26 Pregnancy and Postpartum Considerations
After reading this chapter, you should be able to:
• identify the core physiological adaptations of pregnancy pertinent to critical care nursing
• describe the antenatal assessment that would be required when caring for a woman 28 weeks pregnant in ICU
• describe the priorities of management for a postpartum woman admitted to ICU with preeclampsia
• outline the main causes of obstetric haemorrhage
• outline the standard postnatal care required by a woman in ICU, for the 48 hours following birth
• consider the resources and equipment available in your workplace that are specifically required for the care of pregnant and postpartum women
Introduction
The admission of a pregnant or postpartum woman to ICU often extends ICU staff outside of their comfort zone. Pregnant and postpartum women undergo substantial physiological adaptations. Nursing staff also need to consider the fetus and be aware of, and manage, obstetric conditions. This chapter provides an overview of the epidemiology of critical illness in pregnancy, describes the physiological adaptations of pregnancy and the puerperium, outlines some key medical conditions and their interaction with pregnancy and describes the major obstetric conditions that are associated with critical illness. Additionally, we include guidance on specific practices relating to the care of pregnant and postpartum women in ICU, for example assessment of fetal wellbeing and establishment of lactation. Further details on these topics can be found in textbooks that specifically deal with critical care obstetrics.1,2 Research into critical care obstetrics is limited and at times the evidence being drawn on is dated, but still considered to be valid.
Epidemiology of Critical Illness In Pregnancy
Most women experience a healthy, normal pregnancy and the development of critical illness associated with pregnancy is usually sudden and unexpected. Approximately 1 in 370 births result in a maternal ICU admission, making up about 1% of the ICU population; more than three-quarters of admissions occur following the birth of the baby.3,4 Admission of a pregnant woman to ICU is infrequent and more likely to be related to a non-obstetric diagnosis such as pneumonia or a motor vehicle crash. Conversely, in postpartum women, a condition directly associated with pregnancy is more likely, usually preeclampsia or obstetric haemorrhage.3 However, pregnant and postpartum women may be admitted to ICU with any diagnosis, which may or may not be associated with pregnancy.
Pregnant and postpartum admissions to ICU are usually short with most lengths of stay less than 24 hours. There is a vast variation in the threshold for admission to ICU with one European study of severe maternal morbidity reporting ICU admission proportions of between 0 and 50% across different regions.5 Additionally there are many women who, when admitted to ICU, do not receive any notable specific ICU intervention (Table 26.1) and the need for ICU admission for these women has been questioned.6 In general, about a third of women who experience severe maternal morbidity are admitted to ICU.7 It is feasible that admission to ICU is preventable by upskilling midwifery services6 and by early identification of severe illness resulting in prompt and appropriate treatment.6,8,9 There has been limited study of the long term outcomes for pregnant and postpartum women admitted to ICU in relation to their ongoing health and wellbeing, partner relationship and infant bonding. In developed countries like Australia, the mortality of pregnant and postpartum women admitted to ICU is relatively low at around 3% compared to the 15% mortality observed in the regular ICU population.3
Adapted Physiology of Pregnancy
Conception results in extensive physiological adaptations across most body systems (Table 26.2). The physiological adaptations most relevant to critical care nursing include cardiovascular, respiratory, renal, gastrointestinal and coagulation and the role of the placenta as the maternal–fetal interface. The uterus and breasts obviously undergo major change in pregnancy and any basic midwifery or obstetric textbook, such as Myles’ Textbook for Midwives or Midwifery: preparation for practice will describe these in detail.11,12 The physiological adaptations described in this chapter refer to a singleton pregnancy only, as women with a multiple pregnancy (i.e. twins) may undergo further changes.13 The physiological changes described refer to a non-labouring pregnant woman. Labour induces further changes to physiology, such as increased cardiac output.14
| Parameter | Change during pregnancy |
|---|---|
| Cardiovascular system: | |
| Heart rate | ↑ 10–15 beats/min |
| Blood pressure | |
| Systolic | ↓ 5–9 mmHg |
| Diastolic | ↓ 6–17 mmHg |
| Cardiac output | ↑ 30–50% |
| Systemic vascular resistance | ↓ up to 35% |
| Central arterial and venous pressures | Unchanged |
| Blood and associated components: | |
| Blood volume | ↑ 40–50% |
| Plasma volume | ↑ 40–50% |
| Red blood cells | ↑ 20–40% |
| White blood cells | ↑ 100–300% |
| Platelets | Unchanged |
| Fibrinogen | ↑ 100% |
| Serum albumin level | ↓ 10–15% |
| Respiratory system | |
| Respiratory rate | Unchanged |
| Tidal volume | ↑ 25–40% |
| Minute volume | ↑ 40–50% |
| Oxygen consumption | ↑ 15–20% |
| Arterial blood gas analysis values | |
| PaO2 | 80–110 mmHg |
| PaCO2 | 28–32 mmHg |
| pH | 7.40–7.45 |
| HCO3− | 18–21 |
| SaO2 | ≥95% |
| Vital capacity | Unchanged |
| Functional reserve capacity | ↓ 17–20% |
| Airway compliance and resistance | Unchanged |
| Renal system | |
| Glomerular filtration rate | ↑ 40–50% |
| Serum urea and creatinine | ↓ Unknown |
| Urine output | <300mg/day |
| Proteinuria |
Cardiovascular System
Anatomical Changes
The heart undergoes anatomical change during pregnancy including left ventricular hypertrophy and the cross-sectional areas of the aortic, pulmonary and mitral valves increase by 12–14%. ECG changes include non-specific ST segment changes, the development of a Q wave in Lead III and a left-axis deviation pattern.15 These are evident by the end of the first trimester and remain throughout the pregnancy.16 As with the interpretation of any ECG, consider other information like the patient presentation (signs and symptoms) and blood test results to form a complete assessment of the woman’s condition.
Blood Volume
Very early in the pregnancy there is generalised vasodilatation resulting in sodium and water retention. The causes of the vasodilatation are likely to include hormonal factors (e.g. progesterone), peripheral vasodilators like nitric oxide, and potentially, an as-yet unidentified pregnancy-specific vasodilatory substance.17 The end result is a 40–50% increase in blood volume as well as reduced normal serum sodium level, from 140 to 136 mmol/L and a reduced plasma osmolality from 290 to 280 mosmol/kg. These changes persist throughout pregnancy and the osmoreceptor system resets to accept these values as normal.18
The red cell mass increases 20–40% whilst the plasma volume increases 40–50%. The resultant physiological haemodilution produces a relative anaemia which is thought to be beneficial for utero-placental perfusion. Venous haematocrit typically falls from a non-pregnant value of 40% to 34% near term.19 The increase in blood volume is evident from seven weeks’ gestation and peaks at around 30–32 weeks’ gestation, normally remaining at a stable level until delivery.17,20 Women who do not experience this normal increase in blood volume are more prone to adverse outcomes such as preeclampsia or small- for-gestational-age infant.21 The additional blood volume is also thought to accommodate the normal blood loss associated with birth (<500 mL). Pregnant women are renowned for being able to maintain stable vital signs, with blood losses as much as 1500 mL, before acutely deteriorating.
Blood Pressure
Blood pressure reduces in pregnancy, with the lowest normal blood pressure recorded during the second trimester (16–28 weeks), and returns to pre-pregnancy levels near term (see Table 26.2). Blood pressure begins dropping as early as 8 weeks’ gestation, in association with the generalised vasodilatation occurring at this time. If a woman does not experience the characteristic lowering of blood pressure, particularly during the second trimester, it is viewed with suspicion and as a potentially abnormal sign.
Heart Rate, Stroke Volume and Cardiac Output
Maternal heart rate increases by 10–15 beats per minute during pregnancy with an increase noted as early as 5 weeks’ gestation.16,22 The increase in heart rate may be a compensatory response related to the generalised vasodilatation, although a hormone-related effect cannot be ruled out.23 Tachycardia (>100 beats/min) is an abnormal sign and warrants further investigation.24 The stroke volume is noted to increase between 18 and 32%, beginning as early as 8 weeks’ gestation.25,26 An increase in cardiac output is detectable from 5 weeks gestation and continues to be 30–50% higher by 32 weeks gestation.17,26 Hence, a normal cardiac output in pregnancy may be as high as 8 L/min. The increased cardiac output is achieved by a combination of the increases in heart rate and stroke volume.
Systemic Vascular Resistance
The generalised vasodilatation observed in early pregnancy reduces systemic vascular resistance by up to 35%, with some reduction already detectable by 8 weeks’ gestation.27 The development of the low-resistance utero-placental junction was thought to act as an arteriovenous shunt and contribute to the lowered SVR seen in pregnancy. However, the very-early-observed decrease in SVR argues against this theory and perhaps circulating substances that exert a vasodilatory effect on the vasculature is a more likely proposition.
Effect of Posture on Maternal Haemodynamics
It is evident that from as early as 5–8 weeks’ gestation, pregnancy is characterised by general vasodilatation, increased blood volume, increased cardiac output and is generally a hyperdynamic state. As the pregnancy advances, the bulk of the uterus begins to have an impact on maternal haemodynamics. After 20 weeks’ gestation, a woman lying flat on her back may experience supine hypotension, secondary to compression of the inferior vena cava and aorta with subsequent reduction in venous return, cardiac output and placental flow. A reduction in placental flow may occur even without a recorded drop in blood pressure. Consequently, it is inadvisable to nurse a pregnant woman more than 20 weeks’ gestation, flat on her back. A left lateral lying position results in the best cardiac output, although manually displacing the uterus to the left whilst the woman remains supine is also effective in relieving the aorto-caval compression.28 Otherwise, the use of a wedge or pillows to maintain a left lateral tilt of at least 15 degrees is recommended to minimise aorto-caval compression.29
Postpartum Cardiovascular Changes
Heart rate returns to pre-pregnancy levels by 10 days postpartum; blood pressure has normally returned to pre-pregnancy levels by term and does not change during the puerperium.23,27 The first few days of the puerperium are associated with a diuresis which reduces the circulating volume and results in haemoconcentration of blood. Consequently a postpartum haemoglobin level will increase over the first few days and the risk of thromboembolism is higher during the postpartum period than during pregnancy. Due care should be paid to postpartum women in ICU to prevent deep vein thrombosis, particularly as many of these women are in ICU with complications of preeclampsia or severe obstetric haemorrhage, both of which further increase the likelihood of thromboembolism.30
Cardiac output increases briefly in the immediate postpartum period to compensate for blood losses and tends to increase by 50% of the pre-delivery value, at this point in the post partum phase stroke volume is increased while the maternal heart rate is often slowed.23 For most women, the immediate postpartum elevation in cardiac output only lasts for an hour or so. By 2 weeks postpartum, many haemodynamic parameters have returned to pre-pregnancy levels for the majority of women, although some have been recorded as remaining above pre-pregnancy levels at 12 months postpartum, including cardiac output.14,27 There is increasing acknowledgement that for many women following childbirth, there is a permanent modification to the cardiovascular system, although whether this persists into the menopausal era is not known and whether it impacts on cardiovascular disease risk is also unknown.27
Respiratory System
Changes to the Upper Airways and Thorax
Changes also occur to the chest wall with relaxation of ligaments resulting in an outwards flaring of the lower ribs and a 50% increase in the subcostal angle.31 Both the diameter and the circumference of the thorax increase by 2 cm and 5–7 cm respectively.31,32 These physical changes are thought to cause the diaphragm to rise by 5 cm, with this occurring early in pregnancy and well before there is any pressure from the advancing uterus.32 Respiratory muscle function does not change significantly during pregnancy and rib cage compliance is unaltered.31 The functional reserve capacity (the amount of air left in the lungs after expiration) is reduced 17–20% making the pregnant woman more vulnerable to hypoxaemia during any apnoeic period. Chest X-ray interpretation is unchanged during pregnancy, despite the variety of changes to cardiovascular and respiratory flows.23
Changes to the Physiology of Breathing
From as early as 5 weeks’ gestation, multiple factors result in an increased respiratory drive. The increase in progesterone levels is thought to lower the PaCO2 threshold in the respiratory centre to stimulate respiration resulting in hyperventilation.15 Other related factors include an oestrogen-mediated progesterone response, lower serum osmolality, strong ion difference and increased level of wakefulness that are also present in pregnancy.33–35 Increased minute ventilation begins soon after conception and peaks at 40–50% at term.15 The increase in minute ventilation is achieved by a 30–50% increase in tidal volume (e.g. an increase of 200 ± 50 mL at term), with no increase in respiratory rate.15
Due to the altered respiratory function, normal arterial blood gas values are different in pregnancy compared to the non-pregnant values (see Table 26.2). The reduced PaCO2 level creates the necessary gradient for the fetal CO2 to passively cross the placenta for maternal excretion. PaO2 normally increases by 10 mmHg, although the PaO2 level is affected by posture, particularly as the pregnancy progresses.36 In advanced pregnancy, the supine position is associated with a reduction in PaO2 of up to 10 mmHg when compared with the same woman in the sitting position.37 The kidneys compensate for the lowered PaCO2 by increasing bicarbonate excretion, which serves to maintain a normal pH.36,38,39 Normal oxygen saturation in pregnancy has not been well investigated, however, it is likely to be 97–100% at sea level, with a healthy pregnant woman’s saturation not dropping below 95% during moderate exercise.40,41
The notable hyperventilation of pregnancy is associated with a feeling of breathlessness in up to 75% of healthy pregnant women when attending to activities of daily living.33 Distinguishing what is considered ‘physiological dyspnoea’ from pathological dyspnoea, for example developing cardiomyopathy, can present a challenge in pregnancy. Dyspnoea at rest is usually an abnormal sign in pregnancy.42
Postpartum Respiratory Changes
There is complete resolution of the spirometry and arterial blood gas changes by 5 weeks postpartum.36 Unfortunately there has been no study reporting the daily transition of these parameters over the first week postpartum – the timing when a postpartum woman is likely to be in ICU. One very old study reported that CO2 levels took between two and five days to return to normal non-pregnant values postpartum.43 Regardless, with the fetus delivered, it is probable that no harm will be done to a woman by the titration of her ventilation requirements according to non-pregnant conventions and arterial blood gas values.
Renal System
All smooth muscle dilates in early pregnancy, most likely in response to progesterone. This includes the renal tract, involving the renal pelvis, calyces, ureters and urethra. The placental hormone, relaxin, has also been shown to have an effect on renal tract dilatation.44 Each kidney lengthens by about 1 cm, which is explained by the dilatation and associated mild hydronephrosis and increased vascularity of the kidneys, with no hypertrophy of renal tissue.17 Another effect of widespread dilatation is urinary stasis and an increased likelihood of urinary tract infection. Acute pyelonephritis is one of the most common renal complications of pregnancy and is associated with the onset of preterm labour.45
The kidneys receive a proportion of the additional cardiac output resulting in a 30% increase in renal blood flow. The glomerular filtration rate (GFR) increases 40–50% during the first trimester and then reduces slightly towards the end of the third trimester.18 The increase in GFR may result in the tubule active transport systems for both glucose and proteins to be exhausted, with both glycosuria and proteinuria common in pregnancy. Glycosuria is not related to blood sugar levels and is unhelpful in monitoring diabetes. Proteinuria, up to 300 mg per 24 hours, is considered normal in pregnancy. Conversely, the high GFR results in lowered serum levels of both urea and creatinine. A plasma urea level exceeding 4.5 mmol/L and plasma creatinine level higher than 75 µmol/L, should be viewed as abnormal and indicative of potential renal impairment.18,46 There is conflicting information regarding normal urine output during pregnancy, with some studies suggesting no difference to that during non-pregnancy and others reporting an increase in 24-hour urine volume after 12 weeks’ gestation.45,47
Postpartum Renal Changes
The most significant renal change is the diuresis that occurs in the 1–3 days postpartum. This diuresis serves to offload the additional blood volume that the woman has had circulating for the duration of the pregnancy. There has been little examination of ‘normal urine output’ with the standard 0.5 mL/kg/hr reported as a minimum acceptable level, however a true ‘normal’ level is likely to be closer to 0.8 mL/kg/hr.48 Creatinine levels are within the normal non-pregnancy range within 24 hours postpartum, whilst the lower urea levels remain for at least 48 hours.46 The bladder returns to the pelvis in the early postpartum period as the uterus and other organs resume their pre-pregnancy position.
Gastrointestinal System And Liver
The uterus pushes abdominal organs aside as it advances making assessment and diagnosis of an acute abdomen difficult. For example, the appendix is progressively displaced upwards and laterally from McBurney’s point at the third month, reaching the level of the iliac crest by late pregnancy.49 The bowel and other organs are generally displaced by the enlarging uterus; women with prior abdominal surgery and adhesions are predisposed to intestinal obstruction as a result.50 Additionally, there is an increase in intraabdominal pressure which may contribute to another common pregnancy symptom, heartburn.
Hepatobiliary Changes in Pregnancy
There is no significant increase in hepatic arterial blood flow during pregnancy, despite the 40–50% increased cardiac output.51 There is, however, a doubling of bloodflow to the liver supplied by the portal vein,51 which may have an impact on oral medication metabolism in the liver. There are also changes in other hepatic enzymes responsible for drug metabolism, resulting in a change in pharmacokinetics of some medications, e.g. higher plasma levels of midazolam. Serum albumin levels reduce to 30–40 g/L for the majority of pregnancy, with levels as low as 25 g/L normal during the second postpartum week.46 This low albumin level reduces colloid osmotic pressure that contributes to the dependent oedema, for example swollen ankles, that is common in pregnancy.
Haemostasis System
During pregnancy, the woman’s body prepares for the separation of the placenta, a time of potential large blood loss. The blood flow to the placental bed at term is in the range of 600–800 mL/min. Both elements of the haemostasis system are activated during pregnancy (coagulation and fibrinolysis), with pregnancy and particularly the postpartum period associated with an increased risk of thrombus formation. Thromboembolic events remain a leading cause of maternal death in developed countries.24,52 A number of changes to the haemostatic system occur during pregnancy (Table 26.3).
| Haemostatic component | Changes during pregnancy |
|---|---|
| Platelets: | |
| Count Function and lifespan | UnchangedUnchanged |
| Clotting factors: | |
| Factors VII, VIII & IX Fibrinogen Other clotting factors | IncreasedDoubles by termMainly unchanged |
| Fibrinolysis: | |
| D-Dimer level | Progressively increases throughout pregnancy By term, level >0.5 mg/L common |
Of note, gestational thrombocytopenia – a platelet level between 80–150 × 109/L – occurs in 6–8 % of women.53,54 It generally has no negative impact on the woman or fetus at these levels, as there is no pathology associated with the low platelet count.55
Changes In White Blood Cells And The Immune System
There is continued debate on whether the pregnant state increases vulnerability to infection, secondary to some protective mechanism that prevents the woman’s body from reacting to the fetus as a foreign body.17 Pregnant women have increased innate immune system activity (non-specific response) and a lowered adaptive immune system (specific antibody response), with pregnant women more vulnerable to some infections like malaria and varicella.17,59,60 Pregnant women are often in contact with small children and potentially have an increased exposure to various infections. The white blood cell number increases throughout pregnancy, peaking around delivery when a normal level may be as high as 25 × 109/L.46
The Maternal–Fetal Interface
Placenta
The placenta develops from the trophoblastic layer of the fertilised ovum and is completely formed and functioning ten weeks following fertilisation.61 The chorionic villi constitute the undersurface of the placenta and attach to the uterine wall via the decidua. The end result is an interface whereby maternal blood fills a space in which the nutritive villi float and are bathed in the maternal blood (Figure 26.1). A few villi are more deeply anchored in the decidua and these are referred to as anchoring villi.61 The blood drains back into the maternal circulation via maternal sinuses and the endometrial veins. Approximately 150 mL of maternal blood, replenished three to four times per minute, bathes the villi in the intervillous space.61 The chorionic villi maximise the available surface area to optimise the exchange of products across the maternal–placental interface. By term, this surface area is said to be as large as 13 m2.62 Initially, four layers of cells separate the maternal blood from the fetal blood, reducing to three after 20 weeks’ gestation; these cell layers are collectively referred to as the ‘placental membrane’ or ‘placental barrier’.63 Damage to villi, such as a threatened abortion or blunt trauma, may result in mixing of the blood circulations.
Role of the Placenta
The placenta provides six major functions to sustain the pregnancy and fetus: respiration, nutrition, storage, excretion, protection and endocrine.61 Fetal lungs are filled with fluid and all oxygenation and removal of carbon dioxide must be provided via the placenta. Fetal haemoglobin has a slightly different structure to adult haemoglobin and has a higher affininity for oxygen. Both oxygen and carbon dioxide cross the placental membrane by simple diffusion. Nutrients are actively transported across the placental membrane, with the placenta able to select the substances needed by the fetus, even at the expense of the mother if necessary.61 The placenta is able to store glucose by converting it to glycogen and reconverting it to glucose as required and is also able to store iron and some fat-soluble vitamins.
Impact of Impaired Utero–placental Gas Exchange
Effective gas exchange across the placental membrane depends on sufficient maternal blood pressure and adequate O2 and CO2 gradients for passive diffusion to occur. In response to hypoxaemia, a fetal brain-sparing mechanism goes into effect that increases fetal arterial pressure and redirects blood delivery to the main organs, namely the brain, heart and adrenal glands.64 This centralisation of fetal blood flow is more apparent in response to maternal hypoxaemia than to reduced utero–placental blood flow. It appears that a less mature fetus (i.e. earlier gestation) may be less susceptible to asphyxia than a fetus at term.64
Clinical Implications Of The Physiological Adaptations Of Pregnancy
The beginning point of any nursing practice is an understanding of normal anatomy and physiology. The normal physiological adaptations of pregnancy can be used to explain the so-called ‘minor discomforts’ of pregnancy, including constipation, varicose veins, indigestion, breathlessness and fatigue. For a critically ill pregnant woman being nursed in ICU, these normal physiological changes are also highly relevant for her care. ICU nurses need to accommodate for, and take into account, the likely impact of the normal physiology of pregnancy on common ICU monitoring, interventions and care (Table 26.4).
TABLE 26.4 Clinical relevance of physiological adaptations in pregnancy
| Effects of the normal physiology of pregnancy | Clinical implications |
|---|---|
| Cardiovascular system | |
• Nasal passages more likely to bleed on instrumentation (e.g. nasal intubation, nasogastric insertion)
• More likely to bleed from the gums
• More prone to hypoxaemia during apnoea e.g. when being intubated
• All pregnant women are considered to have a high-risk airway:
• Nasal-tracheal intubation is not usually an option
• Have a doctor experienced with intubation on hand when a pregnant woman is being intubated
• Ensure that the artificial airway is protected and guard against accidental extubation
• Review the ‘failed intubation’ protocol in the ICU
• Pre-oxygenate with 100% O2 prior to intubation or suctioning unless contraindicated
• Titrate fluid resuscitation carefully – especially in women with severe preeclampsia
• Check diaphragm location prior to ICC insertion for haemothorax/pleural effusion
• Maintain cricoid pressure throughout CPR and intubation until the person obtaining an artificial airway instructs its release
• Chart bowel actions and ensure a bowel management strategy is implemented
• Early consideration of non-obstetric causes of an acute abdomen
• Consult with a dietician early to ensure that the woman receives adequate nutrition during ICU admission
Diseases and Conditions Unique to Pregnancy
Preeclampsia
The umbrella term ‘hypertension in pregnancy’ is used to describe a myriad of conditions in pregnancy where hypertension is a major feature. These include gestational hypertension, pre-existing essential hypertension and preeclampsia which incorporates eclampsia and Haemolysis Elevated Liver enzymes and Low Platelets (HELLP) syndrome (Table 26.5). Comprehensive descriptions of these conditions and their management have been published by the Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) and the Society of Obstetric Medicine Australia and New Zealand (SOMANZ).65,66
TABLE 26.5 Definitions of conditions characterised by hypertension in pregnancy
| Term | Definition |
|---|---|
| Hypertension in pregnancy | |
| Essential hypertension | |
| Gestational hypertension | |
| Preeclampsia (Also referred to as pregnancy induced hypertension (PIH), toxaemia) |
DIC – disseminated intravascular coagulopathy; HELLP – haemolysis, elevated liver enzymes and low platelets.
Preeclampsia is a condition unique to human pregnancy in that, whilst characterised by hypertension and proteinuria, it is a multisystem disorder consisting of variable clinical features caused by widespread vasospasm. The basis for preeclampsia remains unknown. The indication for ICU admission is usually related to organ failure, caused by the widespread vasospasm and reduced organ perfusion that characterises the disease.67 Preeclampsia can be a very serious condition and remains a leading cause of maternal death in both developed and developing countries.68
Aetiology
The placenta is strongly implicated in the cause of preeclampsia; its removal is the only definitive treatment for the condition. However, the exact mechanisms of the aetiology of the disease remain elusive and are likely to be complex and multifactorial. Theories explaining the pathophysiology of preeclampsia include immune maladaptation, abnormal trophoblast embedding, endothelial activation and excessive inflammatory response, and a genetic susceptibility (Box 26.1).71 The contribution of each component and whether all components are relevant in all cases of preeclampsia is not known. It is feasible that there are differing types of pathophysiology for mild preeclampsia that occurs at term, compared with severe preeclampsia that often occurs prior to 34 weeks’ gestation.
Box 26.1
Theories on the pathophysiology of preeclampsia71
Placentation and the immune theory of preeclampsia:
Placental debris hypothesis: syncytiotrophoblast shedding
• increased syncytiotrophoblast shedding
• placental ischaemia and reperfusion with subsequent oxidative stress
• increased circulating levels of inflammatory cytokines, corticotropin-releasing hormone, free-radical species and activin A
Endothelial activation and inflammation:
• enhanced vascular sensitivity to angiotensin II and noradrenaline with subsequent vasoconstriction and hypertension
• a fall in production and activity of vasodilator prostaglandins, especially prostacyclin and nitric oxide
Genes, the genetic-conflict hypothesis, and genetic imprinting:
Preeclampsia is associated with impaired remodelling of the uterine spiral arteries and abnormal placental implantation. It is thought that maternal–fetal immune maladaptation could be the main cause for this superficial placentation.71 Placental flow defects are detected as early as 12 weeks in some women who go on to develop preeclampsia.72 Placental ischaemia and reperfusion with subsequent oxidative stress have been regarded as major pathogenetic drivers. It is likely that there is an excessive or atypical maternal immune response to trophoblasts and the disease represents a failed interaction between the mother’s and fetus’ genetic make-up.68 The excessive systemic inflammatory response and associated endothelial dysfunction and enhanced vascular reactivity, results in widespread vasospasm which precedes the onset of clinical signs, such as hypertension.68 Other common clinical manifestations in preeclampsia include enhanced endothelial-cell permeability and platelet aggregation, explaining the increased likelihood for oedema and thrombosis.71
In summary, preeclampsia presents post 20 weeks’ gestation, but the foundation for the disease relates to abnormal placentation early in the first trimester. Whilst a number of ‘biomarkers’ attempting to predict the onset of preeclampsia have been identified, there is no reliable predictive test in clinical use.68
Risk Factors
A number of maternal characteristics are associated with an increased likelihood for the development of preeclampsia; these include:71,73
• preexisting medical conditions including diabetes, chronic hypertension, chronic renal disease, antiphospholipid antibodies
• preeclampsia in a prior pregnancy, particularly if the previous preeclampsia presented prior to 34 weeks
• family history of preeclampsia, particularly on the maternal side of the family
• multiple pregnancy e.g. twins
• body mass index >25 prior to pregnancy
• a new fathering partner for the index pregnancy
• achieving conception using assisted techniques, such as in vitro fertilisation.
Incidence
The incidence of preeclampsia is reported between 2–8%, with variations based on severity of the disease.73 The incidence of eclampsia in developed countries has reduced since the routine use of magnesium sulphate has been adopted; in the UK, the rate is about 3 cases of eclampsia for every 10,000 births.74 A prospective binational study on the incidence of eclampsia in Australia and New Zealand is underway by the Australasian Maternity Outcomes Surveillance System (AMOSS), and intends to document Australian and New Zealand population-based incidences for the first time.75 The incidence of HELLP syndrome is reported to be between 0.11% and 0.67% of all pregnancies.76,77 Preeclampsia is one of the most common indications for ICU admission at approximately one ICU admission for every 1000 deliveries.3
Clinical Presentation and Diagnosis
The clinical presentation of preeclampsia is often subtle, resulting in delayed diagnosis and treatment. Common symptoms include feeling ‘generally unwell’, headache, heartburn, nausea and vomiting, and oedema; all non-specific symptoms experienced by many pregnant women who do not have preeclampsia. Severe preeclampsia is associated with severe headache, hypereflexia, vision disturbances, severe epigastric pain, right upper quadrant pain and even blindness. There is also evidence of impaired systolic and diastolic myocardial function. Diagnosis is made when the woman has hypertension (BP ≥140/90), in association with evidence of multisystem involvement (Box 26.2). Severe preeclampsia is diagnosed when the BP is ≥160/110, in association with multisystem involvement. Additionally, eclampsia and HELLP syndrome are considered severe variants of preeclampsia even if the woman is normotensive.
Box 26.2
Diagnostic features of preeclampsia
Hypertension ≥140/90 accompanied by one or more of the following:
This clinical diagnosis has replaced the traditional triad of signs of hypertension, proteinuria and oedema, in accordance with the increased understanding of the multisystem nature of the disease. Raised blood pressure is commonly, but not always, the first sign of the condition. Although proteinuria is the most commonly recognised additional feature after hypertension, it is not mandatory to make a clinical diagnosis. Oedema is no longer a specific sign of preeclampsia, though women who develop non-dependent oedema, such as facial oedema, should be investigated for evidence of preeclampsia.66 Common investigations include urea, creatinine and electrolytes, full blood examination, liver function tests, serum uric acid, spot urine protein/creatinine ratio and 24 hour urine collection. Additional tests, such as coagulation studies, may be required as indicated by the clinical condition. Intra-uterine fetal growth restriction is a sign of placental involvement (i.e. impairment) and investigation into fetal wellbeing, including an ultrasound for fetal growth estimation and amniotic fluid volume, and umbilical artery Doppler flow patterns should be done routinely following a diagnosis of severe preeclampsia.
The presentation of preeclampsia is usually restricted to women ≥20 weeks’ gestation unless they have a co-existing condition that is known to be associated with the <20 weeks presentation of preeclampsia including hydatidiform mole, multiple pregnancy, fetal triploidy, severe maternal renal disease or antiphospholipid antibody syndrome.66
The old adage is that approximately one-third of eclampsia occurs during pregnancy, one-third during labour and one-third postpartum; the UKOSS study found 45% of first eclamptic fits were during pregnancy, 19% during labour and 36% postpartum.74 The majority of postpartum eclampsia occurs in the first 48 hours, although late-onset eclampsia may occur at two to three weeks postpartum. Despite the nomenclature, eclampsia can occur without any preceding signs and symptoms of preeclampsia. In the UKOSS eclampsia study, only 38% of women had established hypertension and proteinuria in the week preceding the eclamptic fit and 21% of women had no sign or symptom prior to the first eclamptic fit.74 HELLP syndrome commonly presents during pregnancy with about 30% postpartum.78
Management Priorities
Women with mild preeclampsia at term may be managed with induction of labour and delivery and experience few complications. The management of women with severe preeclampsia is focused on stablising the woman’s condition, optimal timing of delivery of the baby (and placenta) and preventing complications of the condition. Women with eclampsia and HELLP syndrome require the same treatments as other women with severe preeclampsia, even though they may or may not have the same degree of hypertension.69,79
Prevention of eclampsia
Magnesium sulphate has received the most attention as an anticonvulsant in preeclampsia, with its mechanism of action thought to be connected to the release of prostacyclin from the endothelium, reversing the vasoconstriction that is the basis of the disease.80,81 Magnesium is the anticonvulsant of choice to reduce the incidence of eclampsia.82,83 A common magnesium regimen is:68,82
• 4g IV loading dose given over 15–20 minutes
• an ongoing infusion of 1 g/hr
• an additional 2–4 g IV loading dose should be administered over 10 minutes to treat a recurrent eclamptic seizure
• continue infusion until 24 hours following delivery or 24 hours following the last eclamptic fit; whichever occurs the later.
The optimal therapeutic level of magnesium required to reduce the risk of fitting is not well understood and many advocate against the need to monitor serum magnesium levels on the basis that clinical assessment of deep tendon reflexes, urine output and respiratory rate is adequate to identify potentially toxic magnesium levels,68,82 although evidence is inconsistent. Other opinions suggests a therapeutic serum magnesium level of 2 mmol/L but there is no rationale provided for this level.84
Control hypertension
Obtaining control of high blood pressure remains a priority not only to improve organ perfusion but to minimise the risk of cerebral haemorrhage, a well-demonstrated hazard of hypertension in preeclampsia.24 Both systolic and diastolic pressures are important and care should be taken to ensure a controlled lowering of blood pressure, as a rapid drop can compromise fetal wellbeing. There is no evidence for the superiority of any specific antihypertensive, although there is some evidence that diazoxide may result in a potentially-harmful rapid drop in the woman’s blood pressure, and that ketanserin may not be as effective as hydralazine.83 Intravenous hydralazine is the most common drug used to treat very high blood pressure with IV labetalol increasingly being used. Severe hypertension may be treated with IV GTN or nitroprusside. The target blood pressure is not well described, other than to avoid precipitous drops in BP and to maintain adequate placental perfusion. Research has used a target diastolic BP of 85–95 mmHg.85
Optimal fluid management
Despite being hypertensive, preeclamptic women are usually plasma-volume depleted.86 In the past, intravenous fluid was administered in an attempt to restore the deficit, with no advantage noted between colloids and crystalloids. More recently, there has been a move towards more conservative plasma volume expansion due to the risk of pulmonary oedema. In reviews of maternal deaths associated with preeclampsia, it was noticed that some women were dying from complications of fluid overload. Careful titration of intravenous fluid is required with the use of pulmonary artery catheters advocated by some to guide the administration of fluid in women with severe preeclampsia, to optimise plasma volume and organ perfusion without the development of pulmonary oedema.87 Central venous pressure is universally accepted as unhelpful to guide fluid management in preeclampsia. See also Box 26.3.
Box 26.3
Management of women with HELLP syndrome using steroids
The use of steroids has been evaluated in the management of HELLP syndrome in the belief that steroids may mitigate the severity of the disease. However, a Cochrane Review concluded that there was insufficient evidence to determine whether steroid use as a treatment for HELLP syndrome had a favourable outcome for mothers and babies, although steroids may be beneficial if an increase in platelet count was imperative.88
Thrombophylaxis
Preeclampsia is an independent risk factor for thromboembolic disease and when combined with prolonged bed rest, as may occur with caesarean section, ICU admission, obesity and age ≥35 years, due consideration must be made on the need for thrombophylaxis (in the absence of any contraindications). Thus women with severe preeclampsia admitted to ICU may meet the requirements for treatment with compression stockings and low molecular weight heparin for a minimum of 7 days.30
Betamethasone
Women in late pregnancy with severe preeclampsia diagnosed prior to 34 weeks’ gestation are normally prescribed a single dose of betamethasone (11.4 mg IM), to promote fetal lung maturity and surfactant production. A Cochrane Review has shown that treatment with antenatal corticosteroids reduces the risk of neonatal death, respiratory distress syndrome, cerebroventricular haemorrhage, necrotising enterocolitis, infectious morbidity, need for respiratory support and neonatal intensive care unit admission, with no adverse effects on the mother.89
Optimal timing of delivery
Women with severe preeclampsia can only be definitively cured by delivery, no matter what the gestation. A number of studies have trialled ‘temporising treatments’, aimed at prolonging the pregnancy especially when a woman develops early onset severe preeclampsia (<34 weeks’ gestation). Whilst some have found that treatment with vasodilators and fluid administration prolongs pregnancy with no adverse effect, the general belief is that prolonging the pregnancy is associated with an increased chance of the maternal complications of preeclampsia, such as eclampsia, pulmonary oedema and cerebral haemorrhage.68,90 Consequently, a woman with severe preeclampsia is usually stabilised (magnesium sulphate commenced and hypertension controlled) and arrangements for delivery are made. Ideally, women <34 weeks’ gestation should be transferred to a tertiary obstetric centre prior to delivery.
Obstetric Haemorrhage
Obstetric haemorrhage is a leading cause of maternal mortality across the world and directly accounts for an estimated 127,000 deaths each year. Postpartum haemorrhage (PPH) is responsible for the majority of these maternal deaths. The past decade has seen an increase in both the incidence and severity of obstetric haemorrhage, with more women requiring a blood transfusion for postpartum haemorrhage than in the past.91 Severe bleeding in childbirth is estimated to occur once in every 200–250 births, although incidence is highly dependent on how ‘severe bleeding’ is defined.24 Major obstetric haemorrhage is often sudden and unexpected, and is frequently associated with an acute coagulopathy. Early recognition and treatment of major obstetric haemorrhage is vital to ensure the best outcome for mother and fetus. A repeated finding in maternal death reviews is a delay by obstetric providers in recognising the severity of haemorrhage and a consequent deterioration in maternal condition.24
Obstetric haemorrhage may occur after the 20th week gestation up to the birth (antepartum haemorrhage) and after the birth of the baby (postpartum haemorrhage). Severe obstetric haemorrhage is a common reason for postpartum women to be admitted to ICU at 0.7/1000 deliveries, with many women experiencing haemorrhage before and after the birth of the baby.3 Although not classified technically as an obstetric haemorrhage, ruptured ectopic pregnancy can also result in life-threatening haemorrhage and result in ICU admission. The common causes of antepartum and postpartum haemorrhage are described below with common management strategies presented at the end of the section. See also Box 26.4.
Antepartum Haemorrhage
Antepartum haemorrhage (APH) is defined as any bleeding from the genital tract occurring between the 20th week of gestation and the birth of the baby and occurs in 2–5% of all pregnancies.92 Bleeding from the vagina prior to 20 weeks’ gestation is referred to in terms of miscarriage (e.g. threatened) and is not classified as an APH. The two main causes of APH are placental abruption and placenta praevia.
Placental abruption (or abruptio placentae)
Placental abruption is premature separation (i.e. before the birth of the baby) of a normally-sited placenta from the uterine wall and is responsible for about 25% of APH.92 Only a portion of the placenta separates with two-thirds separation considered severe. There are two relevant matters to consider with placental abruption: how much blood the woman has lost and how much placenta remains attached and functionally able to support the fetus. If the placenta partially separates along an edge of the placenta, blood loss is usually visible via the vagina. In some cases the centre part of the placenta detaches, leaving the rim attached all the way around (like the rim of a dinner plate) and in these cases, the blood loss is usually not visible via the vagina (i.e. is concealed). However, the woman may have lost substantial blood volume and be in hypovolaemic shock. This type of placental abruption is usually accompanied by severe abdominal pain and DIC commonly develops in response to blood being forced into uterine muscle tissue; referred to as a couvelaire uterus. Once half to two-thirds of the placenta is detached, the likelihood of fetal survival is low, especially if the woman is also hypotensive. In the majority of cases, only women with severe placental abruption are admitted to ICU and usually admission occurs following an emergency caesarean section. Understanding of the aetiology of placental abruption is not complete with approximately 20% of cases unexplained. For most women, placental abruption is associated with a known related factor like preeclampsia, blunt trauma (e.g. car crash) and sudden reduction in uterine volume (e.g. after delivery of the first baby in a twin pregnancy).
Placenta praevia
Placenta praevia is when some or the entire placenta is abnormally sited in the lower segment of the uterus, often referred to as a low-lying placenta. Placenta praevia is graded into four categories of severity according to the location of the placenta in relation to the cervix (Box 26.5). A vaginal birth is not possible with Grades III and IV as the placenta blocks the passage for the baby, necessitating a caesarean section. The lower uterine segment does not fully form until 28–32 weeks’ gestation and the shearing stress as the lower uterine segment forms may precipitate detachment of the placenta from the uterine wall causing maternal bleeding. However, bleeding can occur at any time, is usually painless and may be massive. Placenta praevia is the main cause of APH accounting for 30% of cases.92 As with placental abruption, management is dictated by the size of the blood loss and maternal condition, how much functioning placenta remains and fetal wellbeing, and whether bleeding is ongoing. In severe cases, the woman is usually taken to theatre for an emergency caesarean section.
Box 26.5
Categories of severity of placenta praevia
• Type I (low-lying placenta): The placenta is located in the lower uterine segment but does not impede on the internal cervical os.
• Type II (marginal): The placenta edge is aligned with the internal cervical os.
• Type III (partial): The placenta lies over and partially covers the internal cervical os.
• Type IV (complete): The placenta is centrally located over the cervix and completely covers it.
Placenta accreta is a serious complicating condition that may occur in conjunction with placenta praevia. The attachment of the placenta to the uterine wall is abnormal and is considered morbidly adherent. There are three levels of severity, although often all three are referred to as placenta accreta (Box 26.6). Placenta accreta is strongly associated with prior caesarean section and a woman with an anterior placenta praevia and a prior caesarean section should be actively screened for placenta accreta (by ultrasound or MRI) prior to any elective caesarean section. Placental tissue can be very invasive and may infiltrate local structures like the bladder. Many women with placenta accreta undergo emergency hysterectomy at the time of caesarean section, as a means to remove the placenta and control bleeding. An alternative management is to deliver the baby by caesarean section and leave the placenta in situ.93 As long as a portion of the placenta does not detach, there will be no bleeding and in most cases, the placenta will autolyse and be re-absorbed by the woman.
Postpartum Haemorrhage
Postpartum haemorrhage (PPH), a major cause of maternal death in developed and developing countries, is defined as ≥500 mL blood loss from the genital tract after the birth of the baby. The incidence and severity of PPH is increasing, in both caesarean and vaginal births.91,94–96 PPH rates commonly sit at around 10% of all births. Severe PPH lacks an agreed definition, with published definitions ranging from ‘≥1000 mL’ to ‘estimated blood loss ≥2500 mL or transfused ≥5 units of blood or received treatment for coagulopathy during the acute event’.12,97 Consequently, the incidence of severe PPH varies depending on how it has been defined and ranges from 3.7/1000 deliveries to 4.6/1000 deliveries.5,97 Additionally, PPH is also classified according to the timing of the haemorrhage in relation to the birth. Primary PPH occurs within the first 24 hours after birth whilst secondary PPH occurs from 24 hours up to six weeks following birth. Primary PPH is often caused by uterine atony, whilst secondary PPH is more likely to be associated with retained products and associated infection.
The causes of PPH are varied and have been classified by the four ‘Ts’: tone, tissue, trauma and thrombin (Box 26.7). The cause of the PPH should be identified and targeted with specific management, in conjunction with the general principles of haemorrhage management. See also Box 26.8.
Severe Obstetric Haemorrhage Management Priorities
Whilst it is feasible for a pregnant woman in ICU to develop placental abruption, for example, the vast majority of women admitted to ICU with obstetric haemorrhage will be transferred following birth, and are thus postpartum on admission to ICU. These priorities focus on postpartum management. As with any major haemorrhage (see Chapter 20), the principles of treatment are:
• restore an adequate circulating volume and maintain oxygen and perfusion to vital organs
See Box 26.9 for acute immediate treatment.
Box 26.9
Summary of acute immediate treatment for PPH
Resuscitation and immediate management:
• 2 large bore cannulae and send bloods for rapid crossmatch
• Administer oxytocics e.g. syntocinon98
• Transfuse blood (O-negative in the first instance then type specific)
Maintaining circulating volume, oxygenation and perfusion
Haemodynamic instability following substantial blood loss is a frequent reason for admission to ICU.99 Accurate estimation of blood loss is difficult as bleeding can be concealed, and the presence of amniotic fluid makes accurate blood volume loss estimation a challenge, potentially leading to an underestimation of fluid resuscitation needs. Furthermore, peripartum women are at an increased risk of acute pulmonary oedema, which further complicates fluid resuscitation.100,101 Standard resuscitation fluids, such as normal saline, should be infused according to routine practice of the non-obstetric haemorrhage, remembering that large volumes of blood products may also be required.
Achieving haemostasis and correct coagulopathy
Specific interventions to control haemostasis include radiological arterial embolisation or balloon occlusion of the internal iliac arteries and emergency hysterectomy. It is not uncommon for women to need to return to theatre for abdominal packing for ongoing ‘ooze’ that may continue after a hysterectomy. Most women with severe obstetric haemorrhage in ICU have developed DIC that requires treatment with the appropriate blood products.102 DIC is particularly common in these women in part because of the normal changes in the clotting factors during pregnancy and in part due to the potential for an amniotic fluid embolism to have been the triggering event for the haemorrhage.86,103,104
Large volumes of blood products, such as packed red cells, fresh frozen plasma, platelets and cryoprecipitate are often required. Guidelines recommending the ratio of red blood cells:fresh frozen plasma:platelets in acute major haemorrhage are under development in many countries. Increasingly it is thought that more aggressive use of fresh frozen plasma and platelets in line with red blood cell usage is needed to prevent and/or correct haemorrhage coagulopathy. A recent large trauma study found that a 1 : 1 ratio for both red blood cells/fresh frozen plasma and red blood cells/platelets, if given early following a major blood loss, resulted in significantly improved mortality.105 There has been no similar study conducted in obstetric patients, although it is likely that obstetric patients may also benefit from more liberal early use of fresh frozen plasma and platelet transfusions. Importantly, the large trauma study found the increased ratios of FFPs and platelets were not associated with an increase in transfusion-related acute lung injury and acute respiratory distress syndrome from inflammatory mediators.105 Finally, recombinant Factor VIIa has been used successfully in the management of severe obstetric haemorrhage and should be considered for use early in the management of the bleeding woman, with treatment more likely to be effective if administered before the woman becomes hypothermic and acidotic.106
Preventing complications
Strategies to prevent the following complications should be implemented:
• Complications of major blood transfusion: these are similar in the obstetric patient as the non-obstetric patient and include: acid–base disturbance, transfusion related acute lung injury (TRALI), hypocalcaemia, hyperkalaemia and hypothermia. Standard monitoring and treatment of these complications should be used.
• Increased risk of thrombosis: particularly in the early postpartum period as the risk is exacerbated by lengthy theatre procedures, bed rest associated with ICU admission and following major haemorrhage with an associated massive blood transfusion. Suitable thromboprophylaxis should be considered as soon as feasible and thromboembolic stockings and/or sequential compression devices should be applied.
• Acute renal failure: irreversible renal failure has been reported as a sequela of acute renal failure following severe postpartum haemorrhage.107 Routine monitoring and management of renal impairment is required, keeping in mind that a pregnant patient has a lower urea and creatinine level than non-pregnant patients. Careful titration of fluid for renal purposes is needed due to the increased propensity for pulmonary oedema.
• Rh isoimmunisation: the potential to develop Rh isoimmunisation in Rh-negative women who have experienced antepartum haemorrhage should be considered.108 A Kleihauer-Betke test should be done to quantify the amount of fetal cells in the maternal circulation and determine the dose of anti-D immunoglobulin required.
• Sheehan’s syndrome: necrosis of the pituitary gland is a very rare complication of severe obstetric haemorrhage. The anterior lobe is most often affected due to physiological changes that occur during pregnancy. Whilst the syndrome may go undetected for many years, one of the earliest symptoms is a failure to establish lactation, due to the absence of prolactin secretion. Sheehan’s syndrome can be prevented by maintaining adequate circulating volume, oxygenation and perfusion.
Use of Intra-operative Cell Salvage for Obstetric Haemorrhage
The introduction of cell salvage in obstetrics has been delayed compared to other surgeries for two key reasons: the theoretical risk of amniotic fluid embolism (AFE) and the risk of rhesus isoimmunisation.109 New technologies, combined with an increasing obstetric haemorrhage rate, has seen cell salvage being introduced since the mid-1990s, now becoming common practice.109,110 Historical understanding of amniotic fluid embolism argued against the risk of infusing blood that potentially contained amniotic fluid. The more recent understanding that AFE is more aligned with an anaphylactic reaction has lessened these concerns as a woman has already been exposed to the contents of the fluid that are infused following cell salvage and in practice, there has been no confirmed case of AFE following use of cell salvage infusion.111 Regardless, it is common practice to use a different suction device from the time of amniotic membrane rupture until after delivery (which is not re-used) with blood aspirated from the surgical field collected by the cell salvage device.110 A leukocyte depletion filter should always be used during the re-infusion of salvaged maternal blood to filter any remaining foreign proteins.109 None of the currently available cell saver equipment is able to discern fetal from adult red blood cells and any present fetal cells are transfused to the woman. It is important for Rhesus-negative women to have a post-infusion Kleihauer-Betke test to quantify the amount of fetal red cells in the maternal circulation to ensure that an adequate dose of anti-D immunoglobulin can be given to prevent isoimmunisation.
Amniotic Fluid Embolism
Amniotic fluid embolism (AFE) is a rare and incompletely understood obstetric emergency that usually occurs during labour or pregnancy termination, or shortly after delivery. Traditional understanding of the condition was based around the notion that amniotic fluid entered the maternal blood stream via the endocervical veins or placental bed, with amniotic fluid, fetal cells, hair, or other fetal debris functioning as an embolus, and resulting in the dramatic cardiorespiratory collapse seen with the condition. However, not all women diagnosed with AFE have evidence of fetal squames/amniotic fluid substances in the pulmonary vasculature and many women who do not develop AFE have fetal cells found in the maternal circulation.112
More recently, improved understanding of the mechanics of labour and the interaction of amniotic fluid and maternal blood, as well as the striking similarities between clinical and haemodynamic findings in AFE and both anaphylaxis and septic shock, have led to a belief that a common pathophysiological mechanism is likely to be responsible for all these conditions.113 As AFE resembles an anaphylactic reaction to fetal material rather than an embolic event, the term ‘anaphylactoid syndrome of pregnancy’, instead of AFE, has been proposed.113 AFE has also been likened to systemic inflammatory response syndrome, with the related inappropriate release of endogenous inflammatory mediators.112 The trigger for AFE is not well understood, although it is thought to be a fetal antigen (which may arise from amniotic fluid). It is possible that all labouring women are exposed to the fetal antigen, with those affected by AFE exhibiting a rare and abnormal maternal immune response.112 One of the difficulties blocking improved understanding of AFE is the lack of a diagnostic test.
Regardless of the level of understanding, the abnormal mediator release gives rise to acute lung injury, resulting in acute dyspnoea and hypoxia and often the development of acute respiratory distress syndrome. Within 30 minutes of the antigen insult, there is evidence of severe pulmonary hypertension with acute right ventricular failure.114 It is thought that inflammatory mediators are a more likely cause of pulmonary vasoconstriction, with physical obstruction to the pulmonary vasculature (embolism) not the main mechanism.112,115 The left ventricular failure seen in AFE is considered a secondary response due to poor left ventricular filling pressures. Concomitantly, substances in the amniotic fluid trigger a profound consumptive coagulopathy.
Incidence and Risk Factors
The incidence of AFE is thought to be in the range of 2–8 women per 100,000 deliveries making it a very rare event.116 However, the lack of a diagnostic test is a serious limiting factor for accurate determination of incidence, as clinical diagnoses vary and the accuracy of hospital codes that may be used to count the incidence are fraught with potential error.116 There has been geographical variation in incidence reported, with AFE more common in North America (1 in 15,200 deliveries) than in Europe (1 in 53,800 deliveries);115 this may represent a true difference in incidence or reflect differences in clinical diagnosis or methods of case identification.
Diagnosis remains one of exclusion and there is a long list of differential diagnoses, including air or thrombotic pulmonary emboli, septic shock, cardiomyopathy, acute myocardial infarction, anaphylaxis, transfusion reaction, aspiration, placental abruption, eclampsia, uterine rupture, local anaesthetic toxicity and primary postpartum haemorrhage.113 Older obstetric literature quote mortality rates above 80%.117 More recent larger studies have shown that mortality in developed countries is more likely to be in the range of 13–30%.115,116,118 However, AFE remains a major contributor to maternal death, accounting for 5–15% of all maternal deaths in developed countries.52,115
Although controversy exists, the factors that have been proposed as contributing to an increased likelihood for AFE include:112,113,115,116,118
Presentation
The symptoms associated with AFE have been well described and usually comprise premonitory symptoms, such as restlessness, agitation and numbness/tingling prior to more severe maternal compromise such as hypotension, dyspnoea, hypoxia, altered mental status and haemorrhage.115 Additionally, in pregnant women, collapse of the maternal cardiovascular system leads to fetal distress as the placenta is deprived of maternal oxygen, quickly leading to fetal demise unless the fetus is delivered swiftly. There is variation in the signs and symptoms, and in the timing of their presentation in individual women. Premonitory symptoms, shortness of breath and fetal distress have been reported as the early signs in a UK study.116 Overall, haemorrhage and associated coagulopathy, hypotension and shortness of breath were the most commonly recorded symptoms.116 Cardiac arrest was documented in 40% of cases and seizure in 15%. Haemorrhage and coagulopathy may not be immediately apparent, some women die before it develops, however these clinical features usually develop in women who survive the initial insult.
Treatment
There is no specific treatment for AFE; all therapy is supportive with the aim to maintain adequate oxygenation and perfusion, control haemorrhage and correct any coagulopathy. Common interventions include:116
Peripartum Cardiomyopathy
Peripartum cardiomyopathy, sometimes referred to as postpartum cardiomyopathy, is new onset heart failure in association with pregnancy. Diagnosis is usually dependent on all four of the following criteria: (1) the development of the disease in the last month of pregnancy or within five months of delivery; (2) absence of any other identifiable cause of heart failure; (3) absence of recognisable heart disease before the last month of pregnancy; and (4) left ventricle systolic dysfunction.119 However, time of onset outside of the above criteria does occur occasionally. Peripartum cardiomyopathy is considered to be a dilated cardiomyopathy, resulting in a dilated left atrium and ventricle, and a reduced left ventricular ejection fraction (< 45%).120 Women commonly present with New York Heart Association Class III or IV heart failure.121 The incidence of peripartum cardiomyopathy varies widely from 1:100 in a small region of sub-Saharan Africa to 1:4000 in the US, though many studies on peripartum cardiomyopathy were conducted on data that had been gathered retrospectively.119,122 A relatively recent prospective population-based study in the Netherlands found that 1 in 20,000 pregnancies required ICU admission for peripartum cardiomyopathy.7
The exact cause of peripartum cardiomyopathy is not well understood and a variety of factors have been implicated, including viral infection, autoimmune mechanisms, cytokine-mediated inflammation, increased myocyte apoptosis, increased oxidative stress, genetic disposition and/or cultural habits, and abnormal hormonal regulation.121,123 Maternal mortality associated with peripartum cardiomyopathy is around 15%, however, it may be as low as 2% in developed countries.124 Studies show that approximately 20–40% of women recover their left ventricular function, usually within six months though it may take up to two years.120,125 Women who never fully recover their cardiac function require ongoing medical management; a small proportion of women go on to require a mechanical-assist device and heart transplantation.
Management and Treatment Priorities
Women with peripartum cardiomyopathy present with varying degrees of left heart failure. Signs and symptoms of heart failure including dyspnoea, persistent cough, abdominal discomfort, palpitations and oedema may be mistaken for ‘discomforts of pregnancy’ and lead to a delay in diagnosis. The diagnosis of peripartum cardiomyopathy is one of exclusion requiring systematic investigation to exclude both cardiac and non-cardiac differential diagnoses such as pulmonary embolism, acute myocardial infarction, severe preeclampsia and pneumonia.126 Echocardiography is a useful diagnostic tool with a left ventricular end-diastolic diameter >60 mm predictive of poor recovery, as is a LVEF <30%.120 When available, a cardiac MRI allows for better chamber volume and functional assessment and is a more sensitive tool to identify a left ventricular thrombus.120
Management of peripartum cardiomypothy is centred on optimising cardiac function and preventing complications. The principles of managing acute heart failure in women with peripartum cardiomyopathy are no different to the management of heart failure from any other cause, and aims to reduce preload and afterload and to increase cardiac contractility (see Chapter 10 for a full description). Unfortunately, ACE inhibitors and angiotensin antagonists are contraindicated in pregnancy, and are usually not prescribed.
Bromocriptine, a relatively new and novel treatment for peripartum cardiomyopathy, is still undergoing investigation and as such is not routinely prescribed. Recent advances in understanding the aetiology of peripartum cardiomyopathy have suggested that increased oxidative stress plays a significant role and bromocriptine is directly able to reduce oxidative stress by blocking the release of prolactin.127 Animal and early human studies show promise, with relapse of peripartum cardiomyopathy prevented in women in a subsequent pregnancy and rapid recovery in new-onset peripartum cardiomyopathy observed.128–130 Larger studies confirming these findings are required before this specific therapeutic intervention is adopted for routine management.
For women diagnosed with peripartum cardiomyopathy whilst pregnant, timing and mode of delivery are two other management decisions to be made. A multidisciplinary team, including cardiologist, obstetrician, anaesthetist and nursing/midwifery staff, should consider and plan for delivery dependent on maternal and fetal condition and the woman’s known preferences. Ergometrine-containing drugs, used to contract the uterus post-delivery, are contraindicated because they cause vasoconstriction and the associated increase in afterload may be detrimental for maternal heart function. Synthetic preparations, such as oxytocin, are advised instead to prevent postpartum haemorrhage. Finally, given the postulated role of prolactin in the aetiology of peripartum cardiomyopathy, recent guidelines advise against breastfeeding in women who have been diagnosed with peripartum cardiomyopathy.120
Subsequent Pregnancy
Family planning counselling is an important part of the care of women as they recover from peripartum cardiomyopathy. As indicated earlier, left ventricular function may take over two years to recover and women, after a diagnosis of peripartum cardiomyopathy, are at risk of a relapse in any subsequent pregnancy. Generally speaking, women who become pregnant following a diagnosis of peripartum cardiomyopathy have approximately a 30% risk of relapse.125,131 Peripartum cardiomyopathy remains an important cause of maternal death and this may occur in association with subsequent pregnancies.
Exacerbation of Medical Disease Associated with Pregnancy
Women with preexisting medical conditions pose additional challenges during pregnancy. In a population-based prospective study of all pregnant and postpartum admissions to ICU in the Netherlands, 28% of women had at lease one chronic disease.7 However, this preexisting medical condition may not have been related to the need for ICU admission. For example, in an Australian study 39% of admissions to ICU had a medical history, but the preexisting illness was related to the ICU admission in 24% of women.10 Occasionally pregnant and postpartum women are admitted to ICU with exacerbation of an underlying medical condition and two of the most common conditions, asthma and cardiac disease, are outlined in this section.
Asthma
Epidemiology and Course of Asthma During Pregnancy
Asthma is the most common chronic health disease in pregnant women, affecting 4–8% of all pregnancies in the US.132 However, the incidence in Australia may be higher given the higher prevalence, 12–14%, of ‘current asthma’ in women of childbearing age.133 The course of asthma during pregnancy is highly variable and not predictable for any individual woman. Approximately one-third of women experience an improvement in asthma symptoms, one-third report no change and one-third experience exacerbation of asthma.134 Curiously, the fetal gender may be a relevant factor with the female fetus associated with a worsening of asthma symptoms.135 Generally speaking, the more severe asthma symptoms a woman exhibits pre-pregnancy, the more likely she will experience an exacerbation during pregnancy resulting in hospitalisation.136,137 Very severe exacerbations of asthma during pregnancy requiring ICU admission are rare. A persisting problem in pregnant women with asthma is the potential for reluctance to treat (by physicians) and decreased medication compliance (by women), based on concerns about the safety of medication during pregnancy, with a substantial number of asthma exacerbations in pregnancy associated with non-adherence to prescribed drugs.138,139 Studies comparing medication use have shown that pregnant women are also less likely to be prescribed systemic corticosteroids than non-pregnant asthmatics.138,140 The second and third trimesters are commonly the time when a worsening of asthma symptoms will develop, although women tend to have an improvement in symptoms for the last four weeks of a term pregnancy.138
Effect of Asthma on Pregnancy
The relationship between asthma in pregnancy and adverse maternal and neonatal outcomes including preeclampsia, gestational diabetes, small-for-gestational-age neonates and preterm birth is inconsistent. The general belief is that poor maternal and neonatal outcomes are associated with poor asthma management and not a result of the treatment itself.137
Management and Treatment Priorities
A pregnant woman admitted to ICU with asthma may be experiencing new-onset asthma or an exacerbation of preexisting asthma. Regardless, the management and treatment priorities are the same. Accurate diagnosis and evaluation of the disease is necessary and should involve the advice of a thoracic medicine specialist and an obstetrician, who will continue the care of the woman once discharged from ICU. Methacholine testing, used as a diagnostic tool for asthma, is contraindicated in pregnancy, and a woman with a clinical picture consistent with new-onset asthma, should be treated as such, until diagnostic testing can be conducted postpartum.141 Treatment of severe asthma in pregnancy is no different to the treatment in non-pregnant patients (see Chapter 14), apart from the additional needs to monitor fetal wellbeing and consider the normal respiratory parameters in pregnancy (Figure 26.2). Severe hypoxaemia associated with an exacerbation of asthma places the fetus at risk and should be avoided; maternal SaO2 should remain ≥95%. Peak flow measures are recommended to be used during pregnancy to assess and monitor the woman’s condition, with the normal values unchanged in pregnancy.137 The risks associated with current asthma medication use in pregnancy are far less than the risks associated with uncontrolled asthma, and the regular schedule of asthma medications should be prescribed in pregnancy according to asthma symptom level.141 Likewise, none of the common drug categories such as inhaled corticosteroids, long-acting β-agonists and leukotriene-receptor antagonists, is contraindicated during lactation.141
Cardiac Disease
Cardiac disease in pregnancy consists of women who have congenital heart disease and women who have acquired heart disease, such as rheumatic heart disease. Congenital heart disease is one of the more common forms of congenital birth defects with the four most serious congenital cardiac defects having a combined rate in Australia of 12.4/10,000 births.142 Increasing numbers of those affected with congenital heart disease are surviving into adulthood with the greatest increase in survival benefit seen in people with severe disease.143 The Canadian Cardiovascular Society estimates that in their population of 24 million, 96,000 adults will be living with congenital heart disease.143 The additional load placed on the cardiovascular system in pregnancy is poorly tolerated by some women and cardiac disease in pregnancy remains a leading cause of death in Australia.52
Rheumatic heart disease is the most frequently acquired heart disease and is a condition normally associated with developing countries.144 In Australia, rheumatic heart disease is a significant concern in Aboriginals and Torres Strait Islanders with rates in Indigenous communities in the Northern Territory noted to be the highest in the world, and are over 30 times higher than non-Indigenous Australians.145,146 Similarly in New Zealand, Māori and Pacific Islanders have a much higher incidence of rheumatic heart disease than New Zealanders of European ancestry. Refugee and immigrant women who have migrated from developing countries also have a higher risk for rheumatic heart disease in pregnancy. Rheumatic heart disease is a delayed complication of acute rheumatic fever, and results from untreated Group A streptococcus bacterial infection. It most commonly affects the mitral valve, though may also affect the aortic valve and usually involves restricted leaflet mobility, focal or generalised valvular thickening and abnormal subvalvular thickening, resulting in regurgitation and, rarely, stenosis.147
A cardiac condition increasingly presenting in pregnancy is acute myocardial infarction (AMI), thought to be related to the changing demographics of the pregnant population, such as older women becoming pregnant.148 AMI is the leading cardiac cause of maternal death in the UK, mostly related to undiagnosed ischaemic heart disease.24 Additionally, spontaneous aortic dissection and coronary artery dissection may also occur in pregnant women with no preexisting disease.149 Signs and symptoms of heart failure and complaints of chest pain must be investigated and not put down to the ‘minor discomforts’ of pregnancy, such as breathlessness, heartburn, fatigue and dependent oedema. Given that the cardiac output is expected to increase 40–50% in a normal pregnancy, any cardiac condition resulting in poor left ventricular function and/or restricted left ventricular outflow are particularly associated with poor outcomes in pregnancy.
Also relevant for the outcome of both mother and baby is whether any valvular disease has been repaired and whether a tissue or mechanical valve has been inserted. Use of anticoagulants is of particular concern during pregnancy, with warfarin contraindicated for use in pregnancy. However, the risk of thrombosis is relatively high in pregnant women and some women remain on warfarin despite the risk of associated congenital anomaly and the increased likelihood of miscarriage.150
Treatment Priorities
All women with cardiac disease are considered to have a ‘high risk’ pregnancy and should receive maternity care by a multidisciplinary team including as a minimum, obstetrician, midwife, cardiologist and anaesthetist.151 The timing and location of delivery, choice of anaesthesia and delivery mode should each be discussed by the team with the woman, and planned well in advance. If a pregnant woman with cardiac disease is admitted to ICU, this multidisciplinary team should be consulted about her care. Priorities of care include:
• Pre-pregnancy counselling: this should allow a full and frank discussion about the likely risks of pregnancy for the individual and to discuss a treatment path. This is of particular importance for women who are on potentially teratogenic medication, such as warfarin, and for women who may benefit from surgery or interventional treatment prior to conceiving. Additionally, women with congenital heart disease may require genetic counselling to determine the likelihood of congenital heart disease in any offspring.
• Diagnosis: standard investigations including chest X-ray, ECG, CT scan and MRI should be attended to as indicated by the clinical condition. In general, diagnostic imaging of a critically ill woman should not be withheld due to concerns about the fetus, with abdominal shielding used whenever possible.152
• Heart failure: as was outlined in the section on peripartum cardiomyopathy, the principles of treatment for heart failure in pregnancy are the same as for the non-pregnant population.
• Arrhythmias: commonly used drugs including digoxin, lignocaine, flecainide, verapamil, sotalol, propranolol, adenosine and amiodarone; although limited studies exist in the pregnant population, all have been used safely and effectively during pregnancy.153 Transient neonatal hypothyroidism has been described in women on amiodarone and monitoring of neonatal thyroid function is recommended.154
• Cardiac surgery: interventions such as valvuloplasty may be required. Open-heart surgery is only performed during pregnancy when the maternal condition is critical, for example coronary artery dissection or severe dysfunctioning valve, because of the high chance of fetal loss associated with the woman going on bypass. Standard care should be provided to a pregnant woman, with care to nurse the woman ≥20 weeks’ gestation with a 15 degree left lateral tilt if possible, to reduce the negative effects of aorto-caval compression. Open-heart surgery and ECMO have been used successfully in pregnant women with good outcomes for mother and baby.155,156
• Thrombus prevention: this is a priority in women with valvular disease/prosthetic valves, atrial fibrillation or dilated heart chambers at risk of thrombus formation, especially because of the normal hypercoagulopathy associated with pregnancy. Warfarin embryopathy, a recognised collection of developmental anomalies such as nasal hypoplasia and epiphysis stippling, is associated with warfarin use in the first trimester, consequently warfarin use is contraindicated. However, pregnant women with mechanical valves experience unacceptably high rates of valve thrombosis and embolism when switched to heparin, and so many cardiologists consider the risks associated with the continued use of warfarin in pregnancy to be lower than the risks of stopping it. Therefore a regimen that balances the risk of thrombosis with that of fetal loss and risk of haemorrhage should be implemented, with some variation stopping warfarin for the whole first trimester or from 6–12 weeks gestation and then resumed until close to delivery; replacing warfarin with unfractionated or low molecular weight heparin for the whole pregnancy or continuing warfarin throughout pregnancy and replacing it with heparin for delivery only. Appropriate dosing schedules for heparin have not been confirmed with low-dose heparin considered inadequate and high doses of unfractionated heparin not researched.157
• Secondary prevention of rheumatic heart disease: monthly IM penicillin, e.g. 1,200,000 units of benzyl penicillin, to minimise repeat acute rheumatic fever and associated further valve degeneration.158
Special Considerations
Trauma In Pregnancy
The term ‘trauma’ refers to any accidental or intentional event resulting in injury, with motor vehicle crashes, falls and domestic violence most prevalent amongst the pregnant trauma population. Although pregnancy is considered a period of low risk for traumatic injury as most women choose not to embark on risk-taking behaviour when pregnant, those who do continue to engage in risk-taking behaviour, such as misuse of alcohol and other substances, experience more injury.159 Overall, the incidence of trauma in pregnancy is estimated to be in the range of 5–8% of all pregnancies, with motor vehicle crashes responsible for about half, and falls and assault accounting for roughly one quarter each.160,161
Specific causes of injury in pregnant women include:
• Domestic violence: for women who experience domestic violence, 30% occurs for the first time during pregnancy; homicide during pregnancy and the postpartum period also occurs and intentional violence by an intimate partner is a relatively common reason for presentation to the emergency department.24 The ‘story’ of the injury should be considered in relation to the presenting injury and likely mechanism of injury; another potential sign is when the woman appears evasive or reluctant to speak or disagree in front of her partner.24 Pregnancy-related violence is associated with low birth weight babies, premature labour and fetal trauma.162
• Musculoskeletal injuries: pregnancy hormones, predominantly relaxin, oestrogen and progesterone, affect joints and ligaments making them more lax and pliable. This increased joint mobility explains why pregnant women are more likely to experience joint injury, pelvic instability, back pain and strained and dislocated joints, and combined with the altered centre of balance with the advancing uterus, explains why pregnant women readily fall off ladders, for example when decorating the nursery.
• Motor vehicle trauma: is the most common reason for a pregnant woman to present to an emergency department with trauma. Unfortunately, some pregnant women believe there is no legal requirement to wear a seatbelt when pregnant and this places them and their fetus at increased risk.163 Additionally, many pregnant women are not informed on the correct positioning of a seatbelt during pregnancy, and incorrect positioning can increase the likelihood of placental abruption in a crash (Figure 26.3).
FIGURE 26.3 Positioning of a seatbelt during pregnancy. (A) incorrect positioning; (B) correct positioning.163
Trauma in pregnancy presents a number of challenges, in part due to consideration of the fetus, but also given the impact of the physiological changes of pregnancy. Overwhelmingly, the single principle of management is to treat the mother. Trauma assessment of the pregnant woman should include all the usual elements (see Chapter 23) with the following additional components.
Initial Evaluation of the Pregnant Patient: The Primary Survey
Consideration should be given to all women of childbearing age as to whether she may be pregnant. Determination of the presence of a pregnancy and the estimated gestation may be obtained from the woman (or friend/relative) or may require blood tests/physical examination/ultrasound.160 If the woman is obviously pregnant, a rough estimate of gestation can be made by measuring the height of the fundus from the symphysis pubis. The height in cm equates to the number of weeks’ gestation, e.g. 22 cm = 22 weeks’ gestation. The presence of fetal movement is a quick assessment of fetal wellbeing and if the woman is conscious and over 18–20 weeks’ gestation, she should be able to communicate if she feels fetal movements. The physiological adaptations of pregnancy may initially mask serious injury, with vital signs and patient symptoms not reflective of the underlying injuries.163 A pregnant woman’s condition can rapidly deteriorate.
Use of Imaging in Pregnancy
All radiological investigations and imaging that are clinically indicated by the maternal condition should be attended to without delay over concerns for the fetus.163 When possible and appropriate, use of a pelvic/abdominal lead shield may be used to protect the developing embryo/fetus. If a chest tube is necessary for a haemothorax, care should be taken to position the catheter 1–2 spaces higher than normal due to the raised diaphragm.
Obstetric Assessment in Trauma
If the woman’s gestation is estimated to be 22–24 weeks or more, then a CTG should be conducted to assess fetal wellbeing (see section on fetal assessment). If there has been any likelihood of blunt trauma to the abdomen (i.e. by the steering wheel or seatbelt position), then a continuous four-hour duration CTG should be done to identify any fetal distress resulting from a potential placental abruption. An abdominal ultrasound is commonly done to assess fetal wellbeing and to identify any trauma to the fetus. Ultrasound is also useful in detecting free peritoneal fluid, maternal haemorrhage and may assist in the diagnosis of placental abruption.163 The possibility of uterine rupture should also be considered even though it is rare (<1% of pregnant trauma patients).163 Also remember that the bladder becomes an abdominal organ after 12 weeks’ gestation and is more prone to traumatic injury.
Pneumonia
Pneumonia in pregnancy is one of the more common reasons why a pregnant woman may be admitted to ICU. Although studies have shown that pregnant women are not more likely to contract pneumonia than non-pregnant women, the severity of pneumonia experienced by women in these studies has not been well examined.164 It is not known whether the ICU admission rate for pneumonia is higher in the pregnant population as opposed to the hospitalisation rate. Anecdotally, it is rare for a well woman of childbearing age to be admitted to ICU with community-acquired pneumonia; women living in disabled support accommodation and pregnant women are the exception. Varicella pneumonia is also more prominent in the pregnant population. It would appear from studies on pregnant admissions to ICU and maternal death reports that severe community-acquired pneumonia in previously well women is a persisting concern in the pregnant population.
It is not fully understood why pregnant women may be vulnerable to severe pneumonia though the adaptations to the mechanics of breathing and changes in the immune response may be contributing factors.164 Additionally, it has been postulated that pregnant women are amongst small children more often and may have an increased likelihood of exposure to infective agents. Regardless, the treatment and management of pneumonia in pregnancy is no different to pneumonia in non-pregnant women: identify causative organism and administer appropriate antibiotics/antiviral agents as indicated, maintain oxygenation and prevent complications (see Chapter 14). Assessment of fetal wellbeing and awareness of the changed respiratory parameters in pregnancy are the obvious additional requirements.
Pregnancy and Influenza
The H1N1 2009 flu epidemic killed seven pregnant/postpartum women in Australia and New Zealand in three months.165 Over 60 women were admitted to ICU and a number of their babies died (see the Research vignette at the end of the chapter for more details on this study). Women in the second half of pregnancy were over 13 times more likely to be admitted to ICU with H1N1 influenza than non-pregnant women of child-bearing age. Pregnant and postpartum women admitted to ICU with H1N1 influenza were particularly unwell, with 14% of women requiring ECMO.166
Mental Health Disorders
Preexisting Mental Health Disorders
The underlying principles of management of pregnant women with a preexisting mental health disorder are the same as for non-pregnant women: safety of the woman, stabilisation of the mental illness and empowerment of, and support for, the woman to make her own choices. A considerable additional challenge is maintaining stability of the mental health disorder if changes to medication are required due to potential teratogenesis or contraindication for use during pregnancy. Generally speaking, if the indication for treatment is unchanged, then treatment should be continued during pregnancy.167
Pregnant women with preexisting mental health disorders may require admission to ICU due to acute deterioration in their mental health. This is most likely to be as a result of cessation or alteration of their regular medications.168,169 Most relapses occur in the first trimester and many women who initially stop their medication, recommence it during the pregnancy.168 Routine care should be provided as clinically indicated, keeping in mind the additional requirements to monitor fetal wellbeing, conduct standard antenatal assessment and consider the impact of the physiological adaptations on treatments.
Mental Health Disorders Related to Pregnancy
Suicide related to unwanted pregnancy remains a cause of maternal death in countries like Australia and New Zealand, especially in adolescents and in women from cultures where childbirth outside of marriage is unacceptable.52 Depression may arise during pregnancy (antenatal depression) although is more likely to present during the postpartum (postpartum depression). The most severe mental health disorder related to pregnancy is puerperal psychosis.
Puerperal psychosis
Puerperal psychosis is a rare mental health complication of pregnancy, said to occur in 1/1000 births, though the incidence seems to be reducing with a modern incidence of 0.19/1000 deliveries reported.170,171 The majority of cases occur in women with preexisting mental illness, such as bipolar disorder, with just 0.03/1000 deliveries occurring in women with no preexisting mental health disorder.171 It usually presents within two weeks postpartum and is associated with an increased risk of suicide and infanticide.172 Women with puerperal psychosis are frequently delusional, suffer hallucinations and require acute hospitalisation for treatment.
Postpartum depression
Postnatal depression (PND) is defined as a non-psychotic depressive illness; most definitions specify occurrence within three months postpartum although some specify a shorter period of only one month.173,174 Risk factors include prior mental illness, poor social supports, relationship disharmony and recent life events.173 Depression in the postpartum period raises treatment issues for the nursing mother and the developing infant.173
Caring For Pregnant Women In ICU
Any pregnant woman in ICU is considered to be carrying an ‘at-risk’ fetus. This means that fetal wellbeing may be compromised and that he/she is at risk of sustaining injury/suboptimal growth and development or death in utero. There are circumstances when the woman’s clinical status would improve by delivery of the fetus, and times when the fetus needs to be delivered to increase the likelihood of its’ own survival. Consideration of both the maternal condition and fetal wellbeing contribute to the decision on when to deliver a fetus. Delivery prior to 24 weeks’ gestation is only an option if the maternal condition is very critical and considered necessary to potentially save the woman’s life; it is likely that the neonate’s care in this instance would be palliative, even though babies have been know to survive when born as early as 22 weeks.175 Once gestation reaches 28 weeks, the neonate has more than a 90% chance of survival when cared for in a neonatal intensive care unit.175
Mechanical Ventilation Of The Pregnant Woman
The provision of mechanical ventilation to a pregnant woman occurs rarely and there is very little evidence to guide practice. Pregnancy is considered a ‘high risk airway’ with the reported ‘failure to intubate’ ranging from 1 in 250 to 1 in 750, or approximately eight times more likely than in the non-pregnant population.176 Physiological changes of pregnancy that contribute to the increased difficulty in intubation include generalised vasodilatation of pregnancy, increased fat deposition around the neck and an increase in mucosal oedema. The vasodilation increases the vascularisation of the upper airways in pregnancy, increasing the likelihood of bleeding with any instrumentation. Consequently, nasal intubation is not usually an option for pregnant women. Women with preeclampsia may also have substantial pharyngeal oedema.
• ensure target endpoints reflect the normal ABGs for pregnancy
• remember that a small reduction in maternal oxygenation can severely impact on fetal oxygenation because of the left shift in the oxyhaemoglobin dissociation curve associated with fetal haemoglobin177
• permissive hypercapnia has not been evaluated in pregnancy (remember that the fetal carbon dioxide is higher than the maternal level, given the gradient across the placental membrane)
• Normal tidal volumes in pregnancy are increased by up to 40–50% of non-pregnant values, although the mechanical provision of these larger tidal volumes with respect to volutrauma has not been examined; in practice often respiratory rate is increased first and then increases in tidal volume are only used when necessary100
• a nurse caring for a ventilated pregnant patient should be alert to any patient restlessness or increasing sedation requirements and ask for midwifery assistance to assess for the presence of labour contractions.
Other, less common, methods to support gas exchange have been reported in the literature in the form of case studies. Of note, nitrous oxide, hyperbaric oxygen treatment and extracorporeal membrane oxygenators have all been used successfully to treat acute conditions, such as pulmonary embolus, in pregnant women.178–180
Fetal Assessment
Assessment of fetal wellbeing in ICU presents a number of challenges. Most notable is that many pregnant women in ICU receive sedative medication which has the effect of sedating the fetus. The standard methods for monitoring and assessing fetal wellbeing include presence of fetal movements, continuous cardiotocograph (CTG) monitoring, intermittent auscultation of the fetal heart rate, ultrasounds and fetal biophysical profiles. These assessments are based on the pattern and rate of the fetal heart beat, the breathing and swallowing action of the fetus in utero, the volume of amniotic fluid and on fetal movements.181 Uterine artery doppler flow measurements have also proven useful.182,183 Critical illness and its treatment induce circumstances that make it difficult to interpret these tests of fetal wellbeing with any certainty, for example, morphine decreases the biophysical profile of the fetus.184 With fetal mortality in pregnant women admitted to ICU as high as 20%,6 the assessment of fetal wellbeing during maternal critical illness is of prime importance, in part to optimise timing of delivery.
Cardiotocograph
Cardiotocographs (CTGs) consist of two pieces of information: a Doppler recording fetal heart rate pattern and a pressure transducer detecting uterine muscle contraction. Both elements are recorded on a timed graph so that one may consider the fetal response to uterine contraction (Figure 26.4). Thus, CTGs provide information about the fetal heart rate and whether there is any uterine contraction. A normal fetal heart rate is 120–160 beats/min with variability in the rate. Details of the patient’s condition and treatment, and the date and time the recording was taken should be documented on the trace. Many tertiary obstetric hospitals offer a fax CTG interpretation service for general hospitals without maternity staff to assist with interpretation of CTGs. A CTG provides superior information to an intermittent fetal heart rate (by stethoscope or Doppler) and should be used when possible. The required frequency and duration of a CTG recording will vary according to clinical condition. For example, suspected placental abruption following blunt trauma may require four hours of continuous monitoring. A CTG is recommended during and following elective cardioversion and any other major procedure. CTGs are usually only indicated if the fetus is >22–24 weeks’ gestation and there is the potential to act on adverse findings, such as emergency delivery. The CTG is an indication of fetal wellbeing at the time the trace is recorded and the fetal condition can change rapidly according to changes in maternal condition.
Ultrasound
Practice tip
There is a legal requirement in both Australia and New Zealand for all births to be registered with the Registry of Births, Deaths and Marriages. A birth in both countries is defined as the delivery of a baby of at least 20 weeks’ gestation or, if gestation is unknown, weighing at least 400 g, who is either live born or stillborn.185,186
Modifications To Basic And Advanced Life Support
Generally speaking, all standard basic and advanced life support algorithms can be used with only minor adaptations for the pregnant and postpartum woman (Box 26.10).187 First, for women over 20 weeks’ gestation, the sheer bulk of the uterus and contents impair any ability to obtain adequate circulation using cardiac compressions. Left lateral displacement of the uterus is necessary to enable optimal venous return and cardiac output. Regardless, it is very difficult to obtain adequate perfusion during CPR of an obviously pregnant woman and arrangements should be made for an emergency caesarean section. Delivery of the fetus within five minutes of a witnessed arrest is generally desired. Second, expect a difficult intubation and try to have an experienced person intubate the trachea. Third, consider the list of obstetric conditions that may have precipitated the arrest and provide any specific appropriate treatment. Cardiac arrest in pregnancy is a rare event and the chance of a successful resuscitation is about the same as a non-pregnant arrest.
Box 26.10
Maternal cardiac arrest algorithm
• Activate cardiac arrest team e.g. Code Blue and note time
• Commence chest compressions as per standard BLS algorithm. Place hands slightly higher on the sternum than usual due to raised diaphragm
• Apply standard BLS and ALS algorithms
• Commence documentation of cardiac arrest management e.g. time of onset
• Give standard ALS drugs and doses
• Start IV above the diaphragm
• Assess for hypovolaemia and treat appropriately but cautiously
• Anticipate a difficult airway
• If the woman is on a magnesium infusion, cease and consider administration of calcium chloride 10 mL in 10% solution or calcium gluconate 30mL in 10% solution to treat hypermagnesaemia
• Continue all elements of resuscitation effort during and after caesarean section
Women with an obviously gravid uterus e.g. >20 weeks’ gestation:
• To relieve aorto-caval compression and enable more effective CPR, manually displace the uterus towards the left
Consider and treat any possible contributing factors:
• Haemorrhage with or without DIC
• Embolism, e.g. pulmonary, amniotic fluid
• Anaesthetic complications, e.g. high spinal block
Adapted from (187).
Prevention Of Rhesus Disease
During pregnancy, a small amount of the fetal blood can enter the maternal circulation. If the mother is Rh-negative and the fetus is Rh-positive, the mother produces antibodies against the Rhesus D antigen on her baby’s red blood cells. During this, and subsequent pregnancies, the anti-D antibodies are able to pass across the placenta to the fetus and if the level is sufficient, cause destruction of Rhesus D-positive fetal red blood cells, leading to the development of Rhesus disease. The disease ranges from mild to severe; when the disease is mild the fetus may develop mild anaemia with reticulocytosis. When the disease is moderate or severe the fetus can have a more marked anaemia and erythroblastosis. When the disease is very severe it can cause morbus haemolyticus neonatorum, hydrops fetalis or stillbirth. Management of Rhesus disease is outlined in Table 26.6.
| Blood tests and management | Rationale |
|---|---|
| Kleihauer-Betke test or flow cytometry | Confirms that fetal blood has passed into the maternal circulation, also estimates the amount of fetal blood that has passed into the maternal circulation |
| Indirect Coombs test | Screens maternal blood for anti-D antibodies that may pass through the placenta and cause haemolytic disease of the newborn |
| Fetal blood (or umbilical cord blood) tests | |
| Direct Coombs test | Confirms that maternal anti-D antibodies are present in the fetal/newborn circulation |
| Full blood count | Specifically, the haemoglobin level and platelet count to assess for anaemia |
| Bilirubin | Both total and indirect |
| Antenatal Care | |
| Serial ultrasound and Doppler examinations | Detect signs of fetal anaemia such as increased blood flow velocities and monitor hydrops fetalis |
| Quantitative analysis of maternal anti-RhD antibodies | An increasing titre level suggests fetal Rhesus disease |
| Intrauterine blood transfusion | Blood transfused into fetal umbilical vein, method of choice since the late 1980s, more effective than intraperitoneal transfusion |
| Early delivery | Usually post 36 weeks gestation |
| Postnatal | |
| Phototherapy for neonatal jaundice in mild disease | Converts fat-soluble unconjugated bilirubin to water-soluble bilirubin that can be excreted by the newborn |
| Newborn exchange transfusion | Used if the neonate has moderate or severe disease; the blood for transfusion must be less than a week old, Rh negative, ABO compatible with both the fetus and the mother, and be cross matched against the mother’s serum |
Most Rhesus disease can be prevented by treating the Rh-negative mother during pregnancy or promptly (within 72 hrs) post childbirth.188 The mother is given an intramuscular injection of 500 IU of anti-D immunoglobulin which destroys any Rh D positive fetal red blood cells in her circulation before the maternal immune system can discover them and produce antibodies. This is passive immunity and the effect of the immunity will diminish post injection at around 4 to 6 weeks. Anti-D immunoglobulin is used to prevent the development of anti-D antibodies and is of no use once the antibodies are present. Administration of 500 IU of anti-D immunoglobulin to all Rhesus D-negative pregnant women at 28–34 weeks is now routine care, even in the absence of any vaginal bleeding.
Medication Administration In Pregnancy
There are a number of anatomical, physiological, cellular and molecular changes in pregnancy that affect the pharmacokinetic and pharmacodynamic mechanisms of drugs administered during pregnancy.189 These include reduced serum protein levels (reduced protein binding capacity), increased circulating volume (potential for dilution), delayed gut motility (potential for increased gut absorption), increased glomerular filtration rate (potential for increased excretion) and changes to maternal drug-metabolising enzymes (difficult to predict metabolism pattern of regular drugs).190 Medication may be classified according to the likelihood for teratogenesis, however there may be little understanding about efficacy in pregnancy; standard adult doses may be inadequate or toxic during pregnancy due to the adapted physiology of pregnancy.189
Potential for Teratogenesis
A teratogen is any agent that increases the incidence of a congenital anomaly.191 The major organs are developed by 10 weeks’ gestation, however, the recommendation is to avoid any teratogenic drug throughout the first trimester (14 weeks).192 Some medications exert an adverse effect in the second or third trimesters of pregnancy, such as ACE inhibitors (fetal anuria and stillbirth), indomethacin (potential premature closure of the ductus arteriosus) and selective serotonin uptake inhibitors (neonatal withdrawal syndrome).192 Medical staff prescribing drugs and nursing staff administering them should each check the potential impact of the medication in pregnancy, and consult a pharmacist when possible.
Immediately Prior to Delivery
Besides effects on the structural development of the fetus in the first trimester, the other key time for consideration of drug administration is immediately prior to delivery. Common sedative agents like midazolam, morphine, fentanyl and propofol cross the placenta readily and exert an action on the fetus.193–195 Consequently, even mature term babies may be born sedated and require assistance with breathing. Planning for delivery of a pregnant woman in ICU should include the involvement of a paediatrician/neonatologist or the local newborn emergency transport service (NETS) if no paediatricians are on site.
Therapeutic Routine Drug Therapy in Pregnancy
For women admitted to ICU for prolonged periods of time, for example those with Guillain–Barré syndrome, consideration may be given to routine therapeutic medication in pregnancy. For example, folic acid (400 µg daily) is recommended pre-conception and throughout the first trimester to prevent neural tube defects.192 Similarly, iron and Vitamin D supplementation may be indicated dependent on blood levels. Vitamin D deficiency is common, yet often unrecognised in critically ill patients.196 Maternal vitamin D deficiency is associated with childhood asthma and increased risk of osteoporotic fracture in their offspring.197,198 Due attention should be paid to a pregnant woman’s nutritional status in ICU as poor nutrition during pregnancy is associated with many poor birth outcomes and pregnancy is associated with increased nutritional requirements.199
Caring For Postpartum Women In Icu
Women admitted to ICU during the postpartum phase are often separated from their newborn, possibly even transferred to another hospital, and may not even set eyes on their child for days, until they are discharged from ICU.3 Specific care that should be provided to the postpartum woman includes observations, assistance to establish lactation as required and support for the mother by early nurturing of a mother–infant bond. Finally, attention to psychological needs of both the woman and her partner is an important part of care.
Routine Postpartum Observations
Ongoing surveillance of a postpartum woman is essential in addition to any ad hoc visits provided by a midwife. Routine maternity observations include assessment of the fundus, PV loss and perineum, assessment of the breasts and nipples, consideration of deep vein thrombosis and thrombophylaxis, and evaluation of her psychological wellbeing and transition to motherhood (Box 26.11).
Box 26.11
Routine postnatal observations
• Examination of breasts, looking for signs of engorgement, mastitis, cracked nipples
• Height, depth and texture of fundus, to ensure involution is happening
• Lochia, inspection of PV loss
• Examination of perineum/wound for signs of healing
• Examination for signs of deep vein thrombosis; thrombophylaxis is often indicated in a postpartum ICU woman
• Mictrition and bowels; to ensure bowel and urinary pattern returning to normal
Uterine Involution
• day 1 postnatal, the height of the fundus is usually at the belly button
• there is a steady decrease in size of around 1 cm per day
• as the uterus reduces, it also recedes and is deeper to palpate
• by postnatal day 7, the fundal height is often only 2–3 cm above the symphysis pubis and by day 10 it is usually not palpable at the symphysis pubis
• the rate of involution is slower in multiparity women, if there is an infection present, or retained placental tissue/clots.
Lochia and Perineal Care
The changes in the appearance of the lochia are described in three stages: lochia rubra, lochia serosa and lochia alba.200 Lochia rubra consists of blood coming chiefly from the placental site, mixed with shreds of the decidua. Three or four days post delivery, the lochia changes to brownish in colour, and consists of altered blood and serum containing leucocytes and organisms; this is called lochia serosa. Seven days post delivery the lochia again changes, the PV loss is now yellowish in appearance, and consists mainly of cervical mucus, leucocytes and organisms; this is called lochia alba. Normal lochia is not offensive in odour. Offensive lochia coupled with or without maternal pyrexia may indicate a uterine infection. High and low vaginal swabs for culture and sensitivity, and the commencement of antibiotic cover, should be initiated. Offensive lochia coupled with a high non-involuting (and boggy) uterus may require ultrasound to exclude retained placental tissue. An infected placental site may result in a secondary postpartum haemorrhage.
Increased Potential for Deep Vein Thrombosis
All postpartum women have an increased likelihood for DVT. Preeclampsia and obstetric haemorrhage are additional risk factors, as is an emergency operative procedure and postpartum immobility. Most postpartum women admitted to ICU would fulfil the criteria that recommend medical thrombophylaxis. Routine postpartum care involves examining the legs for signs of DVT and appropriate use of thromboembolic stockings, sequential compression devices and thrombophylaxis as required (see www.rcog.org.uk for more details).30
Breast Care And Breast Feeding
Initiation and Establishment of Lactation
The establishment and maintenance of lactation is a hormone-mediated process. The physiological trigger for the establishment of lactation is a fall in progesterone combined with maintained levels of prolactin and cortisol.201 In the initial postnatal period colostrum is produced. The normal timing for milk to ‘come in’ is between 3 and 4 days post-delivery,202 although establishment and ‘coming in’ of breast milk may be delayed in critically ill women. Additionally, the drug dopamine may hinder lactation, as it inhibits prolactin secretion.203 It is not likely that the severity of maternal illness plays much of a role in the initial capacity to produce milk; anecdotally, 100 mL of breast milk has been expressed 4 hourly from a postpartum woman on ECMO. The initial regularity of hand expression and milk removal provide the stimulus to produce milk.
For women who prefer to breastfeed the infant, reasonable attempts to support this decision should be made. Most women make a decision regarding infant feeding either before becoming pregnant or during the first trimester and in all pregnant women, the breasts have developed and are capable of producing milk from 22 weeks onwards.204,205
There is some debate regarding how crucial the first 24–48 hours are for the successful establishment of lactation.206,207 In many cultures, colostrum is considered poisonous and breastfeeding is withheld until after 48 hours and so clearly the absence of breast stimulation in the first 48 hours does not prohibit the establishment of lactation.208 Hand expressing is recommended for the first few days until the milk ‘comes in’, and then to start and finish each expressing episode along with the use of a breast pump209 (see Box 26.12 for principles and Figure 26.5 for process). It is not uncommon for only a few drops of colostrum to be expressed each time in the first couple of days.210 It is believed that even a small total expressed volume of 5–10 mL per day of colostrum may be of value to stimulate the ‘coming in’ of full milk production.201 The two key factors that support the establishment of lactation are breast stimulation (infant suckling, hand expression) and milk removal. The more often you express and remove milk, the positive feedback mechanisms ensure that more milk is produced. Frequent, short expression of the breasts is more effective than prolonged infrequent expressing. Overnight expression is also important.
Box 26.12
Principles of expressing breast milk
Oxytocin is most commonly known for its role in the ‘let-down reflex’ of milk during breastfeeding, but also has known effects on brain areas involved in emotion and stress response, increased levels of oxytocin lower blood pressure among mothers who breast feed their babies. this is known to improve a mother’s mood, increases pain tolerance, and also has a possible positive association in wound healing. Prolactin may also be responsible for intense ‘mothering’ feelings.203
If the mother’s intention was to formula feed, or if the baby has died, then the lactation process may be suppressed. In practice, this means providing no stimulation to the breasts (i.e. no hand expression). Although used in the past, medications are no longer used to influence this process. With no breast expression, some women may still experience milk ‘coming in’ at or after Day 4 postpartum and comfort measures may assist if the breasts become very uncomfortable. Cold compresses may be of use209 and it is important for the critical care nurse to observe for signs like reddened hot areas on the breast that may be an indication of mastitis.
Medication Administration and Lactation
Many drugs are safe to use in breastfeeding, although most common critical care drugs have not been well evaluated.211 Even if the woman is receiving a medication that is contraindicated during breastfeeding, you can still express (and discard) the milk to establish the process of lactation, unless the woman is likely to stay on the medication long term.
The safety of the expressed milk for the baby depends on three factors: the amount of the medication in the milk, the oral bioavailability of the medication, and the ability of the infant to metabolise the medication.212 The gestation and condition of the infant are relevant as the function of the gut, liver and kidney varies with maturity and illness. Consequently, advice from the baby’s neonatologist or paediatrician can help determine whether the neonate can receive the expressed breast milk, or whether it should be discarded.
Psychology Of The Puerperium
Major emotional changes take place in the majority of women during the puerperium, but there is a wide variation in the amount of distress caused by these changes. The first three days post delivery are known as the latent period because functional mental illness is very unlikely to occur at this time interval. The woman is usually in state of euphoria, excitement and restlessness, extreme tiredness is also present. Days 3–10 are often referred to as the ‘baby blues’ and are characterised by emotional lability (mood swing).213 The ‘baby blues’ are usually characterised by thoughts of inadequacy and generalised panic that there is something wrong with either their baby or themselves. A very severe ‘baby blues’ response may herald the onset of postnatal depression.
The Family Unit
Promoting Maternal–infant Attachment
Promoting maternal–infant attachment depends on the condition of both the mother and her baby, and their physical locations. The best case scenario is that the baby is able to ‘room in’ with the mother for periods of time in ICU. Skin to skin contact is usually recommended to promote bonding.214 Alternatively, the baby may be able to visit the mother in ICU or the mother may be able to visit the baby in NICU. Physically seeing and touching the baby may be an important step for the mother. Newer technologies, like Skype, have been used by some ICUs to enable the mother to see her baby in a different hospital and to watch significant events, such as the first bath.
Summary
Research vignette
Critique
A prospective, collaborative study was rapidly established following the onset of the H1N1 influenza pandemic in 2009. Every ICU in Australia and New Zealand (n = 187) was involved with every confirmed H1N1 influenza ICU admission prospectively entered in to the INFINITE database. Over 700 affected patients were admitted to Australian and New Zealand ICUs during the three months of winter (June–Aug 2009). Of these, 64 (9%) were noted to be pregnant or postpartum; thus pregnant and postpartum admissions were an over-represented cohort.166
Learning activities
1. List the key physiological adaptations of the cardiovascular and respiratory systems during pregnancy.
2. Interpret the following ABG with reference to the normal ranges for pregnancy. PaO2 = 79; PaCO2 = 45; pH = 7.31; HCO3− = 18
3. Outline the key management priorities when caring for a woman in ICU with severe preeclampsia.
4. Explain to a colleague what placenta praevia and placenta accreta are.
Activities 5 to 11 relate to the case study.
5. You are assigned to Carly when she is admitted to ICU. Outline the key elements of your admission assessment including those related to midwifery assessment.
6. Within an hour of Carly’s admission to ICU, her husband John arrives and asks to see his wife. John wants to know what has happened and is very worried about her. What would you say to John? What can John expect over the next couple of days?
7. Outline the minimum postnatal assessment that Carly should have each day she is in ICU.
8. John tells you that Carly breastfed their first child and was planning to breastfeed this child. What can you do to support the process of lactation? What milk production would you expect over the first 2 days in ICU?
9. Consider the support that John and their first child might need whilst Carly is in ICU. How would you support the integration of the new family member?
10. Write a transfer letter that will accompany Carly back to the tertiary obstetric hospital for the midwives who will be continuing her care. Ensure that you include relevant details of her ICU stay, including midwifery progress.
11. Discuss the potential implications of this unexpected serious event for Carly and her family as she recovers and ‘life goes back to normal’.
3 Centres collaboration http://3centres.com.au
Australasian Maternity Outcomes Surveillance System (AMOSS) www.amoss.com.au and
British Thoracic Society British guideline on asthma management http://www.brit-thoracic.org.uk/clinical-information/asthma/asthma-guidelines.aspx
Centre for Maternal and Child Enquiries (CMACE) http://www.cemach.org.uk/Home.aspx
National Perinatal Statistics Unit http://www.preru.unsw.edu.au/PRERUWeb.nsf/page/AIHW+National+Perinatal+Statistics+Unit
National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand http://www.racgp.org.au/Content/NavigationMenu/ClinicalResources/RACGPGuidelines/DiagnosisandmanagementofacuterheumaticfeverandrheumaticheartdiseaseinAustralia/NHFA-CSANZ_ARF_RHD_2006.pdf
Perinatal and Maternal Mortality Review Committee (PMMRC) http://www.pmmrc.health.govt.nz/
Royal College of Obstetricians and Gynaecologists http://www.rcog.org.uk/files/rcog-corp/GT37ReducingRiskThrombo.pdf
United Kingdom Obstetric Surveillance System (UKOSS) https://www.npeu.ox.ac.uk/ukoss
Belfort MA, Saade GR, Foley MR, Phelan JP, Dildy GA. Critical care obstetrics, 5th edn, Hoboken: Wiley-Blackwell, 2010.
Foley M, Strong T, Garite T. Obstetric intensive care manual, 3rd edn, Columbus: McGraw-Hill, 2010.
Fraser D, Cooper M. Myles’ textbook for midwives, 15th edn, Oxford: Churchill Livingstone/Elsevier, 2009.
Pairman S, Tracy S, Thorogood C, Pincombe J. Midwifery preparation for practice, 2nd edn, Chatswood: Churchill Livingstone, Elsevier, 2010.
Pearlman M, Tintinalli J, Dyne P. Obstetric and gynecologic emergencies: diagnosis and management. Chicago: McGraw-Hill Professional Publishing, 2004.
1 Belfort MA, Saade GR, Foley MR, Phelan JP, Dildy GA. Critical care obstetrics, 5th edn, Hoboken: Wiley-Blackwell, 2010.
2 Foley M, Strong T, Garite T. Obstetric intensive care manual, 3rd edn, Columbus: McGraw-Hill, 2010.
3 Pollock W, Rose L, Dennis CL. Pregnant and postpartum admissions to the intensive care unit: a systematic review. Intens Care Med. 2010;36(9):1465–1474.
4 Harrison D, Penny J, et al. Case mix, outcome and activity for obstetric admissions to adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database. Critical Care. 2005;9(Suppl 3):S25–S37.
5 Zhang WH, Alexander S, et al. Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG. 2005;112(1):89–96.
6 Hazelgrove JF, Price C, et al. Multicenter study of obstetric admissions to 14 intensive care units in southern England. Crit Care Med. 2001;29(4):770–775.
7 Zwart J, Dupuis J, et al. Obstetric intensive care unit admission: a 2-year nationwide population-based cohort study. Intens Care Med. 2010;36(2):256–263.
8 Lawton B, Wilson L, Dinsdale R, Rose S, Brown S, et al. Audit of severe acute maternal morbidity describing reasons for transfer and potential preventability of admissions to ICU. Aust N Z J Obstetrics & Gynaecology. 2010;50(4):346–351.
9 Geller SEM, Adams G, et al. Reliability of a preventability model in maternal death and morbidity. Am J Obstet Gynecol. 2007;196(1):57.e1–57.e6.
10 Pollock W. Critically ill pregnant and postpartum women in Victoria: characteristics, severity of illness and the provision of acute health services. PhD thesis. Melbourne: The University of Melbourne; 2007.
11 Fraser D, Cooper M. Myles’ textbook for midwives, 15th edn, Oxford: Churchill Livingston/Elsevier, 2009.
12 Pairman S, Tracy S, Thorogood P, Pincombe J. Midwifery preparation for practice, 2nd edn, Chatswood: Churchill Livingstone, 2010.
13 Norwitz ER, Edusa V, et al. Maternal physiology and complications of multiple pregnancy. Seminars in Perinatology. 2005;29(5):338–348.
14 Robson SC, Dunlop W, Moore M, Hunter S. Haemodynamic changes during the puerperium: a Doppler and M-mode echocardiographic study. BJOG. 1987;94(11):1028–1039.
15 Crapo RO. Normal cardiopulmonary physiology during pregnancy. Clinical Obstetrics and Gynecology. 1996;39(1):3–16.
16 Hunter S, Robson S. Adaptation of the maternal heart in pregnancy. British Heart Journal. 1992;68(6):540–543.
17 Norwitz E, Robinson J, Malone F. Pregnancy-induced physiologic alterations. In: Dildy GA, Belfort MA, Saade GR, et al. Critical care obstetrics. 4th edn. Massachusetts: Blackwell Science; 2004:19–42.
18 Davison J.M. The kidney in pregnancy: a review. J Royal Society of Medicine. 1983;76(6):485–501.
19 Hytten F. Blood volume changes in normal pregnancy. Clinical Haematology. 1985;14(3):601–612.
20 Duvekot JJ, Peeters L. Renal hemodynamics and volume homeostasis in pregnancy. Obstetrical & Gynecological Survey. 1994;49(12):830–839.
21 Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47(2):203–208.
22 Nevo O, Soustiel JF, Thaler I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am J Obstet Gynecol. 2010;203(5):e471–e476.
23 Duvekot JJ, Peeters L. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstetrical & Gynecological Survey. 1994;48(12):S1–14.
24 Lewis G, ed. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer – 2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH, 2007.
25 Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994;170(3):849–856.
26 Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol Heart Circ Physiol. 1989;256(4):H1060–H1065.
27 Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469–1473.
28 Bamber JH, Dresner M. Aortocaval compression in pregnancy: the effect of changing the degree and direction of lateral tilt on maternal cardiac output. Anesthesia & Analgesia. 2003;97(1):256–258.
29 Kinsella SM. Lateral tilt for pregnant women: why 15 degrees? Anaesthesia. 2003;58(9):835–836.
30 Royal College of Obstetricians and Gynaecologists (RCOG). Reducing the risk of thrombosis and embolism during pregnancy and the puerperium. Green-top Guideline no. 37. London: RCOG; 2009.
31 Contreras G, Gutiérrez M, Beroíza T, Fantín A, Oddó L, et al. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis. 1991;144(4):837–841.
32 Weinberger SE, Weiss ST, Cohen WR, Weiss JW, Johnson TS. Pregnancy and the lung. Am Rev Respir Dis. 1980;121(3):559–581.
33 Jensen D, Webb KA, O’Donnell DE. Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Applied Physiology, Nutrition and Metabolism. 2007;32(6):1239–1250.
34 Jensen D, Duffin J, Lam YM, Webb KA, Simpson JA, et al. Physiological mechanisms of hyperventilation during human pregnancy. Respiratory Physiology & Neurobiology. 2008;161(1):76–86.
35 Weissgerber TL, Wolfe LA, Hopkins WG, Davies GAL. Serial respiratory adaptations and an alternate hypothesis of respiratory control in human pregnancy. Respiratory Physiology & Neurobiology. 2006;153(1):39–53.
36 Templeton A, Kelman GR. Maternal blood-gases, (PAO2-PaO2): physiological shunt and VD/VT in normal pregnancy. Brit J Anaesthesia. 1976;48(10):1001–1004.
37 Prodromakis E, Trakada G, Tsapanos V, Spiropoulos K. Arterial oxygen tension during sleep in the third trimester of pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2004;83(2):159–164.
38 MacRae DJ, Palavradji D. Maternal acid-base changes in pregnancy. BJOG. 1967;74(1):11–16.
39 Andersen GJ, James GB, Mathers NP, Smith EL, Walker J. The maternal oxygen tension and acid-base status during pregnancy. BJOG. 1969;76(1):16–19.
40 Richlin S, Cusick W, Sullivan C, Dildy G, Belfort M. Normative oxygen saturation values for pregnant women at sea level. Primary Care Update for OB/GYNS. 1998;5(4):154–155.
41 Langford E, Khwanda A, Langford K. Oxygen saturation response to exercise in healthy pregnant women: a simple protocol and normal range. Obstetric Med. 2010;3(2):65–68.
42 Zeldis SM. Dyspnea during pregnancy: distinguishing cardiac from pulmonary causes. Clinics in Chest Medicine. 1992;13(4):567–585.
43 Boutourline-Young H, Boutourline-Young E. Alveolar carbon dioxide levels in pregnant, parturient and lactating subjects. BJOG. 1956;63(4):509–528.
44 Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, et al. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107(11):1469–1475.
45 Davison JM, Vallotton MB, Lindheimer MD. Plasma osmolality and urinary concentration and dilution during and after pregnancy: evidence that lateral recumbency inhibits maximal urinary concentrating ability. BJOG. 1981;88(5):472–479.
46 Klajnbard A, Szecsi PB, Colov NP, Andersen MR, Jorgensen M, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clinical Chemistry and Laboratory Medicine. 2010;48(2):237–248.
47 Lindheimer M. Polyuria and pregnancy: its cause, its danger [Editorial]. Obstetrics & Gynecology. 2005;105(5, Part 2):1171–1172.
48 Mackenzie MJ, Woolnough MJ, Barrett N, Johnson MR, Yentis SM. Normal urine output after elective caesarean section: an observational study. Int J Obstetric Anesthesia. 2010;19(4):379–383.
49 Baer J, Reis R, Arens R. Appendicitis in pregnancy: with changes in position and axis of the normal appendix in pregnancy. JAMA. 1932;98(16):1359–1364.
50 Augustin G, Majerovic M. Non-obstetrical acute abdomen during pregnancy. Euro J Obstetrics & Gynecology and. Reproductive Biology. 2007;131(1):4–12.
51 Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Archives of Gynecology and Obstetrics. 2002;266(1):25–29.
52 Sullivan E, Hall B, King J. Maternal deaths in Australia 2003–2005 [Maternal Deaths Series no. 3. Cat. no. PER 42]. AIHW National Perinatal Statistics Unit: Sydney, 2008.
53 Salnlo S, Kekomaki R, Rllkonen S, Teramo K. Maternal thrombocytopenia at term: a population-based study. Acta obstetricia et gynecologica Scandinavica. 2000;79(9):744.
54 Burrows RF, Kelton JG. Incidentally detected thrombocytopenia in healthy mothers and their infants. New Eng J Med. 1988;319(3):142–145.
55 Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29(2):125. 130
56 Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thrombosis and Haemostasis. 2010;103(4):718–727.
57 Paniccia R, Prisco D, Bandinelli B, Fedi S, Giusti B, et al. Plasma and serum levels of D-dimer and their correlations with other hemostatic parameters in pregnancy. Thrombosis Research. 2002;105(3):257–262.
58 Uchikova EH, Ledjev II. Changes in haemostasis during normal pregnancy. Euro J Obstetrics & Gynecology and Reproductive Biology. 2005;119(2):185–188.
59 Miller EM. Changes in serum immunity during pregnancy. Am J Human Biology. 2009;21(3):401–403.
60 Rogerson SJ, Hviid L, Duffy PE, Leke RFG, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infectious Diseases. 2007;7(2):105–117.
61 Vance M. The Placenta. In: Fraser D, Cooper M. Myles’ Textbook for Midwives. 15th edn. Oxford: Churchill Livingstone/Elsevier; 2009:147–156.
62 Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Euro J Obstetrics & Gynecology and Reproductive Biology. 2000;92(1):35–43.
63 Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thrombosis Research. 2004;114(5–6):397–407.
64 Low J. Fetal asphyxia and brain damage. Fetal and Maternal Medicine Review. 2001;12(2):139–158.
65 Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust & NZ J Obstetrics & Gynaecology. 2000;40(2):139–155.
66 Lowe SA, Brown MA, Dekker G, Gatt S, McLintock C, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. PLACE: Society of Obstetric Medicine of Australia and New Zealand; 2008.
67 Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–1451.
68 Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644.
69 Rath W, Faridi A, Dudenhausen JW. HELLP syndrome. J Perinatal Med. 2000;28(4):249–260.
70 Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes levels and low platelets): much ado about nothing? Am J Obstetrics & Gynaecology. 1990;162(2):311–316.
71 Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799.
72 Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound in Obstetrics and Gynecology. 2007;30(5):742–749.
73 Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565–572.
74 Knight M. Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
75 Australasian Maternity Outcomes Surveillance System Project website. [Cited Dec 2010]. Available from www.amoss.com.au
76 Abraham KA, Connolly G, Farrell J, Walshe JJ. The HELLP syndrome, a prospective study1. Renal Failure. 2001;23(5):705–713.
77 Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66(5):657–660.
78 Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy and Childbirth. 2009;9(1):8.
79 Vigil-De Gracia P. Pregnancy complicated by pre-eclampsia-eclampsia with HELLP syndrome. Int J Gynecology and Obstetrics. 2001;72(1):17–23.
80 Young BC, Levine RJ, Karumanchi SA. Pathogenesis of Preeclampsia. Annual Review of Pathology: Mechanisms of Disease. 2010;5(1):173–192.
81 Sontia B, Touyz RM. Role of magnesium in hypertension. Archives of Biochemistry and Biophysics. 2007;458(1):33–39.
82 The Magpie Trial Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
83 Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2006. CD001449.
84 Rugarn O, Moen S, Berg G. Eclampsia at a tertiary hospital 1973–99. Acta Obstetricia et Gynecologica Scandinavica. 2004;83(3):240–245.
85 Ganzevoort W, Rep A, Bonsel GJ, Fetter WPF, van Sonderen L, et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG. 2005;112(10):1358–1368.
86 Dildy GA, Belfort MA, Saade GR, Phelan JP, Hankins GD, Clark SL. Critical care obstetrics, 4th edn, Massachusetts: Blackwell Science, 2004.
87 Smith CV, Phelan JP. Determinants for invasive monitoring in severe preeclampsia. Contemporary Obstetrics and Gynaecology. 1986, January:109–124.
88 Woudstra DM, Chandra S, Hofmeyr GJ, Dowswell T. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database of Systematic Reviews 2010. CD008148.
89 Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006. CD004454.
90 Visser W, Wallenburg HCS. Maternal and perinatal outcome of temporizing management in 254 consecutive patients with severe pre-eclampsia remote from term. Euro J Obstetrics & Gynecology and Reproductive Biology. 1995;63(2):147–154.
91 Cameron CA, Roberts CL, Olive EC, Ford JB, Fischer WE. Trends in postpartum haemorrhage. Aust NZ J Public Health. 2006;30(2):151–156.
92 3 Centres Collaboration. Antepartum haemorrhage clinical practice guidelines 2010. [Cited December 2010]. Available at http://3centres.com.au/library/public/file/guidelines/Complications_in_Pregnancy_and_Birth/Antepartum_Haemorrhage.pdf
93 Bretelle F, Courbiere B, Mazouni C, Agostini A, Cravello L, et al. Management of placenta accreta: Morbidity and outcome. Euro J Obstetrics & Gynecology and Reproductive Biology. 2007;133(1):34–39.
94 Ford JB, Roberts CL, Simpson JM, Vaughan J, Cameron CA. Increased postpartum hemorrhage rates in Australia. Int J Gynecology & Obstetrics. 2007;98(3):237–243.
95 Henry A, Birch MR, Sullivan EA, Katz S, Wang YPA. Primary postpartum haemorrhage in an Australian tertiary hospital: a case-control study. Aust NZ J of Obstetrics & Gynaecology. 2005;45(3):233–236.
96 Roberts CL, Ford J, Algert CS, Bell J, Simpson JM, Morris JM. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy and Childbirth. 2009;9(1):7.
97 Brace V, Kernaghan D, Penney G. Learning from adverse clinical outcomes: major obstetric haemorrhage in Scotland, 2003–05. BJOG. 2007;114(11):1388–1396.
98 Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2007. CD003249.
99 Lapinsky S, Kruczynski K, Seaward G, Farine D, Grossman R. Critical care management of the obstetric patient. Canadian Journal of Anaesthesia. 1997;44(3):3259.
100 Campbell L, Klocke R. Implications for the Pregnant Patient. Am J Respir. Crit. Care Med. 2001;163(5):1051–1054.
101 Huang WC, Chen CP. Pulmonary edema in pregnancy. Int J Gynecology and Obstetrics. 2002;78(3):241–243.
102 Chamberlain G, Steer P. ABC of labour care: Obstetric emergencies. BMJ. 1999;318(7194):1342–1345.
103 Letsky E. Coagulation defects. In: de Swiet M, ed. Medical disorders in obstetric practice. 4th edn. Oxford: Blackwell Publishing; 2002:61–96.
104 Slaytor EK, Sullivan EA, King JF. Maternal deaths in Australia 1997–1999. Sydney: AIHW National Perinatal Statistics Unit (Maternal Deaths Series No 1); 2004.
105 Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surgery. 2009;197(5):565–570.
106 Phillips L, McLintock C, Pollock W, Gatt S, Popham P, et al. Recombinant activated Factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand haemostasis registry. Anesthesia and Analgesia. 2009;109(6):1908–1915.
107 Wang HY, Chang CT, Wu MS. Postpartum hemorrhage complicated with irreversible renal failure and central diabetes insipidus. Renal Failure. 2002;24(6):849–852.
108 Fung Kee Fung K, Eason E, Crane J, Armson A, De La Ronde S, et al. Prevention of Rh alloimmunization. J Obstet Gynaecol Canada. 2003;25(9):765–773.
109 Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J of Obstetric Anesthesia. 2008;17(1):37–45.
110 King M, Wrench I, Galimberti A, Spray R. Introduction of cell salvage to a large obstetric unit: the first six months. Int J of Obstetric Anesthesia. 2009;18(2):111–117.
111 Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J of Obstetric Anesthesia. 2007;16(3):241–249.
112 Clark S. Amniotic fluid embolism. Clinical Obstetrics and Gynecology. 2010;53(2):322–328.
113 Tuffnell DJ, Hamilton S. Amniotic fluid embolism. Obstetrics, Gynaecology & Reproductive Medicine. 2008;18(8):213–216.
114 Shechtman M, Ziser A, Markovits R, Rozenberg B. Amniotic fluid embolism: early findings of transesophageal echocardiography. Anesth Analg. 1999;89(6):1456–1458.
115 Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol. 2009;201(445):e1–13.
116 Knight M, Tuffnell D, Brocklehurst P, Spark P. Kurinczuk JJ on behalf of the UKOSS. Incidence and risk factors for amniotic-fluid embolism. Obstetrics & Gynecology. 2010;115(5):910–917.
117 Aguillon A, Andjus T, Grayson A, Race GJ. Amniotic Fluid Embolism: A Review. Obstetrical & Gynecological Survey. 1962;17(5):619–636.
118 Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States. Am J Obstetrics and Gynecology. 2008;199(1):e41–e49.
119 Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) Workshop Recommendations and Review. JAMA. 2000;283(9):1183–1188.
120 Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Euro J Heart Failure. 2010;12(8):767–778.
121 Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–693.
122 Ntusi N, Mayosi B. Aetiology and risk factors of peripartum cardiomyopathy: a systematic review. Int J of Cardiology. 2009;131(2):168–179.
123 Cruz M, Briller M, Hibbard J. Update on peripartum cardiomyopathy. Obstet Gynecol Clin N Am. 2010;37(2):283–303.
124 Mielniczuk LM, Williams K, Davis DR, Tang ASL, Lemery R, et al. Frequency of peripartum cardiomyopathy. Am J of Cardiology. 2006;97(12):1765–1768.
125 Fett JD, Sannon H, Thélisma E, Sprunger T, Suresh V. Recovery from severe heart failure following peripartum cardiomyopathy. Int J of Gynecology & Obstetrics. 2009;104(2):125–127.
126 Egan DJ, Bisanzo MC, Emergency HutsonHR. Department Evaluation and Management of Peripartum Cardiomyopathy. Emerg Med. 2009;36(2):141–147.
127 Hilfiker-Kleiner D, Sliwa K, Drexler H. Peripartum cardiomyopathy: recent insights in its pathophysiology. Trends in Cardiovascular Medicine. 2008;18(5):173–179.
128 Ichida M, Katsurada K, Komori T, Matsumoto J, Ohkuchi A, et al. Effectiveness of bromocriptine treatment in a patient with peripartum cardiomyopathy. J Cardiology Cases. 2010;2(1):e28–e31.
129 Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16-kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600.
130 Hilfiker-Kleiner D, Meyer GP, Schieffer E, et al. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol. 2007;50(24):2354–2355.
131 Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. New Eng J Med. 2001;344(21):1567–1571.
132 Kwon H, Belanger K, Bracken M. Asthma prevalence among pregnant and childbearing-aged women in the United States: Estimates from National Health surveys. Annals of Epidemiology. 2003;13(5):317–324.
133 Australian Centre for Asthma Monitoring. Asthma in Australia 2008 [AIHW Asthma Series no. 3]. Australian Institute of Health and Welfare: Canberra, 2008.
134 Juniper E, Newhouse M. Effect of pregnancy on asthma: A critical appraisal of the literature. In: Schatz M, Zeiger RS. Asthma and allergy in pregnancy and early infancy. New York: Marcel Dekker; 1993:223–249.
135 Beecroft N, Cochrane GM, Milburn HJ. Effect of sex of fetus on asthma during pregnancy: blind prospective study. BMJ. 1998;317(7162):856–857.
136 Belanger K, Hellenbrand M, Holford T, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010;115(3):559–567.
137 Hardy-Fairbanks AJ, Baker ER. Asthma in pregnancy: pathophysiology, diagnosis and management. Obstetrics and Gynecology Clinics of North America. 2010;37(2):159–172.
138 Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin N Am. 2006;26(1):63–80.
139 Murphy VE, Gibson P, Talbot PL, Clifton VL. Severe asthma exacerbations during pregnancy. Obstetrics and Gynecology. 2005;106(5):1046–1054.
140 Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA, Jr. Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med. 1999;160(3):887–892.
141 Schatz M, Dombrowski MP. Asthma in Pregnancy. New Eng J Med. 2009;360(18):1862–1869.
142 Abeywardana S, Sullivan EA. Congenital anomalies in Australia 2002–2003 [Birth anomalies series no. 3]. Australian Institute of Health and Welfare National Perinatal Statistics Unit: Sydney, 2008.
143 Silversides CK, Marelli A, Beauchesne L, Dore A, Kiess, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: executive summary. Canadian J Cardiol. 2010;26(3):143–150.
144 Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373(9672):1382–1394.
145 Australian Institute of Health and Welfare (AIHW). 2004. Rheumatic heart disease: all but forgotten in Australia except among Aboriginal and Torres Strait Islander peoples [Bulletin no 16]. AIHW: Canberra, 2004.
146 Australian Institute of Health and Welfare (AIHW). Australia’s health 2008. Canberra: AIHW; 2008.
147 Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. New Eng J Med. 2007;357(5):470–476.
148 Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: A population-based study. Obstetrics and Gynecology. 2005;105(3):480–484.
149 Koul AK, Hollander G, Moskovits N, Frankel R, Herrera L, Shani J. Coronary artery dissection during pregnancy and the postpartum period: two case reports and review of literature. Catheterization and Cardiovascular Interventions. 2001;52(1):88–94.
150 Sadler L, McCowan L, White H, Stewart A, Bracken M, North R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. Brit J Obstetrics and Gynaecology. 2000;107(2):245–253.
151 Bowater SE, Thorne SA. Management of pregnancy in women with acquired and congenital heart disease. Postgraduate Med J. 2010;86(1012):100–105.
152 Cusick SS, Tibbles CD. Trauma in pregnancy. Emerg Med Clin N Am. 2007;25(3):861–872.
153 Yankowitz J. Fetal effects of drugs commonly used in critical care. In: Dildy GA, Belfort MA, Saade GR, et al. Critical care obstetrics. 4th edn. Massachusetts: Blackwell Science; 2004:612–619.
154 Bartalena L, Bogazzi F, Braverman LE, Martino E. Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinological Investigation. 2001;24(2):116–130.
155 Arnoni RT, Arnoni AS, Bonini RCA, de Almeida AFS, Neto CA, et al. Risk factors associated with cardiac surgery during pregnancy. Annals of Thoracic Surgery. 2003;76(5):1605–1608.
156 King P, Rosalion A, McMillan J, Buist M, Holmes P. Extracorporeal membrane oxygenation in pregnancy. Lancet. 2000;356(9223):45–46.
157 Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160(2):191–196.
158 National Heart Foundation of Australia (RF/RHD guideline development working group) and the Cardiac Society of Australia and New Zealand. Diagnosis and management of acute rheumatic fever and rheumatic heart disease in Australia – an evidence-based review. National Heart Foundation of Australia; 2006.
159 Patteson SK, Snider CC, Meyer DS, Enderson BL, Armstrong JE, et al. The consequences of high-risk behaviors: trauma during pregnancy. Trauma. 2007;62(4):1015–1020.
160 Mattox KL, Goetzl L. Trauma in pregnancy. Crit Care Med. 2005;33(10):S385–S389.
161 Connolly AM, Katz VL, Bash KL, McMahon MJ, Hansen WF. Trauma and pregnancy. Amer J Perinatol. 1997;14(6):331. 336
162 Jasinski J. Pregnancy and domestic violence: a review of the literature. Trauma Violence Abuse. 2004;5(1):47–64.
163 Brown HL. Trauma in pregnancy. Obstetrics & Gynecology. 2009;114(1):147–160.
164 Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med Critical Illness of Pregnancy. 2005;33(10):S390–S397.
165 The ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279.
166 The ANZIC Influenza Investigators. Critical Care Services and 2009 H1N1 Influenza in Australia and New Zealand. New Engl J Med. 2009;361(20):1925–1934.
167 Klinger G, Merlob P. Selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. Isr J Psychiatry Relat Sci. 2008;45(2):107–113.
168 Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
169 Kulkarni J. Special issues in managing long-term mental illness in women. International Review of Psychiatry. 2010;22(2):183–190.
170 Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363(9405):303–310.
171 Tschinkel S, Harris M, Le Noury J, Healy D. Postpartum psychosis: two cohorts compared, 1875–1924 and 1994–2005. Psychological Medicine. 2007;37(4):529–536.
172 Sharma V, Mazmanian D. Sleep loss and postpartum psychosis. Bipolar Disorders. 2003;5(2):98–105.
173 Craig C, Howard L. Postnatal depression. BMJ Clinical Evidence. 2009;1:1407.
174 Wylie L, Hollins Martin CJ, Marland G, Martin CR, Rankin J. The enigma of post-natal depression: an update. J Psychiatric Mental Health Nurs. 2010;18(1):48–58.
175 ANZNN (Australian and New Zealand Neonatal Network). Report of the Australian and New Zealand Neonatal Network 2006. Sydney, ANZNN; 2009.
176 Samsoon GLT, Young JRB. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42(5):487–490.
177 Cousins L. Fetal oxygenation, assessment of fetal well-being, and obstetric management of the pregnant patient with asthma. J Allergy and Clinical Immunol. 1999;103(2, Supplement 1):S343–S349.
178 Bugge JF, Tanbo T. Nitric oxide in the treatment of fulminant pulmonary failure in a young pregnant woman with varicella pneumonia. Euro J Anaesthesiology. 2000;17(4):269–272.
179 Silverman RK, Montano J. Hyperbaric oxygen treatment during pregnancy in acute carbon monoxide poisoning. A case report. J Reproductive Medicine. 1997;42(5):309–311.
180 Plotkin JS, Shah JB, Lofland GK, DeWolf AM. Extracorporeal membrane oxygenation in the successful treatment of traumatic adult respiratory distress syndrome: case report and review. Trauma. 1994;37(1):127–130.
181 Manning FA. Fetal biophysical profile. Obstetrics and Gynecology Clinics N Am. 1999;26(4):557–577.
182 Bobby P. Multiple assessment techniques evaluate antepartum fetal risks. Pediatr Ann. 2003;32(9):609–616.
183 Harman CR, Baschat AA. Comprehensive assessment of fetal wellbeing: which Doppler tests should be performed? Current Opinion in Obstetrics and Gynecology. 2003;15(2):147–157.
184 Kopecky EA, Simone C, Knie B, Koren G. Transfer of morphine across the human placenta and its interaction with naloxone. Life Sciences. 1999;65(22):2359–2371.
185 New Zealand Health Information Service. Report on Maternity: Maternal and Newborn Information 2003. Wellington: Ministry of Health; 2006.
186 Laws PJ, Li Z, Sullivan EA. Australia’s mothers and babies 2008. Perinatal statistics series no 24. Canberra: Australian Institute of Health and Welfare; 2010.
187 Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, et al. Part 12: Cardiac Arrest in Special Situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S829–S861.
188 Percival P. Jaundice and infection. In: Fraser D, Cooper M. Myles’ textbook for midwives. 15th edn. Oxford: Churchill Livingstone/Elsevier; 2009:901–928.
189 Malek A, Mattison DR. Drug development for use during pregnancy: impact of the placenta. Expert Review of Obstetrics & Gynecology. 2010;5(4):437–454.
190 Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy. Expert Opinion on Drug Metabolism & Toxicology. 2007;3(4):557–571.
191 Turner T, Simpson J. Congenital abnormalities. In: Fraser D, Cooper M. Myles’ textbook for midwives. 15th edn. Oxford: Churchill Livingstone/Elsevier; 2009:877–900.
192 Rutherford J. Pharmacology and childbirth. In: Fraser D, Cooper M. Myles’ textbook for midwives. 15th edn. Oxford: Churchill Livingstone/Elsevier; 2009:945–958.
193 Bacon RC, Razis PA. The effect of propofol sedation in pregnancy on neonatal condition. Anaesthesia. 1994;49(12):1058–1060.
194 Kopecky EA, Ryan ML, Barrett JFR, Seaward PGR, Ryan G, et al. Fetal response to maternally administered morphine. Am J of Obstetrics and Gynecology. 2000;183(2):424–430.
195 Littleford J. Effects on the fetus and newborn of maternal analgesia and anesthesia: a review. Canadian Journal of Anesthesia. 2004;51(6):586–609.
196 Lee P, Eisman J, Center J. Vitamin D deficiency in critically ill patients. New Engl J Med. 2009;360(18):1912–1914.
197 Camargo C, Rifas-Shiman S, Litonjua A, Rich-Edwards J, Weiss S, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795.
198 Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43.
199 Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32(1):5–25.
200 Sherman D, Lurie S, Frenckle E, et al. Characteristics of normal lochia. Am J Perinatol. 1999;16(8):399–402.
201 Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutrition. 2001;131(11):S3005–S3008.
202 Neville MC, Keller RP, Seacat J, Lutes V, Neifert M, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clinical Nutrition. 1988;48(6):1375–1386.
203 Grattan DR. Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction. 2002;123(4):497–506.
204 Arora S, McJunkin C, Wehrer J, Kuhn P. Major factors influencing breastfeeding rates: Mother’s perception of father’s attitude and milk supply. Pediatrics. 2000;106(5):E67.
205 Hartmann P, Cregan M, Ramsay DT, Simmer K, Kent JC. Physiology of lactation in preterm mothers: initiation and maintenance. Pediatric Annals. 2003;32(5):351–355.
206 Sozmen M. Effects of early suckling of cesarean-born babies on lactation. Biology of the Neonate. 1992;62(1):67–68.
207 Woolridge M, Greasley V, Silpisornkosol S. The initiation of lactation: the effect of early versus delayed contact for suckling on milk intake in the first week post-partum: a study in Chiang Mai, Northern Thailand. Early Human Development. 1985;12(3):269–278.
208 Morse JM, Jehle C, Gamble D. Initiating breastfeeding: a world survey of the timing of postpartum breastfeeding. Int J Nursing Studies. 1990;27(3):303–313.
209 . Breastfeeding: Best Practice Guidelines. James JP, ed. The Royal Women’s Hospital: Melbourne, 2004.
210 Meier PP. Breastfeeding in the special care nursery. Prematures and infants with medical problems. Pediatric Clin N Am. 2001;48(2):425–442.
211 Hale T. Breastfeeding pharmacology. [Cited February 2011]. Available from http://www.infantrisk.com/
212 Hale TW. Medications in breastfeeding mothers of preterm infants. Pediatric Annals. 2003;32(5):337–347.
213 Swyer G. Postpartum mental disturbances and hormone changes. BMJ. 1985;290(6477):1232–1233.
214 Christensson K, Cabrera T, Christensson E, et al. Separation distress call in the human neonate in the absence of maternal body contact. Acta Paediatr. 1995;84(5):468–473.

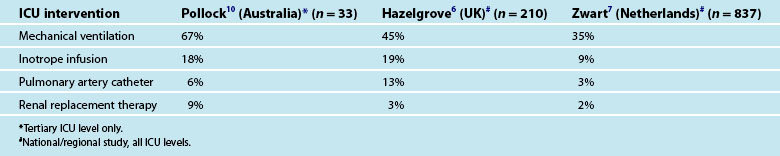
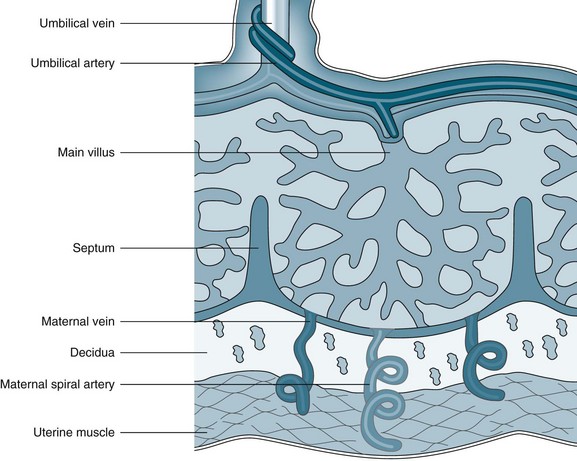
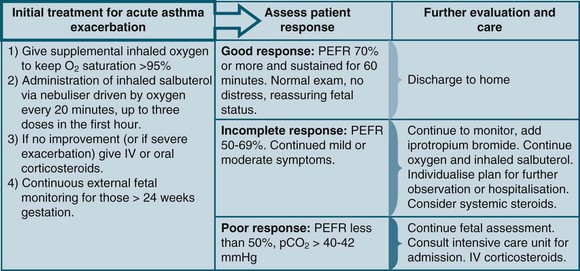
 .
.