CHAPTER 8 Preimplantation development
Understanding the spatial and temporal developmental processes that take place within an embryo as it develops from a single cell into a recognizable human is the challenge of embryology. The control of these processes resides within the genome: fundamental questions remain concerning the genes and interactions involved in development.
STAGING OF EMBRYOS
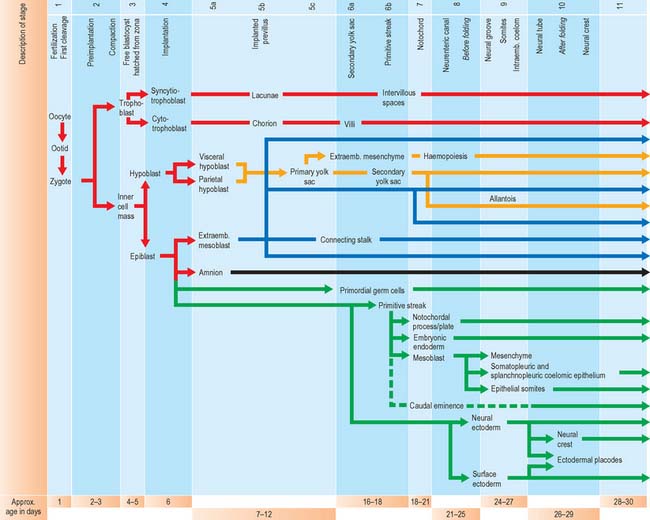
For the purposes of embryological study, prenatal life is divided into an embryonic period and a fetal period. The embryonic period covers the first 8 weeks of development (weeks following ovulation and fertilization resulting in pregnancy). The ages of early human embryos have previously been estimated by comparing their development with that of monkey embryos of known postovulatory ages. Because embryos develop at different rates and attain different final weights and sizes, a classification of human embryos into 23 stages occurring during the first 8 weeks after ovulation was developed most successfully by Streeter (1942), and the task was continued by O’Rahilly & Müller (1987). An embryo was initially staged by comparing its development with that of other embryos. On the basis of correlating particular maternal menstrual histories and the known developmental ages of monkey embryos, growth tables were constructed so that the size of an embryo (specifically, the greatest length) could be used to predict its presumed age in postovulatory days (synonymous to postfertilizational days). O’Rahilly & Müller (2000) emphasize that the stages are based on external and internal morphological criteria and are not founded on length or age. Ultrasonic examination of embryos in vivo has necessitated the revision of some of the ages related to stages, and embryos of stages 6–16 are now thought to be up to 3 to 5 days older than the previously used embryological estimates (O’Rahilly & Müller 1999). Within this staging system, embryonic life commences with fertilization at stage 1; stage 2 encompasses embryos from two cells, through compaction and early segregation, to the appearance of the blastocele. The developmental processes occurring during the first 10 stages of embryonic life are shown in Fig. 8.1.
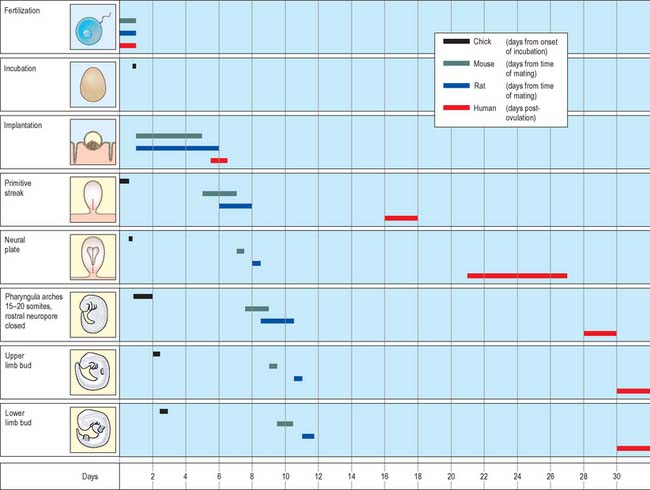
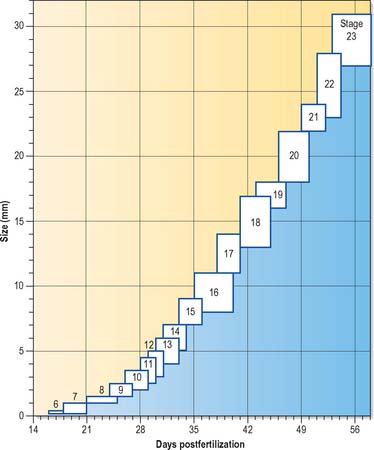
Much of our knowledge of the early developmental processes is derived from experimental studies on amniote embryos, particularly the chick, mouse and rat. Figure 8.2 shows the comparative timescales of development of these species and human development up to stage 12. The size and age, in postovulatory days, of human development from stage 10 to stage 23 is given in Fig. 8.3.
Information on developmental age after stage 23 (8 weeks postovulation) is shown in Fig. 14.3, where the developmental staging used throughout this text is juxtaposed with the obstetric estimation of gestation that is used clinically. A critique of staging terminology and the hazards of the concurrent use of gestational age and embryonic age is given in Chapter 14; sizes and ages of fetuses towards the end of gestation are illustrated in Fig. 14.7.
FERTILIZATION
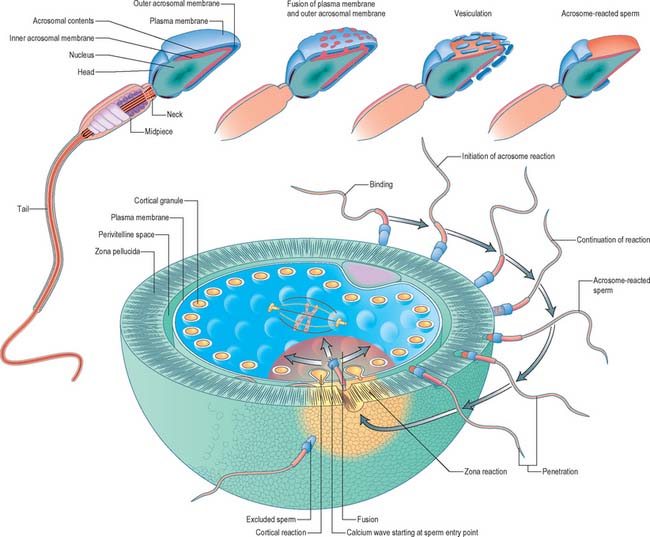
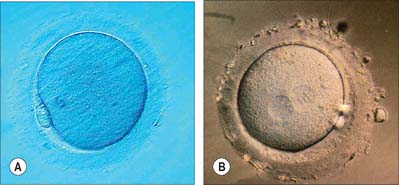
Fertilization normally occurs in the ampullary region of the uterine tube, probably within 24 hours of ovulation. Very few spermatozoa reach the ampulla to achieve fertilization. They must undergo capacitation, a process which is still incompletely understood, and which may involve modifications of membrane sterols or surface proteins. They traverse the cumulus oophorus and corona radiata, then bind to specific glycoprotein receptors on the zona pellucida, ZP3 and ZP2. Interaction of ZP3 with the sperm head induces the acrosome reaction, in which fusion of membranes on the sperm head releases enzymes, such as acrosin, which help to digest the zona around the sperm head, allowing the sperm to reach the perivitelline space. In the perivitelline space, the spermatozoon fuses with the oocyte microvilli, possibly via two disintegrin peptides in the sperm head and integrin in the oolemma (Fig. 8.4 and Fig. 8.5A).
The sperm head undergoes its protamine → histone transition as the second polar body is extruded. The two pronuclei grow, move together and condense in preparation for syngamy and cleavage after 24 hours (Fig. 8.5B). Nucleolar rRNA, and perhaps some mRNA, is synthesized in pronuclei. A succeeding series of cleavage divisions produces eight even-sized blastomeres at 2.5 days, when embryonic mRNA is transcribed.
PREIMPLANTATION DEVELOPMENT
Cleavage
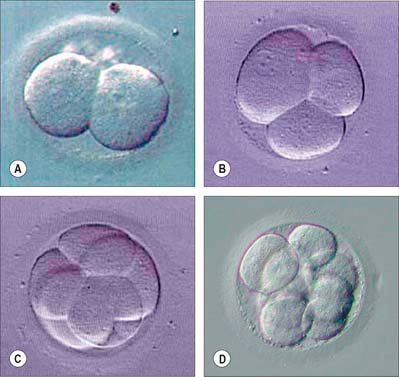
The first divisions of the zygote are termed cleavage. They distribute the cytoplasm approximately equally among daughter blastomeres, so although the cell number of the preimplantation embryo increases, its total mass actually decreases slightly (Fig. 8.6). The cell cycle is quite long, the first two cell cycles being around 24 hours each, thereafter reducing to 12–18 hours. Cell division is asynchronous and daughter cells may retain a cytoplasmic link through much of the immediately subsequent cell cycle via a midbody, as a result of the delayed completion of cytokinesis. No centrioles are present until 16 to 32 cells are seen, but amorphous pericentriolar material is present and serves to organize the mitotic spindles, which are characteristically more barrel- than spindle-shaped at this time.
The earliest time at which different types of cells can be identified within the cleaving embryo is when 8 to 16 cells are present. Up to the formation of eight cells, cells are essentially spherical, touch each other loosely, and have no specialized intercellular junctions or significant extracellular matrix; the cytoplasm in each cell is organized in a radially symmetric manner around a centrally located nucleus. Once eight cells have formed, a process of compaction occurs. Cells flatten on each other to maximize intercellular contact, initiate the formation of gap and focal tight junctions, and radically reorganize their cytoplasmic conformation from a radially symmetric to a highly asymmetric phenotype. This latter process includes the migration of nuclei towards the centre of the embryo, the redistribution of surface microvilli and an underlying mesh of microfilaments and microtubules to the exposed surface, and the localization of endosomes beneath the apical cytoskeletal mesh. As a result of the process of compaction, the embryo forms a primitive protoepithelial cyst, which consists of eight polarized cells, in which the apices face outward and basolateral surfaces face internally. The focal tight junctions, which align to become increasingly linear, are localized to the boundary between the apical and basolateral surfaces. Gap junctions form between apposed basolateral surfaces and become functional.
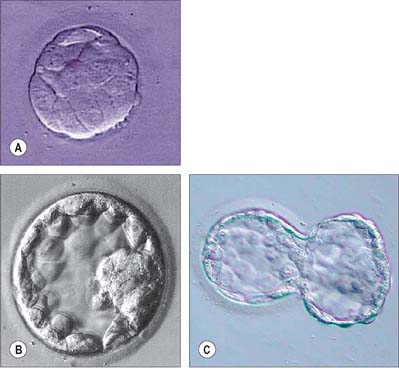
In cleavage the generation of cell diversity, to either trophectoderm or inner cell mass, occurs in the 16-cell morula and precedes the formation of the blastocyst. During the 16-cell cycle, the outer polar cells continue to differentiate an epithelial phenotype, and display further aspects of polarity and intercellular adhesion typical of epithelial cells, while the inner apolar cells remain symmetrically organized. During the next cell division (16 to 32 cells), a proportion of polar cells again divide differentiatively as in the previous cycle, each yielding one polar and one apolar progeny, which enter the trophectoderm and inner cell mass lineages, respectively. Although differentiative division at this time is less common than at the 8- to 16-cell transition, it has the important function of regulating an appropriate number of cells in the two tissues of the blastocyst. Thus, if differentiative divisions were relatively infrequent at the 8- to 16-cell transition, they will be more frequent at the 16- to 32-cell transition, and vice versa.
After division to the 32 cells, the outer polar cells complete their differentiation into a functional epithelium, display structurally complete zonular tight junctions and begin to form desmosomes. The nascent trophectoderm engages in vectorial fluid transport in an apical to basal direction to generate a cavity that expands in size during the 32- to 64-cell cycles and converts the ball of cells, the morula, to a sphere, the blastocyst (Fig. 8.7). Once the blastocyst forms, the diversification of the trophectoderm and inner cell mass lineages is complete, and trophectoderm differentiative divisions no longer occur. In the late blastocyst, the trophectoderm is referred to as the trophoblast, which can be divided into polar trophoblast, which lies in direct contact with the inner cell mass, and mural trophoblast, which surrounds the blastocyst cavity (Fig. 8.8).
Blastocyst
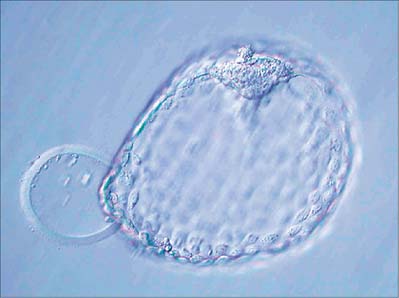
The blastocyst ‘hatches’ from its zona pellucida at 6–7 days, possibly assisted by an enzyme similar to trypsin (Figs 8.7C, 8.8). Trophoblast oozes out of a small slit; many embryos form a figure-of-eight shape bisected by the zona pellucida, especially if it has been hardened during oocyte maturation and cleavage. Such half-hatching could result in the formation of identical twins. Hatched blastocysts expand and differentiation of the inner cell mass proceeds (Fig. 8.8).
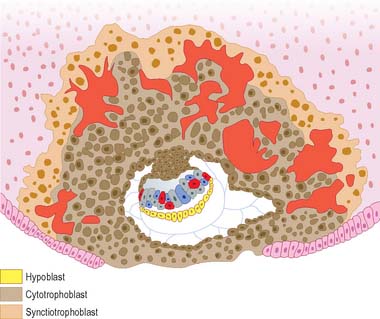
The outer cells of the blastocyst, the trophoblast or trophectoderm, are flattened polyhedral cells, which possess ultrastructural features typical of a transporting epithelium. The trophoblast covering the inner cell mass is the polar trophoblast and that surrounding the blastocyst cavity is the mural trophoblast. The free, unattached blastocyst is assigned to stage 3 of development at approximately 4 days postovulation, whereas implantation (before villus development) occurs within a period of 7–12 days postovulation and over the next two stages of development. Even at this early stage, cells of the inner cell mass are already arranged into an upper layer (i.e. closest to the polar trophoblast), the epiblast, which will give rise to the embryonic cells, and a lower layer, the hypoblast, which has an extraembryonic fate. Thus the dorsoventral axis of the developing embryo and a bilaminar arrangement of the inner cell mass are both established at or before implantation. (The earliest primordial germ cells may also be defined at this stage.)
Twinning
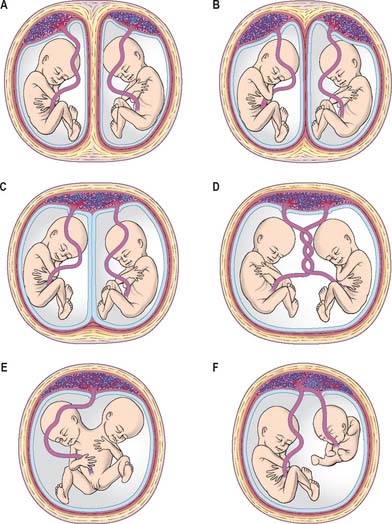
The range of separation of twin embryos is reflected in the separation of the extraembryonic membranes. The types of placentation that can occur are shown in Fig. 8.9. Monoamniotic, monochorionic placentae are associated with the greatest perinatal mortality (50%), caused both by entanglement of the umbilical cords impeding the blood supply and by various vascular shunts between the placentae, which may divert blood from one fetus to the other. Artery–artery anastomoses are the most common, followed by artery–vein anastomoses. If the shunting of blood across the placentae from one twin to the other is balanced by more than one vascular connection, development may proceed unimpaired. However, if this is not the case, one twin may receive blood from the other, leading to cardiac enlargement, increased urination and hydramnios in the recipient, and anaemia, oligohydramnios and atrophy in the donor.
FORMATION OF EXTRAEMBRYONIC TISSUES
The earliest developmental processes in mammalian embryos involve the production of those extraembryonic structures that will support and nourish the embryo during development. Production of these layers begins before implantation is complete. At present it is unclear where the extraembryonic cell lines arise. The trophoblast was considered to be a source, but evidence now points to the inner cell mass as the site of origin. Figure 8.1 shows the sequence of development of various tissues in the early embryo.
Epiblast and amniotic cavity
Epiblast cells, which are closest to the implanting face of the trophoblast, have a definite polarity, being arranged in a radial manner with extensive junctions near the centre of the mass of cells, supported by supranuclear organelles. A few epiblast cells are contiguous with cytotrophoblast cells; apart from this contact a basal lamina surrounds what is initially a spherical cluster of epiblast cells, and isolates them from all other cells. Those epiblast cells adjacent to the hypoblast become taller and more columnar than those adjacent to the trophoblast, and this causes the epiblast sphere to become flattened and the centre of the sphere to be shifted towards the polar trophoblast. Amniotic fluid accumulates at the eccentric centre of the now lenticular epiblast mass, which is bordered by apical junctional complexes and microvilli. As further fluid accumulates, an amniotic cavity forms, roofed by low cuboidal cells that possess irregular microvilli. The cells share short apical junctional complexes and associated desmosomes and rest on an underlying basal lamina. The demarcation between true amnion cells and those of the remaining definitive epiblast is clear. The columnar epiblast cells are arranged as a pseudostratified layer with microvilli, frequently a single cilium, clefted nuclei and large nucleoli; the cells have a distinct, continuous basal lamina. Cell division in the epiblast tends to occur near the apical surface, causing this region to become more crowded than the basal region. At the margins of the embryonic disc, the amnion cells are contiguous with the epiblast; there is a gradation in cell size from columnar to low cuboidal within a two- to three-cell span (Fig. 8.10; see Fig. 9.1). Further development of the amnion and amniotic fluid is described on pages 180 and 181.
Hypoblast and yolk sac
A series of modifications of the original blastocystic cavity develops beneath the hypoblast later than those developing above the epiblast. While the amniotic cavity is enlarging within the sphere of epiblast cells, the parietal hypoblast cells are proliferating and spreading along the mural trophoblast until they extend most of the way around the circumference of the blastocyst, converging towards the abembryonic pole. At the same time, a space appears between the parietal hypoblast and the mural trophoblast that limits the circumference of the hypoblastic cavity. A variety of terms have been applied to the parietal hypoblast layer: extraembryonic hypoblast and later extraembryonic endoderm or the exocoelomic (Heuser’s) membrane. The cavity that the layer initially surrounds is termed the primary yolk sac, or alternatively the primary umbilical vesicle. The resultant smaller cavity lined by hypoblast is termed the secondary yolk sac. It has been suggested that it forms in a variety of ways, including cavitation of visceral hypoblast (a method similar to formation of the amnion), rearrangement of proliferating visceral hypoblast and folding of the parietal layer of the primary yolk sac into the secondary yolk sac. Further development of the yolk sac is described on page 180. The visceral hypoblast cells are now believed to be important in many aspects of the early specification of cell lines. The cells induce the formation of the primitive streak, thus establishing the first axis of the embryonic disc. They are also believed to be necessary for successful induction of the head region and for the successful specification of the primordial germ cells. With the later formation of the embryonic cell layers from the epiblast, the visceral hypoblast appears to be sequestered into the secondary yolk sac wall by the expansion of the newly formed embryonic endoderm beneath the epiblast. Hypoblastic cells remain beneath the primitive streak: their experimental removal causes multiple embryonic axes to form.
After the formation of the secondary yolk sac, a diverticulum of the visceral hypoblast, the allantois, forms towards one end of the embryonic region and extends into the local extraembryonic mesoblast. It passes from the roof of the secondary yolk sac to the same plane as the amnion. Further development of the allantois is described on page 180.
Extraembryonic mesoblast
By definition, extraembryonic tissues encompass all tissues that do not contribute directly to the future body of the definitive embryo and, later, the fetus. At stage 5, blastocysts are implanted but do not yet display trophoblastic villi (Fig. 8.10); they range from 7 to 12 days in age. A feature of this stage is the first formation of extraembryonic mesoblast, which will come to cover the amnion, secondary yolk sac and the internal wall of the mural trophoblast, and will form the connecting stalk of the embryo with its contained allanto-enteric diverticulum. The origin of this first mesoblastic extraembryonic layer is by no means clear, and it may arise from several sources, including the caudal region of the epiblast, the parietal hypoblast and subhypoblastic cells. The trophoblastic origin of extraembryonic mesoblast is questioned, because there is always a complete basal lamina underlying the trophoblast: the migration of cells out of an epithelium is usually associated with previous disruption of the basal lamina. Certainly, the origin of extraembryonic cells will change over time as new germinal populations are established.
The first mesoblastic extraembryonic layer gives rise to the layer known as extraembryonic mesoblast, arranged as a mesothelium with underlying extraembryonic mesenchymal cells; this also appears to form an extracellular structure corresponding to the magma reticulare, between the mural trophoblast and the primary yolk sac in the stage 5 embryo. Later extraembryonic mesoblast populations mushroom beneath the cytotrophoblastic cells at the embryonic pole, forming the cores of the developing villus stems, and villi (see p. 175) and the angioblastic cells that will give rise to the capillaries within them and the earliest blood cells.
O’Rahilly R, Müller F. Developmental Stages in Human Embryos. Washington: Carnegie Institution, 1987.
O’Rahilly R, Müller F. The Embryonic Human Brain. An Atlas of Developmental Stages. 2nd edn.. New York: Wiley-Liss; 1999.
O’Rahilly R, Müller F. Minireview: Prenatal ages and stages – measures and errors. Teratology. 2000;61:382-384.
Streeter GL. Developmental horizons in human embryos. Descriptions of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. Contrib Embryol Carnegie Inst Washington. 1942;30:211-245.