Prenatal Musculoskeletal Imaging
Skeletal Dysplasias
Etiology: Dysplasias are often the result of genetic mutations that encode for proteins important to development of the growth plate.1,2 Current clinical application of “skeletal dysplasia testing panels” is, however, very limited. Both the range of included mutations and the sensitivities of their detection are expected to increase with time. In utero testing in sporadic cases remains controversial.3
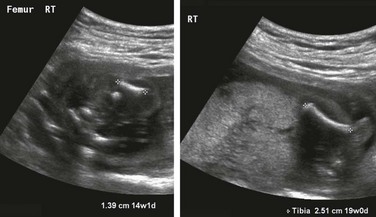
Imaging: Over 450 dysplasias have been described in the literature. Only a small percentage is considered diagnosable in utero with imaging, the first investigation for which is ultrasonography.3,4 Because of the lack of specificity of many of the findings, which have generally to do with abnormal bone shape (formation) or length (growth), efforts at prenatal evaluation have focused on identification of cases in which the neonates are not expected to survive. Identification of these fetuses allows counseling and for the parents and appropriate care planning. To this end, various ultrasonography criteria have been suggested to separate those conditions that are thought to be lethal from those that are not, with very high reliability (Box 131-1).5–11 Most of these criteria correlate limb length with survival. Emphasis is also placed on measures predicting lethal pulmonary hypoplasia. For example, the thoracic circumference is measured in the axial plane at the level of the four-chamber view of the heart; a thoracic circumference at or below the lower limit of the 95% confidence interval for gestational age is considered predictive of lethality in the setting of a micromelic bone dysplasia.12,13 Other findings that may be detected in association with lethality and that increase diagnostic certainty are polyhydramnios and nonimmune hydrops fetalis. It is important to avoid making a diagnosis of lethality in borderline cases and to use the criteria in combination for the most accurate discrimination. The fetus with intrauterine growth retardation should not be assigned a diagnosis of generalized skeletal dysplasia on the basis of proportionate but short bones. Questionable cases in either category deserve follow-up ultrasonography for interval growth.
The three most common lethal dysplasias are (1) thanatophoric dysplasia, (2) achondrogenesis, and (3) osteogenesis imperfecta type II. These three diagnoses together constitute the majority of cases. Therefore, a familiarity with their ultrasonographic features is essential (Box 131-2).14,15
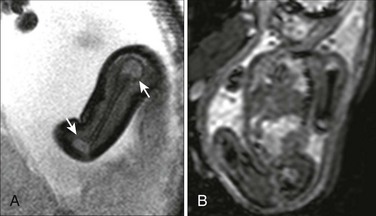
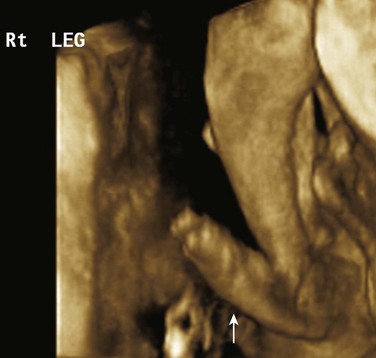
Certain skeletal morphologic findings on ultrasonography suggest particular diagnoses in the setting of a suspected dysplasia. Long bone fractures, for example, are indicative of osteogenesis imperfecta or hypophosphatasia, and rib fractures are rarely seen with other diagnoses (Fig. 131-1). Kleeblattschadel, or cloverleaf skull, may be present in several of the acrocephalosyndactyly syndromes (Apert, Carpenter, Crouzon, and Pfeiffer syndromes), campomelic dysplasia, osteocraniostenosis, and thanatophoric dysplasia type II (Fig. 131-2). Hypoplastic scapular bodies are a feature of campomelic dysplasia and Antley-Bixler syndrome.16 However, many findings such as platyspondyly are not specific and must be considered in relation to other features (short ribs, abnormal mineralization, solid organ malformations) to arrive at a more narrow differential diagnosis (Fig. 131-3).17 Three-dimensional ultrasonography can be very helpful when an appropriate volume of fluid is present around the fetus to display the morphology of specific segments, including the face, hands, and feet.18
Figure 131-1 Long bone fractures in a fetus with severe micromelia.
Diagnosis is osteogenesis imperfecta type II.
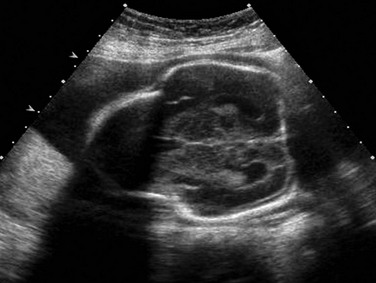
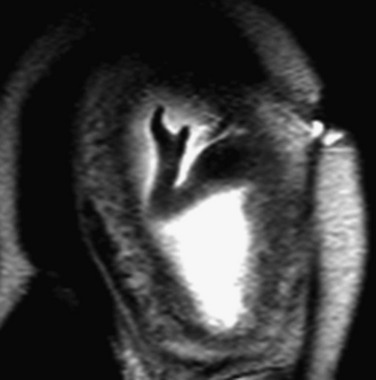
Figure 131-2 Kleeblattschadel, or cloverleaf skull (pancraniosynostosis).
In the setting of a lethal bone dysplasia, the differential diagnosis includes thanatophoric dysplasia type II, campomelic dysplasia and osteocraniostenosis.
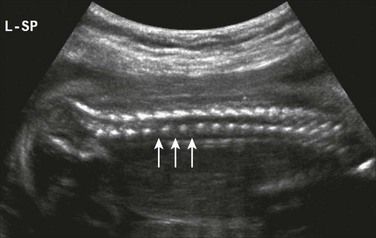
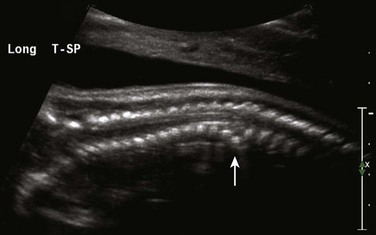
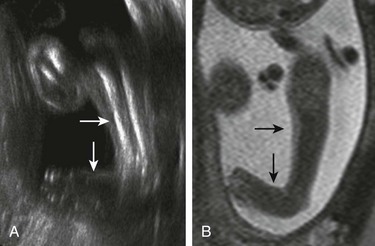
Figure 131-3 Platyspondyly.
Disc spaces (arrows) are wider than the ossification centers for the vertebral bodies.
Fetal MRI, in general, has not been pursued routinely in the evaluation of fetal dysplasia because of the difficulty in imaging the signal-poor ossified skeleton. However, it may have a role in quantification of fetal lung volumes in cases in which ultrasonography has limitations.19 In addition, fetal MRI has been used successfully to make the diagnosis of dysplasias that primarily involve the epiphyses, taking advantage of the high water content and, therefore, the high T2-weighted signal characteristics of cartilage.20
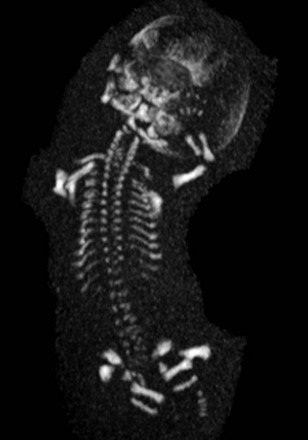
The use of ionizing radiation is avoided during pregnancy unless the need to make a diagnosis is critical to patient care. In such a circumstance, low-dose computed tomography techniques have been used to reconstruct highly detailed three-dimensional models of the fetal skeleton (Fig. 131-4).21,22 The features described would be compared with an atlas of postmortem radiographs in identified syndromes to aid in diagnosis.23 This strategy can be offered at radiation doses of 3 to 5 millisievert with superb resolution of individual skeletal components.22
Treatment and Follow-up: When a lethal skeletal dysplasia is detected by using ultrasonography, the parents and fetal care team can make informed decisions about the continuation of the pregnancy and the circumstances of delivery. An opportunity exists for neonatal hospice care coordination, when appropriate. Even though ultrasonography is reliable at separating lethal dysplasias from nonlethal dysplasias, neonatal physical examination and radiographs, appropriate genetic testing, and autopsy, if relevant, are crucial in arriving at a specific diagnosis, which, in turn, is necessary for accurate family counseling.
Amniotic Band Syndrome and Limb Reductions
Etiology: Limb reductions may be caused by a variety of insults. Some of these are from amputation, such as in the amniotic band sequence spectrum. Although the initiating event in this sequence has not been incontrovertibly established, the most commonly supported theory is that a tear is made in the amnion, exposing the fetus to the more adhesive chorion, with subsequent loss of tissue or the development of a constrictive band.24 In other cases, primary vascular insults are blamed for segmental limb loss, and teratogens (e.g., thalidomide) are highly associated in some instances.25,26 Limb reduction abnormalities can be chromosomal, particularly with Trisomy 18.27 Proximal focal femoral deficiency is a particular form of reduction that affects the femoral head and neck and is associated with fibular aplasia or hypoplasia. It may be unilateral or bilateral (Fig. 131-5).
Imaging: Limb reduction anomalies may be subtle in the case of isolated, very distal defects involving fingers or toes. Ultrasonography can characterize the shape of the affected limb well and is diagnostic, and MRI is usually not necessary (Fig. 131-6). Associated findings may include edema of the extremity distal to a constricting band (Fig. 131-7 and Video 131-1). Cine ultrasound sequences can capture the abnormal motion that is a consequence of subluxation or dislocation if the affected segment involves a joint. Random, multiple defects are more likely secondary to amniotic band sequence than are symmetric defects, which tend to be related to chromosomal abnormalities or syndromes (Fig. 131-8).28 As amniotic bands can cause defects in any body part, a thorough evaluation of the entire fetus and its umbilical cord is indicated. Umbilical cord involvement is often overlooked but has consequences because of the risk of cord occlusion.29
Treatment and Follow-up: Successful cases of fetoscopic lysis of amniotic bands have been reported when it has been thought that release could improve blood flow to and consequent growth of an affected limb segment.30 Otherwise, plastic surgery consultation is sought for limb salvage following delivery of the fetus.
Primary Anomalies of the Spine
Etiology: Primary anomalies of the spine are some of the more common antenatally detected skeletal abnormalities. These anomalies typically manifest as an unusual curvature, either scoliotic or kyphotic. Care must be taken to ensure that this finding is a fixed abnormality and not positional or the result of a lack of space (e.g., in anhydramnios). Fixed scoliosis is abnormal and may be the result of tethering, as with limb–body wall complex (Fig. 131-9), a severe and lethal form of amniotic band sequence; rib fusions, as in Jarcho-Levin syndrome (Fig. 131-10); or fetal akinesia or hypokinesia.31 Often, the finding is subtle and secondary to segmentation anomalies of the spine, such as hemivertebrae or butterfly vertebrae (Fig. 131-11). Complex spinal anomalies can be seen with neural tube defects and with the Currarino triad, which is a heritable complex of anomalous sacrum, presacral mass, and anorectal malformation.32 Absence of the sacrum, with variable involvement of the more proximal spine, occurs in caudal regression syndrome, which is significantly associated with elevated glucose levels in the fetus of a mother with diabetes.33
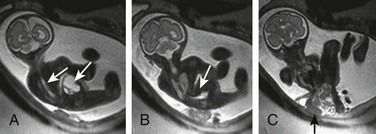
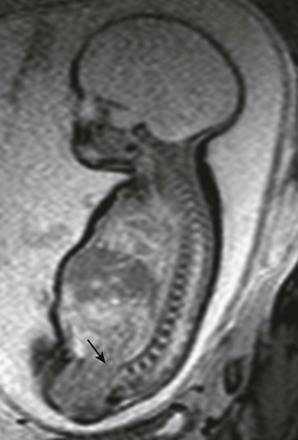
Figure 131-9 Limb–body wall complex.
Multiple images had shown fixed scoliosis and contortion in a fetus with extrusion of abdominal contents adherent to the amnion (black arrow). White arrows indicate the spinal canal in this fetus, who also has a closed neural tube defect.
The most common spinal anomaly in the fetus involves the dysraphism of posterior elements in a neural tube defect. This group of lesions is discussed in detail in Chapter 42.
Imaging: A spinal anomaly may be an isolated fetal finding, but it does prompt a careful search for associated syndromic abnormalities, including the various components of the VACTERL complex (vertebral anomaly, anorectal atresia, cardiac lesion, tracheoesophageal fistula, renal anomaly, limb defect) (Fig. 131-12). The presence of an anal dimple can be confirmed on ultrasonography, where attention is also directed specifically to the size of the stomach and the morphology of the radial ray segments. Devoted echocardiography is recommended in suspicious cases. MRI has been used to examine for a distended esophageal pouch, although this is seen inconsistently. A higher sensitivity may be present for transient esophageal distension using repetitive dynamic steady-state free precession (SSFP) sequences in the sagittal midline.34 The use of the multiplanar capabilities of MRI may be advantageous in sorting out the components of complex spinal anomalies (Fig. 131-13). T2-weighted sequences, whether single-shot fast spin echo or gradient-echo SSFP, are most useful. Fast T1-weighted gradient sequences may help identify ectopic fat.
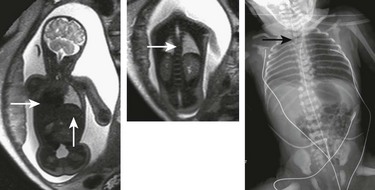
Figure 131-12 VACTERL complex (vertebral anomaly, anorectal atresia, cardiac lesion, tracheoesophageal fistula, renal anomaly, limb defect).
Coronal T2-weighted single-shot fast spin echo images show a right-sided heart, absence of fluid in the stomach, and thoracic scoliosis from hemivertebrae (white arrows), with subsequent neonatal image. Note the Repogle tube in the proximal esophageal pouch (black arrow).
Treatment and Follow-up: Correlation with imaging and physical examination after delivery is critical to confirm the suspected anomaly and to guide therapy. Because both ultrasonography and MRI have limitations in the evaluation of the spine, prenatal imaging findings may not be straightforward, and it should not be assumed that the most complete diagnosis has been described antenatally.
Neuromuscular Disorders
Etiology: Several conditions that cause generalized hypotonia in the fetus and neonate are heritable: the most important, at least from the point of view of obstetric management, is congenital myotonic dystrophy. This is an autosomal dominant muscular disease, in which expansion of abnormal genomic repeats inserted during cell division will be greater in an affected child than in the carrier parent. Milder cases in adults often go undiagnosed, and a carrier mother may not display obvious manifestations of muscular insufficiency until administered respiratory-depressant anesthetics, to which these patients are exquisitely sensitive. Sudden death from cardiac conduction abnormalities during sedation has also been reported.35 Therefore, if myotonic dystrophy is suspected as part of the differential diagnosis in a fetus, maternal genetic testing should be performed and a meticulously careful anesthetic strategy planned for the delivery.36,37
Most of the time, a specific diagnosis is difficult to establish for a fetus with limited movement, fixed position of the extremities, multiple contractures, or all of these. An unsatisfactory term, arthrogryposis multiplex congenita, has been used to describe this combination of findings, but the etiologies are likely to be multiple and various. Muscular, neurologic, and connective tissue disorders may all play roles. Differential considerations include multiple pterygium syndrome, in which skin webs tend to tether the limbs at the joints and restrict fetal movement. The major cause of arthrogryposis is fetal akinesia sequence, which has been associated with Pena-Shokeir syndrome (pseudo-trisomy 18), nemaline rod myopathy, and maternal myasthenia gravis.38–40
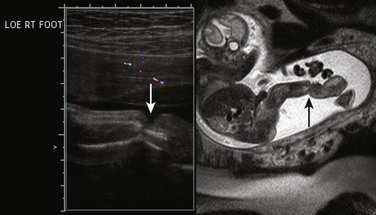
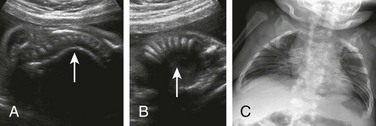
“Clubfoot” is a term that tends to be used generically to describe abnormal foot position relative to the leg; the more accurate term is talipes, the most common presentation of which is fixed plantar flexion with internal rotation at the ankle (talipes equinovarus, or clubfoot), though several forms of talipes (combinations of calcaneo- or equino- varus/valgus) are described.41 If unilateral, it is often an isolated finding with a low likelihood of syndromic association. Bilateral clubfoot, however, is typically secondary to spinal cord abnormalities such as meningomyelocele; anhydramnios or oligohydramnios, as with fetal renal or bladder obstruction; fetal akinesia; or genetic anomalies. Rocker-bottom foot (congenital vertical talus, or rigid flatfoot) is nearly always bilateral and is highly associated with aneuploidy (Trisomies 9, 13, or 18), though most cases are associated with neurologic disorders such as myelomeningocele (Fig. 131-14).42
Imaging: Most fetuses move during ultrasonography, and assessment of fetal posture and movement is an essential part of determining fetal well-being.43,44 Those that do not move as expected may be just sleeping and therefore are reimaged after a time interval to avoid further unnecessary evaluation. Movement limitation should be determined as generalized versus focal, and an effort should be made to look for tethering, constricting bands or a neural axis lesion, and muscle bulk should be assessed. Polyhydramnios is likely if the fetus is not swallowing; a lack of breathing will result in a small chest and pulmonary hypoplasia. Fetal MRI may be indicated to evaluate the neural axis for a cause of generalized hypotonia or contractures and can help measure lung volumes. Alterations on MRI of muscular contour, thickness, and signal have suggested atrophy from neuromuscular disease.45
Clubfoot is diagnosed on ultrasonography or MRI when both the foot and leg are imaged in a coronal plane together (Fig. 131-15). Care should be taken to ensure that this is a fixed position over time; spontaneous flexion at the ankle on real-time ultrasonography suggests the finding is positional.
Treatment and Follow-Up: Neonatal treatments for hypotonia, arthrogryposis, or both are supportive. No fetal treatments are described, though testing for myotonic dystrophy should be undertaken in appropriate cases to avoid maternal complications. Treatment for clubfoot is generally started in the first days of life, and surgery depends on the success of manipulation and casting.46
Tumors
Etiology: Tumors of the fetal musculoskeletal system are extraordinarily rare, except for sacrococcygeal teratoma (SCT). Still, this entity is uncommon, with a reported incidence of only 1 per 40,000.47 SCT is thought to arise from primitive cells associated with the fetal coccyx, which must be removed in entirety with the tumor at excision to prevent recurrence. The Altman classification of SCT applies to the fetus but may change during gestation (Box 131-3).48
Imaging: As with teratoma anywhere in the fetal body, tumors are characteristically mixed cystic and solid, with arterial vessels identifiable in the solid components. Ultrasonography is excellent for identifying the abnormal mass at the pelvis and involvement of superficial soft tissue just dorsal to the anus, which is invariably displaced ventrally when an external tumor component is present. Color Doppler interrogation is used to assess the vascularity of the tumor. Because of tumoral vascular shunting, a fetus may develop signs of high cardiac output failure, with the typical findings of hydrops fetalis that occurs if intervention is not undertaken. Close-interval follow-up with serial ultrasonography for the evolution of cardiac dysfunction and hydrops is indicated in a fetus with SCT if intervention is to be considered.49 Because pelvic bones may interfere with clear visualization of the deep structures on ultrasonography, MRI is advantageous for better defining the extent of a lesion, its relationship to the bladder and rectum, and calculating a tumor–body weight ratio that some institutions use for prognosis (Fig. 131-16).50 Both single-shot T2- and T1-weighted sequences for meconium–rectal position and hemorrhage may be helpful. Fetal MRI can supplant neonatal MRI in some cases. Although much more rare, neuroblastoma in the low pelvis has been reported to mimic the appearance of SCT.51
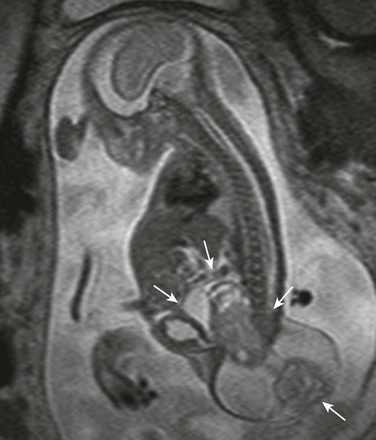
Figure 131-16 Sacrococcygeal teratoma.
Mixed cystic-solid mass in the fetal pelvis between the bladder and the spine, extending externally (arrows).
Rhabdomyoma, rhabdomyosarcoma, fibrosarcoma, or myofibroma (myofibromatosis) are extraordinarily rare in the fetus. These are, when prospectively diagnosed, characteristically solid masses with some internal heterogeneity that grow on serial examination; they are typically found in the fetal neck or pelvis but may be in the extremities, retroperitoneum, or chest wall.52–58 They may be confused with vascular tumors such as a kaposiform hemangioendothelioma, although the latter are typically more infiltrative. Lymphatic malformation may also be infiltrative and multicompartmental and may have both solid (microcystic) and cystic (macrocystic) components. The differential diagnosis for solid tumors includes teratoma (which is more common, generally has some cysts, and might be distinguished by a fat component) and potentially neuroblastoma. Either of these tumors can contain calcifications.
Treatment and Follow-up: Prior to viability, should a fetus develop cardiac failure and hydrops fetalis, in utero surgical resection of SCT has been offered in some centers as an alternative to inevitable demise. The procedure involves a hysterotomy with partial delivery of the fetus, tumor resection, and return of the fetus to the uterus with myometrial closure. A significant complication is preterm labor, and efforts are therefore being directed toward less invasive surgeries.59 This is clearly a rare circumstance, but it has been successfully managed. After the age of viability, a fetus in distress could be delivered for further ex utero treatment options.
Treatment for SCT in the neonate is complete surgical excision of the tumor; the risk of malignancy is lowest with fetal or neonatal presentation and therapy.60 Recurrence rates are negligible as long as the coccyx is also removed with the tumor.61
Avni, FE, Massez, A, Cassart, M. Tumours of the fetal body: a review. Pediatr Radiol. 2009;39:1147–1157.
Avni, FE, Rypens, F, Zappa, M, et al. Antenatal diagnosis of short-limb dwarfism: sonographic approach. Pediatr Radiol. 1996;26(3):171–178.
Cassart, M. Suspected fetal skeletal malformations or bone diseases: how to explore. Pediatr Radiol. 2010;40:1046–1051.
Dighe, M, Fligner, C, Cheng, E, et al. Fetal skeletal dysplasia: an approach to diagnosis with illustrative cases. RadioGraphics. 2008;28:1061–1077.
Teele, R. A guide to the recognition of skeletal disorders in the fetus. Pediatr Radiol. 2006;36(6):473–484.
References
1. Ikegawa, S. Genetic analysis of skeletal dysplasia: recent advances and perspectives in the post-genome-sequence era. J Hum Genet. 2006;51(7):581–586.
2. Alman, BA. Skeletal dysplasias and the growth plate. Clin Genet. 2008;73(1):24–30.
3. Krakow, D, Lachman, R, Rimoin, D. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med. 2009;11(2):127–133.
4. Warman, ML, Cormier-Daire, V, Hall, C, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011;155A(5):943–968.
5. Hersh, JH, Angle, B, Pietrantoni, M, et al. Predictive value of fetal ultrasonography in the diagnosis of a lethal skeletal dysplasia. South Med J. 1998;91(12):1137–1142.
6. Rahemtullah, A, McGillivray, B, Wilson, RD. Suspected skeletal dysplasias: femur length to abdominal circumference ratio can be used in ultrasonographic prediction of fetal outcome. Am J Obstet Gynecol. 1997;177:864–869.
7. Ramus, RM, Martin, LB, Twickler, DM. Ultrasonographic prediction of fetal outcome in suspected skeletal dysplasias with use of the femur length-to-abdominal circumference ratio. Am J Obstet Gynecol. 1998;179(5):1348–1352.
8. Yoshimura, S, Masuzaki, H, Gotoh, H, et al. Ultrasonographic prediction of lethal pulmonary hypoplasia: comparison of eight different ultrasonographic parameters. Am J Obstet Gynecol. 1996;175(2):477–483.
9. Kurtz, AB, Needleman, L, Wapner, RJ, et al. Usefulness of a short femur in the in utero detection of skeletal dysplasias. Radiology. 1990;177(1):197–200.
10. Parilla, BV, Leeth, EA, Kambich, MP, et al. Antenatal detection of skeletal dysplasias. J Ultrasound Med. 2003;22(3):255–258. [quiz 259-261].
11. Ishikawa, S, Kamata, S, Usui, N, et al. Ultrasonographic prediction of clinical pulmonary hypoplasia: measurement of the chest/trunk-length ratio in fetuses. Pediatr Surg Int. 2003;19(3):172–175.
12. Nimrod, C, Nicholson, S, Davies, D, et al. Pulmonary hypoplasia testing in clinical obstetrics. Am J Obstet Gynecol. 1988;158:277–280.
13. Ohlsson, A, Fong, K, Rose, T, et al. Prenatal ultrasonic prediction of autopsy-proven pulmonary hypoplasia. Am J Perinatol. 1992;9(5-6):334–337.
14. Orioli, IM, Castilla, EE, Barbosa-Neto, JG. The birth prevalence rates for the skeletal dysplasias. J Med Genet. 1986;23(4):328–332.
15. Camera, G, Mastroiacovo, P. Birth prevalence of skeletal dysplasias in the Italian Multicentric Monitoring System for Birth Defects. Prog Clin Biol Res. 1982;104:441–449.
16. Mortier, GR, Rimoin, DL, Lachman, RS. The scapula as a window to the diagnosis of skeletal dysplasias. Pediatr Radiol. 1997;27(5):447–451.
17. Rouse, GA, Filly, RA, Toomey, F, et al. Short-limb skeletal dysplasias: evaluation of the fetal spine with sonography and radiography. Radiology. 1990;174(1):177–180.
18. Krakow, D, Williams, J, III., Poehl, M, et al. Use of three-dimensional ultrasound in the diagnosis of prenatal onset skeletal dysplasias. Ultrasound Obstet Gynecol. 2003;21:467–472.
19. Tanigaki, S, Miyakoshi, K, Tanaka, M, et al. Pulmonary hypoplasia: prediction with use of ratio of MR imaging-measured fetal lung volume to US-estimated fetal body weight. Radiology. 2004;232(3):767–772.
20. Yazici, Z, Kline-Fath, BM, Laor, T, et al. Fetal MR imaging of Kniest dysplasia. Pediatr Radiol. 2010;40(3):348–352.
21. Ulla, M, Aiello, H, Cobos, MP, et al. Prenatal diagnosis of skeletal dysplasias: contribution of three-dimensional computed tomography. Fetal Diagn Ther. 2011;29(3):238–247.
22. Cassart, M, Massez, A, Cos, T, et al. Contribution of three-dimensional computed tomography in the assessment of fetal skeletal dysplasia. Ultrasound Obstet Gynecol. 2007;29(5):537–543.
23. Spranger, JW, Brill, PW, Poznanski, A. Bone dysplasias: an atlas of genetic disorders of skeletal development, 2nd ed. Oxford, UK: Oxford University Pres; 2002.
24. Lockwood, C, Ghidini, A, Romero, R, et al. Amniotic band syndrome: reevaluation of its pathogenesis. Am J Obstet Gynecol. 1989;160(5 Pt 1):1030–1033.
25. Gold, NB, Westgate, MN, Holmes, LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genet A. 2011;155A(6):1225–1235.
26. McGuirk, CK, Westgate, MN, Holmes, LB. Limb deficiencies in newborn infants. Pediatrics. 2001;108(4):E64.
27. Christianson, AL, Nelson, MM. Four cases of trisomy 18 syndrome with limb reduction malformations. J Med Genet. 1984;21(4):293–297.
28. Duckworth, HL, Leather, AT, Jessop, F. Intrauterine death and growth restriction at term, secondary to an umbilical cord amniotic band. J Obstet Gynaecol. 2011;31(6):547.
29. Stoll, C, Rosano, A, Botto, LD, et al. On the symmetry of limb deficiencies among children with multiple congenital anomalies. Ann Genet. 2001;44(1):19–24.
30. Keswani, SG, Johnson, MP, Adzick, NS, et al. In utero limb salvage: fetoscopic release of amniotic bands for threatened limb amputation. J Pediatr Surg. 2003;38(6):848–851.
31. Hunter, AG, Seaver, LH, Stevenson, RE. Limb-body wall defect. Is there a defensible hypothesis and can it explain all the associated anomalies? Am J Med Genet A. 2011;155A(9):2045–2059.
32. Emans, PJ, Kootstra, G, Marcelis, CL, et al. The Currarino triad: the variable expression. J Pediatr Surg. 2005;40(8):1238–1242.
33. Chan, BW, Chan, KS, Koide, T, et al. Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes. 2002;51(9):2811–2816.
34. Brugger, PC. Methods of fetal MRI. Berlin, Germany: Springer-Verlag; 2011. [65-80].
35. Barash, PG, et al. Clinical anesthesia, 4th ed. Hagerstown, MD: Lippincott, Williams & Wilkins; 1997. [32-34, 493-494].
36. Dufour, P, Berard, J, Vinatier, D, et al. Myotonic dystrophy and pregnancy. A report of two cases and a review of the literature. Eur J Obstet Gynecol Reprod Biol. 1997;72(2):159–164.
37. Mori, K, Mizuno, J, Nagaoka, T, et al. [Combined spinal-epidural anesthesia for cesarean section in a parturient with myotonic dystrophy. Masui. 2010;59(8):1000–1003.
38. Brueton, LA, Huson, SM, Cox, PM, et al. Asymptomatic maternal myasthenia as a cause of the Pena-Shokeir phenotype. Am J Med Genet. 2000;92(1):1–6.
39. Lammens, M, Moerman, P, Fryns, JP, et al. Fetal akinesia sequence caused by nemaline myopathy. Neuropediatrics. 1997;28(2):116–119.
40. Hall, JG. Pena-Shokeir phenotype (fetal akinesia deformation sequence) revisited. Birth Defects Res A Clin Mol Teratol. 2009;85(8):677–694.
41. Ozonoff, MB. Pediatric orthopedic radiology. Philadelphia, PA: Saunders; 1992.
42. Ogata, K, Schoenecker, PL, Sheridan, J. Congenital vertical talus and its familial occurrence: an analysis of 36 patients. Clin Orthop Relat Res. 1979;139:128–132.
43. Alfirevic, Z, Neilson, JP. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev. (2):2000. [CD000038].
44. de Vries, JI, Fong, BF. Changes in fetal motility as a result of congenital disorders: an overview. Ultrasound Obstet Gynecol. 2007;29(5):590–599.
45. Nemec, SF, Brugger, PC, Nemec, U, et al. The skeleton and musculature. In: Prayer D, ed. Fetal MRI. Berlin, Germany: Springer-Verlag, 2011.
46. Steinman, S, Richards, BS, Faulks, S, et al. A comparison of two nonoperative methods of idiopathic clubfoot correction: the Ponseti method and the French functional (physiotherapy) method. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):299–312.
47. Avni, FE, Massez, A, Cassart, M. Tumours of the fetal body: a review. Pediatr Radiol. 2009;39(11):1147–1157.
48. Altman, RP, Randolph, JG, Lilly, JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg. 1974;9(3):389–398.
49. Cass, DL, Olutoye, OO, Ayres, N, et al. Defining hydrops and indications for open fetal surgery for fetuses with lung masses and vascular tumors. J Ped Surg. 2012;47(1):40–45.
50. Rodriguez, MA, Cass, DL, Lazar, DA, et al. Tumor volume to fetal weight ratio as an early prognostic classification for fetal sacrococcygeal teratoma. J Pediatr Surg. 2011;46(6):1182–1185.
51. Tanaka, K, Kanai, M, Yosizawa, J, et al. A case of neonatal neuroblastoma mimicking Altman type III sacrococcygeal teratoma. J Pediatr Surg. 2005;40(3):578–580.
52. Yang, HJ, Lee, KA, Park, CW, et al. Prenatal US and MRI findings of pelvic embryonal rhabdomyosarcoma. Ultrasound Obstet Gynecol. 2008;32(3):442.
53. Esfahani, SA, Montaser-Kouhsari, L, Saeedi, P, et al. An antenatally diagnosed rhabdomyosarcoma of the bladder treated without extensive surgery, Nat. Rev Urol. 2009;6(8):449–453.
54. Gupta, SS, Singh, O, Sharma, SS, et al. Congenital fibrosarcoma of the chest wall: report of a case. J Cutan Aesthet Surg. 2010;3(3):177–180.
55. Valdez, TA, Desai, U, Volk, MS. Recurrent fetal rhabdomyoma of the head and neck. Int J Pediatr Otorhinolaryngol. 2006;70(6):1115–1118.
56. Huang, SY, Wang, CW, Wang, CJ, et al. Combined prenatal ultrasound and magnetic resonance imaging in an extensive congenital fibrosarcoma: a case report and review of the literature. Fetal Diagn Ther. 2005;20(4):266–271.
57. Govender, L, Moodley, J. Soft tissue tumour of the fetal thigh. S Afr Med J. 2006;96(3):187–188.
58. Wataganara, T, Ngerncham, S, Kitsommart, R, et al. Fetal neck myofibroma. J Med Assoc Thai. 2007;90(2):376–380.
59. Hirose, S, Farmer, DL. Fetal surgery for sacrococcygeal teratoma. Clin Perinatol. 2003;30(3):493–506.
60. Holterman, AX, Filiatrault, D, Lallier, M, et al. The natural history of sacrococcygeal teratomas diagnosed through routine obstetric sonogram: a single institution experience. J Pediatr Surg. 2003;33(6):899–903. [198].
61. Tapper, D, Sawin, R. Teratomas and other germ cell tumours. In: O’Neil JA, Jr., Rowe MI, Grosfeld JL, et al, eds. Pediatric surgery. 5th ed. St. Louis, MO: Mosby Yearbook Inc; 1998:447–459.