25 Prevention and Management of Procedural Complications
 Choice of Physician
Choice of Physician
A primary issue regarding morbidity associated with CIED implantation is which medical professional should implant and follow up in these patients. As the indications for CIEDs have broadened, and an increasing number of patients are considered for implantation, physicians of various specialties are being called on to implant the devices and manage the postimplantation care of these patients. Curtis et al.,1 using data from the National Cardiovascular Device ICD Registry, found that the rates of overall complications and major complications were lowest among procedures carried out by electrophysiologist (EP) physicians (3.5% and 1.3%, respectively) and highest among those performed by thoracic surgeons (5.8% and 2.5%). Importantly, EP physicians had the lowest rates of major complications, including cardiac arrest, lead dislodgment, and pneumothorax. In this study, the choice of device was also influenced by the specialty of the physician performing the procedure. Non–EP physicians failed to recommend implantation of an indicated cardiac resynchronization implantable cardioverter-defibrillator (ICD) device (CRT-D).1 In a different study, Al-Khatib et al.2 also identified implantation by non–EP physicians as being associated with an increased incidence of complications.2
 Implantation-Related Complications
Implantation-Related Complications
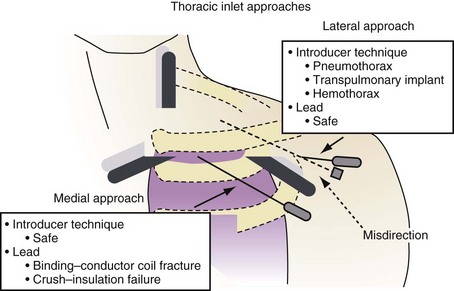
Depending on the physical characteristics of the lead, in terms of both materials and construction, certain approaches to subclavian vein access have been associated with subclavian crush, where the lead is compressed in the space between the clavicle and the first rib. This entrapment of the lead in the subclavius muscle, in the periosteum of the clavicle, or just through the very narrow space that occurs with medial subclavian vein puncture is completely dependent on the proceduralist’s approach to accessing the central circulation. In addition, management of suture tie-down sleeves with overly tight ligatures can damage the outer insulation, inner insulation, or conductor coils, particularly in coaxial leads with a polyurethane inner insulation.3,4
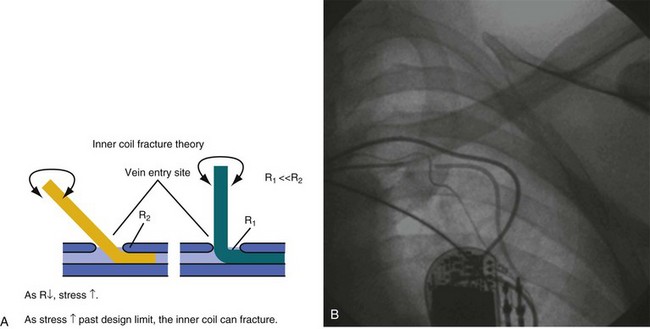
In response to the concerns about medial access of the subclavian system, physicians began to use more lateral access techniques. Unfortunately, extreme lateral access places different stresses on the lead body. Recently popularized implantation techniques within the last 10 years employ access to the subclavian venous system at the point where the axillary vein crosses the first rib, immediately lateral to the clavicle. However, if a more lateral approach into the axillary vein over the second or third rib stresses the leads by a different mechanism. The extreme angle of the lead on entry to the axillary vein at this lateral but also more posterior position, as well as the resultant acute anterior bend following the trajectory of the vein, exacerbated by the suture tie-down sleeve fixing the lead to the muscle, places a torque and compression fatigue stress on the lead conductors. This is most often observed with leads constructed with a thin body, coaxial construction, and active-fixation inner coils (Figs. 25-1 and 25-2).
The more medial axillary vein puncture, over the first anterior rib, avoids both subclavian crush and second-rib fatigue fractures.5 The axillary vein can be accessed under fluoroscopy over the first rib and the area probed posterolaterally until the vein is punctured. Alternatively, venography or ultrasound can be used to guide lead implantation6 (see Chapter 21).
At implantation, the lead must be treated gently, and any mechanical stress should be avoided. Even overuse of the helical screw mechanism can place additional stress on the conductor. It is usually difficult to nail down the precise trauma that later causes a lead failure, but the time of implantation is a relatively dangerous time for a lead and is often a contributor to the ultimate failure. Prolapsing of the lead on itself should not be performed unless absolutely necessary. Again, although difficult to prove, this practice has been implicated in early lead failure, particularly with lower-profile ICD leads.7–12
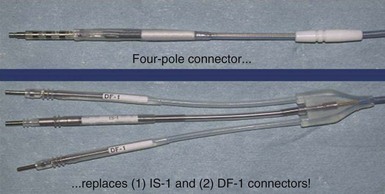
In addition to placing the lead in the heart and affixing the suture sleeve, making the connections to the CIED is another potential problem. Utmost vigilance should be exercised when the leads are connected to the device, to avoid the need for surgical revision, reentering the pocket and increasing the risk of infection. In the Replace trial, five patients needed revision because of generator-lead interface problem, such as loose set screw or misconnected leads.13 With the introduction of the DF-4 ICD leads, in which the two DF-1 and one IS-1 connectors are replaced with a single connector, the incidence of connection error should be significantly reduced.14 Currently, an SJ-4 lead is available and is in clinical use (Fig. 25-3).
 Procedural Complications
Procedural Complications
Lead Dislodgment
Lead dislodgment is the most common complication associated with CIEDs (1.6%-4.4% of cases), with atrial lead dislodgment accounting for most cases. In the MOST trial of more than 2000 patients, Ellenbogen et al.15 found atrial dislodgment in 1.7% of patients in the first 30 postimplant days.15 To prevent dislodgment, it is important to have an adequate intrinsic signal and to ensure contact to the myocardial wall. This is partly assessed by viewing a modest current of injury in the unfiltered electrogram, in addition to the appearance on fluoroscopy. This has the appearance of a QRS complex with ST-segment elevation before fixation of the active helix into the myocardium. Appropriate slack on the lead is also important, to allow for settling of the device when the patient is upright. There may be micro- or macro-dislodgment. Micro-dislodgment has been defined as when there is loss of capture and sensing without radiographic evidence of dislodgment. Macro-dislodgment can be detected by radiography. Lead dislodgment requires reopening the pocket and repositioning of the dislodged lead to ascertain satisfactory function. However, it is best to reaccess the vein, remove the dislodged lead, and ensure that there is no clot or tissue or damage to the lead before reinsertion.
Pneumothorax
Inadvertent entrance into the pleural space, especially with the introducer access technique, can lead to pneumothorax (0.7%-2% of cases).1,2 This is related to the experience of the implanter and also the level of difficulty obtaining access. This complication can be mitigated by using an extrathoracic approach with the introducer technique, accessing the axillary vein over the first rib, which acts as a barrier against entering the lung. Pneumothorax should not occur when using the cephalic vein cutdown technique. Most patients with pneumothorax are asymptomatic and are diagnosed on the postimplant chest radiograph. In patients with a sizable pneumothorax, cardiothoracic surgery should be consulted for possible chest tube insertion, in addition to administering high-flow oxygen. Tension pneumothorax should be recognized as a potential cause of sudden hemodynamic compromise during device implantation. Tension pneumothorax should be treated immediately with a chest tube.
Perforation
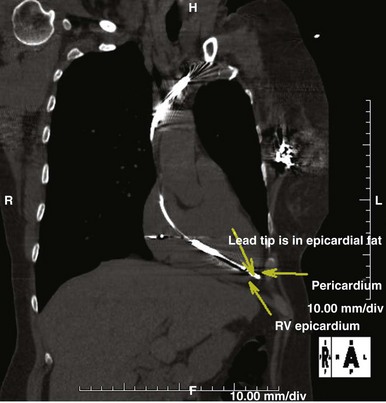
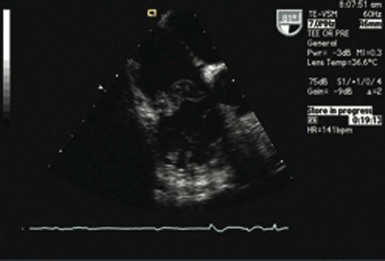
Perforation of the great vessels is very rare during implantation and is more common with lead extraction. Ventricular perforation is rare and reported to occur in 0.3% to 1% of CIED cases7,11,16,17 (Fig. 25-4). Most are self-limited with no sequelae. Acute tamponade is extremely rare but should be quickly recognized as a cause of hemodynamic compromise and easily diagnosed by bedside echocardiography or with fluoroscopy; the enlarged cardiac silhouette is associated with minimal contraction. Immediate pericardiocentesis should be performed and surgical intervention sought if bleeding does not stop.
Most cases of perforation manifest after the procedure with complaints of chest pain, pleurisy, and at times extracardiac pacing. Perforation also may manifest as a change in sensing and pacing characteristics. Delayed perforation has been a concern with the small-profile ICD and pacemaker leads, although careful analysis did not confirm the incidence of perforation.11,17–19 However, when there is a delayed perforation, days to months after implantation, the lead should be extracted with surgical backup in the unlikely event of acute tamponade. According to most reported cases and from experience at our institution, extraction of such perforating leads is uneventful, and reimplantation can be performed at the same time.18 Implantation at the right ventricular septum is associated with less risk of perforation.20 This can be achieved using a stylet with a posteriorly angulated bend.21
Pocket Hematoma
The risk of hematoma is higher in anticoagulated patients, especially when heparin is restarted after the procedure. The use of antiplatelet therapy and anticoagulation may increase the risk of hematoma. In one study, only 4.2% of patients with no antiplatelet or anticoagulation developed hematoma, whereas up to 18% of patients with uninterrupted clopidogrel developed hematoma at the pocket site. Patients taking warfarin (Coumadin) only had a 6.9% risk. Combinations of these increased the risk drastically;22 24% of patients with ongoing aspirin and clopidogrel treatment developed hematoma.23 The use of periprocedural heparin (enoxaparin) was associated with increased risk of bleeding compared with continuation of warfarin (INR >1.5).24 The use of dual-antiplatelet therapy presents a challenge, particularly in that it is difficult to stop these agents in patients with drug eluting stents. Warfarin use seems to be less of an issue. Giudici et al revealed that discontinuation of warfarin was associated with same risk as continuation of anticoagulation with a mean international normalized ratio (INR) of 2.5.25 Most hematomas are treated conservatively. Evacuation of hematoma may be needed with continued bleeding, severe tension, and pain. The optimal approach to patients undergoing therapy with the new factor X antagonists, such as dabigatran, has not been directly approached in those with planned device procedures, but it may provide an alternative pathway to a bridging anticoagulation approach.
Vein Thrombosis
Symptomatic venous thrombosis occurs in up to 5% of patients.26,27 However, the incidence of superior vena cava (SVC) obstruction with symptomatic SVC syndrome is rare, approximating 2 per 1000 implants. Treatment is usually based on symptom relief. Acute deep vein thrombosis (DVT) after implantation can occur in up to 40% of cases, but most are asymptomatic. When subclavian occlusions have not presented with symptoms, they are usually detected when a new lead is required because of lead dysfunction or mode-upgrade procedures. In patients with symptoms, arm elevation is advised and, depending on the severity, anticoagulation started with either warfarin alone or with heparin. Again, the use of factor X antagonists has not been explored.
 Infection
Infection
Increased implantation of CIEDs has led to a greater awareness of implant infection. The increasing incidence of CIED infections, now reported at 1% to 7%, presumably is a result of the increasing number of nonprimary implantations (e.g., generator changes, upgrades). Infection occurs in 1.9 to 2.11 per 1000 device-years, with most infections being pocket infections.28 CIED infection is a serious problem with high morbidity and mortality. In the LExICon study the all-cause in-hospital mortality in infected patients was 3%, with the highest mortality associated with device-related endocarditis (4.3%).29 Tarakji et al.30 demonstrated all-cause mortality as high as 4.6% in infected patients. After extraction of the infected device, mortality remained high in that cohort, with 1-year mortality after removal of 17%. One-year mortality was significant in both the pocket infection group (12%) and the endovascular infection group (25%).
Risk Factors
Patient-associated risk factors for infection include diabetes mellitus, renal failure, and heart failure. In one study, oral anticoagulation with the development of pocket hematoma increased the risk of infection, although this has not been confirmed in larger studies.31
Procedural factors associated with infection are reoperation, prolonged procedure time, and previous temporary pacing. In the long term, generator changes and device upgrades continue to pose significant risk for increasing rates of infection.28
Prevention
Because of the high morbidity and mortality associated with CIED infection, especially in the heart failure population, who also tend to have comorbidities, prevention of infection is probably the most important aspect of device therapy. Universal infection prevention techniques, including hand scrubbing, procedure room and instrument preparation, and double gloving should be observed during CIED implantation. Skin preparation is crucial, but even with skin preparation and periprocedural antibiotics, the overall infection rate during replacement procedures was 1.4% in the Replace Registry.13 Preimplantation antibiotic administration is standard care and has been recently reinforced. In a randomized double-blind study of 649 patients, de Oliveira et al.32 demonstrated that 1.0 g of cefazolin given before CIED implantation was associated with an 80% decrease in infections at 6-month follow-up. The infection rate was 0.64% in the cefazolin group and 3.28% in the control group (P = .016). All infections were caused by Staphylococcus species, with about two-thirds being S. aureus.32
The choice of antibiotic agent depends on the patterns of infections and antibiotic resistance in individual treatment centers. Approximately half of all device-related infections are caused by methicillin-resistant S. aureus (MRSA). At our institution, we recommend the use of vancomycin for preoperative antibiotic prophylaxis. We also screen patients for MRSA using nasal swabs and, if the test is positive, pretreat patients with mupirocin. Although not proven in the literature on CIED use, several studies of patients undergoing cardiac surgery have shown that treatment of carriers of MRSA with mupirocin decreases the rate of postoperative infection.33 No matter what antibiotic is used, it is important to have the infusion completed by the time of the surgical incision and initiated within 1 hour if agent is not vancomycin and within 2 hours if vancomycin.34–36 Not only is this important to reduce the incidence of postsurgical infections. but it is a U.S. national quality measure.37
Presentation
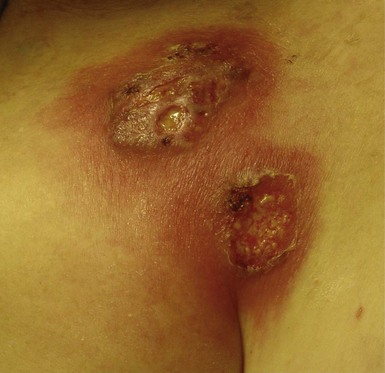
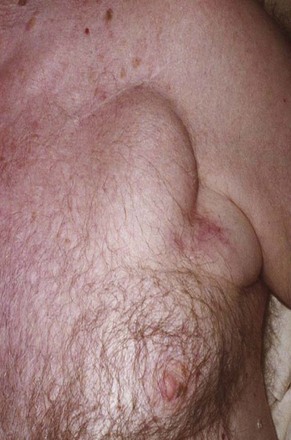
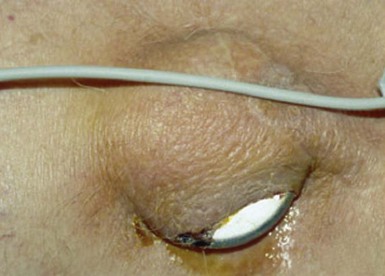
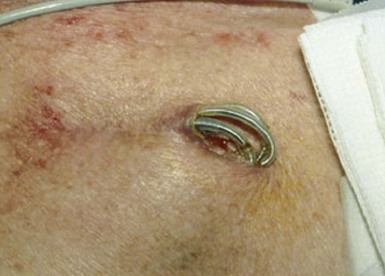
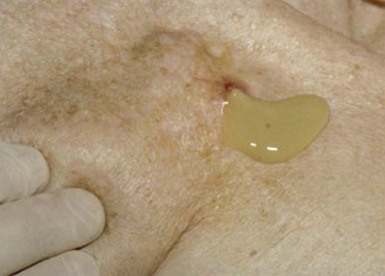
The most common presentation of infection continues to be pocket infection with a variety of appearances (Figs. 25-5 to 25-9). In a recent study by Tarakji et al.,30 89% of patients presented with pocket infection compared to patients with systemic signs of infection with an intact pocket. This is in line with historical data. Patients with pocket infection tend to present earlier, whereas patients with systemic infection have more comorbidities, such as heart failure, renal failure, diabetes, or previous episodes of endocarditis.
Pocket infection has a diverse and continuum of presentation. Although frank, purulent discharge is the obvious presentation, pocket infection should be considered present with warmth, erythema, dehiscence, and skin adherence. Erosion of the overlying skin is by definition a pocket infection. It is important to recognize that patients with pocket infection can also harbor endovascular vegetations (Fig. 25-10), Tarakji et al.30 reported that vegetations were detected in almost half the patients (56) in whom transesophageal echocardiography (TEE) was performed in a group of 241 patients with pocket infection. Klug et al.35,38,39 reported evidence of intravascular system involvement in at least 88% of patients with pocket infection. As such, as deduced by the Cleveland Clinic group, pocket infections should be considered a manifestation of an infected system encompassing endovascular infection, rather than only a localized phenomenon.30 CIED infection can also have systemic manifestations, including fever, chills, endocarditis, and persistent bacteremia.
Bacteriology
Staphylococcus species cause most implant infections, with some series reporting 80%. In cases of coagulase-negative staphylococcal infection, it is important to rule out contamination by repeat cultures. Organisms such as Pseudomonas, Klebsiella, Proteus, and Enterococcus are seen less frequently (8%). Fungi and acid-fast mycobacteria are present on rare occasions. Some of these organisms may have caused the infection, and some may be secondary contaminants (e.g., yeast). Infections caused by a mycobacterium are rare, dangerous, and difficult to treat.40–47
1 Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301:1661-1670.
2 Al-Khatib SM, Greiner MA, Peterson ED, et al. Patient and implanting physician factors associated with mortality and complications following implantable cardioverter-defibrillator implantation, 2002-2005: Al-Khatib—patient and physician factors and ICD outcomes. Circ Arrhythm Electrophysiol. 2008;1:240-249.
3 Magney JE. Anatomical mechanisms that cause lead and catheter damage. Pacing Clin Electrophysiol. 1996;19:257-258.
4 Roelke M, O’Nunain SS, Osswald S, et al. Subclavian crush syndrome complicating transvenous cardioverter defibrillator systems. Pacing Clin Electrophysiol. 1995;18:973-979.
5 Ramza BM, Rosenthal L, Hui R, et al. Safety and effectiveness of placement of pacemaker and defibrillator leads in the axillary vein guided by contrast venography. Am J Cardiol. 1997;80:892-896.
6 Belott P. How to access the axillary vein. Heart Rhythm. 2006;3:366-369.
7 Ellis CR, Rottman JN. Increased rate of subacute lead complications with small-caliber implantable cardioverter-defibrillator leads. Heart Rhythm. 2009;6:619-624.
8 Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6(5):605-610.
9 Farwell D, Green MS, Lemery R, et al. Accelerating risk of Fidelis lead fracture. Heart Rhythm. 2008;5:1375-1379.
10 Krahn AD, Champagne J, Healey JS, et al. Outcome of the Fidelis implantable cardioverter-defibrillator lead advisory: a report from the Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2008;5:639-642.
11 Wilkoff BL. Lead failures: dealing with even less perfect. Heart Rhythm. 2007;4:897-899.
12 Hauser RG, Kallinen LM, Almquist AK, et al. Early failure of a small-diameter high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2007;4:892-896.
13 Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results From the Replace Registry. Circulation. 2010;122:1553-1561.
14 Association for the Advancement of Medical Instrumentation: AAMI DF-4 Standard. 2010.
15 Ellenbogen KA, Hellkamp AS, Wilkoff BL, et al. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003;92:740-741.
16 Danik SB, Mansour M, Singh J, et al. Increased incidence of subacute lead perforation noted with one implantable cardioverter-defibrillator. Heart Rhythm. 2007;4:439-442.
17 Epstein AE, Baker JH2nd, Beau SL, et al. Performance of the St. Jude Medical Riata leads. Heart Rhythm. 2009;6:204-209.
18 Khan MN, Joseph G, Khaykin Y, et al. Delayed lead perforation: a disturbing trend. Pacing Clin Electrophysiol. 2005;28:251-253.
19 Porterfield JG, Porterfield LM, Kuck KH, et al. Clinical performance of the St. Jude Medical Riata defibrillation lead in a large patient population. J Cardiovasc Electrophysiol. 2010;21:551-556.
20 Burri H, Sunthorn H, Dorsaz PA, et al. Thresholds and complications with right ventricular septal pacing compared to apical pacing. Pacing Clin Electrophysiol. 2007;30(suppl 1):75-78.
21 Rosso R, Medi C, Teh AW, et al. Right ventricular septal pacing: a comparative study of outflow tract and mid ventricular sites. Pacing Clin Electrophysiol. 2010;33:1169-1173.
22 Thal S, Moukabary T, Boyella R, et al. The relationship between warfarin, aspirin, and clopidogrel continuation in the peri-procedural period and the incidence of hematoma formation after device implantation. Pacing Clin Electrophysiol. 2010;33:385-388.
23 Kutinsky IB, Jarandilla R, Jewett M, et al. Risk of hematoma complications after device implant in the clopidogrel era. Circ Arrhythm Electrophysiol. 2010;3:312-318.
24 Tompkins C, Cheng A, Dalal D, et al. Dual antiplatelet therapy and heparin “bridging” significantly increase the risk of bleeding complications after pacemaker or implantable cardioverter-defibrillator device implantation. J Am Coll Cardiol. 2010;55:2376-2382.
25 Giudici MC, Paul DL, Bontu P, et al. Pacemaker and implantable cardioverter-defibrillator implantation without reversal of warfarin therapy. Pacing Clin Electrophysiol. 2004;27:358-360.
26 Barakat K, Robinson NM, Spurrell RA. Transvenous pacing lead-induced thrombosis: a series of cases with a review of the literature. Cardiology. 2000;93:142-148.
27 Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. Pacing Clin Electrophysiol. 2002;25:1605-1611.
28 Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458-477.
29 Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579-586.
30 Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043-1047.
31 Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715-720.
32 De Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29-34.
33 Trautmann M, Stecher J, Hemmer W, et al. Intranasal mupirocin prophylaxis in elective surgery: a review of published studies. Chemotherapy. 2008;54:9-16.
34 Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851-1859.
35 Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349-1355.
36 Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97(18):1796-1801.
37 Centers for Medicare and Medicaid Services (CMS): Specifications manual for national hospital inpatient quality measures, version 3, 2009.
38 Klug D, Wallet F, Kacet S, et al. Detailed bacteriologic tests to identify the origin of transvenous pacing system infections indicate a high prevalence of multiple organisms. Am Heart J. 2005;149(2):322-328.
39 Klug D, Wallet F, Kacet S, et al. Involvement of adherence and adhesion Staphylococcus epidermidis genes in pacemaker lead-associated infections. J Clin Microbiol. 2003;41:3348-3350.
40 Sharma S, Tleyjeh IM, Espinosa RE, et al. Pacemaker infection due to Mycobacterium fortuitum. Scand J Infect Dis. 2005;37:66-67.
41 Verghese S, Mullaseri A, Padmaja P, et al. Pacemaker implant site infection caused by atypical mycobacteria. Indian Heart J. 1998;50(2):201-202.
42 Toda H, Sato K, Iimori M, et al. [A case of Mycobacterium goodii infection with isolation from blood and a pacemaker lead]. Kansenshogaku Zasshi. 2006;80:262-266.
43 Tam WO, Yew WW, Yam WC, et al. Pacemaker infections due to rapidly growing mycobacteria: further experience. Int J Tuberc Lung Dis. 2007;11:118.
44 Kestler M, Reves R, Belknap R. Pacemaker wire infection with Mycobacterium tuberculosis: a case report and literature review. Int J Tuberc Lung Dis. 2009;13:272-274.
45 Kessler AT, Kourtis AP. Mycobacterium abscessus as a cause of pacemaker infection. Med Sci Monit. 2004;10:CS60-CS62.
46 Cutay AM, Horowitz HW, Pooley RW, et al. Infection of epicardial pacemaker wires due to Mycobacterium abscessus. Clin Infect Dis. 1998;26:520-521.
47 Chrissoheris MP, Kadakia H, Marieb M, et al. Pacemaker pocket infection due to Mycobacterium goodii: case report and review of the literature. Conn Med. 2008;72:75-77.