CHAPTER 11 Pregnancy and Botanical Medicine Use and Safety
—Obstetrician in Friends, The One Where Rachel Is Late
The charge that herbal medications are not well tested in pregnancy is true, but it is just as true that conventional medications are not well tested in pregnancy. The medication-related embryopathies with which we are familiar are nearly all associated with conventional prescription drugs: thalidomide, anticonvulsants, ACE inhibitors, misoprostol, lithium, and isotretinoin. The most common embryopathy affecting humans is fetal alcohol syndrome, which is not associated with an herbal medication. This observation does not mean that herbal medications are safe, but only underscores the need to be well informed when prescribing in pregnancy. Let’s recommend medications in pregnancy that have been adequately studied, without assuming that all conventional medications are on one side of a divide and all herbal medications are on the other side.1
PREVALENCE OF HERB USE DURING PREGNANCY
Herbs have been applied in the treatment of difficulties arising during pregnancy and childbirth since time immemorial, with texts and treatises on the uses of herbs for childbearing problems dating at least back to ancient Egypt. Childbearing women commonly experience minor complaints for which the use of natural remedies may be preferable to the woman who perceives them as gentler and safer than OTC and prescription pharmaceuticals. The appropriate use of herbs medicinally during pregnancy requires specialized knowledge and a healthy dose of caution. This chapter presents a discussion of the prevalence of herb use during pregnancy and childbearing, possible risks, and general guidelines for responsible use. Subsequent chapters discuss the use of herbs for specific pregnancy concerns.
Herbal medicines are commonly used by pregnant women for a variety of complaints, as well as for nutritive and tonic purposes, such as the use of ginger to treat nausea and vomiting of pregnancy or the traditional use of raspberry leaf as a uterine tonic. There is considerable evidence of increasing herb prescribing by obstetric health professionals, particularly certified nurse-midwives (CNMs).2 The introductory quote to this chapter suggests a mainstreaming of herb use even by obstetricians—enough so that it would be mentioned by an OB-GYN on the popular television sitcom Friends when one of the characters is seeking advice for her postdates pregnancy.
Epidemiologic studies and surveys from the United States, United Kingdom, and Australia estimate a range of approximately 7% to 45% of women using herbs during pregnancy. 2 3 4 5 A recent survey of 587 pregnant women by Glover et al. revealed that a total of 45.2% of participants in a rural obstetric population had used herbal medications (95.8% had used prescription and 92.6% had self-prescribed OTCs).6 In another study, a one-page questionnaire examining the use of all prescription and nonprescription medications, including herbal remedies, was sent to parturients expected to deliver within 20 weeks who had preregistered with the hospital’s admissions office. Sixty-one percent of the women responded to the survey, with 7.1% reporting the use of herbal remedies. Only 14.6% of users considered herbs to be medications. Herbal medicine use was most prevalent (17.1%) in parturients in the 41- to 50-year age range (5.6% of parturients). In another study, approximately one-third of 463 postpartum women surveyed in the United States reported having used CAM therapies during pregnancy.7 Of 734 pregnant women that responded to one survey, 46% used herbal remedies at the recommendation of their health care provider; 54% did so at the recommendation of a friend of family member.2
Botanical medicine use is likely even higher in communities observing traditional practices, for example, among Hispanic Americans or Asian Americans, where herb use is an inherent cultural practice. Internationally, traditional herb use during pregnancy is common. For example, a report from the King Edward VIII Hospital in South Africa demonstrated use of a specific traditional herbal formula among 55% of 229 patients randomly selected for interview upon admission in early labor.8
Articles and studies published in prominent nurse-midwifery and obstetric journals (i.e., Journal of Nurse-Midwifery, Obstetrics and Gynecology, and Clinical Obstetrics and Gynecology) indicate that a large number of CNMs use herbal medicines clinically or are interested in learning to do so. 9 10 11 12 A study of CNMs in North Carolina indicated that 90% of midwives recommend CAM therapies to patients, with 80% of respondents suggesting herbal therapies for labor stimulation.9 A survey of 596 health care professionals in Leicestershire, United Kingdom, found that 34% of midwives and 18% of nurses used complementary therapies in their practices.13 According to Jeanne Raisler, Associate Editor of the Journal of Nurse Midwifery, “Herbal healing is probably the complementary therapy most widely used by midwives.”12
WHY ARE HERBS BEING USED DURING PREGNANCY?
One of the primary reasons women cite for stopping conventional medication during pregnancy is concern for risks to the fetus.2 A recent report by Lo and Friedman indicates that greater than 90% of all conventional medications prescribed for pregnant women have not been proved safe for use during pregnancy.14 The specter of the thalidomide and DES disasters are sufficiently recent to remind us of the hazards of “safe” pharmaceutical use during pregnancy. Many pregnant women turn botanical therapies to pharmaceutical medications believing them to be safer and gentler. Women planning natural birth also may feel that the use of herbs is more philosophically harmonious with their overall belief that childbearing is a natural experience and are more likely to use herbal preparations than those who are not preparing for natural birth.2 Thus, the use of herbal medicines represents both a philosophic and medical choice.
Midwives recommend herbal medicines for a variety of reasons, including support of patient choice, a shared belief in the naturalness of birth and the use of herbs as a natural extension of this belief, and as a way to help pregnant and postpartum women avoid more invasive and costly medical interventions that may be perceived as overly aggressive or unnecessary.15 For example, Tiran suggests that the possibility of cephalic version of breech presentation through the use of moxibustion (see Breech Presentation) may avoid the costs (and risks) of cesarean section and adequate management of nausea and vomiting of pregnancy (NVP) may reduce hospital admission for hyperemesis gravidarum.16
Midwives are in a key position to interface with pregnant clients about the use of botanical therapies. The philosophic compatibility between herbal medicine, midwifery, and nursing care philosophies reinforces a perceived “rightness” of botanical medicine use.11,17,18 Unfortunately, few midwives are adequately trained in the use of botanical medicines during pregnancy. Education on the use—or at least safety and risks— of botanicals in the childbearing cycle should be a requisite part of training for all midwifery and obstetric care providers.
LACK OF TRAINING IN OBSTETRIC BOTANICAL MEDICINE USE
The medical and alternative literature on botanicals for pregnancy and birth often contains erroneous or inadequate information on the use of herbs during childbearing.15,19,20 Herbs may be recommended, for example, with insufficient explanation of possible risks, without specified dosage ranges, and may be based on theoretic or academic knowledge rather than training or clinical experience. In one well-cited survey conducted by McFarlin and O’Rear of nurse-midwives on their use of herbal remedies during pregnancy, most midwives who responded reported that they had learned about the use of herbs by word of mouth.21 Several articles report on the need for further training in botanicals for nurse-midwives, citing its absence from curricula.12,16,22
Lack of practitioner training in botanical medicines for pregnancy might mean that practitioners are inappropriately recommending botanical therapies to their patients, either by recommending herbs that might be contraindicated, recommending inappropriate doses, not identifying safe and high-quality botanical products for patients, or recommending inappropriate durations of use. Further, practitioners with limited knowledge cannot accurately evaluate advice patients might receive from other sources, such as the Internet, a common source of misleading information on herbs and pregnancy.23 There is clearly a need to include education on obstetric botanical medicine use in the growing number of CAM education programs in medical and nursing programs. Adequate practitioner knowledge is critical to the well-being of both patients involved in the prenatal and lactation dyad.
HERBS MOST COMMONLY USED DURING PREGNANCY
The herbs cited in the medical literature as those most frequently used for pregnancy complaints varies slightly among studies, but includes echinacea, St. John’s wort, ephedra, peppermint, spearmint, ginger root, raspberry leaf, fennel, wild yam, meadowsweet, blue cohosh, black cohosh, red raspberry leaf, castor oil, evening primrose, garlic, aloe, chamomile, peppermint, ginger, echinacea, pumpkin seeds, and ginseng. In one study, patients cited lower GI problems, anxiety, nausea and vomiting, and urinary tract problems as the most common reasons for using complementary therapies in pregnancy.2,4,7,10,23 Midwives most frequently recommend herbs for nausea and vomiting, labor stimulation, perineal discomfort, lactation disorders, postpartum depression, preterm labor, postpartum hemorrhage, labor analgesia, and malpresentation.9 Most of the herbs cited as commonly used are generally considered safe and gentle, even for use during pregnancy; however, several including blue cohosh, ephedra, aloe (internally), and St. John’s wort (internally) are not appropriate and may even be harmful (see further discussions on blue cohosh throughout in this and subsequent sections of this chapter).
SAFETY, EVIDENCE, AND POTENTIAL ADVERSE EFFECTS OF BOTANICAL USE DURING PREGNANCY
Little is known scientifically about the risk of using herbs during pregnancy, as most have not been formally evaluated and ethical considerations severely limit human clinical investigation during pregnancy.2,5,9,24,25 Much the same can be said for the use of many pharmaceuticals during pregnancy. Bone identifies five primary risks associated with the use of herbs during pregnancy:26
An additional potential risk is the consequences of delayed administration of necessary medical therapy in favor of herbs, regardless of their safety.27 Most of what is currently known about botanical use during pregnancy is based on a significant body of historical, empirical, and observational evidence, and limited pharmacologic and animal studies. There has been little evidence of harm from the use of botanicals during pregnancy. When apparent adverse events have occurred, cause and effect have been difficult to establish because of a wide range of confounding factors.23 Also, adverse events reports typically have involved the consumption of known toxic herbs, adulterations, or inappropriate use or dosage of botanical therapies. In general, there have also been relatively few case reports of adverse drug interactions involving herbal medicines.28 Overall, most herbs have a high safety profile. Many practitioners take this as proof of safety, believing that whole herbs are inherently safer than concentrated pharmaceutical drugs.10,11 However, lack of proof of harm is not synonymous with proof of safety. Some of the harmful effects of herbs may not be readily apparent until long after use has been discontinued, or may only occur with cumulative use.
There is a paucity of human clinical trials on the safety and efficacy of Western botanical therapies during pregnancy. Two human clinical trials evaluated raspberry leaf for its effects on labor outcome with positive findings, a study conducted on echinacea safety after varying lengths of pregnancy use found no harmful effects, and several studies have evaluated the safety and efficacy of ginger root for the reduction of NVP, finding it safe and effective. 29 30 31 Most often, the results of clinical trials are positive.3 Nonetheless, many researchers feel that in the absence of proof of safety, herbs should be entirely avoided during pregnancy.3 However, many midwives and pregnant women continue to use herbs based on satisfaction with their safety, efficacy, and outcomes, and on the knowledge that many pharmaceutical preparations recommended during pregnancy also carry unknown risks.
Controls over the manufacturing of herbs do not entirely protect consumers from the accidental or deliberate adulteration, sophistication, or contamination of herbal products, all of which can pose problems during pregnancy. In one study of 200 different herbal products, 83% were found to be contaminated with undeclared pharmaceuticals or heavy metals, including lead, arsenic, and mercury.5 Adulteration occurs when one herb is accidentally or deliberately substituted for another. Rarely, toxic herbs have been found as adulterants in otherwise completely benign herbal products, for example, adulteration of skullcap (Scutellaria lateriflora) with the toxic Teucrium, or Digitalis spp. (foxglove) with common plantain (Plantago spp.). One case in the literature reports on the substitution of the herb Periploca sepium for Eleutherococcus senticosus.26,32 This substitution resulted in a case of hyperandrogenization of the fetus (“hairy baby syndrome”) as a result of the mother mistakenly taking the adulterated product throughout her pregnancy. Chinese patent products and imported Asian herbal formulae should be viewed with utmost caution during pregnancy, as they are well known to contain adulterants, heavy metals, and added pharmaceutical medications frequently not listed on the label, all of which can pose a threat to the safety of the pregnant woman and her fetus.
Negative outcomes have been reported for a limited number of herbal products used by parturient women. Ernst provides a through review of these in Herbal Medicinal Products during Pregnancy: Are They Safe?3 Causality remains uncertain. Adverse reports cited by Ernst and others include:8,10,33
Other herbal products that have been associated with increased complications include a possible correlation between a German sinus preparation (Sinupret) and increased rates of miscarriage, stillbirth, and malformations; increased rate of meconium-stained amniotic fluid with maternal use of castor oil; and increased meconium staining and possibly related fetal distress with use of a traditional South African herbal pregnancy formula called isihlambezo, and a case of a baby born with veno-occlusive disease after the mother consumed a coltsfoot-containing cough syrup throughout her pregnancy.3,8
USING HERBS DURING PREGNANCY
The following key points are essential to remember when using botanical medicines during pregnancy:
CONTRAINDICATED HERBS FOR PREGNANCY AND LACTATION
There are several specific categories of herbs that are historically avoided during pregnancy to minimize risks to the maternal–embryo/fetal dyad. See Table 11-1 for a summary of these. A discussion follows, as well as an extensive list of contraindicated herbs for pregnancy and lactation.
| CATEGORY | EXAMPLES |
|---|---|
| Abortifacients and emmenagogues |
Note: The herbs listed under each category are representative examples and are not exhaustive. Additional herbs may fall into any of these categories.
* Avoid internal use; external use may be acceptable under the guidance of an experienced botanical medicine practitioner.
Abortifacients and Emmenagogues
Abortifacients are those herbs that may induce miscarriage/abortion (Table 11-2). The amounts required to induce an abortion may pose toxicity risks to the mother, including kidney and liver damage. Because the risks of maternal intake of these herbs to the fetus are unknown, women who have attempted to abort unsuccessfully may require a follow-up clinical abortion. Abortifacients should be entirely avoided during pregnancy and herbal abortion is not a recommended method of intentional pregnancy termination.
TABLE 11-2 Commonly Used Botanicals with Possible Abortifacient, Emmenagogue, or Oxytocic Activity
| COMMON NAME | BOTANICAL NAME |
|---|---|
| Blue cohosh | Caulophyllum thalictroides |
| Coleus | Coleus forskohlii |
| Cotton root bark | Gossypium spp. |
| Eucalyptus | Eucalyptus spp. |
| Goldenseal | Hydrastis canadensis |
| Motherwort | Leonurus cardiaca |
| Mugwort | Artemisia spp. |
| Pennyroyal | Mentha pulegium |
| Tansy | Tanacetum vulgare |
| Yarrow | Achillea millefolium |
Emmenagogues are defined in herbal medicine as herbs capable of stimulating the menstrual flow even when it is not due, and are also to be avoided during pregnancy. For centuries, they have been colloquially defined as “herbs for delayed menses,” sometimes a euphemism for eliminating an unwanted pregnancy. Many emmenagogic herbs are therefore also abortifacients. There is disagreement in the literature as to what actually constitutes an emmenagogue. Certain herbs such as chamomile are frequently and erroneously listed as emmenagogic. Other herbs, such as Dong quai and peony, are considered emmenagogic, and in fact do have uterine stimulatory activity, but are nonetheless used extensively in the Chinese and Japanese formulary for the prevention of miscarriage. Finally, the recorded data on other herbs are contradictory; for example, Ashwagandha has been described as an abortifacient yet was used historically to prevent miscarriage. Many herbs that are high in essential oil content are undisputed emmenagogues, although the presence of essential oils in an herb does not mean it acts as an emmenagogue.26 Commonly accepted abortifacients and emmenagogic herbs include (but are not limited to) tansy, thuja, safflower, scotch broom, rue, angelica, mugwort, wormwood, yarrow, and essential oil of pennyroyal.26
Teratogens and Mutagens
The word teratogen has its origins in the Greek terato, meaning “monster.” Teratogens are substances that cause structural abnormalities in the fetus. The drug thalidomide was a powerful example of a teratogen, and its legacy has left an imprint on those who prescribe medications to pregnant women. There is extremely limited knowledge about which herbs are teratogenic. Most of what is known is derived primarily from animal studies, observation of teratogenesis in grazing cattle, and suspected teratogenicity in humans from ingestion of suspected harmful herbal products. Known teratogens include those plants in the Lupinus, Veratrum, Conium, and Solanum genuses; and suspected teratogenic substances can be found in Astragalus, Nicotiana, Ferula, and Trachymene genuses. Plants in the Datura, Prunus, Sorghum, and Senecio genuses may also contain teratogenic substances.26 The primary means for identifying the propensity for this type of reaction is through general toxicologic screenings or through pharmacovigilance programs. Birth defects that occur between conception and birth are caused by teratogenic agents, whereas mutagens cause direct changes in the nucleus of cells.
Alkaloids
Alkaloids are a highly bioactive category of plant compounds, many of which may have adverse effects on the developing embryo/fetus. One such group is the pyrrolizidine alkaloids (PAs), known to cause veno-occlusive liver disease (VOD), an adverse outcome that has been observed in fetuses when the herb has been ingested regularly by their pregnant mothers (Table 11-3). Comfrey, coltsfoot, and borage herb (not borage oil) are the main herbs that have been implicated, containing varying amounts of PAs. They should therefore be avoided for internal use and should be used only short term topically when there is broken skin, as there is a minimal risk of transdermal absorption into the bloodstream. Germany has established limits for external application of toxic PAs to no more than 100 µg daily. In addition to causing VOD, PAs are carcinogenic and mutagenic. Botanicals containing toxic PAs should not be used in pregnancy or lactation. PAs have been reported to be secreted into the milk of nursing animals. Also, new mothers should be cautioned against excessive or frequent use of salves, oils, and balms made from herbs known to contain toxic PAs, such as comfrey (Symphytum officinale) for sore breasts associated with breastfeeding.
TABLE 11-3 Botanicals Containing Pyrrolizidine Alkaloids (PAs)
| COMMON NAME | BOTANICAL NOMENCLATURE |
|---|---|
| Borage* | Borago officinalis |
| Butterbur | Petasites hybridus |
| Coltsfoot | Tussilago farfara |
| Comfrey | Symphytum officinalis |
| Eyebright | Euphrasia officinalis |
| Life Root | Senecio aureus |
* Borage oil does not contain PAs.
Oral consumption of berberine alkaloid–containing herbs is also typically best avoided during pregnancy. Although there are no documented reports of adverse events in pregnancy associated with their use, they have been implicated in neonatal jaundice when used in pregnancy.34 Herbs in this category include goldenseal, goldthread, barberry, and Oregon grape root. Topical and vaginal applications appear to be safe for use during pregnancy.
Laxatives
Stimulating laxatives, including Cascara sagrada, castor oil, buckthorn, aloes, and rhubarb are to be avoided. Senna is generally contraindicated by midwives and herbalists for use during pregnancy, though Bone and Mills state that is has not been associated with adverse effects and therefore should not be contraindicated in pregnancy.26 Constipation is best addressed with gentle, nonstimulating bulk laxatives (e.g., flax, psyllium) and lifestyle approaches such as dietary changes and exercise.26 Laxatives containing anthraquinone glycosides are considered overly stimulating during pregnancy, as their effects of increasing intestinal peristalsis can lead to sympathetic uterine stimulation. Yellow dock, however, is one anthraquinone-containing laxative that herbalists and midwives frequently except from this rule, as it is much gentler in effects and is commonly added to formulas for treating anemia because of its purported iron-fortifying actions.
Nervous System Stimulants/Depressants
Herbs that strongly affect the nervous system including stimulants such as ephedra, guarana, and large amounts of caffeine-containing herbs, including coffee and green and black teas may have adverse effects on pregnancy and the developing embryonic and fetal nervous system, and should be avoided during pregnancy. Kava kava, a strong anxiolytic and sedative herb, has recently been implicated in cases of hepatotoxicity. Although many of the cases appear not to be causally related to the herb, the herb does appear to have idiosyncratic hepatotoxic effects, and should be avoided during pregnancy and lactation in favor of other, safer botanical options.
A WORD ABOUT PARTUS PREPARATORS
Partus preparators are herbs used during the last weeks of pregnancy to tone and prepare the uterus for labor. Historically, they have been used to facilitate a rapid and easy delivery. Herbs commonly used as partus preparators include blue cohosh (Caulophyllum thalictroides), black cohosh (Actaea racemosa), partridge berry (Mitchella repens), and spikenard (Aralia racemosa), among others. The use of such herbs to prepare women for labor begs the question of why one would use an herbal preparation to prepare the body for something it naturally knows how to do. Furthermore, the safety of these herbs prior to the onset of labor is questionable. Case reports have appeared in the literature suggesting an association between blue cohosh and profound cerebral ischemic episodes or myocardial infarction in the neonate.35,36 Blue cohosh contains a number of potent alkaloids, including methylcysteine and anagyrine, the latter, which is known to have an effect on cardiac muscle activity. Other side effects of blue cohosh include maternal headache and nausea. Yet, as previously stated, the use of blue cohosh represents one of the widely applied botanical medicines by midwives, and one of those most commonly included in late pregnancy formulas self-prescribed by pregnant mothers. The risks associated with extended third trimester ingestion of blue cohosh specifically suggest that it should be avoided as a partus preparatory. It seems that it would be preferable, unless otherwise indicated for the health of the mother and baby, to focus attention on nonpharmacologic methods of preparing for labor.
CONTRAINDICATED HERB LISTS AND BOTANICAL SAFETY CLASSIFICATIONS
The herbal literature is rife with lists of herbs contraindicated in pregnancy and lactation. Limitations are inherent in most of these lists, particularly in their lack of specifics as to how and when each herb is contraindicated. Herbs may sometimes be broadly contraindicated in pregnancy yet in actuality be only contextually contraindicated; for example, they are absolutely contraindicated during the first and second trimesters but may be reasonably used during labor, or they may be safe in small doses for a very limited duration. Culinary herbs, appearing on many contraindicated lists, when used in moderation as food seasonings pose no harm to the fetus or mother. Herbs such as aloe vera, calendula, and even comfrey may be used topically with no risk but are to be avoided for internal use, yet are contraindicated on such lists with no differentiation, leading to confusion about safety. Certain contraindications have become pervasive myths, for example, the frequent contraindication of chamomile in pregnancy owing to its alleged action as an abortifacient.37 In fact, chamomile provides an excellent example of how misapplication of a scientific finding can lead to unjustified contraindication of a safe herb. A study conducted in 1979 found teratogenic effects using a concentrated extract of α-bisabolol at high doses. No teratogenic effects were seen at lower doses and the dose of the oil constituent required to cause teratogenicity are far greater than it would ever be possible for someone drinking the tea to ever approximate. However, based on this single study, chamomile was erroneously contraindicated for consumption during pregnancy.38
The Botanical Safety Handbook has categorized herbs for use in pregnancy and lactation as follows:39
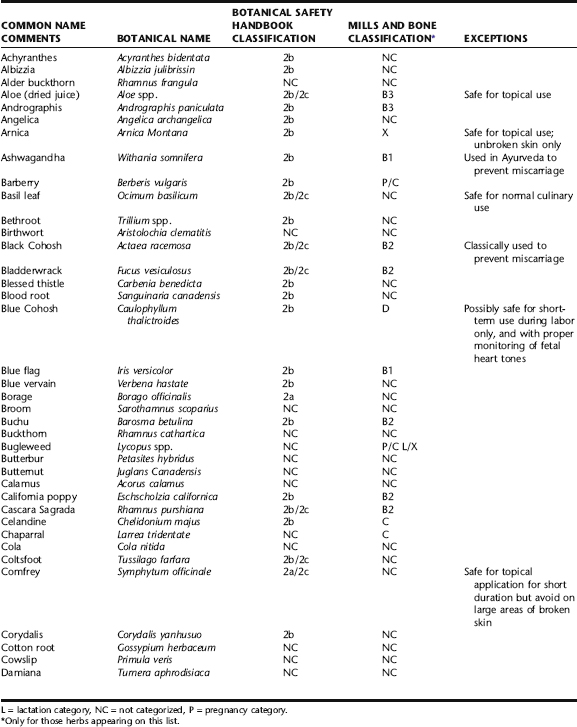
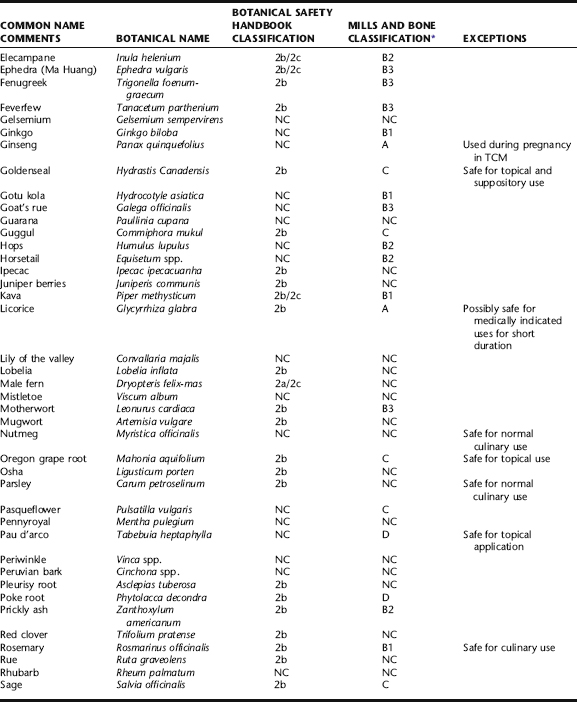
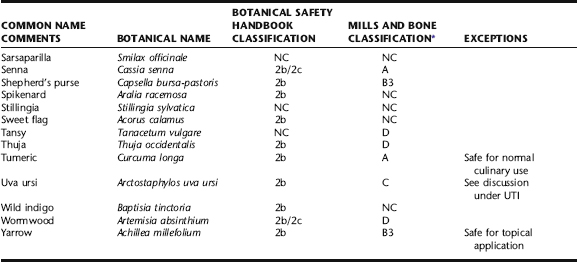
Table 11-4 is an amalgamation of contraindicated herb lists from The Botanical Safety Handbook, The Natural Pregnancy Book, and Women’s Health in Complementary and Integrative Medicine, all compiled by authors with significant knowledge of botanical safety and obstetric botanical use. It is not exhaustive, leaving out many obscure herbs, and focusing on those herbs the clinician is likely to encounter, consider, or question. It also does not cover many herbs from traditional Chinese medicine or the Ayurvedic materia medica. Table 11-4 also includes categoric designations of caution and contraindication according to Mills and Bone, which are subsequently discussed.
Mills and Bone suggest that a descriptive classification scheme for the risk of herbs during pregnancy, similar to that used for drugs in pregnancy, would be more useful than contraindicated lists in helping practitioners sort through some of the inconsistencies in the literature.26 They recommend the use of the Australian Therapeutic Goods Association (TGA) Classification for Drugs in Pregnancy (Table 11-5) as the guideline for a similar classification of herb use and safety during pregnancy and lactation, which they have begun to outline based on available evidence. They suggest that practitioners might use these guidelines to choose at which level(s) they are comfortable prescribing botanical medicines for pregnant women. For example, only those herbs that are considered benign or have been demonstrated to be safe would meet the criteria for category 1 (e.g., red raspberry leaf, ginger, and echinacea); those herbs definitively contraindicated, for example, the internal use of comfrey or other PA-containing herbs would be relegated to categories D or below. More conservative practitioners would have the option of recommending only those herbs in category A; others might prescribe from categories A to B3, herbs from categories C would primarily be avoided throughout pregnancy and would be contraindicated during first trimester, and those from D to X would be contraindicated entirely throughout pregnancy. Much work is still needed to be done to classify herbs according to this scheme, and classifications may shift as new data become available.
TABLE 11-5 The Australian TGA Classification for Drugs in Pregnancy
| CATEGORY | DEFINITION |
|---|---|
| Category A | Drugs that have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed. |
| Category B1 | Drugs that have been taken by only a limited number of pregnancy women and women of childbearing age, without an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have not shown evidence of an increased occurrence of fetal damage. |
| Category B2 | Drugs that have been taken by only a limited number of pregnancy women and women of childbearing age, without an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage. |
| Category B3 | Drugs that have been taken by only a limited number of pregnancy women and women of childbearing age, without an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans. |
| Category C | Drugs that, owing to their pharmacologic effects, have caused or may be suspected of causing harmful effects in the human fetus or neonate without causing malformations. These effects may be reversible. |
| Category D | Drugs that have caused, are suspected to have caused, or may be expected to cause, an increased incidence of human fetal malformations or irreversible damage. These drugs also have adverse pharmacological effects. |
| Category X | Drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy. |
Note: For drugs in B1, B2, or B3 categories, human data are lacking or inadequate and subcategorization is therefore based on available animal data. The allocation of a B category does NOT imply greater safety than the C category. Drugs in category D are NOT absolutely contraindicated in pregnancy (e.g., anticonvulsants). Moreover, in some cases the D category has been assigned on the basis of suspicion.
Based on their review of the literature using sources similar to those accepted in this text, they have developed the categorization that appears in Table 11-6. Mills and Bone clearly take the approach that with proper use and knowledge of risks and contraindications, herbs can be safely applied therapeutically during pregnancy and lactation. Their classification, however, itself introduces further inconsistencies and sometimes contradicts other recognized lists of herbs contraindicated during pregnancy. For example, Mills and Bone include in Category A, “Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed,” licorice, which is contraindicated for use during pregnancy in lists provided by Low Dog and McGuffin et al., among others, and which has recently been associated with premature delivery after regular consumption of licorice candy during pregnancy. 38 39 40 Their support of the use of senna as a category A herb is another example.
TABLE 11-6 Mills and Bone Classification for Herbs in Pregnancy
| CATEGORY | EXAMPLES |
|---|---|
| Category A | Bilberry fruit, Chamomile (German), Cranberry, Echinacea, Garlic, Ginger, Ginseng,* Licorice,* Raspberry leaf, Senna,* Turmeric |
| Category B1 | Astragalus, Blue flag, Boswellia, Bupleurum, Burdock, Butcher’s broom, Chaste tree, Codonopsis, Evening primrose oil, Ginkgo, Goat’s rue, Gotu kola, Hawthorn, Kava, Myrrh, Passionflower, Rosemary, Schisandra, Siberian ginseng, St. John’s wort, St. Mary’s thistle, Valerian, Willow bark, Withania |
| Category B2 | Bacopa, Black cohosh, Black haw, Black walnut, Bladderwrack, Buchu, Calendula, California poppy, Cascara, Celery seed, Chickweed, Cleavers, Corn silk, Couch grass, Cramp bark, Cranesbill root, Damiana, Dandelion, Devil’s claw, Elder flowers, Elecampane, Euphorbia, Eyebright, False unicorn, Fringe tree, Gentian, Globe artichoke, Golden rod, Grindelia, Gymnema, Hops, Horsetail, Hydrangea, Lavender, Lemon balm, Lime flowers, Marshmallow, Mullein, Nettle, Peppermint, Prickly ash, Pygeum, Saw palmetto, Shatavari, Skullcap, Thyme, Wild lettuce, Willow herb, Yellow dock, Ziziphus seed |
| Category B3 | Aloe, Andrographis, Bittersweet, Crataeva, Ephedra, Fennel, Fenugreek, Feverfew, Horsechestnut, Meadowsweet, Motherwort, Rehmannia, Shepherd’s purse, Tribulus, White horehound, Wild cherry, Yarrow |
| Category C | Barberry and Indian barberry, Bearberry, Bugleweed, Chaparral, Dong quai, Golden seal, Greater celandine, Guggul, Oregon grape, Pasque flower, Sage, Tylophora |
| Category D | Blue cohosh, Cat’s claw, Jamaica dogwood, Pau d’arco, Poke root, Tansy, Thuja, Wormwood |
| Category X | Arnica, Boldo |
* Recent literature suggests that the regular use of large amounts of licorice in pregnancy, including licorice candy containing real licorice extract (not just licorice or anise flavor) may lead to preterm birth. These other herbs are also contraindicated for use during pregnancy on other lists.
Strandberg T, Andersson S, Jarvenpaa A, et al.: Preterm birth and licorice consumption during pregnancy, Am J Epidemiol 156(9):803-805, 2002.
FORMS OF ADMINISTRATION APPROPRIATE DURING PREGNANCY
The forms in which herbs are administered (e.g., tincture, tea) can affect their strength and efficacy. During pregnancy, various forms can be chosen to maximize or minimize the volume and availability of more or less desirable constituents in a preparation, as well as exposure to other unwanted substances, for example, using water-based extracts to minimize extraction of bioactive compounds, or avoiding excessive consumption of alcohol in an alcohol-based preparation. Chapter 3 provides a full discussion of forms of administration. The following brief discussion addresses the specific nuances of administration of herbs during pregnancy.
External Forms of Herbal Medicines
Certain constituents, such as the hepatotoxic pyrrolizidine alkaloids present in comfrey, are capable of passing into the bloodstream through the skin. Although the risk is minimal from this small amount of potential exposure, it may be wise to avoid prolonged use of comfrey-containing products on large areas of broken skin during pregnancy.
Vaginal suppositories (see Chapter 3) often may be used safely during pregnancy for the treatment of vaginal infections, providing the herbs they contain are safe for such use during pregnancy. Herbs with suspected or known teratogenicity or mutagenicity, such as thuja, should be avoided, as well as those that might cause uterine stimulation. However, herbs that are typically contraindicated for oral consumption often may be used vaginally in late pregnancy for the treatment of vaginal infection. This should be done with knowledge of the individual herbs in the preparation, or in consultation with a qualified herbalist. Douches should be avoided during pregnancy because of risk of embolism; peri-washes may be used for a vulvar and perineal rinse.