Prenatal Imaging and Therapy of Congenital Heart Disease
Congenital heart disease, the most common birth defect, occurs in 3 to 8 per 1000 newborns.1–3 The incidence is higher prenatally, affecting 5.8% to 16.9% of fetuses undergoing screening echocardiograms.4–6 Despite advances in imaging techniques, routine obstetric ultrasound is only 30% to 50% sensitive for detection of congenital heart defects.7–12 With the addition of careful delineation of outflow tracts, sensitivity improves significantly.13 The most difficult lesions to diagnose prenatally are transposition of the great arteries and outflow tract abnormalities. A complete fetal echocardiogram includes two-dimensional, M-mode, and color Doppler imaging to assess fetal cardiac structure, rhythm, and function. Novel techniques include tissue Doppler and strain analysis.
Fetal Physiology and Flow
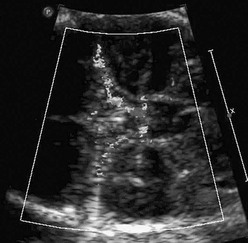
The fetal cardiac circulation has been studied in human and animal models (Fig. 71-1). Fetal and postnatal cardiovascular physiology differs markedly. Key differences include the following:
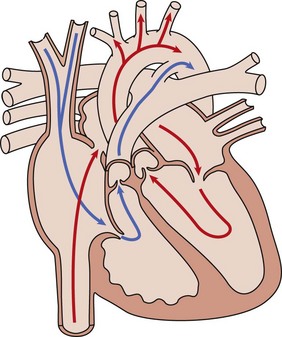
Figure 71-1 Diagram of the normal fetal cardiac circulation.
Oxygenated umbilical venous blood enters the right atrium from the inferior vena cava and is directed across a patent foramen ovale. into the left atrium, from which it enters the fetal systemic circulation. Unoxygenated fetal systemic venous blood enters the right atrium via the superior vena cava. This blood is directed into the right ventricle and then out into the main pulmonary artery. High pulmonary vascular resistance and a patent ductus arteriosus causes preferential flow into the descending aorta.
1. Right ventricular output is greater than left ventricular output.
2. Oxygen saturation of blood to the brain is higher than of blood to the body because maternal blood is directed from the umbilical vein to the ductus venosus and across the foramen ovale by the eustachian valve.
3. A ductus arteriosus is present. Deoxygenated blood from the superior vena cava travels to the right ventricle to the ductus arteriosus and then to the placenta.
4. Pulmonary vascular resistance is increased, resulting in decreased flow to the lungs. This changes after birth to allow an increase in pulmonary blood flow.
5. In utero, afterload for the left ventricle decreases but increases dramatically with umbilical cord clamping.
Cerebral Resistance
Fetal cerebral vessels can vasodilate during stress, which decreases resistance and increases diastolic flow in the middle cerebral artery. Peripheral vessels vasoconstrict to direct blood to the brain; this causes increased resistance and decreased diastolic flow in the descending aorta. This represents an autoregulatory mechanism (Fig. 71-2). This phenomenon of increasing cerebral blood flow has been described in growth-restricted fetuses as a predictor of poor perinatal outcome (Fig. 71-3). This phenomenon also occurs in fetuses with congenital heart disease (Table 71-1), although the clinical significance of this finding as a predictor of outcome is still in question.14
Table 71-1
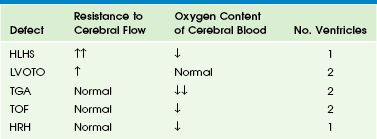
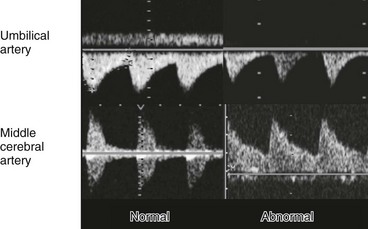
Figure 71-2 Normal and abnormal arterial spectral Doppler signals from the umbilical and middle cerebral arteries.
Fetal compromise results in increased peripheral and placental resistance (decreased diastolic flow in umbilical artery) and decreased cerebral resistance (increased diastolic flow in middle cerebral artery); this is known as the brain-sparing effect.
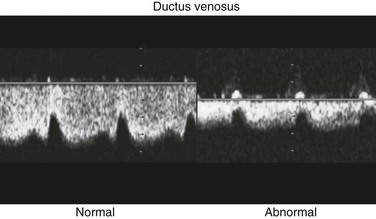
Venous Flow Patterns
Flow patterns in the umbilical vein and ductus venosus can be used to assess right ventricular filling. Impaired relaxation, associated with placental insufficiency or cardiac dysfunction, can cause decreased or reversed diastolic venous flow, particularly in atrial systole (Fig. 71-4). Absent or reversed flow in the ductus venosus and pulsatility of the umbilical vein flow associated with elevated atrial pressure have been recognized as markers of poor outcome in hydropic fetuses.
Fetal Anatomy
Aortic and Ductal Arches
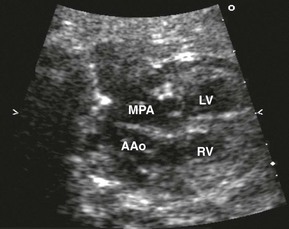
The relationship of the aorta and pulmonary outflow to the ventricles should be determined, and peak velocities in aortic and ductal arches should be obtained. Arch sidedness can be determined from the three-vessel view. The aorta, main pulmonary artery, and the SVC are seen relative to the trachea (Fig. 71-5) An aorta positioned right of the trachea suggests a right aortic arch and calls for careful evaluation for vascular rings and congenital heart disease.
Prenatal Imaging: Timing and Indications
Fetal echocardiography has been in use since the late 1980s. The optimal time for transabdominal imaging of the fetal heart is between 20 and 28 weeks of gestation. Transvaginal imaging can be performed as early as 8 weeks, with successful diagnosis of heart defects possible as early as 11 weeks.15–16 Third-trimester imaging, although possible, is limited by paucity of the amniotic fluid and limited variability in fetal position. Indications for fetal echocardiography include maternal and fetal risk factors (Box 71-1). The most common reasons are family history of congenital heart disease, fetal dysrhythmia, maternal diabetes, and extracardiac defects. Indications that are most predictive of cardiac disease are an abnormal four-chamber view on routine ultrasound (30% to 50%), fetal dysrhythmia (30%), hydrops (30%), and polyhydramnios (25%).
Cardiac Defects
Atrial Septal Defects
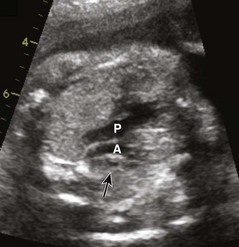
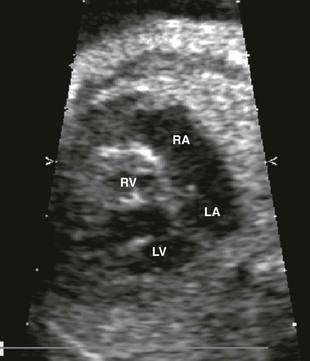
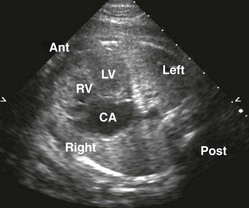
The most common atrial septal defects (ASDs) are ostium secundum defects. Sinus venosus defects (superior or inferior type) are often associated with anomalous drainage of the right pulmonary veins. Ostium primum defects are an endocardial cushion defect, and often associated with Down syndrome. A patent foramen ovale is a normal structure of the fetus and newborn (Figs. 71-6 and 71-7); it may be difficult to distinguish a normal foramen ovale from a secundum ASD prenatally. Secundum defects are amenable to catheter closure; other defects require surgical correction.
Ventricular Septal Defects
Ventricular septal defects (VSDs) are the most common type of congenital heart defect. They can occur in the membranous, AV canal, muscular, or conal (outlet) septum. Perimembranous (around the membranous septum) defects are the most common. Moderate to large defects require surgical intervention in infants with congestive heart failure; small defects often close on their own. Muscular defects are the second most common; small defects usually close on their own, but multiple defects can cause congestive heart failure (Fig. 71-8).
Atrioventricular Canal defects
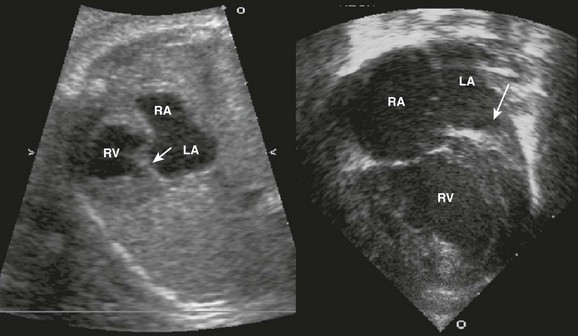
AV canal (endocardial cushion) defects are easily diagnosed prenatally (Fig. 71-9) and can be associated with Down syndrome. They include a primum ASD, inlet VSD, and common AV valve. VSDs can be isolated. The cross-sectional anatomy of the common valve is best determined from short-axis imaging. Partial AV septal defects have a primum ASD with a cleft mitral valve. Transitional AV septal defects have an atrial defect, common AV valve, and restrictive VSD.
Inflow/Outflow
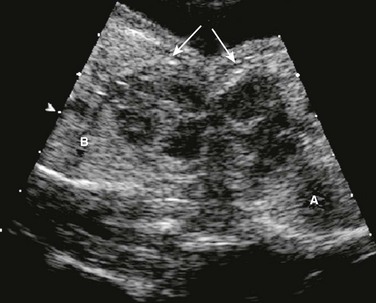
Right ventricular inflow lesions include Ebstein anomaly, tricuspid valve dysplasia, and tricuspid stenosis or atresia. The tricuspid valve orifice is displaced apically in Ebstein anomaly, with abnormal delamination of the septal leaflet and tricuspid valve regurgitation. (Fig. 71-10) Milder disease may not present until the first decade of life with arrhythmias and tricuspid regurgitation; severe forms can cause fetal hydrops, death, or neonatal cyanosis. Ebstein anomaly can be associated with pulmonary stenosis or atresia, causing cyanosis and requiring initiation of prostaglandin therapy to maintain ductus arteriosus patency. Tricuspid valve dysplasia has similar features but without apical displacement of the valve annulus. Tricuspid atresia or stenosis can be associated with hypoplasia of the right ventricle and pulmonary outflow obstruction; without a VSD, this lesion requires postnatal prostaglandin to maintain ductal patency prior to neonatal single-ventricle palliation surgery.
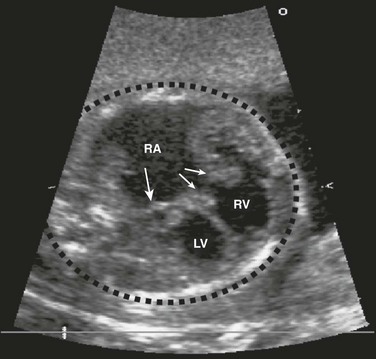
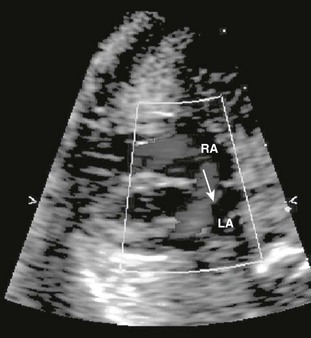
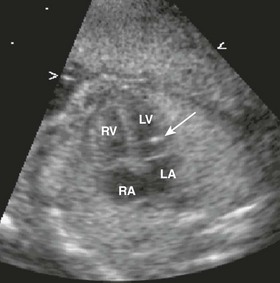
Figure 71-10 Fetal four-chamber image of highly thickened and apically displaced (Ebstein anomaly) tricuspid valve (thin arrows).
Cardiomegaly from tricuspid valve regurgitation is severe, with the cardiac mass taking up most of the cardiothoracic space (dotted black circle). Normally, the cardiothoracic area ratio is less than 33%. The right atrium (RA) is severely dilated, compressing the atrial septum (single thick arrow) and left atrium. LV, left ventricle; RV, right ventricle.
Right-Sided Outflow Lesions
Isolated pulmonary valve stenosis is diagnosed by identifying domed and thickened pulmonary valve leaflets. The annulus may be hypoplastic. Fetal Doppler echocardiography may not accurately predict the postnatal pulmonary valve gradient. This lack of agreement between prenatal and postnatal gradients is attributed to in utero physiology, elevated pulmonary vascular resistance, and right-to-left shunting at the atrial level. Supravalvular pulmonic stenosis is associated with Williams syndrome. Subvalvular obstruction is usually seen in combination with other defects such as tetralogy of Fallot (Fig. 71-11).
Left-Sided Outflow Lesions
Left-sided outflow tract obstruction can occur at the subvalvular, valvular, or supravalvular levels. Infants of mothers with diabetes, especially of mothers with poor glucose control, are at risk for hypertrophic cardiomyopathy (with or without obstruction).17 Even in severe cases, the hypertrophy usually regresses by 3 months of age. Valvular aortic stenosis seen in utero can be associated with left ventricular dysfunction and progressive hydrops. Supravalvular stenosis is associated with Williams syndrome and can occur in combination with right-sided outflow tract obstruction. Left-sided obstructive lesions can be associated with Turner syndrome.18
Conotruncal Defects
Conotruncal defects include abnormalities of the connection between the ventricles and the great vessels, including tetralogy of Fallot, transposition of the great arteries, truncus arteriosus, and double-outlet right ventricle. The lesions can be associated with deletions in chromosome locus 22q11 (DiGeorge sequence, velocardiofacial syndrome, CATCH 22 [cardiac defects, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia]).19
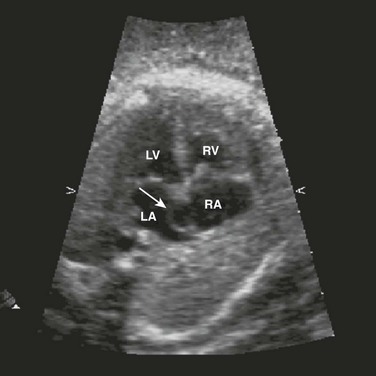
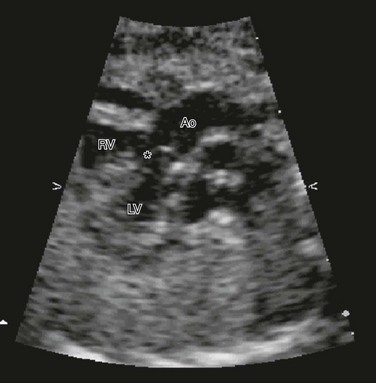
Tetralogy of Fallot (right ventricular outflow obstruction, right ventricular hypertrophy, overriding aorta, and large anterior malaligned VSD) is the most common form of cyanotic congenital heart disease, with transposition of the great vessels being the second most common. In transposition, the aorta originates from the right ventricle, and the pulmonary artery arises from the left ventricle. Echocardiography shows parallel great vessels with aorta located anterior and rightward of the pulmonary artery (Fig. 71-12). A laterally branching vessel (left pulmonary artery) from the great artery is seen related to the left ventricle. Additional abnormalities such as VSDs (one third of cases) also are identified. The four-chamber view is normal, making diagnosis quite difficult.
Ventricular Hypoplasia
Underdevelopment of either ventricle can result in a univentricular heart. After birth, patients require staged surgical palliation and possible heart transplantation later. Hypoplastic right heart syndrome can result from tricuspid atresia or pulmonary atresia with an intact interventricular septum (Fig. 71-13). Hypoplastic left heart syndrome may have severe aortic and mitral stenosis or atresia (Fig. 71-14) and requires intervention or surgery in the neonatal period. Double-inlet left ventricle with a right ventricular outlet chamber is another, more complex form of a single ventricle heart (see Fig. 71-14) and can be associated with a normal or hypoplastic outflow tract.
Fibroelastosis
Endocardial fibroelastosis is an abnormality of the endocardial surface. Prenatally, thickening and fibrosis of the endocardium due to inflammation or hypoxia result in an echobright appearance. These areas can be either focal or diffuse. It has been described in fetuses with maternal Sjogren’s antibody exposure; structural anomalies such as hypolastic left heart syndrome; anomalous coronaries; aortic stenosis; fetal infections such as parvovirus infection; metabolic diseases; and cardiomyopathy (Fig. 71-15).20 Once diagnosed, serial assessment of ventricular function, venous flow patterns, and valve regurgitation is warranted to evaluate for development of fetal hydrops fetalis.
Venous and Aortic Arch Anomalies
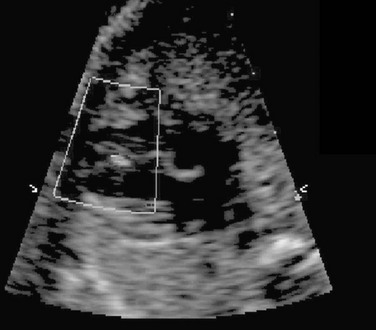
Aortic arch abnormalities include vascular rings, coarctation of the aorta, and interrupted aortic arch. Vascular rings can be associated with a right-sided aortic arch, aberrant left subclavian artery, and Kommerell’s diverticulum or ligamentum arteriosus. Depending on the type of ring, infants may be asymptomatic, have airway narrowing, or have feeding difficulties. Finding a right aortic arch warrants further evaluation for associated anomalies. Coarctation of the aorta may present in early infancy with cardiogenic shock after ductal closure or in later life with hypertension. Coarctation may be difficult to diagnose in the fetal period when the ductus arteriosus is still open. Interrupted aortic arch is a severe type of coarctation, in which the ascending and descending aorta are discontinuous, with the descending aorta supplied by the ductus arteriosus. When associated with a VSD, this form is considered a conotruncal abnormality. Patent ductus arteriosus is a normal fetal structure; its premature closure can lead to right-sided heart failure in utero (Fig. 71-16). Indomethacin and other nonsteroidal antiinflammatory drugs predispose to this condition. The heart normalizes at delivery.21
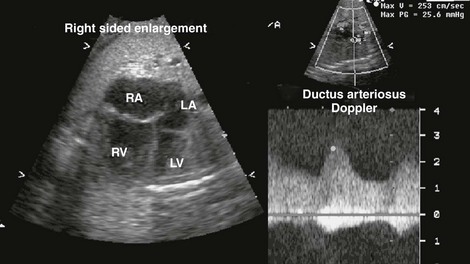
Figure 71-16 Fetal four-chamber (left) and spectral Doppler (right) images of a fetus with a restrictive ductus arteriosus causing significant right-sided enlargement.
The increased Doppler velocity and diastolic “drag” is consistent with a restrictive flow pattern. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Heterotaxy Syndromes: Situs/Cardiac Masses/Arrhythmias
Situs abnormalities and complex congenital heart disease can be diagnosed by carefully evaluating abdominal and cardiac situs and intracardiac relationships (Fig. 71-17).22 Correct determination of abdominal and cardiac situs depends on the delineation of fetal position and right or left orientation. Double-outlet right ventricle, atrioventricular septal defects, and venous anomalies are frequently associated with abnormalities of abdominal situs resulting in asplenia (bilateral “right-sidedness”) or polysplenia (bilateral “left-sidedness”). Abnormal looping of the fetal heart may result in ventricular inversion (right-sided morphologic left ventricle and left-sided morphologic right ventricle) in these patients.
Cardiac Masses
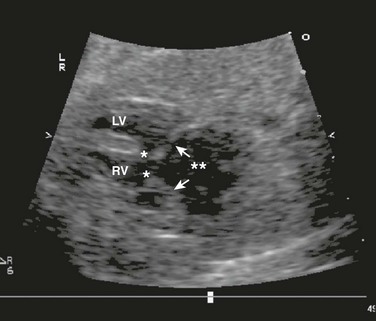
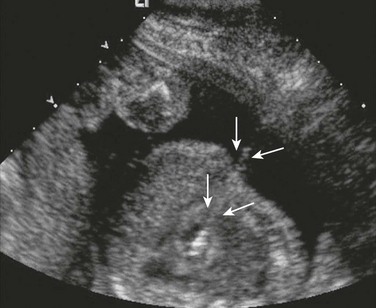
Tiny echogenic objects in the left ventricular papillary muscles are frequently noted on fetal echocardiography (Fig. 71-18) and are considered normal variants.23 Larger, more numerous masses suggest the presence of cardiac tumors. The most common prenatal cardiac tumors are rhabdomyomas (Fig. 71-19). They usually are associated with tuberous sclerosis. Although benign and usually regressive after birth, these tumors can cause obstruction or arrhythmias.24
Fetal Arrhythmias
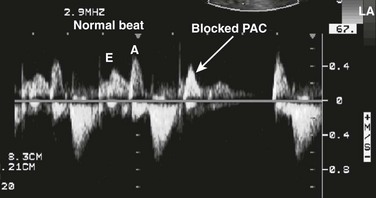
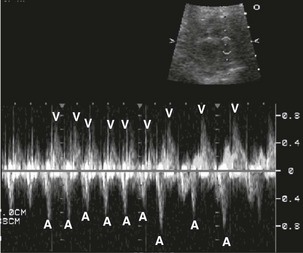
Echocardiography provides accurate diagnosis of fetal arrhythmias.25,26 Premature atrial contractions (Fig. 71-20) are common and usually benign. Supraventricular tachycardia and atrial flutter can be diagnosed with M-mode and Doppler ultrasound (Figs. 71-21 and 71-22). Bradycardia may be benign and is often caused by blocked premature atrial contractions. Complete heart block, a rare complication of maternal Sjögren antibody exposure, can be diagnosed by M-mode or Doppler ultrasound (Fig. 71-23).27 Serial Doppler assessment of the time between atrial and ventricular contractions is used in maternal Sjögren antibody carriers to follow the fetal conduction system (Fig. 71-24).
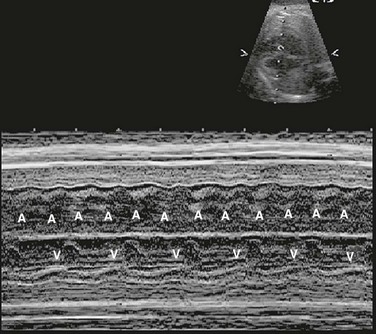
Figure 71-22 Fetal M-mode image of atrial and ventricular contraction showing atrial flutter with 2 : 1 conduction.
The atrial rate is approximately 400 beats per minute, and the ventricular rate is approximately 200 beats per minute. A, atrial wall contraction; V, ventricular wall contraction.
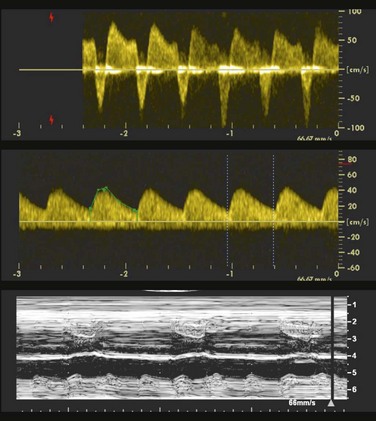
Figure 71-23 Fetal M-mode image of atrial and ventricular contractions showing complete heart block with atrioventricular dissociation.
The atrial rate is approximately 150 beats per minute, and the ventricular rate is approximately 55 beats per minute.
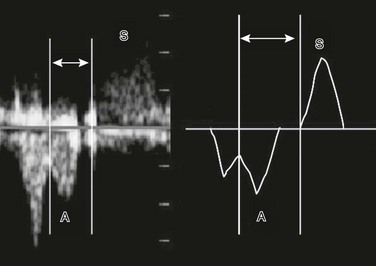
Figure 71-24 Fetal spectral Doppler image and drawing of diastolic atrial contraction (A) and systolic ventricular contraction (S).
The mechanical atrioventricular interval (analogous to the P–R interval on an electrocardiogram) is measured from the beginning of the A wave to the beginning of the S wave (lines and arrows).
Fetal Management
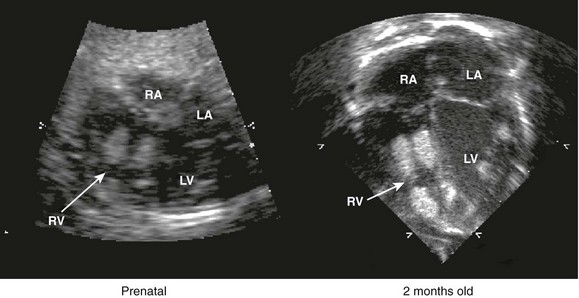
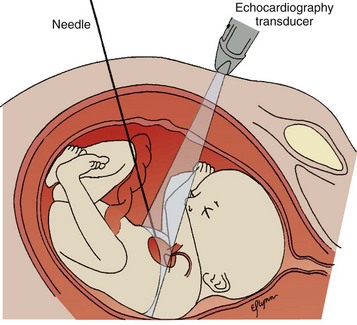
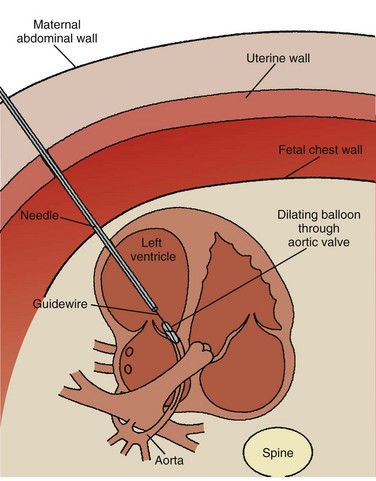
Although the heart develops by 8 weeks’ gestation, fetal flow patterns can affect development of the heart throughout gestation. Disproportionate flow to one side of the circulation may lead to underdevelopment of either side of the heart. Fetuses with aortic or pulmonary valve stenosis at 18 to 20 weeks’ gestation can progress to hypoplastic left or right heart by 30 weeks’ gestation (Fig. 71-25). Fetal catheter-based intervention to relieve aortic stenosis, pulmonary stenosis, or a restrictive atrial septum has been performed.28–31 Technically successful fetal balloon aortic valvuloplasty in fetuses with critical aortic valve stenosis with likely evolving hypoplastic left heart syndrome was reported in 2004. Improved left heart growth and postnatal two-ventricular circulation was seen in some of the successful cases (Figs. 71-26 through 71-28).
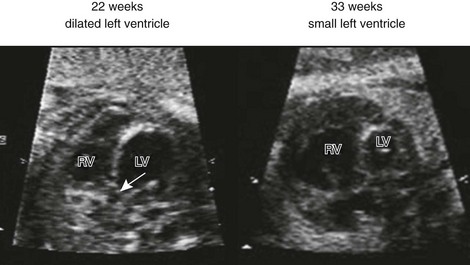
Figure 71-25 Side-by-side four-chamber images of the same fetus with critical aortic valve stenosis at 22 and 33 weeks’ gestational age.
At 22 weeks, the left ventricle (LV) is dilated, and the apex is forming; at 33 weeks, the LV is hypoplastic. Arrow indicates aortic valve. RV, right ventricle. (Courtesy Wayne Tworetzky, MD, Division of Cardiology, Children’s Hospital Boston, Boston, MA.)
Conclusion
Fetal echocardiography is an important adjunct to other prenatal evaluations, including ultrasound and genetic screening. Radiologists, cardiologists, perinatologists, neonataologists, and other pediatric subspecialists must work together to provide a multidisciplinary treatment and counseling approach to patients with complex diagnoses (Fig. 71-29). Newer imaging modalities of the fetal heart, including three-dimensional echocardiography and magnetic resonance imaging, will further contribute to fetal cardiac management. As technology and treatments evolve, fetal cardiology represents an area of tremendous potential for early diagnosis and in utero treatment of abnormalities. In the future, intervention during the fetal period may allow physicians to actually alter the evolution of structural cardiac disease, thus helping improve long-term outcome.
Allan, LD. Fetal cardiology. Ultrasound Obstet Gynecol. 1994;4:441–444.
Ferencz, C, Neill, CA. Cardiovascular malformations: prevalence at live birth. In: Freedom RM, Benson LN, Smallhorn JF, eds. Neonatal heart disease. London: Springer-Verlag; 1992:19–29.
Rychik, J, Ayres, N, Cuneo, B. American Society of Echocardiography guidelines and standards for performance of a fetal echocardiogram. J Am Soc Echocardiogr. 2004;17:803–810.
Simpson, LL. Fetal supraventricular tachycardias: diagnosis and management. Semin Perinatol. 2000;24:360–372.
References
1. Ferencz, C, Rubin, JD, McCarter, RJ, et al. Congenital heart disease: prevalence at live birth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31–36.
2. Ferencz, C, Neill, CA. Cardiovascular malformations: prevalence at live birth. In: Freedom RM, Benson LN, Smallhorn JF, eds. Neonatal heart disease. London: Springer-Verlag; 1992:19–29.
3. Hoffman, JE. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr Cardiol. 1995;16:103–113.
4. Hoffman, JE. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165.
5. Sharony, R, Feigin, M, Biron-Shental, T. Who should be offered echocardiography? One center’s experience with 3965 cases. IMAJ. 2009;11:542–545.
6. Marek, J, Tomek, V, Skovranek, J. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21 year experience. Heart. 2011;97:124–130.
7. Sharland, GK, Allan, LD. Screening for congenital heart disease prenatally: results of a  year study in the South East Thames Region. Br J Obstet Gynaecol. 1992;99:220–225.
year study in the South East Thames Region. Br J Obstet Gynaecol. 1992;99:220–225.
8. Buskens, E, Grobbee, DE, Frohn-Mulder, IM, et al. Efficiency of routine fetal ultrasound screening for congenital heart disease in normal pregnancy. Circulation. 1996;94:67–72.
9. Allan, LD. Fetal echocardiography: confidence limits and accuracy. Pediatr Cardiol. 1985;6:145–146.
10. Meyer-Wittkopf, M, Cooper, S, Sholler, G. Correlation between fetal cardiac diagnosis by obstetric and pediatric cardiologist sonographers and comparison with postnatal findings. Ultrasound Obstet Gynecol. 2001;17:392–397.
11. Skeels, M, Taylor, D, Park, J, et al. Test characteristic of a level I or II fetal ultrasound in detecting structural heart disease. Pediatr Cardiol. 2002;23:594–597.
12. Friedberg, MK, Silverman, NH, Moon-Grady, AJ. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31.
13. Wigton, TR, Sabbagha, RE, Tamura, RK, et al. Sonographic diagnosis of congenital heart disease: comparison between the four-chamber view and multiple cardiac views. Obstet Gynecol. 1993;82:219–224.
14. Donofrio, MT, Bremer, YA, Schieken, RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–443.
15. Dolkart, LA, Reimers, FT. Transvaginal fetal echocardiography in early pregnancy: normative data. Am J Obstet Gynecol. 1991;165:688–691.
16. Persico, N, Montalla, J, Lombardi, CM. Fetal echocardiography at 11-13 weeks by transabdominal high-frequency ultrasound. Ultrasound Obstet Gynecol. 2011;37(3):296–301.
17. Rowland, TW, Hubbel, JP, Nadas, AS. Congenital heart disease in infants of diabetic mothers. J Pediatr. 1972;83:815–820.
18. Surerus, E, Huggon, IC, Allan, LD. Turner’s syndrome in fetal life. Ultrasound Obstet Gynecol. 2003;22:264–267.
19. Marino, B, Digilio, MC, Toscano, A, et al. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet Med. 2001;3:45–48.
20. Trastour, C, Bafghi, A, Delotte, J. Early prenatal detection of endocardial fibroelasatosis. Ultrasound Obstet Gynecol. 2004;26:303–304.
21. Huhta, JC, Cohen, AW, Wood, DC, et al. Premature constriction of the ductus arteriosus. J Am Soc Echocardiogr. 1990;3:30–34.
22. Walmsley, R, Hishitani, T, Sandor, G, et al. Diagnosis and outcome of dextrocardia diagnosed in the fetus. Am J Cardiol. 2004;94:141–143.
23. Petrikovsky, BM, Challenger, M, Wyse, LJ. Natural history of echogenic foci within ventricles of the fetal heart. Ultrasound Obstet Gynecol. 1995;5:92–94.
24. D’Addario, V, Pinto, V, Di Naro, E, et al. Prenatal diagnosis and postnatal outcome of cardiac rhabdomyomas. J Perinat Med. 2002;30:170–175.
25. van Engelen, AD, Weijtens, O, Brenner, J, et al. Management outcome and follow-up fetal tachycardia. J Am Coll Cardiol. 1994;24:1371–1375.
26. Simpson, LL. Fetal supraventricular tachycardias: diagnosis and management. Semin Perinatol. 2000;24:360–372.
27. Buyon, J, Clancy, R. Neonatal lupus: basic science and clinical perspectives. Rheum Dis Clin North Am. 2005;31:299–313.
28. Latson, LA. Aortic valvuloplasty in the fetus: technically possible but is it ready for prime time? J Pediatr. 2005;147:424–426.
29. Marshall, AC, Tworetzky, WA, Bergersen, L, et al. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539.
30. Tworetzky, W, Wilkins-Haug, L, Jennings, R, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome, candidate selection, technique, and results and of successful intervention. Circulation. 2004;110:2125–2131.
31. Huhta, J, Quintero, RA, Suh, E, et al. Advances in fetal cardiac intervention. Curr Opin Pediatr. 2004;16:487–493.