4
Preoperative assessment and perioperative management in oesophageal and gastric surgery
Introduction
Perioperative management strategies have been shown to be important in postoperative outcome following oesophageal and gastric surgery.1 Structured pre- and perioperative management has also been shown to have an important role in outcome from a number of other major surgical procedures.2 The overriding principle of preoperative assessment is to identify comorbidities that may complicate the patient’s operative intervention and perioperative recovery. Identification, recognition and treatment of these comorbidities allow the patient to be optimised prior to undergoing surgery in an effort to reduce the incidence of perioperative mortality and postoperative complications.
Perioperative management is another critical factor that can have a significant impact upon clinical outcome following oesophagectomy or gastrectomy.3 This includes selection of surgical and anaesthetic techniques, methodology of intraoperative monitoring, minimising blood losses and perioperative fluid management, as well as lung isolation techniques and intraoperative organ support. Thus, although surgical technique plays an important role in determining outcome following oesophagectomy and gastrectomy, it remains only one variable amongst many others that play a significant part.4
Recently the role of the multidisciplinary team has become increasingly important in the care of this complex cohort of patients. A collaborative approach fosters an open dialogue between surgeons, anaesthetists, oncologists, radiologists, cancer specialist as well as ward nurses, nutritionists, physiotherapists and critical care teams. This dialogue allows the patient to work with highly specialised medical professionals and ideally be included in validated clinical pathways, in order to provide a high-quality service and successful outcome.5 In this chapter, we will review some of the governing principles of preoperative assessment and perioperative management in the context of oesophagogastric surgery, and examine recent developments in this field.
Physiological stress during the treatment of oesophagogastric malignancy
The multimodal nature of treatment of oesophagogastric malignancy imparts significant physiological stress. There are specific issues that can affect a patient’s tolerance to treatment. These issues classically include cardiac and pulmonary reserve, renal function and any other conditions that limit patient mobility and the potential for patients complying with standardised postoperative goals. Clinical outcome following major surgery involves interplay between patient characteristics (e.g. comorbidities), disease characteristics (e.g. tumour stage, grade and cell type), choice of treatment modality (e.g. surgery, chemotherapy, radiotherapy or combination of several modalities) and postoperative recovery.6,7 The results and interpretation of preoperative testing may affect a patient’s treatment course at multiple levels. Thus the goal of preoperative assessment is to identify relevant risk factors in patients, in order to provide a tailored patient-centred approach to the management of oesophagogastric malignancy.
Surgical resection is one modality in the treatment of oesophagogastric malignancy and remains the most commonly applied approach in physiologically appropriate patients with early and locoregional cancer. Surgery does, however, involve a significant physiological challenge.8 Prolonged operations with blood loss and fluid shifts, large thoracic and abdominal incisions, extensive lymph node and tissue dissection around vital organs, and the potential requirement for single lung ventilation are some of the intraoperative factors that can place significant strain upon the cardiorespiratory system of the patient undergoing surgery.9 Adjunctive therapy, including chemo- and radiotherapy in selected patients, can also result in significant physiological impact.10,11 Prediction of patients with sufficient reserve to undergo multimodality therapies is the most important factor when assigning a treatment approach.
Diagnosis
Standard staging investigations for oesophagogastric malignancy (Box 4.1) include endoscopy, endoscopic ultrasound (EUS), computerised tomography (CT) and positron emission tomography (PET) with or without staging laparoscopy (for oesophagogastric junctional, cardial or gastric tumours). Among the currently available staging modalities, EUS is considered the best for T stage and assessment of regional lymph nodes, whereas PET is the most accurate for the detection of distant nodal and metastatic spread.14 Apart from being increasingly useful in initial staging of oesophageal cancer, [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) scanning has been identified as a potential tool for assessing the therapeutic response after neoadjuvant therapy and detection of recurrent malignancy.15,16
Neoadjuvant therapy
Radiotherapy
Several studies have demonstrated a survival benefit in the use of neoadjuvant chemoradiotherapy in oesophageal cancer.19,20 Although this combination therapy has been shown to be effective, it may result in a significant physiological impact on the patient.21 Changes in myocardial perfusion have been reported following chemoradiotherapy for oesophageal malignancy.22 Hence it is important to identify patients with cardiac comorbidities and impaired preoperative cardiac testing that may be more at risk from resultant myocardial ischaemia. Respiratory reserve as measured by pulmonary function testing can also be adversely affected by the use of thoracic radiotherapy.23 Some chemotherapeutic agents, including 5-fluorouracil and cisplatin, have a radiosensitising effect by decreasing the ability of DNA damage repair mechanisms, thus potentiating both therapeutic and toxic effects of radiotherapy.24 This illustrates the importance of reassessment following completion of neoadjuvant therapy, prior to undertaking surgical resection of the gastro-oesophageal cancer. Timing of surgery around neoadjuvant chemoradiotherapy is also an important consideration as in our institution we would recommend surgery 4–6 weeks following the cessation of radiotherapy; however, surgery within 4–10 weeks would be acceptable.
Chemotherapy
Previous studies have shown a clear benefit to the use of adjunctive chemotherapy in the treatment of advanced stage oesophagogastric malignancy.25 Chapter 9 will discuss in more detail the merits of chemotherapy in this disease. However, it is important to note that chemotherapeutic agents can cause significant side-effects, including vomiting, bleeding, malnutrition, compromised immunity, etc. Thus patients undergoing neoadjuvant chemotherapy must be re-evaluated from a physiological and immunological standpoint prior to undergoing surgical resection.
Nutrition
Nutritional assessment and optimisation is a cornerstone of good pre- and perioperative care in cancer surgery. Preoperative malnutrition and associated immunosuppression have been shown to be well correlated with septic complications and mortality following oesophageal cancer surgery.26 The mechanism of malnutrition (Box 4.2) is often related to dysphagia, disease cachexia or neoadjuvant chemotherapy. Nutritional assessment should be a component of the MDT review.
The relative merits of enteral over parenteral methods of feeding in the malnourished patient have been the subject of debate for several years. The proposed benefits of enteral feeding include improved gut oxygenation, colonisation with gut flora serving to reduce septic complications and a reduced cost compared to parenteral feeding.27 There are several potential approaches to enteral feeding (Box 4.3).
At the time of surgery many surgeons would advocate the routine placement of a feeding jejunostomy to ensure nutrition through the perioperative period and allow a more measured approach to reinstating oral nutrition. This can simplify discharge and avoid postoperative problems during the critical healing period, as in our patients jejunal tube feeding is initiated on postoperative day 1. It is important to emphasise that feeding jejunostomies can still be associated with complications in a proportion of cases,28 which should be discussed with the patient prior to placement. In our own experience, we have found that placing a large 14Fr feeding tube decreases problems with tube obstructions.
Although percutaneous endoscopic gastrostomy (PEG) feeding provides a good method of nutritional supplementation, in oesophageal cancer patients it may compromise the gastric conduit used in surgery. In recent years the development of endoscopic stents has served as a well-tolerated treatment modality to bypass obstructing oesophageal lesions and allow oral enteral feeding either for preoperative optimisation or as a palliative measure. However, despite these benefits, fully covered oesophageal self-expanding metal stents (SEMS) are associated with an increased risk of migration (6–43.8%)29 that may significantly impact upon the patient’s nutritional status and surgical resection. Furthermore, many clinical oncologists are hesitant to use radiotherapy in a patient with an oesophageal metal stent. Thus the future of stents as a nutritional bridge during neoadjuvant therapy remains inconclusive, with further studies required.
Preoperative assessment
In general terms, the most familiar and simple classification of preoperative physical status and risk is that of the American Society of Anesthesiologists (ASA) (Table 4.1). Although the correlation of ASA grade with perioperative risk has limitations, it does provide a useful global assessment tool and its use is universal and familiar. Several other clinical risk indices have been developed, including the Eastern Cooperative Oncology Group (ECOG) performance status, the Karnofsky performance scale index and the Charlson comorbidity index. ECOG performance status allows assessment of the effect of oesophagogastric cancer on the daily living abilities of the patient. The Karnofsky performance scale index allows patients to be classified by their functional impairment, in a similar manner to the ECOG score. The Charlson comorbidity index predicts the 10-year mortality for a patient who may have a range of comorbid conditions such as heart disease, AIDS or cancer (22 conditions in total). This index allows quantitative scoring of a patient’s comorbidities and may provide a useful tool in the preoperative assessment.
Table 4.1
The American Society of Anesthesiologists’ assessment of physical status
| Grade | Definition |
| ASA 1 | Normal healthy patient |
| ASA 2 | Patient with mild systemic disease |
| ASA 3 | Patient with a severe systemic disease that limits activity but is not incapacitating |
| ASA 4 | Patient with incapacitating disease that is a constant threat to life |
| ASA 5 | Moribund patient not expected to survive 24 hours with or without surgery |
Cardiac assessment (Box 4.4)
As described previously, oesophagectomy or gastrectomy places significant physiological stress upon the cardiovascular system. Up to 10% of patients undergoing oesophagectomy will have a significant cardiovascular complication.30 Furthermore, with increasing oesophagogastric surgery being undertaken in the elderly population, accurate identification of patients at risk from cardiovascular complications (associated with ischaemia or dysrhythmia) can help guide treatment planning.
History
A thorough history and appropriate clinical examination will help identify major cardiovascular risk factors. These include ischaemic heart disease, valvular abnormalities, arrhythmias, heart failure, etc. Ischaemic heart disease has been identified as a crucial risk factor predicting severe complications following major surgery.31 Identification of atrial or ventricular tachyarrhythmias can also help identify patients at risk for the most common postoperative complication, atrial fibrillation. Any pertinent findings will help guide further investigations that may be required, including a full cardiology assessment prior to undertaking major surgery.
Functional capacity
Exercise capacity provides a useful measure of functional cardiorespiratory reserve. Poor exercise tolerance correlates with an increased risk of perioperative complications that are independent of age and other patient characteristics.32 However, the ability to climb a flight of stairs does not preclude a patient from having underlying cardiorespiratory disease, and prior to undertaking surgery the majority of oesophagogastric surgeons and most anaesthetists would advocate the use of further cardiac investigation in all elderly patients or patients with multiple risk factors. In the absence of an agreed protocol, exercise testing for oesophagogastric cancer surgery patients remains an important consideration during preoperative evaluation; however, it should not be used as the sole criterion for denying a patient an operation.
Investigations (Box 4.4)
Electrocardiogram (ECG): ECG is the most basic objective cardiac assessment, usually as part of any preoperative work-up prior to major surgery. It remains a useful baseline test to identify electric conductional abnormalities within the heart that may indicate further structural abnormalities that warrant further investigations. Patients with no prior history of cardiac disease but with an abnormal ECG represent a group that must undergo a higher level of investigation and are potentially amenable to intervention and risk reduction prior to surgery.
Cardiopulmonary exercise testing (CPX): The relative merit of cardiopulmonary exercise testing in the setting of oesophagogastric surgery remains controversial. Some surgeons propose the view that CPX testing is expensive, time-consuming and unreliable for the prediction of cardiorespiratory complications following oesophagectomy or gastrectomy. The literature on this subject also fails to resolve the debate. In a retrospective cohort study, Nagamatsu et al.33 divided patients into two groups based on the presence or absence of cardiopulmonary complications. Nagamatsu et al. found significant differences between the two groups in their preoperative VO2max (P < 0.001) and anaerobic threshold (AT; P < 0.001). In the follow-up to this study, Nagamatsu et al.34 performed a retrospective study of CPX testing in 91 patients who underwent radical oesophagectomy with three-field lymphadenectomy. They found VO2max closely correlated with the occurrence of postoperative cardiopulmonary complications. On the basis of their results, Nagamatsu et al. chose a minimally acceptable value of 800 mL/m2 for the VO2max for patients undergoing curative transthoracic oesophagectomy. Forshaw et al.35 undertook a similar study to determine the usefulness of CPX testing before oesophagectomy in a cohort of 78 patients. The study demonstrated there was a significantly reduced VO2peak (P = 0.04) and a non-significant trend towards a reduced AT (P = 0.07), in patients who developed postoperative cardiopulmonary complications following oesophagectomy. Areas under the curve for AT and VO2peak were 0.63 and 0.62, respectively, suggesting that CPX testing did not perform well in predicting postoperative cardiopulmonary complications.
Stress testing: Cardiac stress testing is a well-validated non-invasive modality that has been shown to accurately predict patients at risk of cardiac complications following non-cardiac surgery.36 In addition, stress testing has been shown to identify patients with inducible ischaemia that may benefit from preoperative beta-blockade.37 Preoperative non-invasive stress testing has been recommended for patients with cardiac risk factors (Table 4.2) by the American College of Cardiology and American Heart Association guidelines.38 Exercise-induced hypotension is a sign of possible ventricular impairment secondary to coronary artery disease and warrants further investigation with a coronary angiogram or myocardial perfusion imaging. Several exercise methods for cardiac stress testing exist, including stair climbing, treadmill and shuttle walk testing. Further investigation in patients who are unable to complete exercise testing due to reduced mobility secondary musculoskeletal disease may include pharmacological to stress testing. Commonly used pharmacological agents include adenosine, dipyridamole, dobutamine and propanolol. The choice of pharmacological drug used in stress testing usually depends upon potential drug interactions with other treatments and concomitant diseases. Cardiac stress echocardiography and radioisotope investigation (to measure cardiac perfusion) are also used to provide a more detailed cardiac assessment. The identification of reduced left ventricular ejection fraction by the latter modalities has been significantly associated with the development of cardiac complications following major surgery.39
Optimisation
Preoperative physical cardiopulmonary rehabilitation: Preoperative cardiopulmonary fitness has been shown to be well correlated with postoperative outcome following major surgery.40 The use of intensive preoperative exercise has been shown to improve cardiopulmonary fitness prior to major surgery.41 Although intensive preoperative exercise improves cardiopulmonary fitness, this short-term improvement has not been conclusively shown to correlate with postoperative outcome following major surgery and cancer resection.
Beta-blockade: ACC/AHA guidelines (2006) suggested that beta-blockers should be considered in all patients with an identifiable cardiac risk as determined by the presence of more than one clinical risk factor.38 The hypothesis for this beneficial effect is that adrenergic beta-blockade slows the heart rate and as a result improves ischaemic ventricular dysfunction. Patients on long-term beta-blockade exhibit adrenergic hypersensitivity if the therapy is withdrawn and the intravenous route should be utilised until oral intake can be resumed. The cardioprotective effect of beta-blockers has been reported as persisting for up to 6 months following surgery, even after the cessation of therapy.42 In order for beta-blockade therapy to be most effective, patients should be optimally blocked in the weeks preceding surgery and in the immediate postoperative period. Although not conclusively proven, it is believed that long-acting beta-blockers initiated before surgery are superior to shorter-acting agents.38
Other relevant cardiac medication:
Statins: Current ACC/AHA guidelines on perioperative cardiovascular care recommend that patients should continue statin treatment throughout the perioperative period.38 To date the evidence regarding the cardioprotective effects of statins in the perioperative period is controversial,46 with no studies specifically in the setting of oesophagogastric surgery.
Anticoagulants: In performing oesophagogastric surgery on patients on anticoagulation, the major concern is when is it safe to perform surgery without increasing the risk of haemorrhage or increasing the risk of thromboembolism (e.g. venous, arterial) after discontinuing treatment.
Coronary stents include bare metal and drug-eluting stents, and their placement prior to surgery can significantly impact upon timing of surgical resection. Nuttall et al.48 demonstrated an odds ratio of 3.6 for major cardiac events when surgery was performed within 30 days of bare metal stent placement, which was reduced to 1.6 when surgery was performed between 31 and 90 days. The available data suggest that 30 days should be the minimum interval between placement of a bare metal coronary stent and major non-cardiac surgery. Rabbits et al.49 showed the risk of developing cardiac complications following drug-eluting stent placement is increased (6.4% vs. 3.3%) when surgery is performed within 365 days of stent placement. Thus it is clearly important to discuss the patient’s perioperative plan with the consulting cardiologist prior to any percutaneous cardiac intervention or stent placement if a patient is being scheduled for major oesophagogastric surgery. The timing of when to restart anticoagulants is also a subject of debate, with little clear guidance currently present; however, in our institution we typically reinstitute aspirin on postoperative day 1 following oesophagectomy.
Warfarin: Patients on warfarin are typically told to stop this 4–5 days prior to undergoing major surgery, with the acquisition of an international normalised ratio (INR) assay on the day of surgery. Patients with mechanical heart valves, atrial fibrillation or venous thromboembolism should have an anticoagulation bridging plan with heparin for the perioperative period.49 Patients who have recently sustained a venous thromboembolism should be considered for placement of temporary caval filters prior to radical surgery.
Pulmonary assessment
Oesophageal surgery has significant effects on pulmonary physiology that may predispose to complications. The incidence of postoperative pulmonary complications following oesophagogastric surgery ranges from 15.9% to 30%, with an associated increase in operative mortality.50 Assessment of underlying pulmonary reserve is often recommended for identifying patients more likely to suffer from postoperative pulmonary problems, and then instituting effective aggressive preventative strategies including regular chest physiotherapy, early mobilisation and lung spirometry. For example, a patient with chronic obstructive pulmonary disease (COPD) and sputum retention should be identified as high risk preoperatively to allow the introduction of these preventative strategies early in the postoperative period; if not, this patient may require multiple therapeutic bronchoscopies in the postoperative period to treat mucus accumulation and lobar collapse.
History
A thorough history along with an appropriate examination will help identify pulmonary risk factors that will be important in the perioperative period. Risk factors for postoperative pulmonary complications include age, smoking status and physical activity levels.51 Further pulmonary comorbidities that are important in the recovery following major surgery include COPD, asthma, pulmonary fibrosis or any further restrictive lung disease and previous pulmonary emboli. In the medication history it is important to specifically ask about the use of oral bronchodilator therapy that may be administered as a nebuliser in the postoperative period. Furthermore, the use of oral steroids will need consideration for cover with intravenous hydrocortisone during the perioperative period.
Investigations (Box 4.5)
Arterial blood gas (ABG): Preoperative ABG sampling is commonly used in patients with pulmonary risk factors to gain an idea of baseline respiratory function. Patients with obstructive airway disease (COPD) may show evidence of carbon dioxide retention, which should be taken into account during the postoperative period. Patients with abnormal preoperative ABG results are more likely to suffer from postoperative pulmonary complications following major surgery.52 Interpretation of ABG results in the context of clinical history and examination is important in ensuring optimal perioperative pulmonary care.
Chest X-ray (CXR): Routine preoperative CXR is part of the work-up for most major surgical procedures. Results from a CXR are dependent upon interpretation by clinicians and subsequent action. Chronic disorders such as cardiomegaly and COPD can be detected in upto 65% of cases.53 A preoperative CXR will elucidate obvious chest abnormalities; its greatest value may be as a comparison with postoperative films to act as a reference point.
Pulmonary function testing (PFT): Preoperative PFT with spirometry in conjunction with clinical history and examination can be used to establish baseline lung function, evaluate dyspnoea, detect pulmonary disease, monitor effects of therapies used to treat respiratory disease, evaluate respiratory impairment and evaluate operative risk. Low forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) has been shown to be well correlated with postoperative pulmonary complications.54 Abnormal PFT results will allow identification of patients at risk of postoperative pulmonary complications; this will enable targeted preventative strategies including chest physiotherapy, early mobilisation and lung spirometry to be employed in the perioperative period. However, routine pulmonary function testing can be time-consuming and expensive, and some surgeons would advocate a more measured approach, with PFTs being used in patients with pulmonary risk factors.
Optimisation
As discussed previously, the benefits of preoperative exercise or rehabilitation have been shown to improve cardiopulmonary fitness and postoperative outcome following major cancer resection. Identification of patients at risk from pulmonary complications may provide a justification for altering the method of surgical resection. Patients with very poor pulmonary function who previously would have been deemed unfit to undergo resection may benefit from a minimally invasive approach. Aggressive chest physiotherapy and early mobilisation may also help to reduce the incidence of pulmonary complications associated with oesophagogastric surgery in this cohort. There are several preoperative pulmonary risk factors that may be optimised in patients with impaired lung spirometry who are undergoing upper gastrointestinal surgery (Box 4.6).
Neurological assessment
Identification of patients with neurological risk factors is another crucial element of the preoperative global assessment. These risk factors include previous cerebrovascular accidents (CVAs), transient ischaemic episodes (TIAs), epilepsy, dementia or cognitive decline and neuropsychiatric disorders. These factors can lead to severe neurological complications, including delirium, that may significantly impact upon postoperative recovery. The reported incidence of postoperative delirium following major surgery is highly variable, ranging from 9% to 53%, and more common in the elderly population.55 Previous studies have also shown delirium to be significantly associated with a poor postoperative outcome following major surgery.56 In our own institution delirium affects 9.2% of patients and is the second most common complication following oesophagectomy. Furthermore, delirium in our unit is associated with an increased incidence of postoperative pneumonia, pneumothorax, tracheal re-intubation, length of intensive care unit (ICU) and hospital stay, and increased overall cost.57
Investigations
Several risk factors for postoperative delirium have been identified previously, including age, dementia, functional impairment, depression, psychotropic drug use, increased comorbidity (cardiac, pulmonary, renal and neurological), laboratory abnormalities (electrolyte disturbance, anaemia and low albumin), preoperative visual impairment, hearing impairment, alcohol use, institutional residence and prior postoperative delirium. It is the accurate preoperative identification of these risk factors in vulnerable patients that will allow implementation of interventions to reduce delirium following major surgery.58 Previous studies have attempted with variable success to produce risk scores that can be used to identify postoperative or hospitalised patients at risk from delirium.59 Although creation and validation of these risk scores provides interesting academic points, often they are cumbersome and not designed for widespread clinical application. The presence of pre-existing dementia or cognitive impairment has been shown in a previous study60 to have the strongest correlation with postoperative delirium. The Mini-Mental State Examination (MMSE) provides a means of a quick assessment of a patient’s cognitive state at admission that may allow prediction of patients vulnerable to postoperative delirium.60
Optimisation
The avoidance and treatment of postoperative delirium is a challenge, and thus prediction of vulnerable patients and employment of preventative strategies represent a more attractive option. More recent randomised trials have been aimed at designing multifaceted interventional programmes to prevent postoperative delirium, thus reducing length of hospital stay and mortality.62 The most recent Cochrane review on ‘Interventions for preventing delirium in hospitalised patients’ highlighted the sparse nature of evidence regarding preventative measures against delirium. However, in the context of hip surgery there was a suggestion that proactive geriatric consultation and prophylactic low-dose haloperidol may reduce severity and duration of delirium episodes in vulnerable patients.63 Specifically in the setting of oesophagectomy, bright light therapy or increased bright light exposure has been shown to be useful in reducing the incidence of postoperative delirium.64
Renal assessment
Optimisation
Previous studies have demonstrated that with good preoperative optimisation patients with impaired renal function can undergo gastrectomy with similar results to patients with a normal creatinine clearance.65 Patients with severe renal impairment requiring dialysis are considered inappropriate surgical candidates for major oesophagogastric resection. Active involvement and consultation with a nephrologist will help guide perioperative management strategies, including fluid and electrolyte management, in this complex cohort of patients.
Anaesthetic technique
Anaesthetic agents
Desflurane and sevoflurane have been shown to produce a reduced pulmonary inflammatory response and a decrease in the overall number of adverse events compared to propofol.66 However, a more recent study suggested sevoflurane caused a greater inflammatory response than propofol during thoracic surgery.67 These studies are limited by heterogeneity in patient demographics and comorbidities, along with duration of surgery and one-lung ventilation. Thus, although volatile anaesthetics produce dose- and time-dependent effects upon the inflammatory and immune systems, the nature of these effects in the setting of oesophagogastric surgery requires further investigation.
Airway management
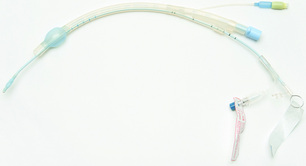
Both gastric and oesophageal surgery can be performed in a patient intubated with a standard endotracheal tube. To allow intraoperative collapse of the right lung (during a two-stage transthoracic oesophagectomy), a left-sided double-lumen endobrochial tube is most commonly used (Fig. 4.1). It is crucial to ensure correct placement of the tube and recognise inadvertent upper lobe occlusion. Usually, endobrochial tube position is confirmed through auscultation of the chest and fibre-optic bronchoscopy.
Single lung ventilation (SLV) is commonly used in oesophageal surgery to facilitate dissection by operating surgeons by increasing the space available within the thoracic cavity. However, SLV has been shown to result in an inflammatory response, with the time period of SLV and surgical manipulation increasing alveolar injury and leucocyte recruitment in the dependent lung. During re-expansion of the collapsed lung, alveolar recruitment and reperfusion lung injury provide an additional source of lung injury.68 Protective strategies aimed at reducing intraoperative lung injury include using small tidal volumes and positive end-expiratory pressure (PEEP) during SLV, and this has been shown to reduce the inflammatory response following oesophagectomy, improve lung function and result in early extubation.69
Endobronchial blockers (Fig. 4.2): In some patients who are difficult to intubate in the presence of irregular dentition or limited temporomandibular joint movement, the use of a double-lumen endobronchial tube can be challenging due to its size. In this situation an endobronchial blocker passed fibre-optically through a single-lumen endotracheal tube may be beneficial to isolate the non-dependent lung. One important limitation to the routine use of endobronchial blockers is their tendency to migrate proximally or distally with mediastinal manipulation, resulting in sudden lung reinflation.
Timing of extubation
The benefits of early extubation have been clearly demonstrated, with reduced mortality and morbidity.71 Early extubation has the additional benefit of reducing the requirement for postoperative ICU admission, instead allowing patients to be managed in a high-dependency setting and reducing overall cost. Postoperative extubation must be predicated on the basis of good pain control and is a prerequisite for early mobilisation.
Fluid management
Perioperative fluid management involves a careful balance between maintaining perfusion pressure and oxygen delivery to vital organs and the newly fashioned anastomosis, and the prevention of excessive fluid accumulation that may delay recovery of gastrointestinal function, impair wound or anastomotic healing, and increase cardiac and respiratory complications.72 Patients undergoing major oesophagogastric surgery have several sources of loss of fluid, including bowel preparation, dehydration secondary to tumour dysphagia, blood loss, insensible and nasogastric losses, wound exudation, urinary output, and evaporative fluid losses from open abdominal and chest cavities.
Recent publications have suggested that a more restrictive approach to fluid management during major surgery may be beneficial, with improved gastrointestinal recovery time and reduced respiratory complications.73
Goal-directed fluid therapy includes minimising blood loss and maintaining haemodynamic stability, and this requires regular communication between surgeon and anaesthetic teams, i.e. during transhiatal dissection, where the blood pressure routinely decreases and anaesthetists typically respond by increasing fluid administration. In oesophagogastric surgery the monitoring of haemodynamic parameters is more challenging, as the most validated method remains oesophageal Doppler, the use of which is not possible in the context of oesophagectomy.76 Other monitoring measures to allow goal-directed fluid administration include arterial lines to monitor arterial pressure variation and the FloTrach/Vigileo system (Edwards Lifesciences, Irvina, CA) to predict intravascular hypovolaemia.77 Clear evidence and the optimal method for monitoring in goal-directed fluid therapy are yet to be determined; however, this approach to intraoperative fluid administration would appear to be beneficial in theory.
Postoperative analgesia
Thoracic epidural analgesia for oesophagectomy has been shown to be a highly effective method of postoperative pain relief.78 Furthermore, the proven benefits of thoracic epidural analgesia following oesophagectomy include earlier recovery of bowel function, reduced pulmonary complications and early extubation.79 Further less well-validated benefits have been described, and these include reduced anastomotic leak and improved gastric conduit microcirculation.80,81 However, aggressive epidural bolus dosing can reduce systolic arterial pressure and thus conduit perfusion. Measures to counteract this effect include changing rate of epidural and avoidance of bolusing, avoidance of hypovolaemia and the judicious use of vasopressor therapy.82
Epidural analgesia has been shown to be a highly effective method of reducing postoperative pain in gastrectomy.83 Epidural analgesic therapies can vary between continuous infusions and patient-controlled analgesia (PCA). The advantages of the latter include subjective titration of a patient’s pain and adequate treatment; however, usually a safety mechanism or lockout is in place to prevent the patient from overusing the PCA. Recent studies have suggested a combined regime of patient-controlled epidural analgesia during the day with a night-time infusion can help to reduce postoperative pain, and specifically pain associated with coughing, and provide a better sleep pattern.84
Extrapleural intercostal nerve blockade is another method of effective pain control following thoracotomy. An indwelling catheter may be left in the extrapleural space, most commonly during the operation, to allow the continuous infusion of local anaesthetic to the thoracotomy region. Extrapleural intercostal nerve blockade has been shown to be as effective as thoracic epidural analgesia in controlling thoracotomy-related pain and allowing recovery of pulmonary function in a randomised controlled trial.85 Despite these benefits, extrapleural intercostal nerve blockade has yet to gain widespread acceptance, with thoracic epidural analgesia remaining the perceived gold standard for pain control following thoracotomy.
Oesophagogastric clinical pathways
Preoperative
In our institution this process begins at the time of initial telephone interview between the patient and the oesophagogastric nurse specialist within 48 hours of referral. This telephone interview will include a review of the patient’s past medical history, their current symptoms (swallowing and weight loss), current investigations, travel arrangements (accommodation), and an initial description of the process of preoperative work-up, surgery and postoperative recovery. This interaction allows specific planning to be made regarding what previous tests have been undertaken and what radiological examinations need to be obtained prior to the initial visit. In addition, specific plans are made to complete all staging and appropriate physiological tests within a specific time period (in our case 48 hours) around the initial trip to the oesophagogastric unit. At the initial visit careful history, examination and organisation of relevant clinical investigations as described previously in this chapter will provide an initial indication of physiological status. This physiological assessment is important in guiding all aspects of the patient’s treatment for their oesophagogastric malignancy. In particular, it is important to be able to adapt the surgical approach according to both tumour location and patient physiology, i.e. in patients with severe coronary artery disease, arrhythmias or cardiomyopathy we typically utilise a right thoracic approach so as to minimise cardiac manipulation during oesophageal mobilisation. Following physiological investigations and tumour staging, all patients should be presented at a multidisciplinary tumour board (see ‘Multidisciplinary team evaluation’ section above) to allow an individually targeted goal-directed treatment plan to be formulated that takes into account both tumour and patient characteristics. A part of this MDT review will include reviewing suitability for enrolment in current clinical trials. The nutritional status of patients should also be discussed, with specific need for either feeding jejunostomy or removable self-expanding metal oesophageal stent (SEMS) being included in the final recommendation (see ‘Nutrition’ subsection above). At the MDT meeting, a plan is made regarding the timing of surgery in patients receiving neoadjuvant chemotherapy, but particularly chemoradiotherapy. Current recommendations indicate the optimum time for resection to be 4–6 weeks following completion of radiotherapy. Contact is maintained with the patient throughout neoadjuvant therapy by the oesophagogastric nurse specialist as well as post-treatment reassessments coordinated before surgery.
Postoperative
Postoperative care pathways allow the introduction of a targeted goal-directed approach to postoperative recovery following major oesophagogastric surgery. They provide a template for all medical personnel interacting with these patients, and can outline a goal-directed recovery for each patient. These pathways, once well established, can provide a framework for quality improvement and improving postoperative outcomes. Previous studies have demonstrated the effectiveness of these pathways in reducing postoperative mortality, pulmonary complications and length of hospital stay following oesophagectomy.86,87 Involvement of the entire healthcare team in the design and implementation of these pathways will help ensure all team members are committed to achieving specific recovery goals.
A simple schematic for a postoperative care pathway is shown in Table 4.3. It is imperative that patients should be provided with this pathway and dietary expectations prior to undergoing surgery, as this will help to guide their expectations regarding their postoperative recovery. The use of specific pathways will help patients, families and their caregivers remain focused on a goal-orientated approach to recovery from major surgery. Through five revisions of our clinical care pathway over the past decade, we have seen our median length of hospital stay decrease from 10 to 8 days (median for past 2 years).
Table 4.3
Clinical care pathway at Virginia Mason Medical Center 2011

Adapted from Low DE, Kunz S, Schembre D et al. Esophagectomy – it’s not just about mortality any more: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007; 11:1395–402.
References
1. Palmes, D., Brüwer, M., Bader, F.G., German Advanced Surgical Treatment Study Group, Diagnostic evaluation, surgical technique, and perioperative management after esophagectomy: consensus statement of the German Advanced Surgical Treatment Study Group. Langenbecks Arch Surg. 2011;396(6):857–866. 21713594
2. Troisi, N., Dorigo, W., Lo Sapio, P., et al, Preoperative cardiac assessment in patients undergoing aortic surgery: analysis of factors affecting the cardiac outcomes. Ann Vasc Surg. 2010;24(6):733–740. 20472385
3. Akutsu, Y., Matsubara, H., Perioperative management for the prevention of postoperative pneumonia with esophageal surgery. Ann Thorac Cardiovasc Surg. 2009;15(5):280–285. 19901880
4. Whooley, B.P., Law, S., Murthy, S.C., et al, Analysis of reduced death and complication rates after esophageal resection. Ann Surg. 2001;233(3):338–344. 11224620
5. Adams, R., Morgan, M., Mukherjee, S., et al, A prospective comparison of multidisciplinary treatment of oesophageal cancer with curative intent in a UK cancer. Eur J Surg Oncol. 2007;33(3):307–313. 17123775
6. Law, S., Wong, K.H., Kwok, K.F., et al, Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004; 240:791–800. 15492560
7. Allum, W.H., Stenning, S.P., Bancewicz, J., et al, Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009; 27:5062–5067. 19770374
8. Morita, M., Egashira, A., Yoshida, R., et al, Esophagectomy in patients 80 years of age and older with carcinoma of the thoracic esophagus. J Gastroenterol 2008; 43:345–351. 18592152
9. Kuppusamy, M.K., Chance, F.D., Helman, J.D., et al, Assessment of intra-operative haemodynamic changes associated with transhiatal and transthoracic oesophagectomy. Eur J Cardiothorac Surg 2010; 38:665–668. 20615723
10. Wakui, R., Yamashita, H., Okuma, K., et al, Esophageal cancer: definitive chemoradiotherapy for elderly patients. Dis Esophagus 2010; 23:572–579. 20459439
11. Mak, R.H., Mamon, H.J., Ryan, D.P., et al, Toxicity and outcomes after chemoradiation for esophageal cancer in patients age 75 or older. Dis Esophagus 2010; 23:316–323. 19788436
12. Portale, G., Peters, J.H., Hsieh, C.C., et al, Esophageal adenocarcinoma in patients ≤ 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg. 2004;70(11):954–959. 15586504
13. Patil, P.K., Patel, S.G., Mistry, R.C., et al, Cancer of the esophagus in young adults. J Surg Oncol 1992; 50:179–182. 1619941
14. Omloo, J.M., Sloof, G.W., Boellaard, R., et al, Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy. 2008;40(6):464–471. 18543134
15. van Westreenen, H.L., Heeren, P.A., van Dullemen, H.M., et al, Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9(1):54–61. 15623445
16. Westerterp, M., van Westreenen, H.L., Reitsma, J.B., et al, Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy – systematic review. Radiology. 2005;236(3):841–851. 16118165
17. Birkmeyer, J.D., Sun, Y., Wong, S.L., et al, Hospital volume and late survival after cancer surgery. Ann Surg 2007; 245:777–783. 17457171
18. Al-Sariria, A.A., David, G., Willmott, S., et al, Oesophagectomy practice and outcomes in England. Br J Surg. 2007;94(5):585–591. 17443856
19. Van der Gaast, A., van Hagen, P., Hulshof, M., et al, Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084 (abstr).. 22646630
20. Lv, J., Cao, X.F., Zhu, B., et al, Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010; 16:1649–1654. 20355244
21. Murthy, S.C., Rozas, M.S., Adelstein, D.J., et al, Induction chemoradiotherapy increases pleural and pericardial complications after esophagectomy for cancer. J Thorac Oncol 2009; 4:395–403. 19247086
22. Gayed, I., Gohar, S., Liao, Z., et al, The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 2009; 25:487–495. 19234869
23. Cerfolio, R.J., Talati, A., Bryant, A.S., Changes in pulmonary function tests after neoadjuvant therapy predict postoperative complications. Ann Thorac Surg 2009; 88:930–935. 19699923
24. Neuner, G., Patel, A., Suntharalingam, M., Chemoradiotherapy for esophageal cancer. Gastrointest Cancer Res 2009; 3:57–65. 19461907
25. Cunningham, D., Allum, W.H., Stenning, S.P., et al, Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–20. 16822992
26. Takagi, K., Yamamori, H., Morishima, Y., et al, Preoperative immunosuppression: its relationship with high morbidity and mortality in patients receiving thoracic esophagectomy. Nutrition. 2001;17(1):13–17. 11165881
27. Gabor, S., Renner, H., Matzi, V., et al, Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr. 2005;93(4):509–513. 15946413
28. Llaguna, O.H., Kim, H.J., Deal, A.M., et al, Utilization and morbidity associated with placement of a feeding jejunostomy at the time of gastroesophageal resection. J Gastrointest Surg. 2011;15(10):1663–1669. 21796458
29. Pellen, M.G., Sabri, S., Razack, A., et al, Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophagal cancer. Dis Esophagus. 2012;25(1):48–53. 21595778
30. Griffin, S.M., Shaw, I.H., Dresner, S.M., Early complications after Ivor–Lewis subtotal esophagectomy with two-field lymphadenectomy. Risk factors and management. J Am Coll Surg 2002; 194:285–297. 11893132
31. Eagle, K.A., Brundage, B.H., Chaitman, B.R., et al, Guidelines for perioperative cardiovascular evaluation for non-cardiac surgery. J Am Coll Cardiol. 1996;27(4):910–948. 8613622
32. Allum, W.H., Blazeby, J.M., Griffin, M., on behalf of the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology, Guidelines for the management of oesophageal and gastric cancer. Gut 2011; 60:1449–1472. 21705456
33. Nagamatsu, Y., Yamana, H., Fujita, H., et al, The simultaneous evaluation of preoperative cardiopulmonary functions of oesophageal cancer patients in the analysis of expired gas with exercise testing. Nippon Kyoba Geka Gakkai Zasshi 1994; 42:2037–2040. 7836813
34. Nagamatsu, Y., Ono, H., Hiraki, H., et al, Pre-operative screening test for lung cancer using the analysis of expired gas with exercise testing – principally VO2max/m2. Nippon Kyobu Geka Gakkai Zasshi. 1994;42(10):1910–1915. 7798708
35. Forshaw, M.J., Strauss, D.C., Davies, A.R., et al, Is cardiopulmonary exercise testing a useful test before esophagectomy? Ann Thorac Surg 2008; 85:294–299. 18154826
36. Wijeysundera, D.N., Beattie, W.S., Austin, P.C., et al, Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. Br Med J 2010; 340:b5526. 20110306
37. Poldermans, D., Boersma, E., Bax, J.J., et al, The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med 1999; 341:1789–1794. 10588963
38. Fleisher, L.A., Beckman, J.A., Brown, K.A., et al, ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol. 2006;47(11):2343–2355. 16750714
39. Healy, K.O., Waksmonski, C.A., Altman, R.K., et al, Perioperative outcome and long-term mortality for heart failure patients undergoing intermediate- and high-risk non-cardiac surgery: impact of left ventricular ejection fraction. Congest Heart Fail 2010; 16:45–49. 20412467
40. Lee, J.T., Chaloner, E.J., Hollingsworth, S.J., The role of cardiopulmonary fitness and its genetic influences on surgical outcomes. Br J Surg. 2006;93(2):147–157. 16302176
41. Kothman, E., Batterham, A.M., Owen, S.J., et al, Effect of short-term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: a pilot study. Br J Anaesth. 2009;103(4):505–510. 19628486
42. Eichhorn, E.J., Beta-blocker withdrawal: the song of Orpheus. Am Heart J 1999; 138:387–389. 10467182
43. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines American Society of Echocardiography, et al, 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–118. 19926002
44. Dunkelgrun, M., Boersma, E., Schouten, O., et al, Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing non-cardiovascular surgery: a randomized controlled trial (DECREASE IV). Ann Surg 2005; 353:349–361. 19474688
45. Lindenauer, P.K., Pekow, P., Wang, K., et al, Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361. 16049209
46. Kapoor, A.S., Kanji, H., Buckingham, J., et al, Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. Br Med J 2006; 333:1149. 17088313
47. Douketis, J.D., Berger, P.B., Dunn, A.S., et al, The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(Suppl. 6):299S–339S. 18574269
48. Nuttall, G.A., Brown, M.J., Stombaugh, J.W., et al, Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008; 109:588–595. 18813036
49. Rabbits, J.A., Nuttall, G.A., Brown, M.J., et al, Cardiac risk of non-cardiac surgery after percutaneous coronary intervention with drug eluting stents. Anesthesiology 2008; 109:596–604. 18813037
50. Avendano, C.E., Flume, P.A., Silvestri, G.A., et al, Pulmonary complications after oesophagectomy. Ann Thorac Surg 2002; 73:922–926. 11899202
51. Quaseem, A., Snow, V., Itterman, N., et al, Risk assessment and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144:575–580. 16618955
52. Poussel, M., Nguyen Thi, P.L., Villemot, J.P., et al, Arterial oxygen partial pressure and cardiovascular surgery in elderly patients. Interact Cardiovasc Thorac Surg 2008; 7:819–824. 18583395
53. Joo, H.S., Wong, J., Naik, V.N., et al, The value of screening preoperative chest x-rays: a systematic review. Can J Anaesth 2005; 52:568–574. 15983140
54. Bapoje, S.R., Whitaker, J.F., Schulz, T., et al, Preoperative evaluation of the patient with pulmonary disease. Chest 2007; 132:1637–1645. 17998364
55. Mercantonio, E.R., Goldman, L., Mangione, C.M., et al, A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994; 271:134–139. 8264068
56. Ganai, S., Lee, K.F., Merrill, A., et al, Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg 2007; 142:1072–1078. 18025336
57. Markar, S.R., Smith, I.A., Karthikesalingam, A., et al, The Clinical and Economic Cost of Delirium Following Surgical Resection for Esophageal Malignancy. Ann Surg 2013; . (Epub ahead of print)
58. Inouye, S.K., Bogardus, S.T., Jr., Charpentier, P.A., et al, A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676. 10053175
59. O’Keeffe, S.T., Lavan, J.N., Predicting delirium in elderly patients: development and validation of a risk-stratification model. Age Ageing 1996; 25:317–321. 8831879
60. Franco, J.G., Valencia, C., Bernal, C., et al, Relationship between cognitive status at admission and incident delirium in older medical inpatients. J Neuropsychiatr Clin Neurosci 2010; 22:329–337. 20686140
61. O’Mahony, R., Murthy, L., Akunne, A., Guideline Development Group, Synopsis of National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med 2011; 154:746–751. 21646557
62. Mouchoux, C., Rippert, P., Duclos, A., et al, Impact of a multifaceted program to prevent postoperative delirium in the elderly: the CONFUCIUS stepped wedge protocol. BMC Geriatr 2011; 11:25. 21592324
63. Siddiqi, N., Stockdale, R., Britton, A.M., et al, Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2) CD005563. 17443600
64. Ono, H., Taguchi, T., Kido, Y., et al, The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs. 2011;27(3):158–166. 21511473
65. Mori, S., Sawada, T., Hamada, K., et al, Gastrectomy for patients with gastric cancer and non-uremic renal failure. World J Gastroenterol 2007; 13:4589–4592. 17729411
66. Schilling, T., Kozian, A., Kretzchmar, M., et al, Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth 2007; 99:368–375. 17621602
67. Abou-Elenain, K., Study of the systemic and pulmonary oxidative stress status during exposure to propofol and sevoflurane anaesthesia during thoracic surgery. Eur J Anaesthesiol 2010; 27:566–571. 20442581
68. Misthos, P., Katsaragakis, S., Milingos, N., et al, Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardiothorac Surg 2005; 27:379–382. 15740942
69. Michelet, P., D’Journo, X.B., Roch, A., et al, Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006; 105:911–919. 17065884
70. Low, D.E., Kunz, S., Schembre, D., et al, Esophagectomy – it’s not just about mortality any more: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007; 11:1395–1402. 17763917
71. Chandrashekar, M.V., Irving, M., Wayman, J., et al, Immediate extubation and epidural analgesia allow safe management in a high-dependency unit after two-stage oesophagectomy. Results of eight years of experience in a specialized upper gastrointestinal unit in a district general hospital. Br J Anaesth 2003; 90:474–479. 12644420
72. Brandstrup, B., Tonnesen, H., Beier-Holgersen, R., et al, Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238:641–648. 14578723
73. Nisanevich, V., Felsenstein, I., Almogy, G., et al, Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005; 103:25–32. 15983453
74. Kita, T., Mammoto, T., Kisis, Y., Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth 2002; 14:252–256. 12088806 This is the first trial to demonstrate significantly reduced length of hospital stay with reduced intraoperative fluid administration specifically in the setting of oesophagectomy.
75. Neal, J.M., Wilcox, R.T., Allen, H.W., et al, Near-total esophagectomy: the influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med 2003; 28:328–334. 12945027 This trial demonstrated a significantly reduced incidence of pulmonary complication and earlier extubation associated with intraoperative fluid restriction.
76. Abbas, S.M., Hill, A.G., Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia 2008; 63:44–51. 18086070
77. Kobayashi, M., Koh, M., Irinoda, T., et al, Stroke volume variation as a predictor of intravascular volume depression and possible hypotension during the early postoperative period after esophagectomy. Ann Surg Oncol 2009; 16:1371–1377. 19219508
78. Rudin, A., Flisberg, P., Johansson, J., et al, Thoracic epidural analgesia or intravenous morphine analgesia after thoracoabdominal esophagectomy: a prospective follow-up of 201 patients. J Cardiothorac Vasc Anesth 2005; 19:350–357. 16130063
79. Popping, D.M., Elia, N., Marret, E., et al, Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 2008; 143:990–999. 18936379
80. Michelet, P., D’Journo, X.B., Roch, A., et al, Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest 2005; 128:3461–3466. 16304300
81. Lazar, G., Kaszaki, J., Abraham, S., et al, Thoracic epidural anesthesia improves the gastric microcirculation during experimental gastric tube formation. Surgery 2003; 134:799–805. 14639359
82. Ng, J.M., Update of anesthetic management for esophagectomy. Curr Opin Anesth 2011; 24:37–43. 21102314
83. Doi, K., Yamanaka, M., Shono, A., et al, Preoperative epidural fentanyl reduces postoperative pain after upper abdominal surgery. J Anesth 2007; 21:439–441. 17680204
84. Komatsu, H., Matsumoto, S., Mitsuhata, H., Comparison of patient-controlled epidural analgesia with and without night-time infusion following gastrectomy. Br J Anaesth 2001; 87:633–635. 11878737
85. Kaiser, A.M., Zollinger, A., De Lorenzi, D., et al, Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg 1998; 66:367–372. 9725371
86. Munitiz, V., Martinez-de-Haro, L.F., Ortiz, A., et al, Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg 2010; 97:714–718. 20187171
87. Cerfolio, R.J., Bryant, A.S., Bass, C.S., et al, Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004; 126:1187–1194. 15486381