Chapter 76 Pregnancy
Pulmonary Physiology
Physiologic Changes in Pregnancy
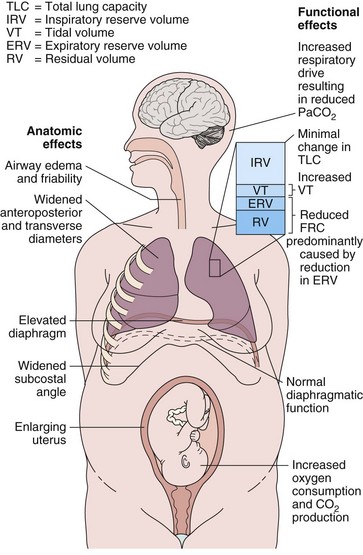
Hormonal changes in pregnancy affect the upper respiratory tract and cause airway hyperemia and edema resulting in symptoms of rhinitis. Estrogens are likely responsible for many of these effects because they produce capillary congestion and mucous gland hyperplasia. Changes to the thoracic cage result from both the enlarging uterus and the hormonal effects producing ligamentous laxity. The diaphragm is displaced cephalad by up to 4 cm, but the potential loss of lung capacity is partially offset by an increase in the anteroposterior and transverse diameters and by widening of the subcostal angle (Figure 76-1). Despite these anatomic changes, diaphragmatic function remains normal, diaphragmatic excursion is not reduced, and the maximum transdiaphragmatic inspiratory pressures that can be generated near term are similar to values generated by patients who are not pregnant. The changes in the chest wall return to normal within 6 months of delivery, although the costal angle may remain widened.
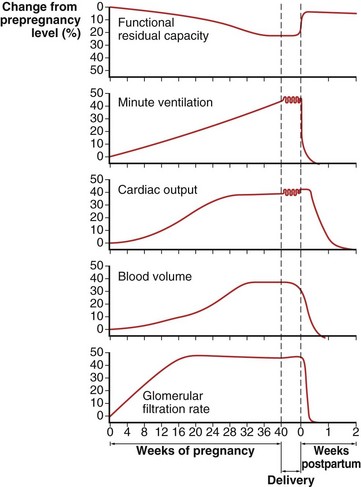
These changes in the thorax produce a progressive decrease in functional residual capacity (FRC) by 10% to 25% by term (Figure 76-2). Residual volume decreases slightly, but the major change is in expiratory reserve volume. These alterations are measurable as early as 16 to 24 weeks of gestation and progress to term. The increased diameter of the thoracic cage and the preserved respiratory muscle function allow the vital capacity to remain unchanged, and total lung capacity decreases only minimally. Measurements of airflow and lung compliance are not affected, but chest wall and total respiratory compliance are reduced in the third trimester because of the chest wall changes and increased abdominal pressure. Inconsistencies in results reported for diffusing capacity during pregnancy likely arise from the effects of anemia, variable changes in intravascular volume, and the increase in cardiac output. A small increase may be noted in early pregnancy with a subsequent decrease to normal values by term.
Minute ventilation increases greatly in pregnancy, beginning in the first trimester and reaching 20% to 40% above baseline at term (Figure 76-2), produced mainly by an increase in tidal volume of approximately 30% to 35%. These changes are mediated by the increase in respiratory drive that results from elevated serum progesterone levels. A respiratory alkalosis with compensatory renal excretion of bicarbonate results, with arterial carbon dioxide tension (PaCO2) falling to 28 to 32 mm Hg and plasma bicarbonate falling to 18 to 21 mEq/L. Alveolar-arterial oxygen tension difference (PO2[A−a]) is similar to nonpregnant values, and mean PaO2 usually exceeds 100 mm Hg at sea level throughout pregnancy. Mild hypoxemia and an increased PO2(A−a) may develop in the supine position because of airway closure, because FRC diminishes near term. One study suggests that shunt is normally increased in the third trimester to approximately 15% and is not changed significantly by posture. Oxygen consumption increases, beginning in the first trimester, and reaches 20% to 33% above baseline by the third trimester because of fetal demands and maternal metabolic processes. The combination of a reduced FRC and increased oxygen consumption lowers oxygen reserve, which renders the pregnant patient susceptible to the rapid development of hypoxia in response to hypoventilation or apnea.
Complications and Disorders of Pregnancy
Pregnancy-Specific Disorders
Amniotic Fluid Embolism
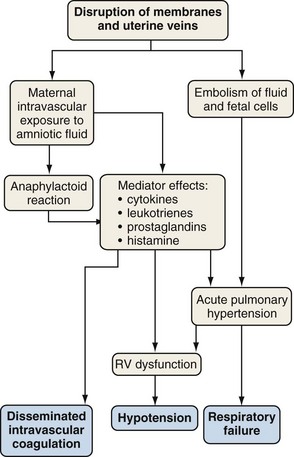
Amniotic fluid embolism is a rare obstetric complication (between 1 in 8000 and 1 in 80,000 live births) that carries a mortality rate of 10% to 86% and may account for 10% of maternal deaths. Amniotic fluid embolism is usually associated with labor and delivery, but it may also occur with uterine manipulations or uterine trauma or in the early postpartum period. The mechanism seems to involve amniotic fluid that enters the vascular circulation through endocervical veins or uterine tears. Particulate cellular contents and humoral factors in the amniotic fluid produce acute pulmonary hypertension, predominantly by causing vascular spasm (Figure 76-3). Acute right ventricular dysfunction results from the increased afterload and myocardial dysfunction mediated by humoral factors, associated with secondary left ventricular (LV) failure. The cardiovascular changes of amniotic fluid embolism may resemble those of anaphylaxis, and sensitivity to amniotic fluid contents may be responsible.
Preeclampsia and Pulmonary Edema
Pulmonary edema may rarely occur in association with preeclampsia (i.e., perhaps 3% of preeclamptic patients). The preeclamptic patient is usually volume-depleted, and pulmonary edema usually occurs in the early postpartum period, often associated with aggressive, intrapartum fluid replacement. Other factors that may contribute to the pathogenesis include reduced serum albumin, elevated LV afterload, and systolic and diastolic myocardial dysfunction (Figure 76-4). Increased capillary permeability may also occur, aggravated by concomitant conditions such as sepsis, placental abruption, or massive hemorrhage.
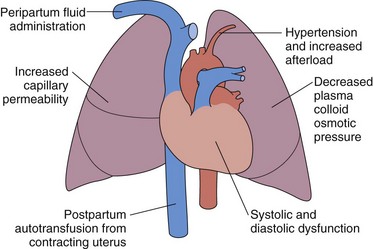
Figure 76-4 Pathophysiologic mechanisms responsible for the development of pulmonary edema in preeclampsia.
Tocolytic Pulmonary Edema
The clinical presentation is of acute respiratory distress with features of pulmonary edema. No specific features characterize tocolytic pulmonary edema. The diagnosis is a clinical one, made in the presence of acute pulmonary edema occurring in the appropriate clinical situation. The differential diagnosis includes cardiogenic pulmonary edema, amniotic fluid embolism, and other conditions (Table 76-1). Failure of the pulmonary edema to resolve in 12 to 24 hours requires a search for alternative causes.
Table 76-1 Acute Respiratory Distress Caused by Complications of Pregnancy and Labor
| Disorder | Distinguishing Features |
|---|---|
| Amniotic fluid embolism | Cardiorespiratory collapse, seizures, disseminated intravascular coagulation |
| Pulmonary edema caused by preeclampsia | Hypertension, proteinuria |
| Tocolytic pulmonary edema | Tocolytic administration, rapid improvement with discontinuation |
| Aspiration pneumonitis | Vomiting, aspiration |
| Peripartum cardiomyopathy | Gradual onset, signs of heart failure |
| Venous thromboembolism | Evidence of deep venous thrombosis; radiologic investigations |
| Pneumomediastinum | Occurs during delivery; subcutaneous emphysema |
| Air embolism | Related to sexual intercourse or cesarean section; associated hypotension |
Other Pulmonary Disorders in Pregnancy
Asthma
Treatment and Prognosis
Although physicians may be reluctant to prescribe medications during pregnancy, poorly controlled asthma is potentially more dangerous for the fetus. Inhaled corticosteroids remain the mainstay of therapy, and are considered safe in pregnancy in moderate or low doses (Table 76-2). One study suggests a small increased risk of congenital malformation in women using high-dose therapy in the first trimester. The use of a spacer device is encouraged to reduce local side effects and systemic absorption. Although animal data suggest a small risk of cleft palate with systemic corticosteroid use, this has not been demonstrated in humans. Short courses of prednisone should be used to manage poorly controlled asthma when clinically indicated. Inhaled β-agonists seem safe and should be used as required for symptomatic relief. GER, a potentially preventable cause of worsening asthma, is frequently overlooked. Antireflux measures may greatly reduce asthmatic symptoms. Acute attacks are treated by ensuring adequate oxygenation, closely monitoring the fetus, and administering appropriate medications. Concerns over fetal effects of drugs should not cause physicians and patients to avoid use of effective pharmacologic therapy. Updated management algorithms are available through the Working Group on Asthma and Pregnancy of the National Asthma Education and Prevention Program (NAEPP).
Table 76-2 Safety Classification of Asthma Therapy in Pregnancy
| Drug | FDA Class |
|---|---|
| Inhaled bronchodilators | |
| Albuterol (salbutamol) | C |
| Terbutaline | B |
| Ipratropium | B |
| Salmeterol | C |
| Formoterol | C |
| Tiotropium | C |
| Inhaled corticosteroids | |
| Beclomethasone | C |
| Budesonide | B |
| Fluticasone | C |
| Leukotriene antagonists | |
| Zafirlukast | B |
| Montelukast | B |
| Other agents | |
| Theophylline | C |
| Cromolyn | B |
| Systemic corticosteroids | C |
Data from U.S. Food and Drug Administration (FDA) classification of drug safety in pregnancy; note that the FDA is in the process of revising this approach to labeling. Categories are as follows: A, Human studies fail to demonstrate fetal harm. B, Animal studies fail to demonstrate harm, but no human studies or animal studies demonstrate risk not shown in human studies. C, Animal studies demonstrate risk, or insufficient data available; drugs may be used if benefit outweighs risk. D, Human studies demonstrate risk; drugs may be used if benefits justify the risks. X, Contraindicated in pregnancy.
Pulmonary Thromboembolic Disease
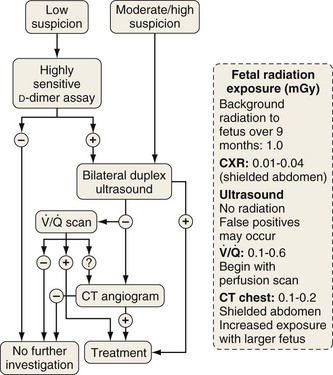
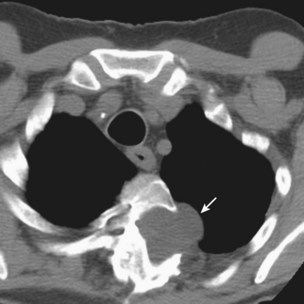
Investigation of suspected pulmonary embolism follows a similar approach to that in the patient who is not pregnant, and the diagnosis must be pursued aggressively. Duplex ultrasonography is useful for the diagnosis of DVT, although venous Doppler can give false-positive results because of venous obstruction by the gravid uterus. Ventilation-perfusion scanning can be performed with less than 0.5 mGy (<50 mrad) exposure to the fetus and, if necessary, a computed tomography (CT) pulmonary angiogram may be performed with similarly low fetal exposure (Figure 76-5). Teratogenicity is generally thought to require exposure greater than 50 to 100 mGy (5-10 rad), but an increased incidence of childhood leukemia may occur with fetal radiation exposures of 20 to 50 mGy (2-5 rad).
Treatment and Prognosis
Warfarin therapy during the first trimester has been associated with an embryopathy, and central nervous system abnormalities have been described with second- and third-trimester exposure. Accordingly, warfarin is usually avoided (Table 76-3). The anticoagulant of choice is heparin, which does not cross the placenta, is not associated with adverse fetal outcome, and can be readily reversed. Low-molecular-weight (LMW) heparins do not seem to cross the placenta and are both safe and effective in pregnancy.
Table 76-3 Management of Thromboembolic Disease in Pregnancy
| Therapy | FDA Class* | Comments |
|---|---|---|
| Heparin | C | Bone demineralization after prolonged use |
| Low-molecular-weight heparin | B/C | Good evidence of safety |
| Warfarin | D/X | Teratogenic, central nervous system abnormalities and bleeding occur, although the risk appears low |
| Sometimes used in second trimester | ||
| Alteplase (r-tPA) | C | Consider in acute, life-threatening situations |
* See Table 76-2 for U.S. Food and Drug Administration classification of drug safety in pregnancy.
Acute Respiratory Distress Syndrome in Pregnancy
The pregnant patient is at risk for development of ARDS from a number of pregnancy-associated problems (Box 76-1). Gastric acid aspiration is a particular risk because of the increased intraabdominal pressure, reduced lower esophageal sphincter tone, and supine position during delivery. Iatrogenic factors such as excessive fluid administration and tocolytic therapy may contribute, as well as reduced albumin level.
Box 76-1
Causes of Acute Respiratory Distress Syndrome in Pregnancy
ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population-based cohort study. BMJ. 2010;340:c1279.
Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30:256–265.
Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol. 201, 2009. 445.e1–455.e13
Dombrowski MP, Schatz M. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2008;111(pt 1):457–464.
Jensen D, Webb KA, Davies GA, O’Donnell DE. Mechanisms of activity-related breathlessness in healthy human pregnancy. Eur J Appl Physiol. 2009;106:253–265.
Lapinsky SE. Concise definitive reviews in critical care: cardiopulmonary complications of pregnancy. Crit Care Med. 2005;33:1616–1622.
Ratnapalan S, Bentur Y, Koren G. Doctor, will that x-ray harm my unborn child? CMAJ. 2008;179:1293–1296.
Scarsbrook AF, Evans AL, Owen AR, et al. Diagnosis of suspected venous thromboembolic disease in pregnancy. Clin Radiol. 2006;61:1–12.
Schatz M, Dombrowski MP. Clinical practice: asthma in pregnancy. N Engl J Med. 2009;360:1862–1869.