Prenatal Imaging
Central nervous system (CNS) anomalies occur in 1.4 to 1.6 per 1000 live births and 3% to 6% of still births.1 Whereas some anomalies can be detected as early as the first trimester (such as anencephaly), others may not develop until—or only become apparent—later in gestation.2
Ultrasound is the initial imaging modality used for the assessment of fetal CNS anomalies. When ultrasound is carefully performed using established guidelines, it can be very sensitive in evaluating the fetal brain.3 Axial images are important for the assessment of biparietal diameter and head circumference measurements, ventricular size, and cerebellar configuration. Coronal and sagittal images can confirm the presence of the cavum septum pellucidum and corpus callosum. However, because of limitations from skull shadowing, fetal lie, maternal obesity, and oligohydramnios, evaluation of the fetal brain by ultrasound may be incomplete. When a fetal CNS anomaly is being considered, magnetic resonance imaging (MRI) is an adjunct that provides additional information.4–7
Advanced techniques include diffusion-weighted imaging, diffusion tensor imaging, and magnetic resonance (MR) spectroscopy.8 The apparent diffusion coefficient normally decreases after 30 weeks’ gestation,9 but higher apparent diffusion coefficient values have been reported in high-risk fetuses.10 Diffusion-weighted imaging can help detect hemorrhage and acute ischemia (Fig. 29-1). Diffusion tensor imaging measures the magnitude and direction of diffusion (fractional anisotropy). Although intrinsic anisotropy is low in the fetal brain, imaging improvements will help understand the onset and timing of delayed white matter connectivity.11,12 Proton MR spectroscopy has advanced the investigation of fetal brain metabolism. Creatine and N-acetylaspartate peaks appear to have a progressive increase, whereas choline decreases in the third trimester.13 Alterations in these peaks may help identify conditions associated with fetal compromise.
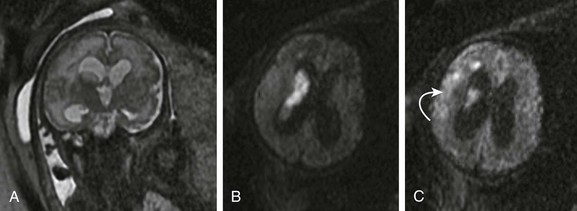
Figure 29-1 A fetus at 32 weeks’ gestation with periventricular hemorrhagic infarction.
A, A coronal single shot fast spin-echo T2-weighted magnetic resonance image shows moderate ventriculomegaly, intraventricular material, and an abnormal high signal in the right periventricular white matter. An axial diffusion-weighted sequence demonstrates increased diffusion signal within the lateral ventricle (B) and deep frontal white matter (C, arrow) consistent with intraventricular hemorrhage and a periventricular hemorrhagic infarct.
Ongoing enhancement of ultrafast MR sequences and postprocessing methodology has resulted in imaging techniques that can evaluate growth, organization, and remodeling processes that occur during fetal brain development.14,15 Three-dimensional volumetric studies have demonstrated the value of quantitative assessment of brain growth in healthy versus high-risk fetuses. Fetuses with congenital heart disease have been shown to have impaired third-trimester brain growth compared with control subjects, offering a method to evaluate timing and progression of abnormal fetal brain growth.16 Three-dimensional reconstruction of the fetal brain can provide cortical measures such as surface area and gyrification indices.
Normal Development of the Fetal Brain
Knowledge of normal fetal morphology and development is important when evaluating anomalies. From 18 to 24 weeks’ gestation, the brain is smooth, with minimal sulcation. The ventricles and extraaxial subarachnoid space, including the cisterna magna, are prominent until the third trimester17 (Fig. 29-2).
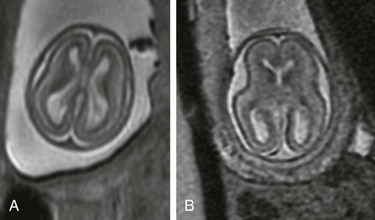
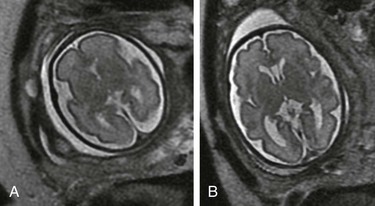
Figure 29-2 Normal development.
An axial single shot fast spin-echo T2-weighted magnetic resonance image at 19 weeks’ gestation (A) shows a smooth cortex, prominent ventricles, and subarachnoid space. Low-signal germinal matrix is present along the lateral ventricular walls. B, At 22 weeks’ gestation, mild infolding of the sylvian fissure has occurred.
Neuronal migration patterns can be documented by MRI.18 Three layers are visualized, including the germinal matrix, cell sparse zone, and cortex. The germinal matrix has a low signal on T2-weighted images along the lateral ventricular walls and involutes from posterior to anterior after 28 weeks’ gestation. The cell sparse zone represents migrating glial cells and eventually becomes the white matter. In the second trimester, the cortical ribbon is intermediate in signal.
The fetal cortical mantle follows a predictable course in maturation. Gyration progresses throughout the second and third gestation and can be used to assess gestational age (Box 29-1). By 32 weeks’ gestation, extensive gyration and sulcation is present (Fig. 29-3).17–19
Fetal Ventriculomegaly
The fetal ventricles are prominent in relation to the brain parenchyma until the third trimester. After 25 weeks’ gestation, the ventricles lose their colpocephalic configuration. Fetal ventriculomegaly is defined as an atrial measurement greater than 10 mm with separation of choroid from the medial wall (i.e., floating choroid). Ventriculomegaly can be due to obstruction, atrophy, maldevelopment, or, rarely, overproduction of cerebrospinal fluid. Ultrasound and MRI should be used to carefully assess for findings that suggest chromosomal anomalies (e.g., trisomy 13, 18, or 21), malformations (e.g., Chiari 2, Dandy-Walker, agenesis of the corpus callosum [ACC], or holoprosencephaly), or destructive lesions (e.g., infarction or infection).19–21
The degree of ventriculomegaly has been shown to be associated with the incidence of live birth and survival beyond the neonatal period. With mild to moderate ventriculomegaly (10 to 15 mm), a close search for other anomalies and chromosome evaluation is important for further assessment. When ventriculomegaly is isolated, abnormal outcome can range from 10% to 25%. If other anomalies are present, outcome is worse, with only 50% to 80% of fetuses having a normal neurodevelopmental outcome.22–24 In a large series evaluating fetal ventriculomegaly, motor outcomes were more severely affected than cognitive or adaptive outcomes, although prenatal atrial diameter was not consistently associated with postnatal developmental outcome.25
Agenesis of the Corpus Callosum
The corpus callosum forms between the eighth and twentieth week from genu to splenium. The rostrum forms last, between 18 to 20 weeks’ gestation. Anomalies can be complete (ACC) or partial (hypogenesis). The corpus callosum may be difficult to visualize sonographically, particularly in the early weeks of gestation and/or with hypogenesis.26 Sonographic and MR findings include colpocephaly of the occipital horns with parallel orientation of the lateral ventricles, an absent septum pellucidum, and a high-riding third ventricle (Fig. 29-4). Coronal images are particularly helpful in demonstrating the presence or absence of the cavum septum pellucidum and corpus callosum. With ACC, the frontal horns tend to be narrow with straight medial borders secondary to the bundles of Probst, which represent the callosal fibers that have not crossed the midline. The third ventricle may extend superiorly into an interhemispheric cyst. The cerebral convolutions have a radial arrangement on sagittal imaging. An associated lipoma may be present, which will be echogenic on ultrasound and isointense to gray matter on T2-weighted MRI (e-Fig. 29-5). If ACC is isolated, there is a 15% to 25% risk of a handicap and a 10% risk of aneuploidy. If associated anomalies are detected, such as Dandy-Walker malformation, cortical dysplasia, or encephalocele, the outcome is poorer.27–29
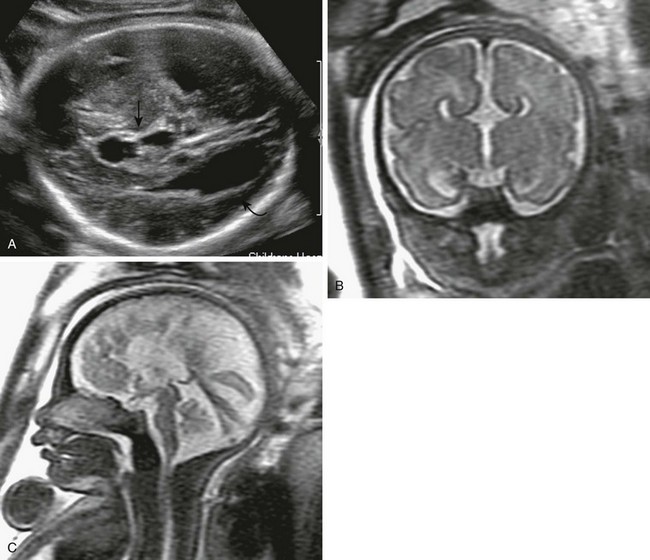
Figure 29-4 Agenesis of the corpus callosum.
A, A transverse sonogram of the fetal brain shows dilatation of the occipital horns (curved arrow) and a high-riding third ventricle (arrow). B, A coronal T2-weighted image shows that the cavum septum pellucidum is absent, along with a high-riding third ventricle. The medial walls of the anterior horns are indented by the bundles of Probst. C, A sagittal T2-weighted image shows medial sulci radiating perpendicular to the expected course of the corpus callosum. The pericallosal sulcus is absent.
Neural Tube Defects
Cranial neural tube defects can be assessed by ultrasound and include anencephaly, iniencephaly (cervical dysraphism and fixed fetal head extension), Chiari 3 malformation (low occipital/high cervical encephalocele), and cranial encephaloceles (Fig. 29-6). MRI is particularly useful to further evaluate the amount of herniated brain and associated cranial anomalies.
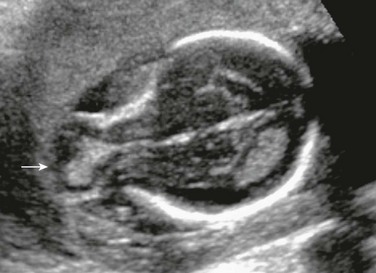
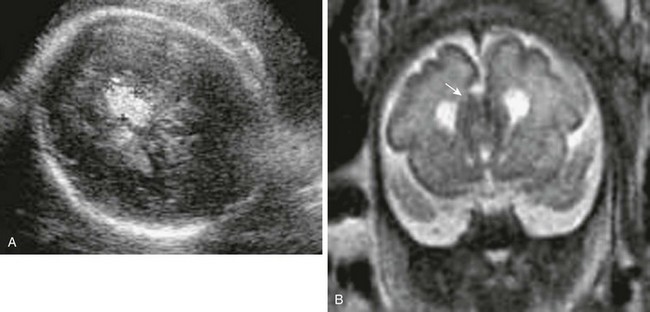
Figure 29-6 Encephalocele.
An axial sonogram of the fetal brain at 16 weeks’ gestation shows a frontal skull defect with brain contents herniated anteriorly (arrow).
Meningomyeloceles associated with Chiari 2 malformation are the most common neural tube defect. The cranial findings in fetuses with Chiari 2 malformation that are identified sonographically include a small or absent cisterna magna, cerebellar herniation (banana sign), frontal concavity (lemon sign), and ventriculomegaly.30,31 MRI can further delineate the amount of brainstem and cerebellar herniation, beaking of the tectum, heterotopias, small subarachnoid space, and callosal dysgenesis. After fetal surgery, hindbrain herniation may reverse, decreasing the need for shunting postnatally.32,33
Holoprosencephaly
Failure of prosencephalic cleavage results in holoprosencephaly; septo-optic dysplasia is the mildest form, and alobar holoprosencephaly is the most severe form.34 These anomalies are associated with several genetic syndromes such as Meckel-Gruber, Smith-Lemli-Opitz, trisomy 13 and 18, and teratogen exposure. Midline structures often are abnormal as well and can include proboscis, trigonocephaly, cyclopia, hypotelorism, and facial clefts (e-Fig. 29-7). Ultrasound can identify the most severe alobar form, but subtle cases of lobar holoprosencephaly may be difficult to diagnose, even with MRI.
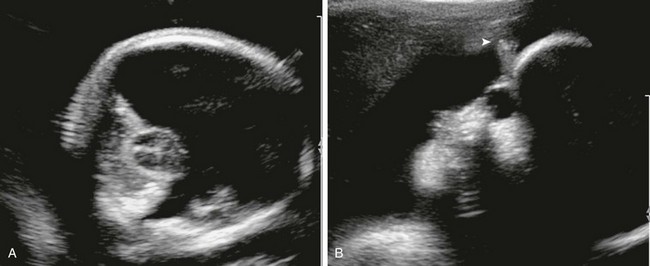
e-Figure 29-7 Cyclopia.
A, An axial sonogram of the fetal brain shows a monoventricle, absent falx, and fused thalami. B, A sagittal image of the fetal face shows a single midline orbit, absent nose, and supraorbital proboscis (arrowhead).
Alobar holoprosencephaly is complete failure of division of the promesencephalic vesicle. A monoventricle is identified with “kissing choroid” by ultrasound. The thalami and basal ganglia are fused. The falx, corpus callosum, and interhemispheric fissure are absent (Fig. 29-8). With semilobar and lobar forms, a posterior interhemispheric fissure is present, with absence of the genu of the corpus callosum. Lobar holoprosencephaly may have a normally formed thalamus and callosal splenium. The anterior frontal lobes are fused and the frontal lobes typically are hypoplastic. The septum pellucidum and anterior falx are absent. Syntelencephaly is a variant in which the anterior parietal lobe or posterior frontal lobes are contiguous across the midline.35,36
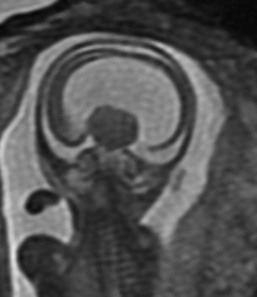
Figure 29-8 An example of 17-week gestation alobar holoprosencephaly.
A coronal single shot fast spin echo T2-weighted magnetic resonance image of the fetal brain shows a single mantle of neural tissue with a monoventricle and fused thalami.
Septo-optic dysplasia may be identified by the presence of ventricular dilation, an absent septum pellucidum, and flat roof of the frontal horns. Schizencephaly, heterotopias, and callosal dysgenesis often are associated with this sporadic anomaly and are better seen by MRI.20
Posterior Fossa Malformations
Malformations of the cerebellum and brainstem can be categorized by posterior fossa size: small, normal, or large. The cisterna magna is measured from the midline posterior aspect of the vermis to the inner occiput and normally measures between 3 to 10 mm. Although ultrasound can identify many posterior fossa anomalies, MRI is particularly useful in evaluating the vermis, brainstem, and location of the tentorium.37–39
When the posterior fossa is normal in size, abnormalities include cerebellar/pontocerebellar hypoplasia and vermian dysgenesis (partial or total absence of the vermis) (e-Fig. 29-9). Rarely, rhombencephalosynapsis (agenesis of the vermis with fusion of cerebellum) can be identified prenatally. The fetal vermis is not completely developed until 18 weeks’ gestation, and the fetal cerebellum continues to form in the third trimester, with cellular migration occurring through the first year of life, and thus mild cerebellar hypoplasia may be difficult to diagnose prenatally.40–43 Volume measurements are available to aid in the diagnosis. Although pontocerebellar hypoplasia has a poor prognosis, the outcome for vermian dysgenesis is not as clear. In these cases, counseling must proceed cautiously and be correlated with associated anomalies and aneuploidy.
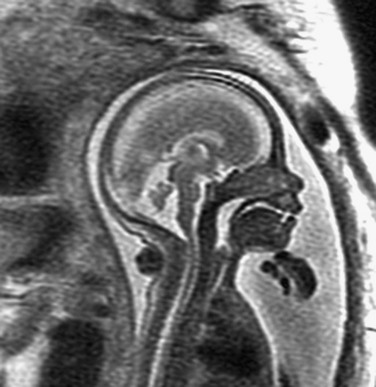
e-Figure 29-9 Hypoplastic inferior vermis.
A sagittal single shot fast spin-echo T2-weighted image shows a hypoplastic inferior vermis at 23 weeks’ gestation. The posterior fossa is normal in size, and no ventriculomegaly is present.
When the posterior fossa is enlarged, the differential diagnosis includes mega cisterna magna, Blake’s pouch cyst, Dandy-Walker malformation, or arachnoid cyst. Mega cisterna magna is a wide cistern (anteroposterior greater than 10 mm) with a normal vermis and cerebellum (e-Fig. 29-10). The tentorium is located in a normal position. Mega cisterna magna can be a normal finding, although it has been associated with aneuploidy. If no additional anomalies are found and chromosomes are normal, the outcome should be good.44
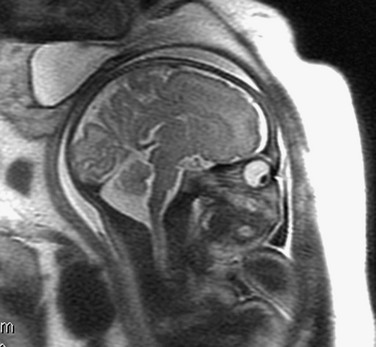
e-Figure 29-10 Mega cisterna magna.
A sagittal single shot fast spin-echo T2-weighted magnetic resonance image shows a prominent cisterna magna. The vermis is intact. The cerebellar volume and brainstem are normal for gestational age.
Blake’s pouch cyst is a posterior protuberance of the inferior medullary velum into the cistern. This fluid collection is posterior inferior to the vermis and communicates with the fourth ventricle. The vermis typically is intact, with mass effect and elevation of the tentorium. The cyst does not communicate with the subarachnoid space.45
Dandy-Walker malformation is a retrocerebellar cyst that communicates with the fourth ventricle. The posterior fossa is enlarged, with an elevated tentorium. The vermis is incomplete, elevated, and rotated (Fig. 29-11). Hydrocephalus usually is present. Prognosis depends on the degree of vermian hypoplasia, brainstem hypoplasia, and associated anomalies such as ACC, heterotopias, and encephaloceles.
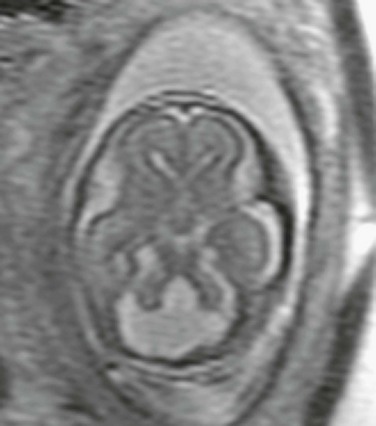
Figure 29-11 Dandy-Walker malformation.
A saggital single shot fast spin-echo T2-weighted magnetic resonance image demonstrates an enlarged posterior fossa, hypoplastic elevated vermis, and retrocerebellar fluid collection connected to the fourth ventricle with elevated torcula.
Arachnoid cysts can develop in the posterior fossa, causing an effect of the mass on the vermis, which otherwise is normally formed (e-Fig. 29-12). These cysts do not communicate with the fourth ventricle. The tentorium may be elevated. Symptoms can occur if hydrocephalus develops or the brainstem is compressed.
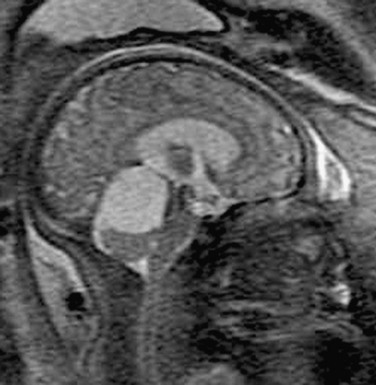
e-Figure 29-12 Arachnoid cyst.
A sagittal single shot fast spin-echo T2-weighted magnetic resonance image of a 37-week fetus shows an enlarged posterior fossa with a cystic structure deviating the vermis inferiorly. The cyst does not connect to the fourth ventricle. A sonogram at 21 weeks of gestation was reported to show normal findings.
Cortical Development
Disorders of neuronal cell migration can be difficult to recognize in the fetal brain both by ultrasound and by MRI.46,47 The cortex is poorly visualized by ultrasound, and whereas MRI demonstrates the cortical mantle relatively well, the cortex is not well developed in the second trimester. Thus a relatively smooth brain at 20 weeks that is normal can be indistinguishable from smooth pachygyria.
Abnormalities of cellular differentiation include unilateral megalencephaly and tuberous sclerosis (TS).48 Subependymal hamartomas are present in up to 80% of patients with TS. Subependymal and subcortical tubers may be noted as intermediate-signal lesions on T2-weighted images and high-signal lesions on T1-weighted images in the third trimester (e-Fig. 29-13).
e-Figure 29-13 Subependymal hamartoma.
An axial single shot fast spin-echo T2-weighted magnetic resonance image shows a low signal subependymal mass (arrow), which was of high signal on T1-weighted imaging. The fetus had rhabdomyomas involving the heart as well. After delivery, tuberous sclerosis was confirmed.
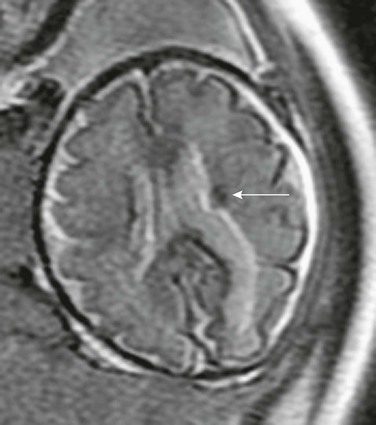
Agyria/pachygyria results from arrest of migration of neuroblasts with abnormal cortical lamination and failure of sulcation. Classic lissencephaly has few sulci, whereas pachygyria is less severe. In fetuses with cobblestone lissencephaly, cellular overmigration is present with hydrocephalus. Prenatally, ventricles typically are enlarged. Fetal MRI may show abnormal cortical sulcation in the third trimester (Fig. 29-14).49
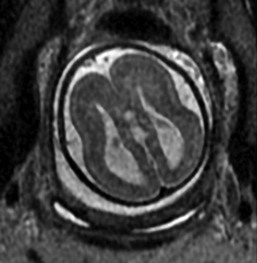
Figure 29-14 Lissencephaly.
A single shot fast spin-echo T2-weighted image of a 36-week gestation fetus demonstrates an abnormal smooth surface with lack of gyri and sulci. The cortical and subcortical layers are disorganized. Colpocephaly is present with dilated atria. The infant was diagnosed with Miller-Dieker syndrome after delivery.
Garel, C. Fetal MRI: what is the future? Ultrasound Obstet Gynecol. 2008;31:123–128.
Garel, C. MRI of the fetal brain: normal development and cerebral pathologies. Berlin: Springer-Verlag; 2005.
Limperopoulos, C, Clouchoux, C. Advancing fetal brain MRI. Targets for the future. Semin Perinatol. 2009;33:289–298.
Twickler, DM, Reichel, T, McIntire, DD, et al. Fetal central nervous system ventricle and cisterna magna measurements by MRI. Am J Obstet Gynecol. 2002;187:927–931.
Vezina, G. Congenital malformation of the brain: prenatal and postnatal imaging. Semin Roentgenol. 2004;39:165–181.
References
1. Chitty, LS, Pilu, G. The challenge of imaging the fetal central nervous system: an aid to prenatal diagnosis, management and prognosis. Prenat Diagn. 2009;29:301–302.
2. Malinger, G, Lerman-Sagie, T, Watemberg, N, et al. A normal second trimester ultrasound does not exclude intracranial structural pathology. Ultrasound Obstet Gynecol. 2009;20:51–56.
3. Malinger, G, Monteagudo, A, Pilu, G, et al. Sonographic examination of the fetal central nervous system: guidelines for performing the “basic examination” and the “fetal neurosonogram.”. Ultrasound Obstet Gynecol. 2007;29:109–116.
4. Salomon, LF, Garel, C. Magnetic resonance imaging examination of the fetal brain. Ultrasound Obstet Gynecol. 2007;30:1019–1032.
5. Giboud, L. Contribution of fetal cerebral MRI for diagnosis of structural anomalies. Prenat Diagn. 2009;29:420–433.
6. Hagmann, DF, Roberson, NJ, Leung, WC, et al. Fetal brain imaging: US ore MRI: a comparison between magnetic resonance imaging and a dedicated multidisciplinary neurosonographic opinion. Acta Paediatr. 2007;97:414–419.
7. Prayer, D. Investigation of the normal organ development with fetal MRI. Eur Radiol. 2006;17:2458–2471.
8. Limperopoulos, C. Advanced neuroimaging techniques: their role in the development of future fetal and neonatal neuroprotection. Semin Perinatol. 2010;34:93–101.
9. Righini, A, Bianchini, E, Parazzini, C, et al. Apparent diffusion coefficient determination in normal fetal brain: a prenatal MR imaging study. AJNR Am J Neuroradiol. 2003;24:799–804.
10. Berman, JI, Hamrick, SE, McQuillen, PS, et al. Diffusion-weighted imaging in fetuses with severe congenital heart defects. AJNR Am J Neuroradiol. 2011;32:E21–E22.
11. Kasprian, G, Brugger, PC, Weber, M, et al. In utero tractography of fetal white matter development. Neuro Image. 2008;43:213–224.
12. Jiang, S, Xue, H, Counsell, S, et al. In-utero three dimension high resolution fetal brain diffusion tensor imaging. Med Image Comput Assist Interv. 2007;10(pt 1):18–26.
13. Borowska-Matwiejczuk, K, Lemancewicz, A, Tarasow, E, et al. Assessment of fetal distress based on magnetic resonance examinations: preliminary report. Acad Radiol. 2003;10:1274–1282.
14. Rousseau, F, Glenn, OA, Iordanova, B, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Acad Radiol. 2006;13:1072–1081.
15. Sandrasegaran, K, Laal, C, Aisen, AA, et al. Fast fetal magnetic resonance imaging. J Comput Assist Tomogr. 2005;29:487–498.
16. Limperopoulos, C, Tworetzky, W, McElhinney, DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33.
17. Blaas, HG, Eik-Nes, SH. Sonoembryology and early prenatal diagnosis of neural anomalies. Prenat Diagn. 2009;29:312–325.
18. Garel, C, Chantrel, E, Birsse, H, et al. Fetal cerebral cortex: normal gestation landmarks identified. AJNR Am J Neuroradiol. 2001;22:184–189.
19. Girard, N, Rayboud, C, Bambarelli, D, et al. Fetal brain MR imaging. Magn Reson Imaging Clin North Am. 2001;9:19–56.
20. Li, Y, Sansgiri, RK, Estroff, JA, et al. Outcome of fetuses with cerebral ventriculomegaly and septum pellucidum leaflet abnormalities. AJR Am J Roentgenol. 2011;196(1):W83–W92.
21. Sadan, S, Malinger, G, Schweiger, AS, et al. Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG. 2007;114:596–602.
22. Pier, DB, Levine, D, Kataoka, ML, et al. Magnetic resonance volumetric assessments of brains in fetuses with ventriculomegaly correlated to outcomes. J Ultrasound Med. 2011;30(5):595–603.
23. Gaglioti, P, Oberto, M, Todrdos, T. The significance of fetal ventriculomegaly: etiology, short and long term outcomes. Prenat Diagn. 2009;29:381–388.
24. Li, Y, Estroff, JA, Mehta, TS, et al. Ultrasound and MRI of fetuses with ventriculomegaly: can cortical development be used to predict postnatal outcome? AJR Am J Roentgenol. 2011;196(6):1457–1467.
25. Beeghly, M, Ware, J, Soul, J, et al. Neurodevelopmental outcome of fetuses referred for ventriculomegaly. Ultrasound Obstet Gynecol. 2010;35(4):405–416.
26. Achiron, R, Achiron, A. Development of the human fetal corpus callosum: a high resolution cross section sonographic study. Ultrasound Obstet Gynecol. 2001;21:116–120.
27. Volpe, P, Paldini, D, Resta, M, et al. Characteristic associations and outcome of partial agenesis of the corpus callosum in the fetus. Ultrasound Obstet Gynecol. 2006;27:509–516.
28. Glenn, OA, Goldstein, RB, Li, KC, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med. 2005;24:791–804.
29. Fratelli, N, Papgeorghious, AT, Prefumo, F, et al. Fetal outcome of prenatally diagnosed agenesis of the corpus callosum. Prenat Diagn. 2007;27:512–517.
30. Cameron, M, Moran, P. Prenatal screening and diagnosis of neural tube defect. Prenat Diagn. 2009;29:402–411.
31. Sepulveda, W, Corral, E, Ayal, C, et al. Chromosomal abnormalities in fetuses with open neural tube defects: prenatal identification with US. Ultrasound Obstet Gynecol. 2004;23:352–356.
32. Bebbington, MW, Danzer, E, Johnson, PM, et al. Open fetal surgery for myelomeningocele. Prenat Diagn. 2011;31(7):689–694.
33. Adzick, NM. Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Semin Fetal Neonatal Med. 2010;15(1):9–14.
34. Volpe, P, Campobsso, G, De Robertis, V, et al. Disorders of prosencephalic development. Prenat Diagn. 2009;29:340–354.
35. Simon, EM, Hevner, RJ, Pinter, JD, et al. The middle interhemispheric variant of holoprosencephaly. AJNR Am J Neuroradiol. 2002;23:151–156.
36. Oba, H, Barkovich, AJ. Holoprosencephaly an analysis of callosal formation and its relation to development of the interhemispheric fissure. AJNR Am J Neuroradiol. 1995;16:453–460.
37. Guiboud, L. Practical approach to prenatal posterior fossa abnormalities using MRI. Pediatr Radiol. 2003;34:700–711.
38. Guiboud, L, des Portes, V. Pleas for an anatomical approach to abnormalities of the posterior fossa in prenatal diagnosis. Ultrasound Obstet Gynecol. 2006;27:477–481.
39. Patel, S, Barkovich, AJ. Analysis and classification of cerebellar malformations. AJNR Am J Neuroradiol. 2002;23:1074–1087.
40. Malinget, G, Lev, D, Lerman-Sagie, T. The fetal cerebellum: pitfalls in diagnosis and management. Prenat Diagn. 2009;20:372–380.
41. Forzano, F, Mansour, S, Ierullo, A, et al. Posterior fossa malformation in fetuses: a report of 56 further case and a review of the literature. Prenat Diagn. 2007;27:495–501.
42. Limperopoulos, C, Roberston, RI, Jr., Khwaja, OS, et al. How accurately does current fetal imaging identify posterior fossa anomalies? AJR Am J Roentgenol. 2008;190:1637–1643.
43. Limperopoulos, C, Robertson, RL, Estroff, JA, et al. Diagnosis of inferior vermian hypoplasia for fetal MRI potential pitfalls and neurodevelopmental outcome. Am J Obstet Gynecol. 2006;194:1070–1076.
44. Malinger, G, Dror, R, Ber Sira, L, et al. Developmental outcome of children with a large cisterna magna diagnosed in utero. Ultrasound Obstet Gynecol. 2008;32:253–259.
45. Calbro, F, Arcuri, T, Jinkins, JR. Blake’s pouch cysts: an entity within the Dandy Walker continuum. Neuroradiology. 2000;42:290–295.
46. Barkovich, AJ, Kuzniecky, RI, Jackson, GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887.
47. Toi, A, Chitayar, D, Blaser, S. Abnormalities of the fetal cerebral cortex. Prenat Diagn. 2009;17:105–114.
48. Wortmann, SB, Reimer, A, Creemers, JW, et al. Prenatal diagnosis of cerebral lesions in tuberous sclerosis complex: case report and review of the literature. Eur J Pediatr Neurol. 2008;12:123–126.
49. Ghai, S, Fong, KW, Toi, A, et al. Prenatal US and MR imaging findings of lissencephaly: review of fetal cerebral sulcal development. Radiographics. 2006;26:389–405.