Preoperative Staging of Ocular Surface Disease
Ocular Factors
Extent of Limbal Stem Cell Deficiency
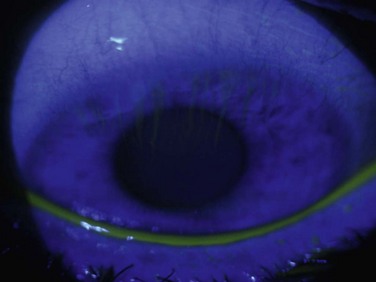
Limbal stem cell deficiency (LSCD) may be partial or total. In congenital aniridia or in diseases with ongoing chronic inflammation, partial LSCD can progress over time to total LSCD. As seen in Figure 38.1, a patient with a severe alkaline chemical injury progresses over time from active conjunctival inflammation to partial LSCD and then total LSCD. If the visual axis remains unaffected in partial LSCD, the patient may not require transplantation and may be treated medically and monitored for progression. In quiet eyes, with small areas of conjunctival invasion, patients may benefit from sequential conjunctival epitheliectomy in the hope that the residual normal stem cells may repopulate the ocular surface. If several clock hours or more of LSCD exists, then a sectoral OSST may be used to treat that area of LSCD. In total LSCD, the only treatment options are complete OSST for restoration of the ocular surface with or without optical keratoplasty, or a KPro for visual rehabilitation.
Extent of Conjunctival Involvement
Activity of Inflammation
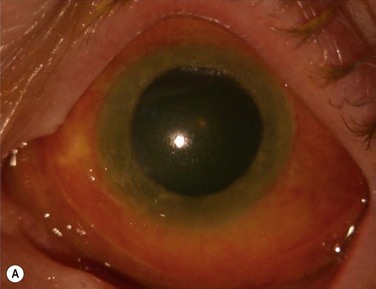
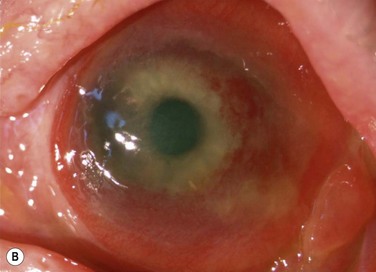
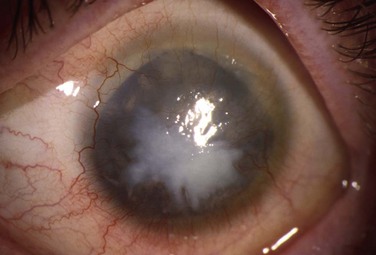
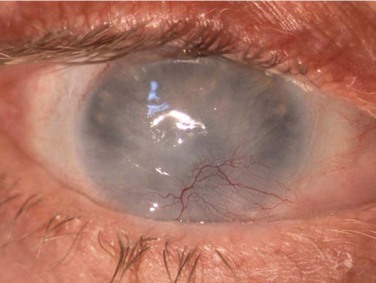
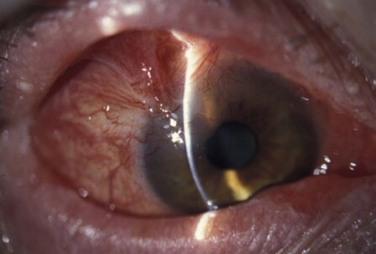
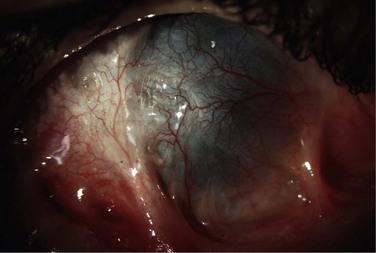
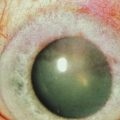
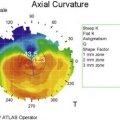
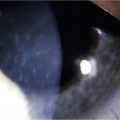
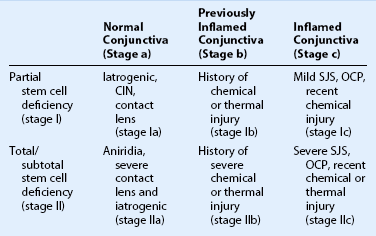
Schwartz et al.1 proposed a classification system of ocular surface disease based on the extent of stem cell deficiency (stage I or II) and the presence or absence of conjunctival inflammation (stage a, b, or c) (Table 38.1). CLIK and aniridia are examples of stage Ia or IIa disease with partial/total stem cell deficiency but normal conjunctiva, as shown in Figures 38.2 and 38.3. A quiet eye with previous chemical or thermal injury would be an example of either stage Ib or IIb, depending on the extent of limbal stem cell loss, as shown in Figure 38.4. A chronically inflamed eye from OCP or SJS would be an example of either stage Ic or IIc disease, as shown in Figures 38.5 and 38.6.
Table 38.1
Classification of Ocular Surface Disease
(Adapted from Schwartz GS et al. Ocular surface disease: medical and surgical management. New York: Springer-Verlag; 2002.)
Keratolimbal allograft (KLAL) replaces limbal stem cells only, whereas a living-related conjunctival limbal allograft (lr-CLAL) replaces both limbal stem cells and conjunctival goblet cells. Therefore, patients with conjunctival, as well as limbal involvement may benefit more from an lr-CLAL or combined KLAL/lr-CLAL (the Cincinnati procedure)2 as opposed to a KLAL alone to help increase mucin production for a healthier ocular surface.
Tear Film Abnormalities
A healthy tear film is essential for epithelial healing after OSST and for prevention of corneal melts after KPro surgery. Tears contain growth factors, immunoglobulins, and electrolytes, and prevent desiccation of the ocular surface.3 The mucin component of tears is produced by the goblet cells of the conjunctiva, which may be deficient in conjunctival disease. The aqueous component is produced by the glands of Wolfring and Krause, which may be damaged by conjunctival disease causing subepithelial fibrosis and forniceal foreshortening. Similarly, conjunctival fibrosis may scar the lacrimal ductules that secrete aqueous from the lacrimal gland, further compounding aqueous deficiency. The lipid component may be deficient from damage caused from scarring of the tarsus.
Extent of Stromal Scarring
More than half the patients undergoing stem cell transplantation will require a future keratoplasty for visual rehabilitation.4 A keratoplasty may not be necessary if the corneal scarring is superficial and removed with the superficial keratectomy when performing stem cell transplantation. In diseases characterized by progressive LSCD, such as aniridia, the authors recommend that OSST be performed prior to the onset of stromal scarring in patients with significant epithelial disease.5 If corneal transplantation is necessary and the endothelium is normal, then a deep anterior lamellar keratoplasty (DALK) is preferred over a penetrating keratoplasty (PK). The epithelium should be allowed to heal completely, and ocular surface inflammation allowed to subside before keratoplasty is performed. Patients should be informed of the longer time-frame required to achieve visual improvement with OSST and keratoplasty, compared to the more immediate visual improvement with KPro.
Mechanical Eyelid Problems
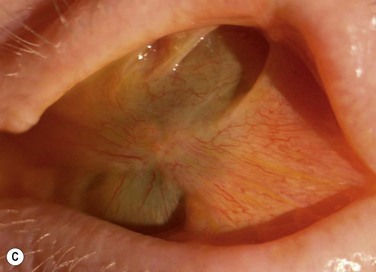
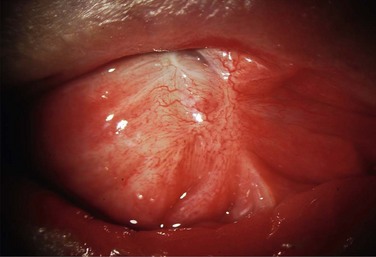
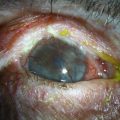
Conjunctival inflammation and scarring may cause foreshortening of the fornices, symblepharon, ankyloblepharon, distichiasis, trichiasis, entropion or ectropion. Disorders of the lid–lash complex may cause microtrauma and perpetuate chronic inflammation through the mechanical rubbing of lashes against the ocular surface.6 Ectropion or lagophthalmos can cause exposure keratopathy and delay healing post-OSST, or predispose to corneal melting post-KPro surgery. Symblepharon and loss of fornices make it difficult to retain a bandage contact lens that is necessary to prevent corneal melting after KPro implantation. Figure 38.7 shows symblepharon formation after chemical injury.
Other Ocular Disease: Glaucoma and Retinal disease
Ocular stem cell transplantation (OSST) and KPro surgery may cause glaucoma or contribute to the progression of pre-existing glaucoma. The UC Davis study7 showed that glaucoma is the major cause of long-term vision loss after KPro implantation. Many patients have pre-existing glaucoma from diseases, such as congenital aniridia or chemical/thermal injuries and may already have had surgical treatment, usually in the form of a glaucoma drainage device. Patients should be assessed by a glaucoma specialist preoperatively, and their IOP should be well controlled prior to OSST or KPro surgery. Given the difficult in measuring IOP after KPro surgery and the high risk of developing glaucoma, some surgeons have a lower threshold for placement of a glaucoma drainage device prior to KPro implantation. End-stage glaucoma is a contraindication for OSST and KPro surgery, since it is possible that postoperative spikes in IOP may snuff out a borderline optic nerve.
Non-Ocular Factors
Although elderly patients are likely to have more co-morbidities, many do not require immunosuppression for OSST, since they have a dampened immune response. In the authors’ experience, younger age is the strongest risk factor for OSST rejection.4
Systemic Health
Immunosuppression is necessary to prevent immune rejection in OSST. Immunosuppression can be co-managed with a renal transplant team or rheumatologist using a steroid-sparing regimen of tacrolimus and mycophenolate mofetil.8 Absolute contraindications for systemic immunosuppression are patients with a history of malignancy within 5 years, as well as significant co-morbidities, such as diabetes, uncontrolled hypertension, renal insufficiency, congestive heart failure, and other organ failure.
Personality Factors
Patients who are candidates for OSST or KPro surgery must be willing and able to comply with long-term regular follow-up. Patients undergoing OSST must be compliant with their immunosuppression medication and laboratory follow-up. Noncompliance with immunosuppression has been shown to increase the risk of immune rejection and surface failure.4 Patients undergoing KPro implantation must be compliant with bandage contact lens wear and the use of long-term prophylactic antibiotics. Patients who have been noncompliant with previous treatment or who do not comprehend the importance of complying with follow-up and medications should not undergo OSST or KPro surgery.
References
1. Schwartz, GS, Gomes, JAP, Holland, EJ. Preoperative staging of disease severity. In: Holland EJ, Mannis MJ, eds. Ocular surface disease: medical and surgical management. New York: Springer-Verlag; 2002:158–167.
2. Biber, JM, Skeens, HM, Neff, KD, et al. The Cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
3. Management and Therapy Subcommittee members of the International Dry Eye Workshop. Management and therapy of dry eye disease: Report of the Management and Therapy Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:163–178.
4. Ang, AY, Chan, CC, Biber, JM, et al. Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea. 2013;32:229–236.
5. Holland, EJ, Djalilian, AR, Schwartz, GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–130.
6. Di Pascuale, MA, Espana, EM, Liu, DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens–Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912.
7. Greiner, MA, Li, JY, Mannis, MJ. Longer-term vision outcomes and complications with the Boston Type 1 keratoprosthesis and the University of California, Davis. Ophthalmology. 2011;118:1543–1550.
8. Holland, EJ, Mogilishetty, GM, Skeens, HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year experience. Cornea. 2012;31:655–661.