anti-inflammatory drugs, are self-limiting and last for less than 1 week. More severe complications occur in less than 5% of patients and include, in decreasing order of frequency: cholecystitis or gallbladder infarction, gastric or duodenal wall necrosis, and acute pancreatitis. These, along with the postembolisation syndrome, have become less frequent with the use of supraselective embolisation. Hepatic abscess formation is rare, occurring in 0.3%, but is associated with a high mortality. The main risk factors are a previous history of bilioenteric anastomosis, large tumours and portal vein thrombosis.
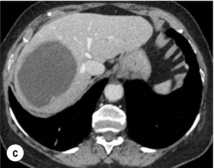
Monitoring: The efficacy of TACE is assessed by CT (usually at 1 month) as the disappearance of the arterial vascular supply to the tumour and a decrease in its diameter. These features do not necessarily evolve in parallel. A decrease in tumour size may, for example, be associated with persistent vascularisation (i.e. residual tumour), whereas compact Lipiodol uptake without residual vascularisation may indicate complete tumour necrosis despite no significant decrease in size (Fig. 5.4).
Efficacy: There is grade A evidence that TACE improves survival.48 One of the largest studies is a prospective Japanese nationwide survey reporting median and 1-, 3-, 5- and 7-year survivals of 34 months, 82%, 47%, 26% and 16%, respectively, with a TACE-related mortality of 0.5%. Independent predictors of survival were, in decreasing order of influence: the degree of liver damage, portal vein invasion, maximum tumour size and number of lesions, and AFP levels.
Percutaneous local ablative therapy:
Technique: Locoregional therapies are percutaneous treatment modalities that allow the injection of a damaging agent or the application of an energy source directly into the tumour. Damaging agents include chemicals such as ethanol (percutanenous ethanol injection, PEI) or acetic acid. Energy sources either aim at increasing temperature by radiofrequency, microwave or interstitial laser photocoagulation or, alternatively, at decreasing temperature (cryoablation). Irreversible electroporation is a new non-thermal ablation therapy that uses a high-voltage direct electrical current to create nanopores in the cellular membrane and results in cell death via apoptosis. Radiofrequency ablation (RFA) has emerged as the most effective of these techniques. It exploits the conversion of electromagnetic energy into heat via a needle electrode (15–18 G) positioned in the tumour, while patients are made into an electric circuit by grounding pads applied to their thighs. The radiofrequency emitted from the tip causes ionic agitation and frictional heat, which leads to cell death from coagulation necrosis. The objective is to maintain a temperature of 55–100 °C throughout the entire target volume for a sufficient period of time. Monitoring the impedance is important because excessive heating results in tissue charring, increased tissue impedance and decreased energy absorption.
Advantages and drawbacks: These ablative methods are minimally invasive, preserve the uninvolved liver parenchyma, have no systemic side-effects, and avoid the mortality and morbidity of major hepatic surgery. On the other hand, only tumours less than 5 cm are likely to be treated successfully but the smaller the diameter, the greater the probability of complete local control. The presence of multiple tumours (more than three) is also a limitation because of the need for repeated punctures. In addition, multiple tumours are either the result of multifocal carcinogenesis or vascular extension, and therefore a focal treatment is unlikely to be very effective. Obviously, a common requirement is also the need to clearly visualise the tumour by US and access it safely. Hence, isoechoic HCC or tumours located in the upper part of segments 4, 7 and 8 or at the edge of the left lateral section if it extends behind the spleen may occasionally be unsuitable for treatment. Finally, whichever technique is used, the needle should not enter the tumour directly but pass through the hepatic parenchyma so as to prevent intraperitoneal bleeding or seeding of tumour cells. This may prove impossible for some superficial or protruding tumours. Recently, an experienced group reported that up to one-third of patients who were theoretically good candidates for ablation could not be treated due to non-visibility of the HCC on US, risk of thermal injury or absence of a safe path.51
Contraindications and limitations: Contraindications to ablation procedures include gross ascites that favours intraperitoneal bleeding, coagulopathy that cannot be corrected, previous history of bilioenteric anastomosis or endoscopic sphincterotomy associated with bile bacterial contamination and therefore a risk of abscess formation. Additional contraindications (more specific to radiofrequency or microwave rather than ethanol injection) come from the proximity of the tumour to the colon, duodenum, stomach or biliary confluence, which may be injured or perforated by the heating process. RFA, unlike microwave ablation, is as a rule contraindicated in patients with a pacemaker. The efficacy of RFA also seems to be more impacted by the proximity of a vascular pedicle (the so-called cooling effect) than microwave ablation. Whereas PEI is a quick and very cheap procedure performed under light sedation, RFA is more costly, prolonged (20–90 minutes) and painful, and therefore generally performed under general anaesthesia. Microwave ablation is also performed under general anaesthesia but the procedure is quicker.
Methods and margins: Ablation should not only target the tumour but also aim to achieve a safety margin so as to control satellite nodules. The incidence of these satellite nodules, as well as their distance from the main tumour, increases as the main tumour enlarges. Both incidence and distance also increase for poorly, compared to well differentiated, tumours. This safety margin should be 5 mm at least; hence, for an HCC measuring 3 cm in diameter, the diameter of the ablation should be 4 cm. This is best achieved with thermal rather than chemical ablation. Methods to further improve tumour and margin control include multipolar ablation (several probes are placed around the tumour) and combining ablation with TACE.52 Treatment response is assessed by CT or MRI no earlier than 1 month after the procedure. RFA may result in a rim of fibrotic tissue (hypervascular on late-phase MRI or CT) at the periphery of the tumour and should not be mistaken for residual tumour tissue. Follow-up thereafter relies on imaging studies at 3-monthly intervals to ensure that there is no recurrence of contrast enhancement.
Indication: Percutaneous ablative therapies have initially been performed in patients who were unsuitable for resectional surgery and both the EASLD and AASLD have recommended this strategy. It has thereafter been used as neoadjuvant treatment in liver transplant candidates and for treatment of recurrences after liver resection.
As the results of ablation improve, due to improved technology and patient selection, it may also be considered as an alternative to surgery or even as a first-line treatment in selected situations. A large multicentre phase 2 trial reported a 97% sustained complete response and a 68% actuarial 5-year survival following ablation in patients with HCC of 2 cm or less.53 The results of two randomised controlled trials comparing ablation and resection in patients with early HCC demonstrated no difference.54,55
However, meta-analyses still favour surgery compared to ablation in terms of 3-year survival and local control.56,57 One additional concern is that both in the USA58 and in Italy59 there has been a recent temporal trend of increased use of ablation as a treatment for HCC with a simultaneous decrease in survival following this treatment, unlike what has been observed for other treatments. These observations suggest that the extension of indications for ablation should be strictly evaluated.
Other palliative treatments
Conventional systemic chemotherapy: Systemic chemotherapy has had very limited value in the past as only a very small number of patients obtained partial response or meaningful palliation using conventional drugs. Therefore, there is no rationale for using chemotherapy in patients with unresectable HCC outside of clinical trials.
Anti-angiogenic targeted therapies:
Sorafenib (Nexavar®) exerts an anti-angiogenic effect by targeting the tyrosine kinases vascular endothelial growth factor (VEGF) receptors 2 and 3, and the platelet-derived growth factor receptor β. In an initial phase 3 trial, the median overall survival of Child–Pugh A cirrhotic patients with histologically proven and advanced HCC was 10.7 months in the treated group versus 7.9 months in the placebo double-blinded controlled arm of the study (P = 0.00058), and the median times to tumour progression were 24 weeks and 12 weeks, respectively (P = 0.000007).60 This efficacy in advanced HCC (unresectable or metastatic) has been confirmed in an Asian randomised placebo-controlled trial that included mostly patients with HBV-related HCC61 and in a large phase 4 study with more than 3000 patients.62 Side-effects included diarrhoea (39%), hand–foot syndrome (21%), anorexia (14%) and alopecia (14%). The antitumour effect, the pharmacokinetic profile and safety profile were similar in Child–Pugh A and B. These results have established sorafenib as the standard of treatment for advanced HCC in Child A (or B) patients.23 Several trials assessing strategies such as combination or sequential treatments are under way.
Radioembolisation: External beam radiation therapy has been of limited value in treating HCC because the normal liver parenchyma is very radiosensitive. Greater interest has therefore been given to injecting radioisotopes such as 131I-iodised oil or 90Y-labelled microspheres directly into the hepatic artery (radioembolisation), which offers the advantage of increased delivery within the tumour and decreased toxicity. The former agent has an efficacy comparable to that of chemoembolisation in patients with HCC not complicated by portal thrombosis but is superior in patients with tumour portal extension. The use of 90Y microspheres is more recent and has been shown in a phase 2 trial to be safe and effective, in particular in patients with portal vein thrombosis.63 These results have been reproduced in three recent studies, but without randomised controlled trials comparing 90Y-labelled microspheres, TACE or other established treatments, defining the role of this expensive treatment in clinical practice is not possible.
Defining a treatment strategy
Uncomplicated HCC associated with chronic liver disease
Treatment algorithms need to take account of availability of treatments.
• Liver transplantation, when available, is considered first and attention is therefore paid to the extent of liver disease, patient age and presence or absence of associated conditions. If a long waiting time (> 6 months) is expected, resection, ablation or TACE are considered prior to liver transplantation.
• If transplantation is not available or not indicated, resection should be considered. Limiting factors are the number of nodules (ideally there should be only one) and the severity of underlying liver disease (patients should be Child–Pugh A and have neither cytolysis, portal hypertension nor impaired ICG tests). If a right hepatectomy is considered it should be preceded by PVE (with or without TACE).
• If resection is not considered due to the severity of the underlying liver disease and the nodule is single (or if there are less than three nodules), ablation is the treatment of choice provided the tumour is less than 3–5 cm. For single tumours 2 cm or less, RFA is becoming a first-line treatment, as an alternative to resection.
• If neither resection nor RFA is considered, TACE is performed provided there is no ascites or liver failure (and in particular that the serum bilirubin is less than 50 μmol/L) and that the tumour burden is not too extensive (no vascular invasion or extrahepatic metastases).
• Remaining patients are currently considered for anti-angiogenic treatments provided there is neither liver failure nor vascular disease.
Treatment of complicated HCC
HCC with macroscopic portal vein invasion: This is a contraindication for liver transplantation and ablative treatments. Traditionally, TACE was also contraindicated (because of the risk of liver necrosis); today it is occasionally performed provided the thrombus is limited to a liver section or less and that embolisation is highly selective, with reduced doses and partial (rather than total) arterial occlusion as the end-point. If thrombus does not extend into the main portal vein, surgical resection can be considered. Radioembolisation and anti-angiogenic therapy is otherwise indicated.
HCC with macroscopic invasion of hepatic veins: This seems to carry an even worse prognosis as the tumour thrombus will extend into the inferior vena cava. When the thrombus is confined to the hepatic vein, resection if possible can be proposed. There is, however, a very high risk of pulmonary metastases developing within 6–12 months of surgery. Extension into the inferior vena cava or the right atrium is usually beyond any treatment.
Ruptured HCC: This should be actively treated unless it occurs as a terminal presentation in patients with multiple tumours, portal thrombosis and end-stage liver failure. The primary aim of treatment is to stop bleeding, ideally by arterial embolisation. Subsequent hepatectomy can be associated with long-term survival. Indeed, (i) bleeding is not necessarily due to tumour rupture, but occasionally due to rupture of an artery at the junction of the tumour and the adjacent parenchyma, and (ii) even if the tumour has ruptured, this is not always associated with peritoneal seeding of tumour cells.
Fibrolamellar carcinoma (FLC)
• FLCs are usually large at the time of diagnosis (8–10 cm), and the common revealing symptoms are a palpable mass, abdominal pain, weight loss, malaise and anorexia.
• The prognosis is better than that of HCC overall. Five-year survival following resection is 50–75%.64
• Resection is preferred to transplantation as the latter has very little or no place.
AFP levels are raised in less than 10% of patients.64 Lymph node invasion within the hepatic pedicle is frequent (60%) and if resection is considered, simultaneous lymphadenectomy is recommended. There is a significant risk of recurrence, not only within the liver but also as lymph node or distant metastases. Close long-term follow-up is mandatory since recurrence and death beyond 5 years are common. Repeat surgery is a reasonable option in this younger patient population due to the relatively indolent course of the disease and the relative inefficacy of non-surgical treatments.
A recent study has suggested that true FLC should be differentiated from mixed FLC–HCC, defined as conventional HCC displaying some distinct area with FLC features.65
Intrahepatic cholangiocarcinoma (ICCA)
Incidence
In the Western world, the incidence of ICCA is 0.3–3 per 100 000, 10 times less than HCC. Recent reports suggest the incidence is increasing, particularly in the USA, UK, France, Italy, Japan and Australia. Although this increase may be real, it is probably mainly explained by improved identification of this tumour and changing rules on how they should be coded according to the International Classification of Diseases for Oncology (ICD).66
Risk factors
New risk factors are emerging, including chronic non-alcoholic liver disease, HBV infection, HCV infection, diabetes and the metabolic syndrome.67 However, in contrast to HCC, most ICCAs develop without a background of liver disease. In surgical series, 75% of patients have normal livers, 16% have chronic hepatitis/liver fibrosis and 9% have cirrhosis.
Classification and staging
Whereas previously ICCAs were staged using a similar system as HCCs, the AJCC implemented a specific classification for ICCAs in 2010.68 The T staging takes into account number of tumours and vascular invasion (the presence of either defines T2), rather than tumour size. The reason for this is that it is very unusual to diagnose ICCA early and size does not independently impact survival in published surgical series. T3 tumours are those perforating the visceral peritoneum or involving local extrahepatic structures by direct invasion, although this is fairly rare. The T staging also aims to take into account the periductal-infiltrating pattern of ICCA and, when present, defines T4. However, this infiltrating pattern may be difficult to identify on imaging studies or even on pathological specimens and there is no standardised definition yet. Lymph node involvement has a major impact on survival and, when present, defines TNM stage III. Prevalence of lymph node extension is high and therefore lymphadenectomy should be routinely performed to achieve accurate staging. Median survival of patients with stage I is greater than 5 years (but these patients are very rare), whereas that of patients with stage II is 53 months and that of patients with stage III is 16 months.69
Pathology and progression analysis
Two distinct conditions that precede invasive cholangiocarcinoma have been identified. The first is a flat or micropapillary growth of atypical biliary epithelium, which has been called biliary dysplasia or biliary intraepithelial neoplasia. The second is an intraductal papillary neoplasm of the bile duct characterised by the prominent papillary growth of atypical biliary epithelium with distinct fibrovascular cores and frequent mucin over-production. These preneoplastic conditions have mainly been analysed in hepatolithiasis and are observed more frequently in large bile ducts as hilar tumours than in small septal–interlobular bile ducts such as with ICCA.70 The dysplasia–carcinoma sequence therefore appears more obvious for hilar lesions than peripheral lesions. This suggests that an alternative source of ICCA could be the canals of Hering or hepatic progenitor cells, which are a target cell population for carcinogenesis in chronic liver disease.
Imaging studies
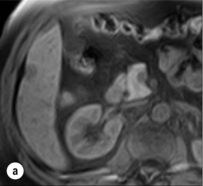
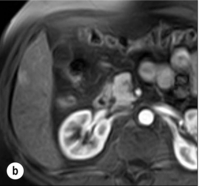
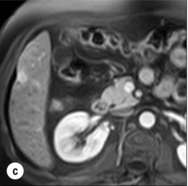
The main characteristic of mass-forming ICCA is that it is a fibrous tumour and therefore displays no enhancement on the arterial phase and delayed enhancement during the late phase. This may be seen both on CT and MRI. On MRI, lesions are hypointense on T1-weighted images and moderately to markedly hyperintense on T2-weighted images (Fig. 5.5). They are typically large, non-encapsulated, heterogeneous, associated with narrowing of adjacent portal veins and retraction of the liver capsule. As the tumour grows, satellite nodules frequently develop in the vicinity of the tumour, and subsequently in the contralateral lobe (Fig. 5.6). When superficial, these satellite nodules may not be visible on imaging. There is a high propensity for lymph node invasion (present in 40% of resected patients if lymphadenectomy is performed routinely), but imaging studies only have a sensitivity of 50% and a specificity of 75% to predict this.
Treatment
According to the series published over the past decade, the 1-, 3- and 5-year survival rates following resection of ICCA are 67%, 38% and 27%, respectively. There are few data on survival beyond 5 years. Variables that influence postoperative survival most are the presence of lymph node invasion and an R1 resection.71 Intraductal growth-type ICCAs are rare but have a better long-term prognosis. Infiltrating-type ICCAs have a worse prognosis than the mass-forming type due to spread along Glisson’s capsule and high incidence of lymph node involvement.
There is little evidence that these figures have improved over the past 10 years. However, the recent demonstration that systemic chemotherapy may be effective in unresectable patients opens the possibility of combining surgery with either adjuvant and/or neoadjuvant chemotherapy.72
Epithelioid haemangioendothelioma (EHE)
EHEs are neoplasms of vascular origin that arise predominantly from soft tissues, bones and visceral organs, in particular the lung and the liver. Hepatic EHE develops from the endothelial cells lining the sinusoids and progresses along the sinusoids and vascular pedicles. It is extremely rare (no more than 200 cases have been reported), with an incidence of less than 1 per million population. It does not arise on a background of liver disease and there is no identified causative factor. Mean age at presentation is 42 years, with a female to male ratio of 3:2.73 Half present with right upper quadrant pain, a quarter incidentally, and the remainder with severe symptoms such as ascites, jaundice, weakness and weight loss. Liver failure as a result of massive infiltration has been described.
The natural history of this tumour is highly variable. Although exceptional, prolonged survival of more than 10 years has been reported without treatment, and both partial and complete spontaneous tumour regression has even been described. On the other hand, some patients die within 2 weeks of diagnosis and 20% are dead within 1 year. Overall, only 20–40% survive more than 5 years.73 Because of the rarity of this tumour and its highly variable course, there is no widely accepted therapeutic strategy.
Partial hepatectomy is rarely feasible due to the invariable multifocal involvement of the liver. Palliative resection is not advocated as some have raised concerns that liver regeneration could promote a flare-up of residual tumours. Reports of favourable outcome with an estimated 5-year survival of 75% probably represent a highly selected subgroup.73
The place of liver transplantation has recently been clarified by a multi-institutional analysis.74 In 59 patients reported to the European Liver Transplant Registry, impressive 5- and 10-year survival rates of 83% and 74%, respectively, were reported. Invasion of lymph nodes and presence of restricted extrahepatic involvement had limited impact on survival and should therefore not be considered as contraindications to transplantation. The current shortage of liver grafts and the prolonged waiting time may dictate that liver transplantation is only indicated in highly selected patients. Experience with locoregional or systemic chemotherapy is small and of limited value, especially as first-line therapy. Neoadjuvant combination therapies using anti-VEGF antibodies, however, deserve investigation.
Angiosarcoma
Other sarcomas, including leiomyosarcoma, tend to have a better prognosis and should be resected if feasible.75
References
1. Ferlay, J., Shin, H.R., Bray, F., et al, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. 21351269
2. Eguchi, S., Kanematsu, T., Arii, S., et al, Recurrence-free survival more than 10 years after liver resection for hepatocellular carcinoma. Br J Surg. 2011;98(4):552–557. 21267990
3. Chen, C.J., Yang, H.I., Su, J., et al, Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. 16391218
4. Braks, R.E., Ganne-Carrie, N., Fontaine, H., et al, Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;13(42):5648–5653. 17948941
5. Lewden, C., May, T., Rosenthal, E., et al, Changes in causes of death among adults infected by HIV between 2000 and 2005: the “Mortalité 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr. 2008;48(5):590–598. 18645512
6. Berretta, M., Garlassi, E., Cacopardo, B., et al, Hepatocellular carcinoma in HIV-infected patients: check early, treat hard. Oncologist. 2011;16(9):1258–1269. 21868692
7. Calle, E.E., Rodriguez, C., Walker-Thurmond, K., et al, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. 12711737
8. El-Serag, H.B., Tran, T., Everhart, J.E., Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. 14762783
9. Paradis, V., Zalinski, S., Chelbi, E., et al, Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851–859. 19115377
10. Jin, F., Qu, L.S., Shen, X.Z., Association between C282Y and H63D mutations of the HFE gene with hepatocellular carcinoma in European populations: a meta-analysis. J Exp Clin Cancer Res 2010; 29:18. 20196837
11. Liang, Y., Yang, Z., Zhong, R., Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012;56(4):1409–1417. 22504852
12. Migita, K., Watanabe, Y., Jiuchi, Y., et al, Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization – autoimmune hepatitis prospective study). Liver Int. 2012;32(5):837–844. 22221966
13. Stoot, J.H., Coelen, R.J., De Jong, M.C., et al, Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12(8):509–522. 20887318
14. Farges, O., Ferreira, N., Dokmak, S., et al, Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60(1):85–89. 21148580
15. Bioulac-Sage, P., Laumonier, H., Couchy, G., et al, Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50(2):481–489. 19585623
16. Dokmak, S., Paradis, V., Vilgrain, V., et al, A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137(5):1698–1705. 19664629
17. Bioulac-Sage, P., Rebouissou, S., Thomas, C., et al, Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46(3):740–748. 17663417
18. Di Tommaso, L., Destro, A., Seok, J.Y., et al, The application of markers (HSP70, GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50(4):746–754. 19231003
19. Lok, A.S., Sterling, R.K., Everhart, J.E., et al, Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493–502. 19852963
20. Kudo, M., Hatanaka, K., Kumada, T., et al, Double-contrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol. 2011;106(2):368–370. 21301463
21. Talbot, J.N., Fartoux, L., Balogova, S., et al, Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51(11):1699–1706. 20956466
22. Kawaoka, T., Aikata, H., Takaki, S., et al, FDG positron emission tomography/computed tomography for the detection of extrahepatic metastases from hepatocellular carcinoma. Hepatol Res. 2009;39(2):134–142. 19208034
23. Bruix, J., Sherman, M., Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. 21374666
24. Forner, A., Vilana, R., Ayuso, C., et al, Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(1):97–104. 18069697
25. Sangiovanni, A., Manini, M.A., Iavarone, M., et al, The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59(5):638–644. 19951909
26. Khalili, K., Kim, T.K., Jang, H.J., et al, Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54(4):723–728. 21156219
27. Zhang, B.H., Yang, B.H., Tang, Z.Y., Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–422. 15042359
28. Thompson Coon, J., Rogers, G., Hewson, P., et al, Surveillance of cirrhosis for hepatocellular carcinoma: a cost–utility analysis. Br J Cancer. 2008;98(7):1166–1175. 18382459
29. Smoot, R.L., Nagorney, D.M., Chandan, V.S., et al, Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg. 2011;98(5):697–703. 21280030
30. Mergental, H., Adam, R., Ericzon, B.G., et al, Liver transplantation for unresectable hepatocellular carcinoma in normal livers. J Hepatol. 2012;57(2):297–305. 22521348
31. Ishizawa, T., Hasegawa, K., Aoki, T., et al, Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134(7):1908–1916. 18549877
32. Delis, S.G., Bakoyiannis, A., Biliatis, I., et al, Model for end-stage liver disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2009;11(4):351–357. 19718364
33. Maithel, S.K., Kneuertz, P.J., Kooby, D.A., et al, Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212(4):638–650. 21463803
34. Ogata, S., Belghiti, J., Farges, O., et al, Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93(9):1091–1098. 16779884
35. Wakai, T., Shirai, Y., Sakata, J., et al, Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14(4):1356–1365. 17252289
36. Shi, M., Guo, R.P., Lin, X.J., et al, Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. 17197963
37. Dagher, I., Belli, G., Fantini, C., et al, Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg. 2010;211(1):16–23. 20610244
38. Laurent, A., Tayar, C., Andreoletti, M., et al, Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(3):310–314. 19280110
39. Lau, W.Y., Lai, E.C., Leung, T.W., et al, Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial – update on 5-year and 10-year survival. Ann Surg. 2008;247(1):43–48. 18156922
40. Breitenstein, S., Dimitroulis, D., Petrowsky, H., et al, Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96(9):975–981. 19672926
41. Miyake, Y., Takaki, A., Iwasaki, Y., et al, Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17(4):287–292. 19732321
42. Shen, Y.C., Hsu, C., Chen, L.T., et al, Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol. 2010;52(6):889–894. 20395009
43. Mazzaferro, V., Regalia, E., Doci, R., et al, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. 8594428
44. Yao, F.Y., Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8(10):1982–1989. 18727702
45. Clavien, P.A., Lesurtel, M., Bossuyt, P.M., et al, Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11–e22. 22047762
46. Song, M.J., Chun, H.J., Song, D.S., et al, Comparative study between Doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57(6):1244–1250. 22824821
47. Qu, X., Chen, C., Wang, J., et al, The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12(1):263. 22721173
48. Llovet, J.M., Bruix, J., Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. 12540794 This meta-analysis of randomised controlled trials demonstrates that TACE should be recommended as first-line non-curative option for intermediate HCC (as defined by the BCLC stagin system) as it improved survival.
49. Lopez, P.M., Villanueva, A., Llovet, J.M., Systematic review: evidence-based management of hepatocellular carcinoma – an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23(11):1535–1547. 16696801 This meta-analysis of four randomised controlled trials shows the efficacy of RFA in terms of local better control in HCC > 2 cm, as compared to PEI.
50. Cho, Y.K., Kim, J.K., Kim, M.Y., et al, Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49(2):453–459. 19065676 This meta-analysis of four randomised controlled trials demonstrates that RFA significantly improved survival for patients with HCC, as compared to PEI.
51. Kim, J.E., Kim, Y.S., Rhim, H., et al, Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79(2):e80–e84. 21514757
52. Wang, W., Shi, J., Xie, W.F., Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30(5):741–749. 20331507
53. Livraghi, T., Meloni, F., Di Stasi, M., et al, Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. 18008357
54. Chen, M.S., Li, J.Q., Zheng, Y., et al, A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. 16495695
55. Lu, M.D., Kuang, M., Liang, L.J., et al, Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86(12):801–805. 16681964
56. Bouza, C., Lopez-Cuadrado, T., Alcazar, R., et al, Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol 2009; 9:31. 19432967
57. Zhou, Y., Zhao, Y., Li, B., et al, Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78. 20618937
58. Massarweh, N.N., Park, J.O., Farjah, F., et al, Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2010;210(4):441–448. 20347736
59. Santi, V., Buccione, D., Di Micoli, A., et al, The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2012;56(2):397–405. 21756850
60. Llovet, J.M., Ricci, S., Mazzaferro, V., et al, Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. 18650514 This is a randomised controlled trial demonstrating an improved survival benefit of sorafenib in patients with advanced HCC.
61. Cheng, A.L., Kang, Y.K., Chen, Z., et al, Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. 19095497
62. Lencioni, R., Kudo, M., Ye, S.L., et al, First interim analysis of the GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66(7):675–683. 22698419
63. Kulik, L.M., Carr, B.I., Mulcahy, M.F., et al, Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71–81. 18027884
64. Stipa, F., Yoon, S.S., Liau, K.H., et al, Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106(6):1331–1338. 16475212
65. Malouf, G.G., Brugières, L., Le Deley, M.C., et al, Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer. 2012;118(20):4981–4990. 22415897
66. Khan, S.A., Emadossadaty, S., Ladep, N.G., et al, Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848–854. 22173164
67. Welzel, T.M., Graubard, B.I., Zeuzem, S., et al, Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54(2):463–471. 21538440
68. Edge, S.B., Compton, C.C., The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. 20180029
69. Farges, O., Fuks, D., Le Treut, Y.P., et al, AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer. 2011;117(10):2170–2177. 21523730
70. Aishima, S., Kuroda, Y., Nishihara, Y., et al, Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31(7):1059–1067. 17592273
71. Farges, O., Fuks, D., Boleslawski, E., et al, Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254(5):824–830. 22042474
72. Valle, J., Wasan, H., Palmer, D.H., et al, Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. 20375404
73. Mehrabi, A., Kashfi, A., Fonouni, H., et al, Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107(9):2108–2121. 17019735
74. Lerut, J.P., Orlando, G., Adam, R., et al, The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246(6):949–957. 18043096
75. Matthaei, H., Krieg, A., Schmelzle, M., et al, Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144(4):339–344. 19380647