CHAPTER 9 Preoperative Preparation
In addition to having a sound understanding of a child’s medical disease and anticipated surgical procedure, the anesthesiologist must also appreciate the emotional stresses that affect both the child and parent. The preoperative meeting with the patient and his or her parents is not only a responsibility of the anesthesiologist but also an important opportunity to learn facts that could otherwise be missed. It is a chance to win the confidence of the patient and the parents, if they are present (Fig. 9-1).
A careful preoperative examination of the child and the child’s medical record enables the anesthesiologist to assess the child’s general state of health and to identify the presence of chronic, acute, or intercurrent diseases, as well as to recognize previous anesthetic problems (Black, 1999). From this knowledge, appropriate subspecialty consultation can be sought, the operative medical condition can be optimized for the surgery, and the anesthetic plans can be made. In addition to monitoring practices and anesthetic techniques, anesthetic plans should include provisions for the patient’s postoperative care, particularly an analgesic plan. It is the general goal of the preanesthetic visit to anticipate potential complications before they occur, to avert them when possible, and, in so doing, to minimize the risks to the health of the child. The risk of anesthesia is assessed during the preoperative visit, and the child’s parents should be informed of the plans for anesthesia and monitoring, and they should be apprised of the anticipated risk.
Preanesthetic Visit
The preanesthetic visit should begin with a careful review of the medical record; particular attention should be paid to previous anesthetic agents and problems encountered, the successful and unsuccessful techniques used in the past for airway management, and any history of cardiorespiratory diseases or airway anomalies. A history of medical or environmental allergies should be elicited, and it should include questions specifically directed toward evaluating the presence of allergy to latex in children at risk, notably those with meningomyelocele or urogenital anomalies, those who undergo bladder self-catheterization, or those whose medical histories indicate a significant amount of latex exposure in the past (Holzman 1997; Porri et al., 1997; Hollnberger et al., 2002; Pires et al., 2002; Eustachio et al., 2003; Dehlink et al., 2009; Rendeli et al., 2006; Bostancy et al., 2007; Dieguez et al., 2007; Garcia 2007; Baker and Hourihane, 2008; De Queiroz et al., 2009). Results of laboratory tests should be reviewed, focusing on hematologic evaluations, renal function, and electrolyte profiles, as well as blood gas analysis and pulmonary function tests when appropriate.
The anesthesiologist must be aware of the child’s current drug therapy and how it may interact with the anesthetic. The perioperative administration of bronchodilators, cancer chemotherapeutic agents, or anticholinesterases has significant implications for anesthesia (Schein and Winoker, 1975; Selvin, 1981; Drummond, 1984). Corticosteroid administration is traditionally recommended for patients who receive chronic corticosteroid therapy and for patients who have received steroids in the past, although evidence for the necessity of doing so is lacking (see Chapter 36, Systemic Disorders). Current drug therapy must also include questions regarding the use of herbal medications. Potential complications in the perioperative period have been attributed to the use of complementary medicines. Table 9-1 summarizes the most commonly used herbal remedies (Ang-lee et al., 2001).
TABLE 9-1 Pharmacologic Effects and Potential Perioperative Complications of Eight Commonly Used Herbal Remedies
| Name of Herb | Common Uses | Potential Perioperative Complications |
| Echinacea, purple cone flower root | Prophylaxis and treatment of viral, bacterial, and fungal infections | Reduced effectiveness of immunosuppressants; potential for wound infection; may cause hepatotoxicity when used with other hepatotoxic drugs |
| Ephedra, ma-huang | Diet aid | Dose-dependent increase in heart rate and blood pressure; arrhythmias with halothane; tachyphylaxis with intraoperative ephedrine |
| Garlic, ajo | Antihypertensive, lipid-lowering agent, anti-thrombus forming | May potentiate other platelet inhibitors; perioperative bleeding |
| Ginkgo, maidenhair; fossil tree | Circulatory stimulant; Alzheimer’s disease, peripheral vascular disease, and erectile dysfunction | May potentiate other platelet inhibitors; perioperative bleeding |
| Ginseng | To protect the body against stress and restore homeostasis | Perioperative bleeding; potential for hypoglycemia |
| Kavakava, pepper | Anxiolytic | Potentiates sedative effects of anesthetic agents; possible withdrawal syndrome after sudden abstinence; kavakava-induced hepatotoxicity |
| St. John’s wort, goatweek, amber, hardhay | Treatment for depression and anxiety | Decreased effectiveness of cyclosporine, alfentanil, midazolam, lidocaine, calcium channel blockers, and digoxin |
| Valerian, vandal root, all heal | Anxiolytic and sleep aid | Potentiates sedative effects of anesthetic agents; withdrawal-type syndrome with sudden abstinence |
From Skinner CM, Rangasami J: Preoperative use of herbal medicines: a patient survey, Br J Anaesth 89:792-795, 2002.
Many unusual syndromes occur in childhood, and they often have multisystem involvement; consequently, they have an important impact on anesthetic management. An important caveat in pediatric medicine is that when one congenital anomaly exists, there is a significant likelihood of anomalies involving other organs. For example, infants with tracheoesophageal fistulas have an increased incidence of congenital heart disease, and some forms of radial dysplasia are associated with thrombocytopenia or atrial septal defects. The topic of congenital anomalies was extensively discussed in a review by Lynn (1985). The remainder of this section is a review of pediatric diseases that may be important to the anesthesiologist. Information regarding these problems may be forthcoming from the child’s medical history, the physical examination, or both.
Physical Examination
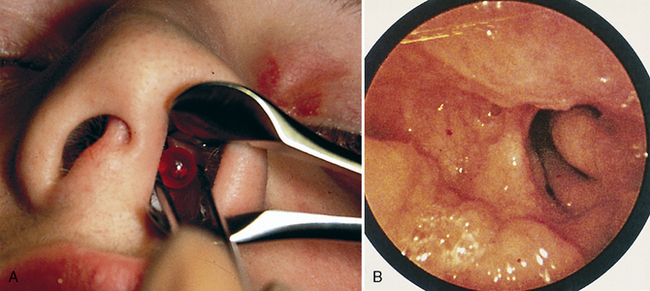
Partial airway obstruction may result from infection, anatomic anomalies, or tumors. When possible, a diagnosis should be made before anesthesia is begun. Unilateral nasal discharge is unusual and suggests a foreign body (or, less often, choanal atresia) (Fig. 9-2).
Review of Body Systems (Table 9-2)
Central Nervous System
TABLE 9-2 Medical History and Review of Systems: Anesthetic Implications
| System | History | Potential Anesthetic Implication |
| Central nervous and neuromuscular systems | Seizure Head trauma Hydrocephalus Central nervous system tumor Developmental delay Neuromuscular disease Muscle disease |
Medications: drug interactions, inadequate anticonvulsant therapy, drug-induced hepatopathology Elevated intracranial pressure Anemia Elevated intracranial pressure Elevated intracranial pressure Chemotherapeutic drug interactions History of steroid useBulbar dysfunction Risk of aspiration Altered response to relaxants Risk of malignant hyperthermia Risk of rhabdomyolysis and hyperkalemia |
| Cardiovascular system | Heart murmur Cyanotic heart defect History of squatting Diaphoresis with feeding or crying Hypertension |
Risk of right-to-left air embolism of intravenous air bubbles Need for SBE prophylaxis Right-to-left cardiac shunt Risk of right-to-left air embolism of intravenous air bubbles Hemoconcentration Need for SBE prophylaxis Teratology of Fallot Congestive heart failure Coarctation of the aorta, renal disease, or pheochromacytoma |
| Respiratory system | Prematurity Bronchopulmonary dysplasia Respiratory infection, cough Croup Snoring Asthma Cystic fibrosis |
Increased risk of postoperative apneaLower airway obstruction Reactive airways disease Subglottic stenosis Pulmonary hypertension Reactive airways and bronchospasm Medication history Subglottic stenosis or anomaly Obstructive sleep apnea Perioperative airway obstructionβ-Agonist or theophylline therapy History of steroid useDrug interactions Pulmonary toilet Pulmonary dysfunction and VQ mismatch Reactive airways disease |
| Gastrointestinal/hepatic systems | Vomiting, diarrhea Growth failure Gastroesophageal reflux JaundiceLiver transplant recipient |
Electrolyte abnormality, especially hypokalemia Dehydration Risk of aspiration Low glycogen reserves/risk of hypoglycemia AnemiaRisk of aspiration Reactive airways disease Anemia Altered drug metabolism Risk of hypoglycemia Coagulopathy Altered drug metabolism Immunosuppression Coagulopathy |
| Renal system | Frequency, nocturia Renal failure/dialysis Kidney transplant recipient |
Occult diabetes mellitus Electrolyte disturbance Urinary sepsis Electrolyte disturbance Hypervolemia or hypovolemia Anemia Medication history Immunosuppression Poor toleration of hypotension Hypertension |
| Endocrine system | Diabetes Steroid therapy |
Insulin requirement Intraoperative hyperglycemia or hypoglycemia Adrenocorticoid suppression |
| Genitourinary system | Pregnancy | Teratogenic effects Risk of spontaneous abortion |
| Hematologic system | Anemia Bruising, history of bleeding Sickle cell disease Human immunodeficiency virus infection |
Transfusion requirement Occult hemoglobinopathy Coagulopathy Anemia Need for hydration Limb tourniquet use Susceptibility to infection Infectious risk to medical personnel |
| Dental system | Loose primary teeth | Risk of aspiration if tooth avulsed |
SBE, Subacute bacterial endocarditis; VQ, ventilation perfusion.
Modified from Coté CJ, Todres ID, Ryan JF: Preoperative evaluation of pediatric patients. In Ryan JF, Todres ID, Coté CJ, et al., editors: A practice of anesthesia for infants and children, New York, 1986, Grune & Stratton. (With permission from Elsevier.)
Trauma is the most common cause of death in children, and most fatal trauma involves injury to the CNS. Head injuries often result in an altered level of consciousness, cerebral edema, and elevated intracranial pressure. Tumors of the brain are the most common solid tumors of childhood and usually occur in the posterior fossa. They generally increase intracranial pressure as a mass effect but also often obstruct cerebrospinal fluid pathways, resulting in hydrocephalus. The anesthetic care of children with elevated intracranial pressure is discussed in Chapter 22, Anesthesia for Neurosurgery.
Neuromuscular diseases, such as congenital myotonia, muscular dystrophy, and the various forms of myositis, contraindicate the use of succinylcholine even in emergency airway management, although it is rarely used in current pediatric anesthetic practice (see Chapter 36, Systemic Disorders). In myotonia, succinylcholine produces a sustained contracture of skeletal muscle that may impede the ability to maintain a patent airway and ventilate the lungs. In other myopathies, such as clinically active dermatomyositis, succinylcholine produces life-threatening hyperkalemia. Some forms of muscular dystrophy (central core disease) are statistically associated with malignant hyperthermia, whereas others may result in a malignant hyperthermia-like syndrome that is equally life threatening (Guis et al., 2004; Rosenberg et al., 2007; Driessen and Snoeck, 2008; Hayes et al., 2008; Puel et al., 2008; Takagi and Nakase, 2008; Schwartz and Raghunathan, 2009). Although not all children with Duchenne’s or other muscular dystrophies are genetically susceptible, Rosenberg and Heiman-Patterson (1983), as well as Takagi and Nakase (2008), recommend that precautions against malignant hyperthermia be taken in patients with this disorder because of rhabdomyolysis and hypermetabolism that may occur in myopathic children after exposure to triggering agents. For further discussion please refer to Chapter 37, Malignant Hyperthermia.
Cardiovascular System
Asymptomatic cardiac murmurs occasionally have implications for anesthesia. If they represent small ventricular septal defects or mild valvular disease, prophylaxis against bacterial endocarditis is indicated for procedures that may result in bacteremia, such as dental surgery, gastrointestinal or urogenital endoscopy, and nasotracheal intubation (Wilson et al., 2007). Atrial septal defects contraindicate the use of the sitting position for suboccipital craniotomies in order to minimize the risk of paradoxical air embolism (Fischler, 1992). The defects may also make intraoperative transesophageal echocardiography desirable in certain cases that have been associated with venous air embolism (e.g., posterior spine fusions or liver transplantation), so the movement of air from the pulmonary to the systemic circulation may be detected. If the anesthesiologist detects a previously undescribed murmur in these circumstances, a consultation with the cardiologist is indicated to further delineate the nature of the lesion (Fig. 9-3). Many congenital anomalies and syndromes are associated with cardiac defects or other cardiovascular problems; Box 9-1 provides an outline of these conditions.
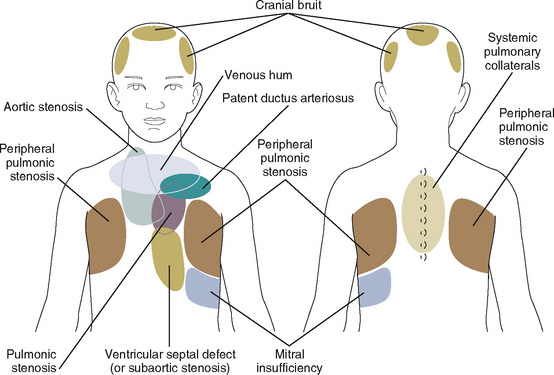
FIGURE 9-3 Sites for auscultation where murmurs are heard best.
(From Beerman LB, Kreutzer J, Park SC: Cardiology. In Zitelli J, Davis HW, editors: Atlas of pediatric physical diagnosis, ed 5, St Louis, 2007, Mosby.)
Box 9-1 Pediatric Syndromes Associated with Cardiac Conditions
Syndromes Associated With Congenital Heart Disease
Respiratory System
Chapter 3, Respiratory Physiology in Infants and Children, describes the anatomic and physiologic differences between the pediatric and adult respiratory systems. The differences in dimension and function predispose the child to perioperative airway obstruction, which mandates a critical preoperative evaluation of the airway. The upper airway of the child may be further compromised by many entities, including: tonsillar or adenoidal hypertrophy or both; craniofacial anomalies such as Crouzon’s disease, Apert’s syndrome, hemifacial microsomia, Goldenhar’s syndrome, Treacher Collins syndrome (or Pierre Robin syndrome); lingular hypertrophy (common in trisomy 21); Beckwith’s syndrome and the various forms of mucopolysaccharidosis (Hurler’s syndrome and Hunter’s syndrome being the most common); isolated airway anomalies, such as cleft palate, laryngeal web or cleft, laryngomalacia, or subglottic stenosis; or tumors, such as hemangiomas and lymphangiomas, which may occur anywhere along the airway. Cutaneous cervicofacial hemangiomas along a beard distribution are suggestive of an association with upper airway or subglottic hemangiomas (Orlo et al., 1997).
Acute upper respiratory tract infections provide a common dilemma for the anesthesiologist. The mere volume of publications that evaluate the risk of adverse events of anesthesia in children with upper airway infections speaks to the ongoing controversy (Tait and Malviya, 2005). In the best of all worlds, no child would be anesthetized electively during an acute respiratory illness. Although not all studies have identified acute respiratory illness as a cause of perioperative complications in children, there is compelling evidence that the occurrence of both intraoperative and postoperative hypoxemia and other airway complications are increased in children with upper respiratory tract infections (DeSoto et al., 1988; Cohen and Cameron, 1991; Kinouchi et al., 1992; Levy et al., 1992; Rolf and Coté, 1992; Parnis et al., 2001; Bordet et al., 2002; Elwood et al., 2003; Tait, 2005). There is also evidence that the incidence of bronchospasm is increased in the presence of upper respiratory infections in children who are intubated (Rolf and Coté, 1992; Rachel Homer et al., 2007; von Ungern-Sternberg et al., 2007). In a prospective study, Tait and colleagues (2001) noted that endotracheal intubation, a history of prematurity, reactive airways disease, parental smoking, airway surgery, and nasal congestion are all risk factors associated with respiratory complications in infants and children who have upper respiratory infections and who are undergoing anesthesia. Furthermore, the child with an acute respiratory disease exposes other patients and health care workers to their contagion, which may not be a trivial concern when these individuals are immunocompromised.
Other considerations, however, must be taken into account before making the decision to postpone surgery. For example, the relatively small risk to the child must be weighed against the expense and effort the family has made to come to the hospital, often from a distant locale and at the cost of lost income. Some children, particularly those seen for otolaryngologic surgery, appear to never be free from respiratory infections during much of the year. Postponement of surgery may not be practical in these circumstances. Indeed, one study indicates that myringotomy is therapeutic in these children and is not associated with an increased incidence of postoperative pulmonary complications (Tait and Knight, 1987).
Chronic diseases of the lower respiratory tract occur in both children and adults. Asthma and cystic fibrosis are the most common chronic pulmonary diseases of childhood. A careful history and physical examination usually suffice in the preoperative evaluation of these diseases. If preoperative impairment is severe, however, or if the planned surgery is extensive, formal pulmonology consultation and pulmonary function testing may provide the anesthesiologist with information that can be used to provide optimal postoperative care. Children with asthma are commonly medicated with β2-adrenergic agents and inhaled corticosteroids. Other first-line drugs include cromolyn sodium and leukotriene receptor antagonists; rarely are theophylline preparations administered at this time. However, when theophylline is a part of the patient’s treatment, serum concentration of theophylline should be measured preoperatively to ensure blood levels in a therapeutic range (10-20 mcg/mL), and the anesthesiologist should be aware of potential interactions among theophylline, β2-adrenergic drugs, and halothane (although it is not often used). Asthmatic children who receive corticosteroids should also be considered for perioperative therapy with stress doses of corticosteroids if steroid therapy has been recent, although as previously stated, the evidence to support this convention is lacking. For those children who have required systemic steroids in the past, a short course of steroids beginning 1 to 2 days before the day of surgery may be beneficial (see Chapter 36, Systemic Disorders).
Severe kyphoscoliosis typically leads to significant restrictive lung disease. This is particularly true in cases of kyphoscoliosis that occur before the teenage years. Particularly in this age group, the cause of the kyphoscoliosis should be assessed, because it typically results from neuromuscular diseases such as cerebral palsy or muscular dystrophy or from anatomic anomalies such as hemivertebrae, which may be part of a syndrome that is associated with other congenital anomalies of importance to the anesthesiologist (e.g., VATER association). Preoperative testing of pulmonary function may be useful in predicting which children will require admission to an intensive care unit with or without mechanical ventilation postoperatively, but usually the decision to mechanically ventilate patients after spinal surgery is based on general preoperative condition, duration and difficulty of surgery, blood loss, and other surgical factors, rather than on the test of pulmonary function itself (see Chapter 26, Anesthesia for Orthopedic Surgery).
An infant who was born prematurely is often left with a residual chronic obstructive pulmonary disease called bronchopulmonary dysplasia, the consequence of both oxygen toxicity and ventilator-induced lung injury to immature lungs. The incidence and severity of this disabling condition have been dramatically reduced by the use of surfactant in neonatal intensive care units. Children with bronchopulmonary dysplasia exhibit a combination of fibrotic and cystic changes in the lung parenchyma with reactive small airways disease, with or without wheezing and air trapping. These children may respond to steroids and bronchodilators in varying degrees. More advanced bronchopulmonary dysplasia is associated with chronic hypoxia, carbon dioxide retention, pulmonary hypertension, and ultimately cor pulmonale (Berman et al., 1982).
Life-threatening apnea and bradycardia may occur after general anesthesia, most commonly in the preterm infant who is still younger than 45 weeks’ or as old as 60 weeks’ postconceptional age (the sum of gestational age and postnatal age) (Liu et al., 1980; Kurth et al., 1986; Wellborn et al., 1986). Hospital admission and respiratory monitoring are necessary for infants at risk, even after brief general anesthesia. Risk factors for postoperative apnea in preterm infants include: a history of mechanical ventilation, history of apnea and bradycardia, and anemia at the time of surgery (Kurth and LeBard, 1991; Wellborn et al., 1991; Spear, 1992; Malviya et al., 1993; Coté et al., 1995). In a meta-analysis of eight studies, Coté et al. (1995) reported that the postconceptual age required to reduce the risk of postoperative apnea to 1% was 54 weeks for infants born at 35 weeks’ gestation and 56 weeks for infants born before 32 weeks’ gestation. For further information, see Chapter 17, Neonatology for Anesthesiologists, Figure 17-10.
Congenital diseases of the lungs are usually recognized and surgically corrected in the newborn period. These conditions and their anesthetic management are discussed in Chapters 18 and 23, Anesthesia for General Surgery in the Neonate, and Anesthesia for General Abdominal, Thoracic, Urologic, and Bariatric Surgery.
Renal System
Milder degrees of renal dysfunction may also affect anesthetic care. In small children with mild or moderate underlying renal disease, clinically significant hypervolemia may occur without compensation by augmented urine output, and an excessive sodium or free-water load further deranges the serum electrolyte level. Particular caution is important in the management of fluids in children, and central venous pressure monitoring is required during major surgery in which significant blood loss or fluid shifts are anticipated (see Chapter 5, Regulation of Fluids and Electrolytes).
Hematologic System
Underlying disorders of the hematologic system are not common. The systems review should include an inquiry into unusual bleeding in the family’s or child’s medical history to explore possible genetic coagulopathies. A report of excessive bleeding from a circumcision or tonsillectomy should raise the possibility of thrombocytopenia, von Willebrand’s disease, or one of the inherited factor deficiencies and is a reason to measure platelet count, bleeding time, and coagulation time (see Chapter 36, Systemic Disorders).
In a report by the Preoperative Transfusion in Sickle Cell Disease Study Group, aggressive treatment (transfusion to a hemoglobin S level of less than 30%) was compared with a more conservative management regimen (hemoglobin maintained at 10 g/dL). The conservative approach was equally as effective as the aggressive approach in preventing serious complications but was associated with half the number of transfusion-associated complications (Vichinsky et al., 1995). A hematologic consultation should be sought or institutional protocols be developed for children with hemoglobinopathies who are undergoing anesthesia (see Chapter 36, Systemic Disorders).
The Child With Physical Or Mental Disabilities
Cerebral Palsy
Special consideration is needed when caring for patients with the diagnosis of cerebral palsy, spastic diplegia, or quadriplegia (Nolan et al., 2000). Inexperienced personnel commonly make the serious mistake of assuming that patients with spastic diplegia or quadriplegia also suffer from cognitive impairment, which may not be true. Cerebral palsy is a general term applied to several different forms of neuromuscular disability, arising from various anatomic lesions of the brain and not always involving intellectual impairment (Stiles, 1981). The treatment of patients with cerebral palsy should include careful assessment of their level of intelligence. When in doubt, as with all patients who have difficulty communicating, one should assume that they can both hear and understand what is said.
Developmental Delay, Cognitive Dysfunction, and Psychological Disorders
The term mental retardation is one of the broadest in medicine, encompassing many different forms, and it is pejorative in its tone. The anesthesiologist is encouraged to use more sensitive terminology, such as cognitive dysfunction, developmental delay, or intellectual impairment, to name a few more acceptable terms. Trisomy 21 (or other trisomies), autism, and phenylketonuria are well-known forms of this affliction; information on more obscure forms may be found in special texts, such as that of Katz and Steward (1993). Developmental delays that are genetic bear no specific outward stigmata or congenital anomalies, and anesthesia is adapted to the child’s level of consciousness and cooperation. Trisomy 21 and other chromosomal forms of intellectual impairment are often associated with congenital heart defects, as well as other congenital organ defects. Trisomy 21 is also commonly associated with blunting of the styloid process of the second cervical vertebra, which combined with the ligamental laxity of the syndrome allows atlanto-occipital subluxation, or dislocation on marked flexion of the head and neck, resulting in spinal cord injury (Moore et al., 1987; Williams et al., 1987) For more on this subject, see Chapter 36, Systemic Disorders.
Children with autism are difficult to deal with and are sometimes wildly resistant to any intervention. These patients may appear to be remarkably alert, but they also appear to be locked within themselves. The management of the autistic child must be individualized to the dynamics particular to each child’s circumstances (Rainey et al., 1998; van der Walt and Moran, 2001). As for other intellectually impaired children, the presence of a parent at induction often has a soothing effect. Premedication with oral midazolam or clonidine is often effective for sedating these children and improving cooperation.
Hyperactivity and Lack of Cooperation
The history is carefully reviewed to determine the extent of the hyperactivity and factors that affect the behavior. Parents or attendants are questioned as to which approaches have succeeded in the past and which have not. Trial-and-error methods are not advisable. Oral premedication with midazolam, clonidine, or ketamine may be helpful. Intramuscular ketamine (2 to 4 mg/kg) may be used as a last resort (Hannallah and Patel 1989).
Drug Abuse In The Child And Adolescent
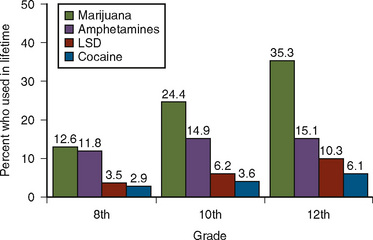
Abuse of illicit drugs is unfortunately not limited to adults. Drugs such as cocaine, marijuana, and lysergic acid diethylamide (LSD) are of social and medical concerns. In 1993, about 1 in 3 high school seniors in the United States (35.5%) had used marijuana in their lifetime (Fig. 9-4) (Johnston et al., 1994). A survey of schoolchildren in Great Britain showed that 15.8% of boys have been offered the drug Ecstasy and that 5.7% have taken it (Milroy, 1999). A survey among college students in Great Britain indicated that the most commonly used drugs are marijuana (59%), amphetamines (19%), cocaine (18%), and LSD (18%) (Christophersen, 2000). Because drug abuse may result in increased morbidity and mortality, a thorough understanding of the consequences of drug abuse is essential for the practicing anesthesiologist.
Cocaine
Cocaine is an alkaloid derived from the leaves of the South American shrub, Erythroxylum coca. Cocaine can be abused via every possible route, including oral, nasal, intravenous, and rectal. The hydrochloride form can be chemically altered to the base form, which is then concentrated by extraction in ether or baking soda (Perez-Reyes et al., 1982; Fleming et al., 1990). The residue from this method is a form of cocaine base commonly called crack (based on the cracking sounds it makes when heated) (Fleming et al., 1990; Julien, 1994). High levels of cocaine may persist in a person for 6 hours after nasal administration (Inaba et al., 1978). The metabolism of cocaine occurs primarily through plasma and hepatic cholinesterase, and patients with pseudocholinesterase deficiency are at increased risk for cocaine toxicity. Less than 5% of ingested cocaine is excreted unchanged in the urine (Inaba et al., 1978). Ecgonine methyl ester and benzoylecgonine constitute over 80% of cocaine metabolites and are detected in the urine for 14 to 60 hours after cocaine use.
Medical Complications
Myocardial ischemia and infarction have been described among young cocaine users with no other known cardiac risk factors (Box 9-2) (Mittleman et al., 1999; Feldman et al., 2000). The pathophysiologic basis for cocaine-related cardiac effects is not clear, and multiple mechanisms have been postulated, including increases in myocardial oxygen demand, accelerated atherosclerosis, thrombus formation, coronary vasospasm and vasoconstriction, and abnormally enhanced platelet aggregation (Pitts et al., 1997). Endothelin-1, a potent vasoconstrictor, is released by cocaine and may play a significant role in vasospastic angina, acute myocardial infarction, and sudden cardiac death (Wilbert-Lampen et al., 1998). Cocaine abuse has also been associated with ventricular hypertrophy, myocardial depression, and cardiomyopathy (Ghuran and Nolan, 2000). Dysrhythmias associated with cocaine use include sinus tachycardia, ventricular premature contractions, ventricular tachycardia or fibrillation, and asystole (Mouhaffel et al., 1995). Dilated cardiomyopathy, myocarditis, and congestive heart failure have also been reported secondary to cocaine use (Kloner et al., 1992; Mouhaffel et al., 1995). In addition, there is an increased incidence of hemorrhagic cerebrovascular accidents in patients who abuse cocaine (Brust, 1993). The smoking of crack cocaine produces alteration in pulmonary function, as would be expected.
Anesthetic Management
Identification of the cocaine user during the preoperative assessment presents a special challenge to the anesthesiologist, because self-reporting of drug abuse is notoriously unreliable. The nasal mucosa may show signs of ulceration. Extremities should be examined for sclerosis of peripheral veins and needle marks from intravenous injections. Auscultation over the lungs is important to exclude cocaine-induced asthma, and a careful cardiovascular and neurologic examination is necessary (Fleming et al., 1990; Kain and Rosenbaum, 1994).
Acutely Intoxicated Patient
General stabilization and hemodynamic control should precede induction of anesthesia. Propranolol was used successfully in the past to treat the β-adrenergic cardiac effects of cocaine (Fleming et al., 1990). Propranolol may worsen coronary vasoconstriction and should not be used if the patient is experiencing chest pain. Intravenous nitroglycerin, which has been shown to reverse both cocaine-induced hypertension and coronary vasoconstriction, may be the preferable drug. Furthermore, Brogan et al. (1991) reported that sublingual nitroglycerin, in a dose sufficient to reduce the mean arterial pressure by 10% to 15%, reverses cocaine-induced coronary artery vasoconstriction. Labetalol, hydralazine, and esmolol have been documented to adequately control cocaine-induced hypertension (Hollander, 1995).
Clinical experience and experimental evidence support the use of benzodiazepines as a first-line treatment for cocaine-intoxicated patients (Hollander, 1995). In addition to anxiolytic effects, benzodiazepines may lower blood pressure and heart rate, thereby decreasing myocardial oxygen consumption. Benzodiazepines are recommended for patients who are experiencing cocaine-associated chest pain and cardiac ischemic changes, as well as for patients who are having convulsions. In addition, although no clinical data exist to support the use of acetylsalicylic acid (aspirin) in patients with cocaine-associated ischemia, some experimental evidence supports the use of aspirin (Rezkalla et al., 1993).
Amphetamine-Related Designer Drugs
The term designer drugs includes compounds that have been chemically altered from federally controlled substances to produce special effects and to bypass legal regulation. The largest group consists of the methylenedioxy derivatives of amphetamine and methamphetamine. Amphetamine designer drugs produce indirect sympathetic activation by releasing norepinephrine, dopamine, and serotonin from terminals in the central and autonomic central nervous system (Albertson et al., 1999; Christophersen, 2000). The best known and most widely used designer drug is 3,4-methylenedioxymethamphetamine (MDMA, or Ecstasy), which chemically resembles a combination of amphetamine and mescaline (Milroy, 1999). The drug can be ingested orally, injected, smoked, or snorted. The onset of action is directly related to the route of administration, and for an oral dose, onset usually occurs in 20 to 45 minutes and lasts up to 6 hours (Milroy, 1999). Ecstasy is metabolized by the liver and excreted by the kidneys.
Most people who use Ecstasy experience no complications, but a number of deaths have occurred as a result of hyperthermia or idiosyncratic reactions to the drug. The most commonly seen reaction to severe or toxic ingestion of Ecstasy is a syndrome of altered mental status, tachycardia, tachypnea, profuse sweating, and hyperthermia (Henry, 2000). This constellation of symptoms closely resembles that caused by acute amphetamine overdose, which is not surprising given the chemical similarity of MDMA. Reported severe complications from ingestion of Ecstasy include hyperthermia, rhabdomyolysis, renal failure, cardiovascular collapse, disseminated intravascular coagulation, hepatic failure, hyponatremia, urinary retention, cerebral infarct, and cerebral hemorrhage (Hall, 1997a; Milroy, 1999; Ghuran and Nolan, 2000; Henry, 2000; Reneman et al., 2000). Liver damage was recognized among the first deaths in the United Kingdom and seems to be a function of hyperthermia and the resulting shock and disseminated intravascular coagulopathy (Henry, 2000).
The usual presentation in cases of acute toxicity includes hyperthermia, muscle rigidity, and a rise in creatinine kinase (CK) (Hall, 1997b). These patients may deteriorate toward multiple-organ failure, requiring intensive cardiovascular and respiratory support. These patients should be treated with active cooling and dantrolene given over 72 hours (Dar and McBrien, 1996). MDMA-induced hyperthermia results from augmentation of central serotonin function by stimulation of neuronal serotonin release (Dar and McBrien, 1996). Interestingly, Rittoo and Rittoo (1992) suggest that serum MDMA concentrations be measured in the admission blood sample of young adults who develop hyperthermia during general anesthesia. They also raise the question of whether patients who develop hyperthermia after Ecstasy use are at higher risk for the development of severe hyperthermia after general anesthesia.
Management is aimed at controlling symptoms. Benzodiazepines are suggested for agitation or seizures, and dopamine or norepinephrine is recommended for hypotension unresponsive to fluid challenges. Also suggested are phentolamine or nitroprusside for hypertension, lidocaine for ventricular dysrhythmias, and aggressive cooling and possibly the use of dantrolene (Milroy, 1999; Ghuran and Nolan, 2000; Henry, 2000). Additionally, the use of bicarbonate for rhabdomyolysis and correction of electrolyte abnormalities may be needed in the management of these patients.
Marijuana
Marijuana is the most commonly used illegal drug. The hemp plant Cannabis sativa, from which marijuana grows throughout the world, flourishes in most temperate and tropical regions. Marijuana is commonly ingested by smoking, which increases the bioavailability of the primary psychoactive constituent, tetrahydrocannabinol (THC) (Musty et al., 1995; Hall and Solowij, 1998). Inhalation of marijuana smoke produces euphoria, signs of increased sympathetic nervous system activity, and decreased parasympathetic nervous system activity (Schwartz, 1987). At high doses, however, sympathetic activity is inhibited and parasympathetic activity is increased, leading to bradycardia and hypotension. Reversible ECG abnormalities have been reported, as have increases in supraventricular and ventricular ectopic activity (Ghuran and Nolan, 2000). The clinical relevance of this finding is unclear.
Phencyclidine and Lysergic Acid Diethylamide Abuse
Phencyclidine (PCP) was developed in 1956, and it was briefly used as an anesthetic in humans before being abandoned because of the high incidence of bizarre and serious psychiatric reactions, including agitation, excitement, delirium, disorientation, and hallucinatory phenomena (Abraham et al., 1996). If taken orally, the effects of PCP develop in 1 to 2 hours and last from 8 to 12 hours. The mechanisms of action of both PCP and LSD are quite complex and include agonist, partial agonist, and antagonist effects at various serotonin, dopaminergic, and adrenergic receptors (Abraham et al., 1996). No data exist that examine PCP and its effect on patients undergoing general anesthesia, but anesthesiologists should be familiar with the management of issues related to PCP, because this product is a structural analogue of ketamine (Jansen, 1993).
The CNS effects of LSD begin approximately 15 to 30 minutes after intake and last about 6 to 12 hours (Abraham et al., 1996). The psychological effects related to LSD are intense and include alterations in mood and emotion, euphoria, dysphoria, and visual hallucinations (Abraham and Aldridge, 1993). LSD abuse is associated with only a mild sympathetic discharge that does not resemble those of cocaine, Ecstasy, or amphetamines (Ghuran and Nolan, 2000). These patients may also experience severe attacks of anxiety and panic. Anesthesia and surgery may precipitate these uncontrollable panic responses; diazepam or midazolam may be useful for the management of such responses. Postoperative hallucinations in patients undergoing general anesthesia have been reported as well (Morris and Magee, 1995).
Preoperative Pregnancy Testing
Although the pregnancy rates for teenagers fell to record lows for the United States in 2002, teen pregnancy rates have since risen, and teen pregnancy remains a significant public health concern and poses a dilemma for pediatric anesthesiologists. The birth rate for the youngest teenagers is about 0.7 birth per 1000 females aged 10 to 14 years and about 44 per 1000 for girls aged 15 to 19 years (Martin et al., 2003). At least 5% of girls are or have been pregnant by the time they reach their nineteenth birthday, and a substantial percentage of girls who are screened for elective or emergency surgery in their teen years have unsuspected or unrecognized pregnancies. General anesthesia and surgery in this population place the patient at risk for spontaneous abortion and the fetus at risk of exposure to known teratogenic substances during both anesthesia and the perioperative period, when the patient is no longer under the control of the anesthesiologist. It is this knowledge that leads many anesthesiology departments to have a policy of routinely screening postmenarchal girls for pregnancy.
Preoperative Orders
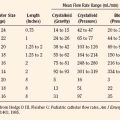
For preoperative fasting, the anesthesiologist must consider the patient’s age, size, and general medical condition, as well as the scheduled time of surgery if it is known (Table 9-3). The younger the child, the smaller the glycogen stores; therefore, the occurrence of hypoglycemia with prolonged intervals of fasting is more likely. For this reason, fasting time is reduced in the infant and young child. In general, solid food and milk products are prohibited within 8 and 6 hours, respectively, before surgery (generally after midnight); breast milk is prohibited within 4 hours of surgery; and clear liquids are prohibited within 2 hours of surgery. Liquids such as apple or grape juice, flat cola, and sugar water may be encouraged up to 2 hours before the induction of anesthesia. Ample experience has shown that shortened fasting times are safe, diminish preoperative anxiety and agitation, and may reduce the volume of gastric contents (Coté, 1990; Miller et al., 1990; Schreiner et al., 1990; Emerson et al., 1998; American Society of Anesthesiologist Task Force on Preoperative Fasting, 1999; Ferrari et al., 1999). When surgery is to be delayed beyond the anticipated time, it is important that small infants, generally those younger than 12 to 18 months old, are offered clear fluids or given intravenous fluids to prevent dehydration or hypovolemia.
TABLE 9-3 Fasting (NPO) Guidelines for Elective Surgery in Infants and Children
| Substance | Minimum Hours of Fasting |
| Solid food | 8 |
| Commercial formula | 6 |
| Milk or milk products | 6 |
| Citrus juices | 6 |
| Breast milk | 4 |
| Clear liquids | 2 |
Two issues related to the NPO guidelines that commonly arise involve the safety of clear-liquid fasting in overweight and/or obese children for the recommended 2 hours before surgery and gastric fluid volume in children who have been chewing gum before the induction of anesthesia. Cook-Sather and colleagues (2009) noted in a study of ambulatory surgical patients, in which 27% of the patients were overweight or obese, that weight and obesity did not change the ratio of the patients’ gastric fluid volume to their ideal body weights. Thus, the 2-hour fasting interval is appropriate in overweight and obese patients.
Chewing gum has the potential to increase gastric fluid volume and acidity. In adults who chewed sugared or sugarless gum before surgery, gastric fluid volume and pH did not change (Dubin et al., 1994). However, Schoenfelder et al. (2006) noted that children who chewed gum had larger gastric volumes than those children who did not. The significance of these findings remains unclear in that the incidence of aspiration is still exceedingly low.
Preanesthetic Medications
Preoperative anxiety not only is an unpleasant experience for the patient but also makes the induction and recovery from anesthesia more complicated (Holm-Knudsen et al., 1998; Aono et al., 1999; Kain et al., 1999; Zuwala and Barber, 2001; Wollin et al., 2003). Indeed, inadequate preoperative premedication not only results in stormier anesthetic inductions, but also greater postoperative pain and behavioral abnormalities in children that persist long after surgical recovery occurs (Kain et al., 2006). In addition to allaying the anxieties of surgery, separation, pain, and body disfigurement, premedication should therefore allow a smoother and safer induction of anesthesia. The issue of which child should be premedicated and what agent should be used, and whether a parent may be present during induction of anesthesia must be considered according to the specific needs of each child and within the context of the anesthetic practice and clinical setting (Coté, 1999; McCann and Kain, 2001; Leelanukrom et al., 2002; Caldwell-Andrews et al., 2005; Kain et al., 2007a; 2007b).
Which is the best agent to use for premedication? The sheer volume of articles on the subject attests to the lack of an ideal agent or combination of agents as premedication for children. Most preoperative medication is dictated by tradition. As Beecher remarked more than 50 years ago, “Empirical procedures firmly entrenched in habits of good doctors seem to have a vigor and life, not to say an immortality of their own” (1959).
Anticholinergic Agents
Oral atropine has been shown to be effective in preventing the adverse cardiovascular changes during induction of anesthesia (Miller and Friesen, 1987). Although injection is avoided with the use of oral atropine, the timing of its use relative to the operative procedure must still be anticipated.
Glycopyrrolate is a quaternary ammonium complex that does not cross the blood-brain barrier. As a result, it has a minimal CNS effect. It causes less tachycardia than atropine, but it is as effective as atropine at half the dose (0.01 mg/kg) as a vagolytic and antisialagogue. Studies in children have shown that glycopyrrolate reduced gastric fluid volume and altered its pH (Brock-Utne et al., 1978; Stoelting, 1978).
Opioids
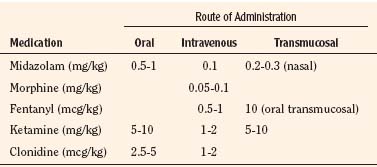
Opioid premedication, once routinely administered as an intramuscular morphine injection in children, is nearly as uncommon and unnecessary as anticholinergic premedication. Opioid premedication can result in unpleasant dysphoria and an increased incidence of preoperative and postoperative vomiting. When given, opioids can be administered via the oral, rectal, intravenous, intramuscular, or transmucosal route. Interest in the use of opioids as preanesthetic medications has focused on the intranasal and oral transmucosal forms of administration (Leiman et al., 1987; Henderson et al., 1988; Nelson et al., 1989). The advantage of the latter technique is that the dose of premedication can be titrated to effect. Oral transmucosal fentanyl (OTFC) appears to have a relatively short onset time without adversely increasing gastric pH, but it does slightly increase gastric volume and delays the time that children need to tolerate postoperative fluids (Stanley et al., 1989; Ashburn et al., 1990).
Pharmacokinetic studies have demonstrated that OTFC is absorbed well through the buccal mucosa (Streisand et al., 1991). In volunteers administered an oral drink of both fentanyl and OTFC, OTFC produced higher and earlier peak plasma concentrations, as well as increased bioavailability (50% versus 30%), compared with swallowed fentanyl. In blinded studies of children in which OTFC (15 to 20 mcg/kg) was compared with placebo, OTFC is superior to placebo in allowing children to separate from parents and undergo inhalation induction of anesthesia (Moore et al., 2000; Tamura, et al., 2003). However, it was associated with considerable untoward side effects, including nausea and vomiting, oversedation, and oxygen desaturation (Nelson et al., 1989; Streisand et al., 1989, 1991; Ashburn et al., 1990; Goldstein-Dresner et al., 1991; Moore et al., 2000). The postoperative nausea and vomiting are not attenuated by intraoperative intravenous droperidol (50 mcg/kg) (Freisen and Lockhart, 1992). Because of the high incidence of reported side effects, as well as the need for continuous pulse oximetry while and after this drug is administered, OTFC as a preoperative sedative in children has not gained widespread use or popularity.
Hypnotics
Midazolam is a water-soluble benzodiazepine with a more rapid onset time and a shorter duration of action than diazepam. Its water solubility allows better absorption after intramuscular injection and eliminates the venous irritant properties associated with diazepam (Ghoneim and Korttila, 1977). Midazolam’s peak plasma concentration occurs 45 minutes after intramuscular injection, but its anxiolytic effects occur in as little as 5 minutes. Its duration of action is usually 2 hours, with a range of 1 to 6 hours (Reves et al., 1985).
Now approved as a premedication for children and marketed as an oral preparation, midazolam has become the most commonly administered premedication before routine surgery in virtually every pediatric center; it is now the gold standard with which other premedications are compared. A national survey of over 5000 anesthesiologists has indicated that midazolam is the preoperative sedative of choice in more than 90% of all routine cases in children (Kain et al., 1997; Brosius and Bannister, 2002). The experience with midazolam is extensive and demonstrates the drug to be highly effective in alleviating anxiety, increasing cooperation, and diminishing antegrade recall without affecting retrograde memory (Payne et al., 1991a; Parnis et al., 1992; Twersky et al., 1993; Gillerman et al., 1996; McCann and Kain, 2001; Millar et al., 2007). Premedication with midazolam is safe and free of side effects, and it does not prolong recovery times (Payne et al., 1991b; Sievers et al., 1991; McMillan et al., 1992; Weldon et al., 1992; Davis et al., 1995; Viitanen et al., 1999; Brosius and Bannister, 2002). Finally, midazolam premedication smoothes the postoperative recovery of children and diminishes the incidence of delirium (Ko et al., 2001).
Nasal midazolam has also been reported to be highly effective in reducing anxiety in children within 10 to 12 minutes of administration (Griffith et al., 1998; Gautam et al., 2007). Furthermore, Davis et al. (1995) demonstrated that nasal midazolam (0.2-0.3 mg/kg) administered to patients undergoing myringotomies led to reduced preoperative anxiety and did not prolong recovery time and hospital discharge time. A drawback of nasal midazolam, however, is that more than 50% of children cry at administration because it irritates the nasal passages. The only rationale for administration of nasal midazolam is if a child without intravenous access cannot or will not swallow the oral preparation. Occasionally, younger children who refuse to take oral or nasal midazolam are more inclined to accept the midazolam rectally (McCann and Kain, 2001). Midazolam administered rectally in doses of 0.5 to 1 mg/kg effectively reduces the anxiety of children before induction of anesthesia.
Other benzodiazepines have not gained the popularity of midazolam nor have shown any advantage over the use of midazolam. Studies of flunitrazepam in children are limited, but it appears to be an effective pediatric premedication (Richardson and Manford, 1979). As with other benzodiazepines, flunitrazepam can be administered as a rectal premedication. In a double-blind, placebo-controlled study, 0.04 mg/kg of rectal flunitrazepam provided better sedation and mask-acceptance scores without prolonging recovery from anesthesia (Esteve and Saint-Maurice, 1990). Triazolam has been used as a preanesthetic medication in adults (Yamakage et al., 2002) but its use in children has not been evaluated, nor has it been compared with conventional agents.
Ketamine
In high doses (4 to 12 mg/kg), intramuscular ketamine has often been used to induce and maintain anesthesia in children (Wyant, 1971). Hannallah and Patel (1989) demonstrated that at low doses (2 mg/kg), intramuscular ketamine can facilitate inhalation induction of anesthesia. In this study of uncooperative children undergoing tympanostomy tube insertion, low-dose intramuscular ketamine was very effective in completing a mask induction with halothane in a shorter time than in cooperative children who did not need premedication. Although the induction time was shorter in the group receiving ketamine, ketamine did prolong the hospital discharge times.
As an alternative to intramuscular administration, rectal, nasal transmucosal, and oral routes of ketamine administration have been described. Rectal administration of ketamine has been reported in children undergoing a wide variety of surgical procedures. Van der Bijl and colleagues (1991) compared rectal administration of midazolam (0.3 mg/kg) with rectal administration of ketamine (5 mg/kg). Thirty minutes after the administration of either drug, good anxiolysis, cooperation, and sedation were achieved. In doses of 8 to 10 mg/kg, Saint-Maurice et al. (1979) noted that the interval from rectal administration to loss of verbal contact and acceptance of the facemask was 7 and 9 minutes, respectively.
Nasal transmucosal ketamine is an effective means of premedicating children. Weksler and colleagues demonstrated that 6 mg/kg of nasal ketamine administered 20 to 40 minutes before surgery achieved satisfactory sedation in 78% of the patients, whereas smaller doses of 5 mg/kg produced comparable sedation and ease of separation from parents as nasal midazolam (Weksler et al., 1993; Gautam et al., 2007).
Oral ketamine, of course the most acceptable route in most situations, has been used for nearly 20 years as a preoperative anesthetic medication in healthy children who are undergoing routine surgical procedures and in children undergoing corrective surgery for congenital heart defects (Stewart et al., 1990). Gutstein et al. (1992), in a double-blind, prospective study, evaluated placebo and oral ketamine at both 3 and 6 mg/kg doses as a preanesthetic medication in children. At some time during the study, 100% of the children administered 6 mg/kg were sedated as opposed to 73% of the children administered 3 mg/kg. In both groups of children who received ketamine, the onset of sedation occurred in 12 minutes, and in the 6 mg/kg group, 67% of patients were sufficiently sedated to have an intravenous cannula inserted. Similarly, the combination of oral midazolam and oral ketamine has a very high success rate of satisfactory anxiolysis compared with a somewhat lower success rate for either drug alone (Funk et al., 2000; Ghai et al., 2005). Postanesthesia care-unit discharge time of children who received orally administered ketamine is reported not to be prolonged compared with orally administered midazolam, provided that the duration of surgery is longer than 30 minutes (Funk et al., 2000). Oral ketamine has also been found to be an effective premedication in alleviating the distress of invasive procedures in pediatric oncology patients (Tobias et al., 1992). An oral transmucosal lozenge containing ketamine was under development for some time as a surgical premedication, and was found to be equally effective as oral midazolam; the present status of this drug development project is unknown at the time of this writing (Horiuchi et al., 2005).
Alpha-2-Adrenoreceptor Agonists
The role of α2-adrenoreceptor agonists for both premedication of children before surgery and as analgesics during and after surgery is continuing to evolve. In adults, α2-adrenoreceptor agonists have been shown to provide perioperative sedation, postoperative analgesia, improved perioperative hemodynamic stability, and reduced anesthetic requirements (Ghignone et al., 1987; Wright et al., 1990; Carabine et al., 1991). The MAC-sparing effect of premedication with 1 to 10 mcg/kg of oral clonidine has been demonstrated (Nishina et al., 1996; 1997; Inomata et al., 2002).
Clonidine
In a double-blind, randomized study, Mikawa and colleagues demonstrated that in children, oral clonidine produces sedation in a dose-dependent manner, and that at a dose of 4 mcg/kg, clonidine provides satisfactory sedation, a better quality of child/parent separation, and better mask acceptance than does standard oral diazepam premedication (0.4 mg/kg) (1993). Fazi and colleagues (2001) showed that oral clonidine was generally inferior to oral midazolam; however, clonidine 4 mcg/kg was associated with faster awakening than midazolam 0.5 mg/kg. These authors found that after clonidine premedication, children were more distressed and agitated during inhalation induction and had higher pain scores and greater analgesic requirements in the postoperative period. Yet few other citations in the literature have found similar results. Virtually all other authors have shown that oral clonidine in doses of 4 to 5 mcg/kg is comparable in efficacy with oral midazolam 0.5 mg/kg; whereas the onset of sedation tends to be less after the use of clonidine, the degree of sedation tends to be greater (Inomata et al., 2002; Bergendahl et al., 2004; Almenrader et al., 2007a; 2007b). Clonidine may be a useful premedication in children who have previously experienced delirium, agitation, or paradoxical reactions to midazolam or other benzodiazepines in the past (Stella and Bailey, 2008).
Importantly, clonidine premedication has beneficial effects that go beyond the preoperative period. It has become clear both from clinical experience and from clinical research that the analgesic effect of clonidine persists well into the postoperative period, reducing measures of surgical pain in children (Schmidt et al., 2007). This may be closely related to why the incidence of emergence delirium is reduced following clonidine premedication (Ko et al., 2001; Bock et al., 2002; Malviya et al., 2006) and is not after midazolam premedication (Breschan et al., 2007), and why the neuroendocrine stress response to surgery is attenuated in those children who were premedicated with clonidine (Kain et al., 2000).
Dexmedetomidine
Dexmedetomidine is another α2-agonist that has an α2 selectivity that is seven to eight times greater than clonidine. Although approved to be used as an infusion for sedation of adult intensive care unit (ICU) patients, dexmedetomidine has also been used as a premedication, an anesthetic adjuvant, a procedural sedation agent, and a treatment for postoperative shivering and agitation resulting from an emergency situation. In addition, when applied to intra-articular surfaces after orthoscopic surgery, dexmedetomidine enhances postoperative analgesia and decreases the need for postoperative analgesic agents (Ibacache 2004; Easley et al., 2007; Yuen et al., 2007, 2008; Mason et al., 2006, 2008; Al-Metwalli et al., 2008; Heard et al., 2008; Mahmoud et al., 2009; Talon et al., 2009). As a premedication, Yuen and colleagues (2007, 2008) have shown that 1.7 mcg/kg of dexmedetomidine intranasally can provide sedation in children but has an onset time and peak effect of 45 minutes and 90 to 150 minutes, respectively. In burn patients, Talon et al. (2009) suggested that 2 mcg/kg of dexmedetomidine was comparable with 0.5 mg oral midazolam with respect to preoperative sedation, conditions at induction, and emergence from anesthesia. The bioavailabilities of dexmedetomidine after oral and buccal administration were 16% and 82%, respectively (Anttila et al., 2003). Dyck et al. (1993) have reported that after intramuscular administration, peak concentrations of dexmedetomidine occur at 12 minutes and that the bioavailability is 73%.
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Abraham H., Aldridge A., Gogia P. The psychopharmacology of hallucinogens. Neuropsycopharma. 1996;14:285-298.
Abraham H., Aldridge A. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
Al-Metwalli R.R., Mowafi H.A., Ismail S.A., et al. Effect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgery. Br J Anaesth. 2008;101:395-399.
Albertson T.E., Derlet R.W., Van Hoozen B.E. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214-219.
Albrecht C.A., Jafri A., Linville L., et al. Cocaine-induced pneumopericardium. Circulation. 2000;102:2792-2794.
Almenrader N., Passariello M., Coccetti B., et al. Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatr Anaesth. 2007;17:1143-1149.
Almenrader N., Passariello M., Coccetti B., et al. Steal-induction after clonidine premedication: a comparison of the oral and nasal route. Paediatr Anaesth. 2007;17:230-234.
American Academy of Pediatrics Committee on Hospital Care. Child life programs. Pediatrics. 1993;91:671-673.
American Society of Anesthesiologist Task Force on Preoperative Fasting. 1999.
Ang-lee M.K., Moss J., Yuan C.S. Herbal medicines and perioperative care. JAMA. 2001;286:208.
Anttila M., Penttila J., Halminen A., et al. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharm. 2003;56:691-693.
Aono J., Mamiya K., Manabe M., et al. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand. 1999;43:542-544.
Ashburn M.A., Streisand J.B., Tarver S.D., et al. Oral transmucosal fentanyl citrate for premedication in paediatric outpatients. Can J Anaesth. 1990;37:857.
Baker L., Hourihane J.O. Latex allergy: two educational cases. Pediatr Allergy Immunol. 2008;19:477-481.
Beecher H. Measurement of subjective responses: a quantitative effect of drugs. New York: Oxford University Press, 1959.
Bergendahl H.T., Lonnqvist P.A., Eksborg S., et al. Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiol Scand. 2004;48:1292-1300.
Berman W., Yabek S.M., Dillon T. Evaluation of infants with bronchopulmonary dysplasia using cardiac catheterization. Pediatrics. 1982;55:783.
Black A.E. Medical assessment of the paediatric patient. Br J Anaesth. 1999;83:3-15.
Bock M., Kunz P., Schreckenberger R., et al. Comparison of caudal and intravenous clonidine in the prevention of agitation after sevoflurane in children. Br J Anaesth. 2002;88:790-796.
Bordet F., Allaouchiche B., Lansiaux S., et al. Risk factors for airway complications during general anaesthesia in paediatric patients. Paediatr Anaesth. 2002;12:762-769.
Bostancy I., Dallar Y., Unsal Sac R., et al. Latex allergy risk assessment in children and adolescents with type I diabetes mellitus. Pediatr Allergy Immunol. 2007;18:687-691.
Breschan C., Platzer M., Jost R., et al. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth. 2007;17:347-352.
Brock-Utne J.G., Rubin J., Wilman S., et al. The effect of glycopyrrolate (Robinul) on the lower esophageal sphincter. Can Anaesth Soc J. 1978;25:14.
Brogan W.C.3rd, Lange R.A., Kim A.S., et al. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991;18:581-586.
Brosius K.K., Bannister C.F. Oral midazolam premedication in preadolescents and adolescents. Anesth Analg. 2002;94:31-36.
Brust J.C. Clinical, radiological, and pathological aspects of cerebrovascular disease associated with drug abuse. Stroke. 1993;24(Suppl I):129-133. discussion 134–135
Caldwell-Andrews A.A., Kain Z.N., Kain Z.N., et al. Motivation and maternal presence during induction of anesthesia. Anesthesiology. 2005;103:478-483.
Carabine U.A., Wright P.MC., Moore J. Preanesthetic medication with clonidine: a dose-response study. Br J Anaesth. 1991;67:79.
Christophersen A.S. Amphetamine designer drugs: an overview and epidemiology. Toxicol Lett. 2000;112/113:127-131.
Cohen M.M., Cameron C.B. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282.
Cook-Sather S.D., Callagher P.R., Kruge L.E., et al. Overweight/obesity and gastric fluid characteristics in pediatric day surgery: implications for fasting guidelines and pulmonary aspiration risk. Anesth Analg. 2009;109:727-736.
Coté C.J. NPO after midnight for children: a reappraisal. Anesthesiology. 1990;72:589.
Coté C.J. Preoperative preparation and premedication. Br J Anaesth. 1999;83:16-28.
Coté C.J., Todres I.D., Ryan J.F. Preoperative evaluation of pediatric patients. In: Ryan JF., Todres ID., Coté CJ., Goudsouzian N., editors. a practice of anesthesia for infants and children. New York: Grune & Stratton; 1986:27.
Coté C.J., Zaslavsky A., Downes J.J., et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809-822.
Dar K.J., McBrien M.E. MDMA induced hyperthermia: report of a fatality and review of current therapy. Intensive Care Med. 1996;22:995-996.
Davis P.J., et al. Preanesthetic medication with intranasal midazolam for brief pediatric surgical procedures: effect on recovery and hospital discharge times. Anesthesiology. 1995;82:2-5.
De Queiroz M., Combet S., Bérard J., et al. Latex allergy in children: modalities and prevention. Paediatr Anaesth. 2009;19:313-319.
Dehlink E., Prandstetter C., Eiwegger T., et al. Increased prevalence of latex-sensitization among children with chronic renal failure. Allergy. 2009;59:734-738.
DeSoto H., Patel R.I., Soliman I.E., et al. Changes in oxygen saturation following general anesthesia in children with upper respiratory infection and symptoms undergoing otolaryngological procedures. Anesthesiology. 1988;68:276.
Dieguez M.C., Pulido Z., de la Hoz B., et al. Latex allergy in healthcare workers: an epidemiological study in a Spanish hospital. Allergy Asthma Proc. 2007;28:564-570.
Driessen J., Snoeck M. Duchenne muscular dystrophy: which is the best anesthetic agent? Paediatr Anaesth. 2008;18:1007-1008.
Drummond J.C. Of venous air embolism, aminophylline, and volatile agents. Anesthesiology. 1984;60:389.
Dubin S.A., Jense H.G., McCranie J.M., Zubar V. Surgarless gum chewing before surgery does not increase gastric fluid volume or acidity. Can J Anaesth. 1994;41:603-606.
Dyck J.B., Maze M., Haack C., et al. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813-820.
Easley B.R., Brady K.M., Tobias J.D. Dexmedetomidine for the treatment of postanesthesia shivering in children. Paediatr Anaesth. 2007;17:341-346.
Elwood T., Bailey K. The pediatric patient and upper respiratory infections. Best Pract Res Clin Anaesthesiol. 2005;19:35-46.
Elwood T., Morris W., Martin L.D., et al. Bronchodilator premedication does not decrease respiratory adverse events in pediatric general anesthesia. Can J Anaesth. 2003;50:277-284.
Emerson B.M., Wrigley S.R., Newton M. Pre-operative fasting for paediatric anaesthesia: a survey of current practice. Anaesthesia. 1998;53:326-330.
Esteve C., Saint-Maurice C. Rectal flunitrazepam as premedication in preschool children: a double-blind, randomized study. Acta Anaesthesiol Scand. 1990;34:662.
Eustachio N., Cristina C.M., Antonio F., et al. A discussion of natural rubber latex allergy with special reference to children: clinical considerations. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:171-180.
Fazi L., Jantzen E.C., Rose J.B., et al. A comparison of oral clonidine and oral midazolam as preanesthetic medications in the pediatric tonsillectomy patient. Anesth Analg. 2001;92:56-61.
Feldman J.A., Fish S.S., Beshansky J.R., et al. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469-476.
Ferrari L.R., Rooney F.M., Rockoff M.A. Preoperative fasting practices in pediatrics. Anesthesiology. 1999;90:978-980.
Fischler M. Patent foramen ovale and sitting position. Anesthesiology. 1992;76:46.
Fleming J.A., Byck R., Barash P.J. Pharmacology and therapeutic applications of cocaine. Anesthesiology. 1990;73:518-531.
Friesen R.H., Lockhart C. Oral transmucosal fentanyl citrate for preanesthetic medication of pediatric day surgery patients with and without droperidol as a prophylactic anti-emetic. Anesthesiology. 1992;76:46.
Funk W., Jakob W., Reidl T., et al. Oral preanaesthetic medication for children: double-blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. Br J Anaesth. 2000;84:335-340.
Garcia J.A. Type I latex allergy: a follow-up study. J Investig Allergol Clin Immunol. 2007;17:164-167.
Gautam S.N., Bhatta S., Sangraula D., et al. Intranasal midazolam vs ketamine as premedication in paediatric surgical procedure for child separation and induction. Nepal Med Coll J. 2007;9:179-181.
Ghai B., Grandhe R.P., Kumar A., et al. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Paediatr Anaesth. 2005;15:554-559.
Ghignone M., Calvillo O., Quintin L. Anesthesia and hypertension: the effects of clonidine on perioperative hemodynamics and isoflurane requirements. Anesthesiology. 1987;67:3.
Ghoneim M.M., Korttila K. Pharmacokinetics of intravenous anaesthetics: implications for clinical use. Clin Pharmacokinet. 1977;2:344.
Ghuran A., Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
Gillerman R.G., Hinkle A.J., Green H.M., et al. Parental presence plus oral midazolam decreases frequency of 5% halothane inductions in children. J Clin Anesth. 1996;8:480-485.
Goldstein-Dresner M.C., Davis P.J., Kretchman E., et al. Double-blind comparison of oral transmucosal fentanyl citrate with oral meperidine, diazepam, and atropine as preanesthetic medication in children with congenital heart disease. Anesthesiology. 1991;74:28.
Griffith N., Howell S., Mason D. Intranasal midazolam for premedication of children undergoing day-case anaesthesia: comparison of two delivery systems with assessment of intra-observer variability. Br J Anaesth. 1998;81:65-69.
Guis S., Figarella-Branger D., Monnier N., et al. Multiminicore disease in a family susceptible to malignant hyperthermia: histology, in vitro contracture tests, and genetic characterization. Arch Neurol. 2004;61:106-113.
Gutstein H.B., Johnson K.L., Heard M.B., et al. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76:28.
Hall A.P. “Ecstasy” and the anaesthetist. Br J Anaesth. 1997;79:697-698.
Hall A.P. Ecstasy: creatinine kinase. Anaesth Intensive Care. 1997;25:586-587.
Hall W., Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611-1616.
Hannallah R., Patel R.I. Low-dose intramuscular ketamine for anesthesia pre-induction in young children undergoing brief outpatient procedures. Anesthesiology. 1989;70:598.
Hayes J., Veyckemans F., Bissonnette B. Duchenne muscular dystrophy: an old anesthesia problem revisited. Paediatr Anaesth. 2008;18:100-106.
Heard C., Burrows F., Johnson K., et al. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. 2008;107:1832-1839.
Henderson J.M., Brodsky D.A., Fisher D.M., et al. Pre-induction of anesthesia in pediatric patients with nasally administered sufentanil. Anesthesiology. 1988;68:671.
Henry J. Metabolic consequences of drug misuse. Br J Anaesth. 2000;85:136-142.
Hollander J.E. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
Hollnberger H., Gruber E., Frank B. Severe anaphylactic shock without exanthema in a case of unknown latex allergy and review of the literature. Paediatr Anaesth. 2002;12:544-551.
Holm-Knudsen R.J., Carlin J.B., McKenzie I.M. Distress at induction of anaesthesia in children: a survey of incidence, associated factors and recovery characteristics. Paediatr Anaesth. 1998;8:383-392.
Holzman R.S. Clinical management of latex-allergic children. Anesth Analg. 1997;85:529-533.
Horiuchi T., Kawaguchi M., Kurehara K., et al. Evaluation of relatively low dose of oral transmucosal ketamine premedication in children: a comparison with oral midazolam. Paediatr Anaesth. 2005;15:643-647.
Ibacache M.E., Muñoz H.R., Brandes V., Moralies A.L. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60-63.
Inaba T., Stewart D.J., Kalow W. Metabolism of cocaine in man. Clin Pharmacol Ther. 1978;23:547-552.
Inomata S., Kihara S., Miyabe M., et al. The hypnotic and analgesic effects of oral clonidine during sevoflurane anesthesia in children: a dose-response study. Anesth Analg. 2002;94:1479-1483.
Jansen K. Non-medical use of ketamine. BMJ. 1993;306:601-602.
Johnston L., O’Malley P., Bachman J. National survey results on drug use from the monitoring the future study, 1975–1993, vol I: Secondary school students. Rockville, MD: National Institute on Drug Abuse, 1994. DHHS Publication No. [NIH] 94–0000
Julien R. Psychostimulants: cocaine and amphetamines, ed 7, New York: W.H. Freeman, 1994.
Kain Z.N. Parental presence and premedication revisited. Curr Opin Anesth. 2001;14:331-337.
Kain Z.N., Mayes L.C., Bell C., et al. Premedication in the United States: a status report. Anesth Analg. 1997;84:427-432.
Kain Z.N., Rosenbaum H. Physiology and management of cocaine exposure preceding emergency anesthesia and surgery. Curr Opin Anesthesiol. 1994;7:293-298.
Kain Z.N., Caldwell-Andrews A.A., Mayes L.C., et al. Family-centered preparation for surgery improves perioperative outcomes in children: a randomized controlled trial. Anesthesiology. 2007;106:65-74.
Kain Z.N., MacLaren J., McClain B.C., et al. Effects of age and emotionality on the effectiveness of midazolam administered preoperatively to children. Anesthesiology. 2007;107:545-552.
Kain Z.N., Mayes L.C., Caldwell-Andrews A.A., et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651-658.
Kain Z.N., Mayes L.C., Caramico L.A., et al. Parental presence during induction of anesthesia: a randomized controlled trial. Anesthesiology. 1996;84:1060-1067.
Kain Z.N., Sevarino F., Pincus S., et al. Attenuation of the preoperative stress response with midazolam: effects on postoperative outcomes. Anesthesiology. 2000;93:141-147.
Kain Z.N., Wang S.M., Mayes L.C., et al. Distress during the induction of anesthesia and postoperative behavioral outcomes. Anesth Analg. 1999;88:1042-1047.
Katz J., Steward DJ., editors. Anesthesia and uncommon pediatric diseases. Philadelphia: WB Saunders, 1993.
Kinouchi K., Tanigami H., Tashiro C., et al. Duration of apnea in anesthetized infants and children required for desaturation of hemoglobin to 95%: the influence of upper respiratory infection. Anesthesiology. 1992;77:1105.
Kloner R.A., Hale S., Alker K., et al. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407-419.
Ko Y.P., Huang C.J., Hung Y.C., et al. Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiol Sin. 2001;39:169-177.
Kurth C.D., LeBard S.E. Association of postoperative apnea, airway obstruction and hypoxemia in former premature infants. Anesthesiology. 1991;75:22.
Kurth C.D., Spitzer A.R., Broennie A.M., et al. Postoperative apnea in preterm infants. Anesthesiology. 1986;66:483.
Leelanukrom R., Somboonviboon W., Sriprachittichai P. Parental presence during induction of anesthesia in children: a study on parental attitudes and children’s cooperation. J Med Assoc Thai. 2002;85(Suppl 1):S186-S192.
Leiman B.C., Walford A., Rawal N., et al. The effects of oral transmucosal fentanyl citrate premedication on gastric volume and acidity in children. Anesthesiology. 1987;87:A489.
Levy L., Pandit U.A., Randel G.I., et al. Upper respiratory tract infections and general anaesthesia in children: perioperative complications and oxygen saturation. Anaesthesia. 1992;47:678.
Liu L.MP., Coté C.J., Goudsouzian N.G., et al. Life-threatening apnea in infants recovering from anesthesia. Anesthesiology. 1980;59:283.
Lynn A.M. Unusual conditions in paediatric anaesthesia. Clin Anaesth. 1985;3:379.
Mahmoud M., Gunter J., Donnelly L.F., et al. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745-753.
Malviya S., Swartz J., Lerman J. Are all preterm infants younger than 60 weeks post conceptual age at risk for postanesthetic apnea? Anesthesiology. 1993;78:1076.
Malviya S., Voepel-Lewis T., Ramamurthi R.J., et al. Clonidine for the prevention of emergence agitation in young children: efficacy and recovery profile. Paediatr Anaesth. 2006;16:554-559.
Malviya S., Voepel-Lewis T., Siewert M., et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
Martin J.A., Hamilton B.E., Sutton P.D., et al. Births: Final data for 2002. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics, publication 52, 2003.
Mason K.P., Zgleszewski S.E., Dearden J.R., et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006;103:57-62.
Mason K.P., Zurakowski D., Zgleszewski S.E., et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403-411.
Mather L., Mackie J. The incidence of postoperative pain in children. Pain. 1983;15:271.
McCann M., Kain Z.N. Management of preoperative anxiety in children: an update. Anesth Analg. 2001;93:98-105.
McMillan C.O., Spahr Schopfer I.A., Sikich N., et al. Premedication of children with oral midazolam. Can J Anaesth. 1992;39:545.
Mikawa K., Mackawa N., Nishina K., et al. Efficacy of oral clonidine premedications in children. Anesthesiology. 1993;79:926.
Millar K., Asbury A.J., Bowman A.W., et al. A randomised placebo-controlled trial of the effects of midazolam premedication on children’s postoperative cognition. Anaesthesia. 2007;62:923-930.
Miller B.R., Friesen R.H. Oral atropine premedication in infants. Anesthesiology. 1987;67:A491.
Miller B.R., Tharp J.A., Isaacs W.B. Gastric residual volume in infants and children following a 3-hour fast. J Clin Anesth. 1990;2:301.
Milroy C.M. Ten years of “ecstasy,”. J R Soc Med. 1999;92:68-71.
Mittleman M.A., Mintzer D., Maclure M., et al. Triggering of myocardial infarction by cocaine. Circulation. 1999;99:2737-2741.
Moore P.A., Cuddy M.A., Magera J.A., et al. Oral transmucosal fentanyl pretreatment for outpatient general anesthesia. Anesth Prog. 2000;47:29-34.
Moore R.A., McNicholas K.W., Warren S.P. Atlantoaxial subluxation with symptomatic spinal cord compression in a child with Down’s syndrome. Anesth Analg. 1987;66:89.
Morris G., Magee P. Anaesthesia and past use of LSD. Can J Anaesth. 1995;42:177.
Mouhaffel A.H., Madu E.C., Satmary W.A., et al. Cardiovascular complications of cocaine. Chest. 1995;107:1426-1434.
Musty R., Reggio P., Consroe P. A review of recent advances in cannabinoid research and the 1994 International Symposium on Cannabis and the Cannabinoids. Life Sci. 1995;56:1933-1940.
Nelson P.S., Streisand J.B., Mulder S.M., et al. Comparison of oral transmucosal fentanyl citrate and an oral solution of meperidine, diazepam, and atropine for premedication in children. Anesthesiology. 1989;70:616.
NIH Consensus Development Panel on Acupuncture. Acupuncture. JAMA. 1998;280:1518-1524.
Nishina K., Mikawa K., Maekawa N., et al. The efficacy of clonidine for reducing perioperative haemodynamic changes and volatile anaesthetic requirements in children. Acta Anaesthesiol Scand. 1996;40:746-751.
Nishina K., Mikawa K., Shiga M., et al. Oral clonidine premedication reduces minimum alveolar concentration of sevoflurane for tracheal intubation in children. Anesthesiology. 1997;87:1324-1327.
Nolan J., Chalkiadis G.A., Low J., et al. Anaesthesia and pain management in cerebral palsy. Anaesthesia. 2000;55:32-41.
Orlo S.J., Isakoff M.S., Blei F. Increased risk of symptomatic hemangiomas of the airway in association with cutaneous hemangiomas in a “beard” distribution. J Pediatr. 1997;131(4):643-646.
Parnis S.J., Barker D.S., van der Walt J.H. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
Parnis S.J., Foate J.A., van der Walt J.H., et al. Oral midazolam is an effective premedication for children having day-stay anaesthesia. Anaesth Intensive Care. 1992;20:9-14.
Payne K.A., Coetzee A.R., Mattheyse F.J. Midazolam and amnesia in pediatric premedication. Acta Anaesthesiol Belg. 1991;42:101.
Payne K.A., Coetzee A.R., Mattheyse F.J., et al. Oral midazolam in paediatric premedication. S Afr Med J. 1991;79:372.
Perez-Reyes M., Di Guiseppi S., Ondrusek G., et al. Free-base cocaine smoking. Clin Pharmacol Ther. 1982;32:459-465.
Pires G., Morais-Almeida M., Gaspar A., et al. Risk factors for latex sensitization in children with spina bifida. Allergol Immunopathol (Madr). 2002;30:5-13.
Pitts W., Lange R., Cigarroa J., et al. Cocaine-induced myocardial ischemia and infarction: physiology, pathology, recognition, and management. Prog Cardiovasc Dis. 1997;40:65-76.
Porri F., Pradal M., Lemière C., et al. Association between latex sensitization and repeated latex exposure in children. Anesthesiology. 1997;86:599-602.
Puel A., Picard C., Lorrot M., et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647-654.
Rachel Homer J., Elwood T., Peterson D., et al. Risk factors for adverse events in children with colds emerging from anesthesia: a logistic regression. Paediatr Anaesth. 2007;17:154-161.
Rainey L., van der Walt J.H. The anaesthetic management of autistic children. Anaesth Intensive Care. 1998;26:682-686.
Rendeli C., Nucera E., Ausili E., et al. Latex sensitisation and allergy in children with myelomeningocele. Childs Nerv Syst. 2006;22:28-32.
Reneman L., Habraken J.B., Majoie C.B., et al. MDMA (“Ecstasy”) and its association with cerebrovascular accidents: preliminary findings. AJNR Am J Neuroradiol. 2000;21:1001-1007.
Reves J.G., Fragen R.J., Vinik H.R., et al. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310.
Rezkalla S.H., Mazza J.J., Kloner R.A., et al. Effects of cocaine on human platelets in healthy subjects. Am J Cardiol. 1993;72:243-246.
Richardson F., Manford M. Comparison of flunitrazepam and diazepam for oral premedication in older children. Br J Anaesth. 1979;51:313.
Rittoo D. Complications of “ecstasy” misuse. Lancet. 1992;340:725-726.
Rolf N., Coté C.J. Frequency and severity of desaturation events during general anesthesia in children with and without upper respiratory infections. J Clin Anesth. 1992;4:200.
Rosenberg H., Davis M., James D., et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21.
Rosenberg H., Heiman-Patterson T. Duchenne’s muscular dystrophy and malignant hyperthermia: another warning [letter]. Anesthesiology. 1983;59:362.
Saint-Maurice C., Laguenie G., Couturier C., et al. Rectal ketamine in paediatric anaesthesia. Br J Anaesth. 1979;51:573.
Saint-Maurice C., Meistelman C., Rey E., et al. The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology. 1986;65:536.
Schein P.S., Winoker S.H. Immunosuppressive and cytotoxic chemotherapy: long-term complications. Ann Intern Med. 1975;82:84.
Schmidt A.P., Valinetti E.A., Bandeira D., et al. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667-674.
Schoenfelder R.C., Ponnamma C.M., Freyle D., et al. Residual gastric fluid volume and chewing gum before surgery. Anesth Analg. 2006;102:415-417.
Schreiner M.S., Triebwasser A., Keon T.P. Ingestion of liquids compared with perioperative fasting in pediatric outpatients. Anesthesiology. 1990;72:593.
Schwartz D., Raghunathan K. Anesthesia and mitochondrial disorders. Paediatr Anaesth. 2009;19:60-61.
Schwartz R. Marijuana: an overview. Pediatr Clin North Am. 1987;34:305-317.
Selvin B.L. Cancer chemotherapy: implications for the anesthesiologist. Anesth Analg. 1981;60:425.
Sievers T.D., Yee J.D., Foley M.E., et al. Midazolam for conscious sedation during pediatric oncology procedures: safety and recovery parameters. Pediatrics. 1991;88:172.
Skinner C.M., Rangasami J. Preoperative use of herbal medicines: a patient survey. Br J Anaesth. 2002;89:792-795.
Spear R.M. Anesthesia for premature and term infants: perioperative implications. J Pediatr. 1992;120:165.
Stanley T.H., Leiman B.C., Rawal N., et al. The effects of oral transmucosal and gastric volume and acidity in children. Anesth Analg. 1989;69:328.
Stella M.J., Bailey G.A. Intranasal clonidine as a premedicant: three cases with unique indications. Paediatr Anaesth. 2008;18:71-73.
Steward D.J. Psychological preparation and premedication. In: Gregory GA., editor. Pediatric anesthesia. New York: Churchill Livingstone, 1983.
Stewart K.G., Rowbottom S.J., Aiken A.W., et al. Oral ketamine premedication for paediatric cardiac surgery: a comparison with intramuscular morphine (both after oral trimeprazine). Anaesth Intensive Care. 1990;18:11.
Stiles C.M. Anesthesia for the mentally retarded patient. Orthop Clin North Am. 1981;12:45.
Stoelting R.K. Responses to atropine, glycopyrrolate, and Riopan on gastric fluid pH and volume in adult patients. Anesthesiology. 1978;18:367.
Streisand J.B., Stanley T.H., Hague B., et al. Oral transmucosal fentanyl citrate premedication in children. Anesth Analg. 1989;69(1):28-34.
Streisand J.B., Varvel J.R., Stanski D.R., et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology. 1991;75:223.
Tait A.R. Anesthetic management of the child with an upper respiratory tract infection. Curr Opin Anaesthesiol. 2005;18:603-607.
Tait A.R., Burke C., Voepel-Lewis T., et al. Glycopyrrolate does not reduce the incidence of perioperative adverse events in children with upper respiratory tract infections. Anesth Analg. 2007;104:265-270.
Tait A.R., Knight P.R. The effects of general anesthesia on upper respiratory tract infections in children. Anesthesiology. 1987;67:930.
Tait A.R., Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesthesiol Analg. 2005;100:59-65.
Tait A.R., Malviya S., Voepel-Lewis T., et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
Takagi A., Nakase H. Malignant hyperthermia-like reactions in Duchenne or Becker muscular dystrophy: review and hypothesis. Rinsho Shinkeigaku. 2008;48:101-105.
Talon M.D., Woodson L.C., Sherwood E.R., et al. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. 2009;30:599-605.
Tamura M., Nakamura K., Kitamura R., et al. Oral premedication with fentanyl may be a safe and effective alternative to oral midazolam. Eur J Anaesthesiol. 2003;20:482-486.
Tobias J.D., Phipps S., Smith B., et al. Oral ketamine premedication to alleviate the distress of invasive procedures in pediatric oncology patients. Pediatrics. 1992;90:537.
Twersky R.S., Hartung J., Berger B.J., et al. Midazolam enhances antegrade but not retrograde amnesia in pediatric patients. Anesthesiology. 1993;78:51.
Van der Bijl P., Roelofse J.A., Stander I.A. Rectal ketamine and midazolam for premedication in pediatric dentistry. J Oral Maxillofac Surg. 1991;49:1050.
van der Walt J.H., Moran C. An audit of perioperative management of autistic children. Paediatr Anaesth. 2001;11:401-408.
Vichinsky E.P., Haberkern C.M., Neumayr L., The Preoperative Transfusion in Sickle Cell Disease Study Group. a comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. N Engl J Med. 1995;333:206-213.
Viitanen H., Annila P., Viitanen M., et al. Midazolam premedication delays recovery from propofol-induced sevoflurane anesthesia in children 1–3 yr. Can J Anaesth. 1999;46:766-771.
von Ungern-Sternberg B.S., Boda K., Schwab C., et al. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology. 2007;107:714-719.
Weksler N., Ovadia L., Muati G., et al. Nasal ketamine for paediatric premedication. Can J Anaesth. 1993;40:119.
Weldon B.C., Watcha M.F., White P.F. Oral midazolam in children: effect of time and adjunctive therapy. Anesth Analg. 1992;75:51.
Wellborn L.G., Hannallah R.S., Luban N.L., et al. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74:1003.
Wellborn L.G., Ramirez N., Oh T.H., et al. Postanesthetic apnea and periodic breathing in infants. Anesthesiology. 1986;65:658.
Wilbert-Lampen U., Seliger C., Zilker T., et al. Cocaine increases the endothelial release of immunoreactive endothelin and its concentrations in human plasma and urine: reversal by coincubation with sigma-receptor antagonists. Circulation. 1998;98:385-390.
Williams J.O., Somerville G.M., Miner M.E., et al. Atlanto-axial subluxation and trisomy-21: another perioperative complication. Anesthesiology. 1987;67:253.
Wilson W., Taubert K.A., Gewitz M., et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736-1754.
Wollin S.R., Plummer J.L., Owen H., et al. Predictors of preoperative anxiety in children. Anaesth Intensive Care. 2003;31:69-74.
Wright P.MC., Carabine U.A., McClune S., et al. Preanesthetic medication with clonidine. Br J Anaesth. 1990;65:628.
Wyant G.M. Intramuscular ketalar (CI-581) in paediatric anaesthesia. Can Anaesth Soc J. 1971;18:72.
Yamakage M., Tsuchiya S., Ohtsuka N., et al. Usefulness of oral hypnotic premedication for volatile induction of anesthesia in adults. J Anesth. 2002;16:194-197.
Yuen V.M., Irwin M.G., Hui T.W., et al. A double-blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105:374-380.
Yuen V.M., Hui T.W., Irwin M.G., Yuen M.K. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715-1721.
Zuwala R., Barber K.R. Reducing anxiety in parents before and during pediatric anesthesia induction. AANA J. 2001;69:21-25.