Chapter 11 Postsurgical Endoscopic Anatomy
![]() Video related to this chapter’s topics: Post-Operative Endoscopic Anatomy
Video related to this chapter’s topics: Post-Operative Endoscopic Anatomy
Introduction
Patients who have undergone surgical procedures that alter the anatomy of the upper gastrointestinal (GI) tract are often referred for endoscopic evaluation.1 If meaningful and accurate diagnostic information is to be obtained in these patients, it is important that the endoscopist fully understand the anatomic changes resulting from the surgical procedures.2 Knowledge of the new anatomy is essential to define the type of endoscope and accessories and to determine the need for supplementary studies during or before the endoscopic procedure.3,4 In addition, accurate interpretation of endoscopic findings may permit the endoscopist to identify an unknown previous surgical procedure. This chapter discusses the most common operations in the upper GI tract that are relevant to the endoscopist. Technical details and common variations are described for each surgical procedure and correlated to the findings and to the anatomic alterations observed endoscopically. Surgical terms are also presented to assist the endoscopist in the interpretation of the surgical reports, which should always be reviewed before the endoscopic examination.
Antireflux Procedures
Nissen Fundoplication
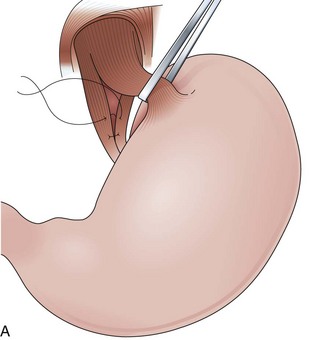
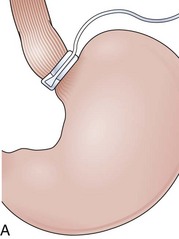
Fundoplications to treat gastroesophageal reflux disease (GERD) are performed without gut resection to restore the competency of the cardia (Fig. 11.1). The plication has to be created over the distal esophagus just proximal to the cardioesophageal junction to be effective.5 Modifications to the original fundoplication described by Nissen decreased the incidence of postoperative gas-bloat syndrome and dysphagia at the same time that the laparoscopic approach proved to be safe and reliable.6–10 Shortening from 5 cm to 2 cm and loosening of the fundoplication resulted in the so-called floppy Nissen, which was performed laparoscopically and became the surgical “gold standard” treatment for GERD.11
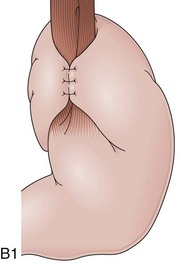
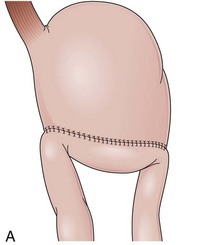
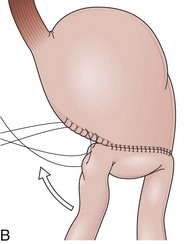
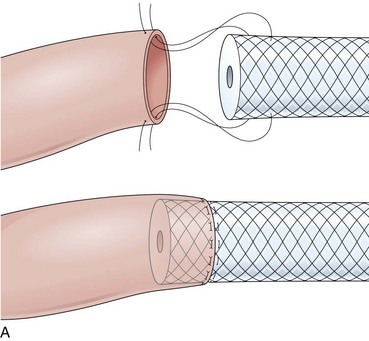
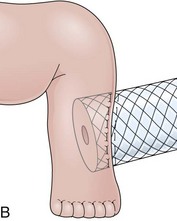
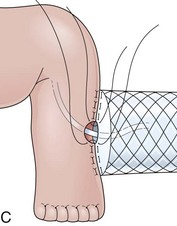
The distal esophagus, the cardioesophageal junction, the gastric fundus, and the right and left crura are dissected in the same way for the open or laparoscopic procedure. Careful dissection is required to avoid transection of the nerve of Latarjet on the right side of the stomach. After reduction of the hernia, the left and right crura are approximated by sutures, gently snuggling the hiatus around the esophagus, which accommodates a previously inserted 60-Fr dilator (Fig. 11.1A). Division of the short gastric vessels may be required to mobilize the fundus.12,13 The gastric fundus is passed behind the esophagus from left to right creating a 360-degree wrap by the placement of two or three sutures involving stomach-esophagus-stomach in the anterior portion of the wrap. The anterior and posterior vagus nervus are usually contained into the wrap, attached to the esophagus. At the end of the procedure, the wrap must lie below the diaphragm without tension (Fig. 11.1B-1).14,15
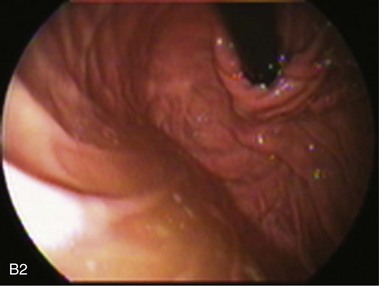
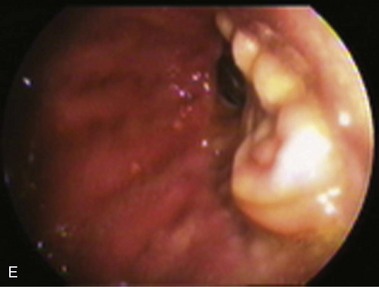
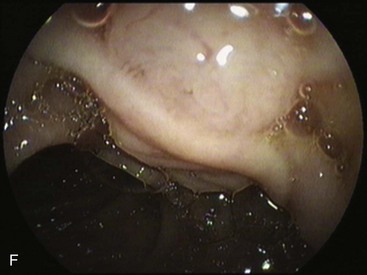
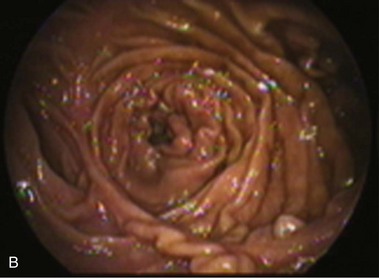
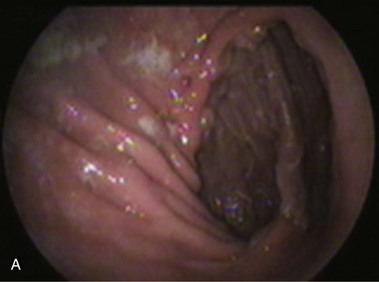
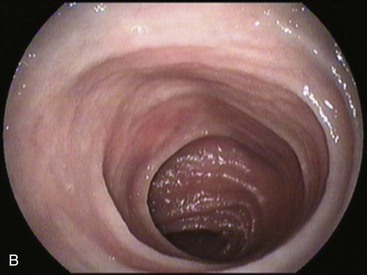
An intact Nissen fundoplication appears to the endoscopist as a narrow, easily transposable distal esophagus, with noninflamed mucosa and reduced distention to air insufflation. An encircling redundant fold overlies the cardia on a retroflexed view and snuggles the endoscope. Edema accentuates the prominent cardia in the early postoperative setting, and the area of the fundus becomes less capacious than normal. Late evaluation of the wrap may reveal several rugal folds parallel to each other and to the markings on the insertion tube of the endoscope (Fig. 11.1B-2). Although this is a 360-degreee wrap, endoscopically the redundant fold appears as a 270-degree free cuff margin because the border continuous with the lesser curvature is not evident.16 The crural closure should maintain the cardia below the diaphragm with the stomach completely insufflated with air. Occasionally, sutures in the distal esophagus may be observed, indicating migration through the wall or inappropriate penetration depth of the stitches during the procedure; this may or may not be associated with symptoms.17
Findings that could be associated with failure of the fundoplication include esophagitis, lack of the encircling fold on a retroflexed view, patulous gap between the endoscope tube and the wrap, migration of the wrap through an enlarged esophageal hiatus, hourglass appearance of the proximal stomach indicating slippage through the valve, and irregularity in the dome shape of the fundus indicating parahiatal hernia. The squamocolumnar junction located more than 1 cm proximal to the margin of the wrap has been reported to be a major endoscopic clue in diagnosis of postfundoplication problems.18 Gastric food retention may be related to damage to the vagus nerves during the procedure.19 Some patients with persistent dysphagia have a tight wrap that causes resistance to the advance of the endoscope, and these patients may benefit from endoscopic dilation.20
Partial Fundoplications (Dor and Toupet)
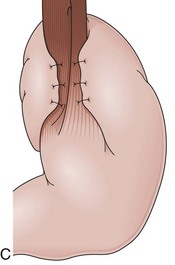
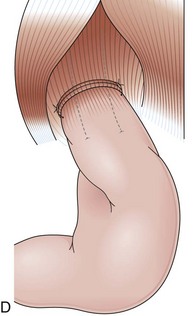
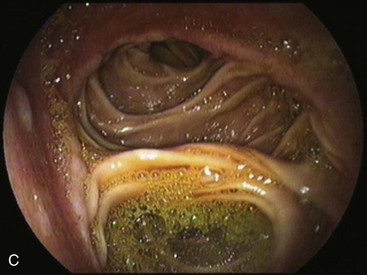
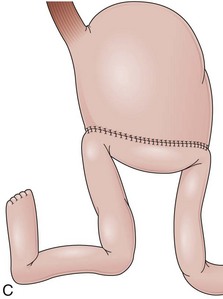
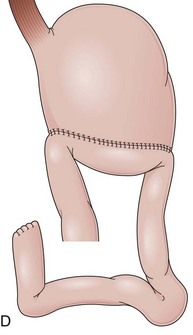
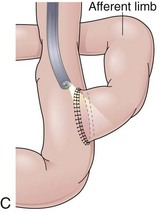
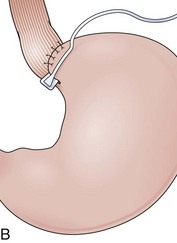
Partial fundoplications are created with the fundus involving partially the distal esophagus. A Dor fundoplication is performed anteriorly, and a Toupet fundoplication is performed posteriorly (Fig. 11.1C and D). Both procedures can be performed to treat GERD; however, they are best indicated in patients who underwent a Heller myotomy (Dor) or with impaired esophageal body motility (Toupet).21–23 Partial fundoplications also have a prominent fold overlying the cardia, which is not less evident than 360-degree wraps when observed endoscopically.24
Belsey Mark IV
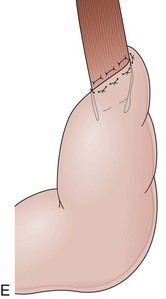
With the advent of laparoscopy, the Belsey Mark IV fundoplication is performed only occasionally because it requires a thoracotomy. A circumferential invagination of the distal esophagus into the proximal stomach is performed with two layers of stitches, including anchoring to the diaphragma in the last one. The crura are also sutured to narrow the esophageal hiatus. The final result is a 270-degree wrap around the distal esophagus, placed in the abdomen, and a hiatal repair (Fig. 11.1E). Endoscopically, Belsey Mark IV and Nissen fundoplications are similar, with folds encircling the endoscope at the level of the cardia. However, coils of gastric rugae as seen after Nissen repair are not evident, and there is an anterior compression that corresponds to the attachment of the esophagus to the diaphragm.16
Collis Gastroplasty
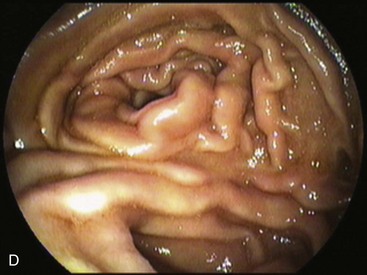
A short esophagus, usually caused by chronic scarring resulting from GERD, can be repaired surgically through a Collis gastroplasty. This gastroplasty creates a tubular segment of stomach in continuity to the esophagus, long enough to be encircled by a 360-degree fundoplication placed below the diaphragm. The fundoplication around this tubular segment within the positive pressure of the abdomen prevents the gastroesophageal reflux.25 Short esophagus is declining possibly because patients with GERD are medically diagnosed and treated earlier.26 Endoscopically, the squamocolumnar junction is observed above a short tubular segment of stomach, which may not distend properly because of the wrap. The Collis gastroplasty resembles the Nissen fundoplication on a retroflexed view with a less capacious fundus.
Operations without Alteration of the Pancreaticobiliary Anatomy
Billroth I
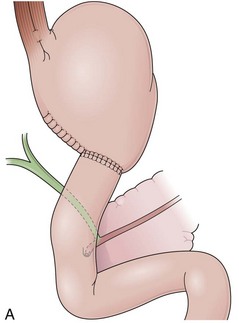
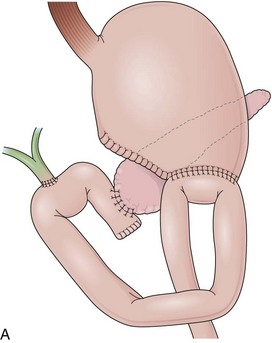
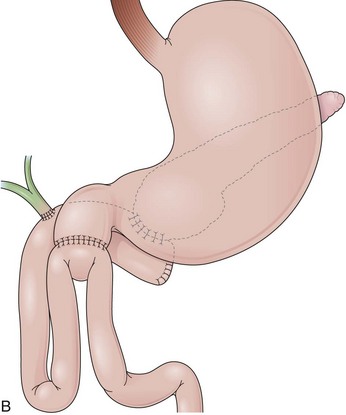
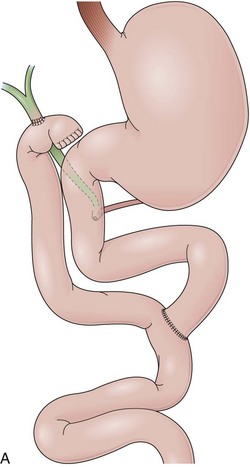
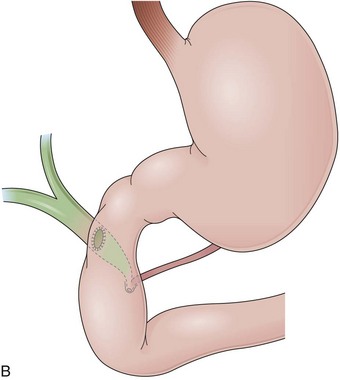
Billroth I is a type of reconstruction after a partial gastrectomy in which the stomach is anastomosed to the duodenum (Fig. 11.2A).27 The stomach resection is usually restricted to the antrum, and a truncal vagotomy is often associated. A considerable amount of remaining stomach with refluxed bile is observed endoscopically. The gastroduodenostomy is found toward the greater curvature. A prominent fold representing the closed part of the stomach is often observed along the lesser curvature ending at the gastroduodenostomy. A mucosal pattern change from gastric folds to flat duodenal surface indicates the anastomosis site. The duodenum is rectified, and the circular folds of the second portion are close to the anastomosis because of the partial resection of the bulb. Major and minor papillae appear to be more proximal in the duodenum than in a patient with intact anatomy.
Billroth II
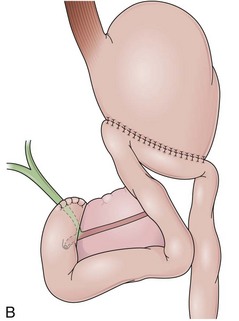
In a Billroth II reconstruction after a partial gastrectomy, the duodenal stump is closed and a gastrojejunostomy is created (Fig. 11.2B). This type of reconstruction, commonly used for complicated ulcer disease in the past, is now used more often in the treatment of gastric neoplasia in which extensive resections are required. The remaining stomach is variable in length allowing the retroflexion maneuver if a long segment is present. The gastric remnant usually contains frothy bile and mucosal erythema from the alkaline reflux.28 The gastrojejunostomy is located at the distal end of the stomach where two stomal openings corresponding to an end-to-side anastomosis can be identified (Fig. 11.2C).
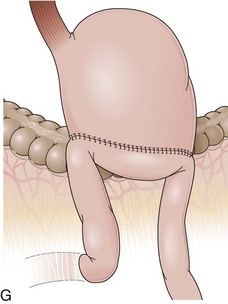
Several surgical techniques are employed to perform the gastrojejunostomy leading to distinct endoscopic presentations. The chosen technique depends on surgeon preference, and there is no consensus for the ideal one. The gastrojejunostomy can vary in the size of the anastomosis, in the orientation of the jejunal loop to the stomach, and in the position of the anastomosis to the transverse colon. If the whole length of the transected stomach is anastomosed to the jejunum (oralis totalis or Polya), several rows of jejunal folds are observed between the two stomal openings (Fig. 11.3A). Conversely, if only a segment of the transected stomach is anastomosed to the jejunum (oralis partialis or Hoffmeister), few or no folds are evident. In this case, the stomach is partially closed, always from the lesser curvature, to reduce the diameter of the anastomosis, which is observed toward the greater curvature. Some surgeons attach the jejunal limb to the suture line that is closing the stomach to prevent dehiscence when performing an oralis partialis anastomosis (Fig. 11.3B).29 In this case, a sharp angulation might be negotiated to enter the corresponding jejunal limb, and a prominent fold may be seen emanating from the lesser curvature to the anastomosis. The small anastomosis diameter in association with the sharp angulation of this type of reconstruction may make the anatomy difficult to define endoscopically.
Gastrojejunostomies performed with staplers are usually oralis partialis. In some cases, the stomach is completely closed at the distal end, and the gastrojejunal anastomosis is performed with a linear or a circular stapler in a side-to-side fashion at the posterior wall, 2 cm proximal to the end of the stomach.30 When observed endoscopically, however, this side-to-side anastomosis is almost indistinguishable from a short end-to-side anastomosis. The jejunum can be anastomosed to the stomach with the afferent limb attached to the greater curvature (isoperistaltic) or to the lesser curvature (antiperistaltic). The afferent limb refers to the jejunal limb that is in continuity with the duodenum, whereas the efferent limb refers to the one that leaves the stomach toward the distal jejunum. The two stomal openings observed endoscopically may represent the afferent or efferent limb depending on how the reconstruction was performed (Fig. 11.3C and D). If the reconstruction is isoperistaltic, the opening linked to the greater curvature corresponds to the afferent limb. If the reconstruction is antiperistaltic, the opening linked to the greater curvature corresponds to the efferent limb. Usually the stomal opening linked to the lesser curvature is more difficult to access with the endoscope because of the relative verticalization of the anastomosis (Fig. 11.3E).31
Gastrectomies usually include the lesser more than the greater curvature in the resection. In addition, the information from surgical notes about the type of reconstruction, peristalsis, and bile flow might help to define the limbs endoscopically. On careful observation of the anastomosis, bile may be seen coming predominantly from the afferent limb. Introducing the endoscope through this opening should reveal an increasing volume of bile as the endoscope advances toward the bulb, although bile may also be observed in the efferent limb. Visible peristaltic waves advancing away from the endoscope suggest that the instrument is in the efferent limb. When the duodenal stump is reached, the flat mucosa of the residual bulb with a scarlike deformity in a cul-de-sac can be identified. A careful withdrawal of the endoscope exposes the major papilla, usually located at the right upper quadrant on the monitor screen (Fig. 11.3F).
In patients with Billroth II anatomy, the papilla is rotated 180 degrees in the endoscopic visual field. This “upside down” position requires distinct techniques to perform endoscopic retrograde cholangiopancreatography (ERCP), including dedicated sphincterotomes, needle-knife cut technique over the stent, or balloon dilation of the papilla.32–36 If the duodenal stump cannot be identified, the endoscope should be withdrawn, and the other limb should be intubated as far as possible. Fluoroscopic guidance may indicate that the efferent limb has been entered when the instrument is seen to pass deep into the pelvis. Conversely, passage of the endoscope into the right upper quadrant toward the liver or previous cholecystectomy clips suggests entry into the afferent limb.37
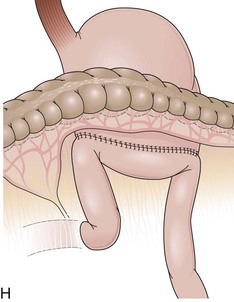
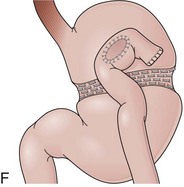
The length of the afferent limb also varies depending on the surgical technique. The afferent limb naturally fixed at the ligament of Treitz and surgically fixed to the stomach should be tensionless but not redundant. There are two ways to position the afferent limb in relation to the transverse colon during a Billroth II reconstruction. If an antecolic anastomosis is performed, the gastrojejunostomy is placed anterior to the transverse colon (Fig. 11.3G). Antecolic reconstructions frequently have long afferent limbs because of the distance between the ligament of Treitz and the remaining stomach, over the mesocolon, omentum, and transverse colon. Conversely, retrocolic reconstructions are performed through an opening in the transverse mesocolon, shortening the distance between the ligament of Treitz and the remaining stomach (Fig. 11.3H).38,39 Antecolic and retrocolic anastomoses are similar endoscopically except for the unspecific observation obtained from the length of the limbs. Caution should be taken if a percutaneous endoscopic gastrostomy is indicated for a patient with a previous partial gastrectomy and retrocolic reconstruction.
Billroth II reconstruction can be associated with a side-to-side jejunojejunostomy, referred to as the Braun procedure (Fig. 11.4A).40 This procedure creates an anastomosis between the afferent and the efferent limb to divert bile from the gastric remnant and to release the pressure of the afferent limb, supposedly preventing duodenal stump fistula.41 The Braun anastomosis is performed 10 to 15 cm distal to the gastrojejunostomy and requires a longer afferent limb to accommodate the jejunojejunostomy.42 Endoscopically, the gastrojejunostomy is similar to a standard Billroth II. Frothy bile is present in the stomach because the Braun procedure only partially diverts biliopancreatic fluids from the gastrojejunostomy. After advancing the endoscope through either opening of the gastrojejunostomy, the side-to-side Braun anastomosis can be found in the afferent and efferent limb, and three openings can be noted (Fig. 11.4B). One leads to the distal jejunum, one leads to the afferent limb, and the third one leads back to the stomach. A complete reverse intubation of the stomach may be carried out through the loop created with the Braun anastomosis. The same anatomic landmarks described for other Billroth II procedures are helpful in directing the endoscope through the limbs. However, a trial-and-error approach may be necessary ultimately to reach the duodenal stump.
A higher rate of perforation has been reported during ERCP while traversing the afferent limb compared with standard ERCP, particularly when a stiff therapeutic duodenoscope is used.43,44 The Braun procedure has also been associated with perforations during ERCP. The use of a forward-viewing endoscope in these patients can reduce the risk of jejunal perforations.45 The ability to use a duodenoscope elevator may increase the success of the procedure, and a flexible diagnostic duodenoscope may be safer than a stiff therapeutic instrument. If the papilla cannot be located with a side-viewing endoscope, a forward-viewing endoscope should be attempted and vice versa. Patients with an excessively long afferent limb may require longer endoscopes to reach the papilla.
Roux-en-Y Gastrectomy
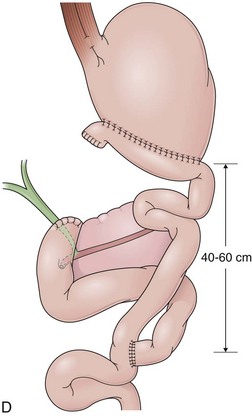
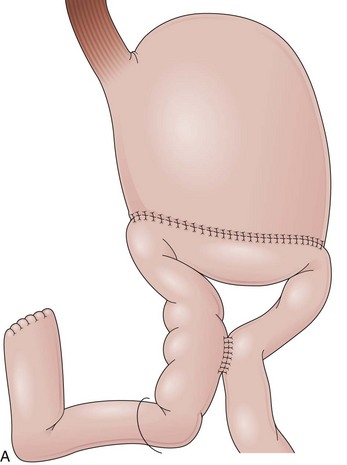
In a Roux-en-Y reconstruction, the jejunum is transected close to the ligament of Treitz, creating two distinct segments. The distal segment is sutured to the gastric remnant (gastrojejunostomy) becoming the efferent limb. The proximal segment is sutured to this efferent limb (jejunojejunostomy) approximately 40 cm below the gastrojejunostomy (see Fig. 11.2D). The proximal segment is called the afferent limb, which connects the duodenum to the efferent limb instead of the stomach as in Billroth II reconstructions. The Roux-en-Y prevents biliopancreatic fluids from refluxing into the stomach in patients who have undergone gastric resection. It can be performed as the initial reconstruction after a gastrectomy or as the treatment for postgastrectomy syndrome resulting from a previous Billroth II reconstruction.46–48 Truncal vagotomy is commonly performed in association with Roux-en-Y to prevent peptic ulcers in the efferent limb, which is no longer washed by the alkaline contents of the biliopancreatic fluid.49
In effective Roux-en-Y reconstructions (Fig. 11.5), the remnant stomach is completely clean of bile (see Figs. 11.3A and B and 11.5A and B). However, because Roux-en-Y gastrojejunostomy increases the risk for delayed gastric emptying, residual contents in the stomach, including bezoars, and in the efferent limb may impair visualization of these segments or progression of the endoscope. The absence of bile in an operated stomach should always alert the endoscopist for a Roux-en-Y reconstruction, and the presence of residual food in this case should not lead to an erroneous conclusion of efferent limb obstruction. Total obstruction of the afferent limb in a Billroth II reconstruction could also prevent bile to reflux to the stomach, mimicking a Roux-en-Y, but this is uncommon.50 Conversely, presence of bile does not exclude a Roux-en-Y reconstruction. In this case, a short-length efferent limb may be responsible for the reflux. To be effective, the efferent limb has to measure at least 40 cm from the gastrojejunal anastomosis to the jejunojejunal anastomosis.51 Longer limbs (up to 60 cm) may also be encountered.52
Intubation through the efferent limb usually follows a straight route with variable looping. The enteroenteric anastomosis is usually end-to-side, but it may be side-to-side with a blind end. In either case, the endoscope has to leave the efferent limb and enter the afferent limb to reach the major papilla in the duodenum (see Fig. 11.5C). If a side-to-side anastomosis is present, three openings can be observed. The opening in continuity with the efferent limb leads to the distal jejunum, the second opening leads to a blind distal end of the afferent limb, and the third one leads to the duodenum through the afferent limb (see Fig. 11.5D). An end-to-side anastomosis has two openings. One is a continuation of the efferent limb and leads to the distal jejunum; the other opening leads to the afferent limb. Different degrees of angulation have to be negotiated to enter the afferent limb depending on the anastomosis configuration. Once the afferent limb is entered, progressively more bile should be seen until the duodenal stump is reached.
A complete visualization of the Roux-en-Y gastrojejunostomy during a routine esophagogastroduodenoscopy (EGD) can be performed with a forward-viewing gastroscope, including the jejunojejunostomy. In contrast, when patients with Roux-en-Y gastrectomy require ERCP, endoscopes with a longer insertion tube are usually needed (pediatric and adult colonoscopes, push-enteroscopes). In addition to the difficulties to reach the papilla, the small diameter of the working channel of these endoscopes and the short length of the endoscopic accessories contribute to the high rate of ERCP failures in these patients (compared with Billroth II patients).53
Gastrojejunostomy without Gastric Resection
Gastrojejunostomy without gastric resection is performed to bypass the distal stomach or the duodenum mostly in cases of malignant obstruction that cannot be resected. In major duodenopancreatic trauma with a high risk for fistulas, a gastrojejunostomy may also be performed in association with a temporary closure of the pylorus as part of the duodenal exclusion.54 Occasionally, the gastrojejunostomy is created prophylactically during the surgical exploration of a patient with unresectable adenocarcinoma of the head of the pancreas to prevent subsequent gastric outlet obstruction.55 The gastrojejunostomy is usually performed along the greater curvature of the distal body or the proximal antrum of the stomach (Fig. 11.6A). It may involve the anterior or the posterior wall at the surgeon’s discretion. In all cases, a side-to-side anastomosis is performed with the first jejunal loop that can be sutured without tension to the stomach. The anastomosis can be isoperistaltic or antiperistaltic, retrocolic or antecolic, as described for a Billroth II gastroenteroanastomosis. The definition for the length of the anastomosis does not apply (oralis totalis or oralis partialis) because this is a side-to-side anastomosis. However, this anastomosis usually resembles an oralis partialis in length.
The gastrojejunostomy appears endoscopically as a vertical anastomosis with two stomal openings that correspond to the afferent and efferent limbs. Either one of the limbs may be in a superior (upper) or inferior (lower) position, depending on the technique used during the surgery. If an isoperistaltic gastrojejunostomy has been created, the opening of the afferent limb should be expected in the upper position. The endoscopist should look carefully for a gastrojejunostomy in a patient with an upper tract obstruction who had undergone surgery. This anastomosis may become easily overlooked because it is typically not large, usually located among edematous gastric folds, and associated with gastric contents resulting from outlet obstruction (Fig. 11.6B). Ulcerations are also common and may impair intubation of the jejunal openings resulting from tissue retraction.56 Access to the papilla can be achieved by passing the endoscope retrograde through the afferent limb when a gastric outlet obstruction has been established. The Braun procedure may be added to the gastrojejunostomy as previously described for Billroth II reconstruction (Fig. 11.6C).
Bariatric Surgery
The National Institutes of Health (NIH) Consensus Conference in 1985 recognized obesity as a health risk, acknowledged the importance of treating this condition, and recommended the body mass index (BMI) to classify patients.57,58 Indications for bariatric procedures are increasing because of the increase in prevalence of obesity, including childhood obesity, and the lack of effective nonsurgical treatments. A growing number of patients with altered anatomy and perhaps new diseases should be expected in endoscopy units because GI complaints are frequent after bariatric surgery. The same complaints in uncomplicated postoperative courses can be present in patients with important surgical complications, which may require surgical revision.59–61
Some endoscopic findings may represent either a normal postsurgical appearance or a complication depending on the surgery that was performed.62 An example is the endoscopic finding of a communication between a short proximal gastric pouch with a normal-size remnant stomach. This communication is normally expected in a vertical banded gastroplasty (VBG), but it represents a failure (gastrogastric fistula) if the surgical procedure was a gastric bypass (GB). Familiarity with the most common bariatric procedures is essential for optimal endoscopic assistance to bariatric patients and surgeons. Surgical procedures to treat obesity have evolved during the last 5 decades. They can be simplified in two types, restrictive and malabsortive.63 Selection of a procedure is based on individual patient characteristics and surgeon preference.64–66
Jejunoileal Bypass
Jejunoileal bypass (JIB) was the first procedure proposed to induce malabsorption in 1954.67 It is technically simple and safe because it involves only enteroanastomosis, and the surgical steps are performed in the middle abdomen. In JIB, the proximal jejunum and the distal ileum are transected. The long jejunoileal segment in between these two transections is excluded from the intestinal transit by suturing the proximal margin in a close end and the distal margin to the sigmoid. An enteroanastomosis is performed between the proximal jejunum and the distal ileum, leaving a short segment of small bowel to absorption (Fig. 11.7). This procedure does not alter the endoscopic anatomy of the upper GI tract. JIB is no longer performed because of severe hepatic complications.68 Patients with intact JIB should be considered to revert the operation.
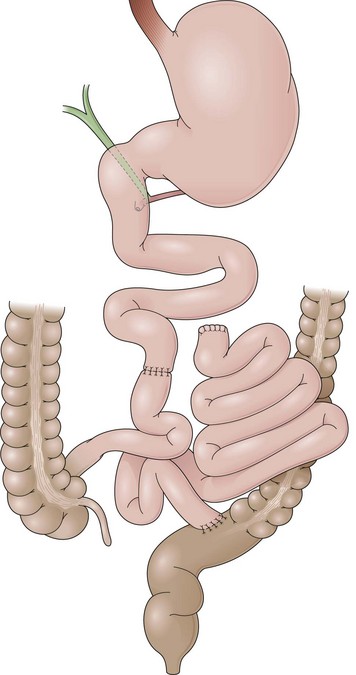
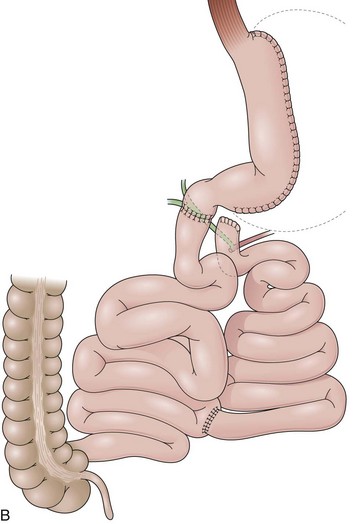
Gastric Bypass
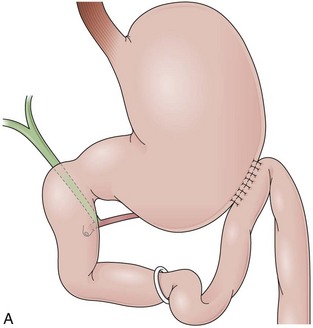
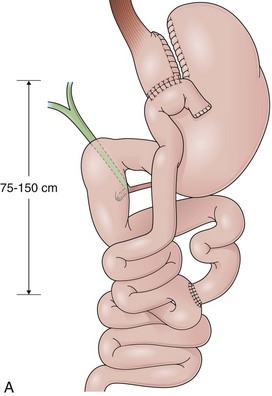
GB includes partition of the stomach creating a small-volume pouch (15 to 50 mL) in the proximal stomach.69,70 With the distal stomach completely disconnected, the proximal gastric pouch is anastomosed with a Roux-en-Y limb that ranges from 50 to 200 cm to reestablish the alimentary transit (Fig. 11.8A).71 For the endoscopist, GB may be compared with a Roux-en-Y gastrectomy. The differences are the size of the proximal gastric pouch, the length of the Roux-en-Y limb, and the fact that the distal stomach is not resected. Surgical technical variations can be observed in GB in regard to the orientation of the pouch (horizontal vs. vertical), partition of the stomach (transection vs. no transection), use of a Silastic ring around the gastrojejunal stoma, length of the Roux-en-Y limbs, and surgical access (laparoscopy vs. open surgery), among others.72–74
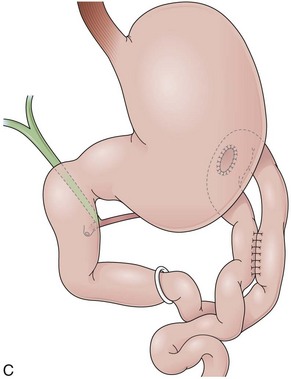
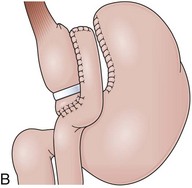
The procedure proposed by Capella and Capella75 incorporates a Silastic ring in the upper gastric pouch to prevent late stretching and the suture of the Roux-en-Y limb to the staple line of the pouch to prevent late gastrogastric fistulas (Fig. 11.8B-D). GB is a restrictive and malabsorptive procedure.76 Upper endoscopy in a patient with GB shows a small proximal pouch immediately after the esophagogastric junction with a narrow stoma leading to the small bowel and a long limb before reaching the jejunojejunal anastomosis, which may be inaccessible depending on the length of the limb (Fig. 11.8E). The gastric partition may include only the staple line, without division of the stomach (undivided bypass), or a complete transection of the stomach (divided bypass) (Fig. 11.8F). Undivided bypass presents a higher rate of fistulas between the pouch and the distal stomach compared with divided bypass. A gastrogastric fistula leads to a failure in weight loss and to a higher incidence of peptic ulcers beyond the gastrojejunal anastomosis.
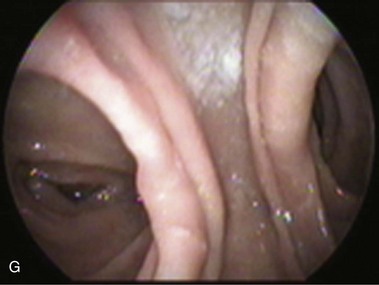
The gastrojejunostomy may be to the side or to the end of the jejunum or stomach. The small gastric pouch makes lateral and terminal gastric anastomoses indistinguishable. However, lateral and terminal anastomoses are different in the jejunal side. Lateral jejunal anastomosis has two openings. One ends blindly shortly after the anastomosis; the other leads to the distal jejunum (efferent limb) (Fig. 11.8G). Terminal anastomosis has one opening that should be readily accessible endoscopically. The blind end of a lateral anastomosis should not be confused with stenosis of the efferent limb, particularly when scarring alters the anatomy. Abnormal endoscopic findings include esophagitis, pouch or esophagus dilation, stomal stenosis, stomal ulceration, prosthesis erosion at the stoma, and breakdown of the partition staple line. Stomal ulceration has been related to staple line dehiscence in which a gastrogastric fistula occurs, although other factors may be involved.77,78
Access to the major papilla and to the disconnected part of the stomach is often impossible per os in patients with GB using regular endoscopes.79–81 A gastrostomy tract created in the distal stomach is used as an alternative to access these areas with the endoscope.82,83 Double-balloon enteroscopy has emerged as an alternative to access the biliopancreatic ducts and the disconnected part of the stomach in these patients.84–87
Biliopancreatic Diversion
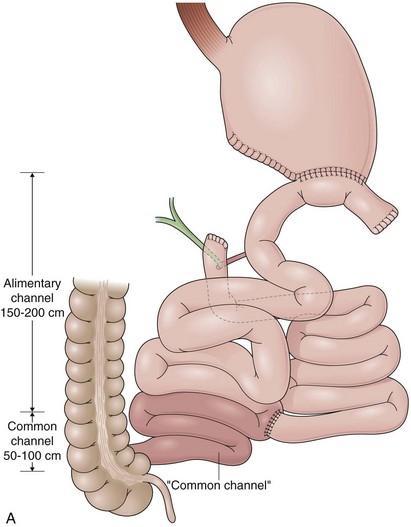
Biliopancreatic diversion (BPD) is a malabsorptive procedure to delay the involvement of bile and pancreatic juice in digestion.88 BPD was first reported in 1979 by Scopinaro and colleagues89 and is also known as the Scopinaro procedure. In BPD, the small bowel is divided creating two limbs. The distal limb is anastomosed to the stomach, and the proximal limb is anastomosed to the ileum. After completion, the small bowel has a new anatomic configuration with three distinct channels: common, alimentary, and biliopancreatic (Fig. 11.9A). BPD requires no small bowel resection and does not leave a nonfunctional small bowel segment. The results of the procedure depend on the length of the channels, which are variable because of the individual patient characteristics and surgeon preferences. Typically, a 50-cm to 100-cm common channel and a 150-cm to 200-cm alimentary channel are created. The remaining small bowel constitutes the biliopancreatic channel.
Duodenal Switch
The duodenal switch (DS) procedure is a variation of BPD. This procedure includes a sleeve gastrectomy preserving the pylorus and anastomosis of the enteric limb end-to-end with the postpyloric duodenum (Fig. 11.9B).88A lower prevalence of side effects has been reported for DS compared with BPD. The same principles described in regard to BPD apply to DS during the endoscopic evaluation except that a duodenojejunostomy rather than a gastrojejunostomy is present.
Vertical Banded Gastroplasty
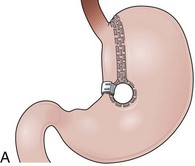
Initial gastroplasty configurations proved inadequate in terms of weight loss, and the procedure was refined by Mason into VBG. VBG, a pure restrictive procedure, is the result of a search for a simpler operation compared with GB.90 VBG involves the creation of a small pouch in the proximal stomach and the encirclement of the outlet channel to prevent dilation. The pouch is created along the lesser curvature with a stapled partition precisely at the angle of His to accommodate a volume of 15 mL or less. The outlet channel of the pouch is stabilized by the encirclement of a 5-cm circumference band or a Silastic ring (Fig. 11.10A). A technical variation includes dividing the stapled partition (Fig. 11.10B).
Upper endoscopy in patients with intact VBG shows a small tubular pouch immediately after the esophagogastric junction with a narrow outlet channel that once transposed leads to the remaining distal stomach. Abnormal endoscopic findings include esophagitis, staple line dehiscence, food impaction, stenosis of the pouch outlet, and erosion of the gastric wall by the material used to encircle the outlet channel.91,92 The remaining stomach, duodenal bulb, and biliopancreatic ducts are readily accessible for endoscopy if the outlet channel permits passage of the endoscope. The outlet channel is ideally 11 mm wide and 15 mm long, and it can be dilated endoscopically in cases of stenosis.
Laparoscopic Adjustable Gastric Banding
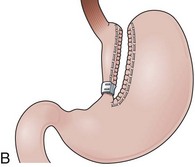
Laparoscopic adjustable gastric banding (LAGB) is a restrictive procedure largely used in European countries and Australia.93 LAGB involves placing a band around the proximal stomach to create a 15-mL pouch without the need of resecting or stapling the stomach (Fig. 11.11A). LAGB is now performed using a silicone material device that can be inflated with saline solution to adjust the gastric-pouch outflow. The inflatable part of the band device is connected by tubing to a reservoir implanted and secured to the abdominal fascia that can be accessed via a needle.94 Adjustable silicone gastric bands reduce the risks of eroding the gastric wall and the incidence of uncontrolled vomiting compared with former types of gastric banding.
Upper endoscopy in a patient with LAGB shows a small gastric pouch at the level of the cardia with a narrow outlet channel that leads to the distal normal stomach. Esophageal dilation, esophagitis, gastric pouch dilation, gastric slippage, outlet channel stenosis, and gastric wall erosion by the band device are the most common abnormal findings observed after LAGB.95,96 Occasionally, a marked gastric fold surrounding the pouch outlet channel can be observed in a retroflexed view within the distal stomach. This fold corresponds to the gastrogastric sutures placed anteriorly over the band device to decrease the risks of gastric herniation (Fig. 11.11B). Similar to VBG, once the endoscope is advanced through the pouch-outlet channel, examination of the distal stomach, duodenum, and biliopancreatic ducts can be performed as in a regular endoscopy.
Operations with Alteration of the Pancreaticobiliary Anatomy
Pancreaticoduodenectomy (Whipple Procedure)
The Whipple procedure is performed to resect malignant or benign lesions in the head of the pancreas or in the second portion of the duodenum.97 The extent of the resection classifies this procedure as classic or pylorus-preserving.
Classic Whipple Procedure
In the classic Whipple procedure, the gastric antrum, duodenum, head of the pancreas, and distal bile duct are resected. This extensive resection has led to the proposal of at least 68 techniques for reconstruction of the alimentary and pancreaticobiliary tract during the evolution of this operation.98 Currently, one well-accepted technique is to create all necessary anastomoses with a single limb of small bowel (Fig. 11.12).99,100 In this case (Fig. 11.12A), a side-to-side gastroenteroanastomosis is encountered endoscopically, usually oralis partialis and with the resection limited to the antrum. All the variations regarding orientation, position to the transverse colon, and stoma size described for the Billroth II gastroenteroanastomosis apply here. On entering the afferent limb, which may range from 40 to 60 cm and include a Braun procedure, the anastomosis with the biliary and pancreatic ducts can be identified. Sharp angulations resulting from fixation to adjacent organs may be encountered before reaching the blind end of the most proximal portion of the afferent limb, where the pancreaticojejunostomy is found.
The pancreaticojejunostomy may be end-to-end or end-to-side. In either case, the pancreaticojejunostomy may also be a mucosa-to-mucosa or a “dunking” anastomosis (Fig. 11.13).101 A mucosa-to-mucosa anastomosis creates a small opening by suturing the pancreatic duct to the jejunal mucosa. The dunking anastomosis differs from the mucosa-to-mucosa anastomosis in that the pancreas is invaginated into the jejunum. The opening of the pancreatic duct varies from a flat, small-diameter anastomosis (mucosa-to-mucosa) to a protuberant, sometimes downward-oriented anastomosis (lateral dunking), making the identification and cannulation of this duct technically challenging. The hepaticojejunostomy is located approximately 10 cm proximal (endoscopically) to the pancreaticojejunostomy. It is always an end-to-side anastomosis located in the antimesenteric border of the limb, occasionally subtle or hidden by a fold.
Pylorus-Preserving Whipple Procedure
The pylorus-preserving Whipple procedure differs from the classic Whipple operation in that the stomach is not resected and a short segment of the proximal bulb remains to be anastomosed with the jejunum (see Fig. 11.12B).102 This modification has proved to decrease the morbidity of pancreaticoduodenectomies without compromising the oncologic principles of the resection. A duodenojejunostomy rather than a gastrojejunostomy is observed in patients with a pylorus-preserving Whipple procedure. After traversing a normal stomach and the pylorus, a two-opening, small-diameter anastomosis is identified in a short segment of bulb. Depending on the orientation of the reconstruction, the afferent limb is to the right (antiperistaltic) or to the left (isoperistaltic). Antecolic or retrocolic anastomosis can also be observed, creating variations on the length of the jejunal limb. Usually, a trial-and-error approach is necessary to define the afferent limb, in which the pancreatic and biliary anastomosis are performed as described for the classic Whipple procedure. In patients who have undergone either Whipple operation, the biliary and pancreatic anastomosis may be reached with a side-viewing or a forward-viewing endoscope, owing to the relatively short afferent limb.
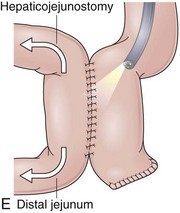
Roux-en-Y Hepaticojejunostomy
Anastomosis of the hepatic duct to a loop of jejunum without disturbing the gastroduodenal anatomy is usually performed for biliary disease or during liver transplantation when the native bile duct cannot be used to create a duct-to-duct anastomosis (e.g., in the setting of sclerosing cholangitis).103 The hepaticojejunostomy is usually end-to-side, but side-to-side anastomosis can also be encountered (Fig. 11.14A). The anatomy of the stomach, duodenum, and pancreas is not altered, and endoscopic evaluation of these organs is similar to a nonoperated stomach. If the bile duct must be accessed, the endoscope has to be advanced through a normal stomach and duodenum before reaching the jejunojejunal anastomosis that leads to a Roux-en-Y limb with the hepaticojejunostomy. Long-length endoscopes are usually necessary, and in most cases balloon enteroscopy systems employing an overtube are now used.104–106
In contrast to the anatomy after a Roux-en-Y gastrectomy, the duodenojejunal limb merges into the small bowel rather than merging with a loop of small bowel. At the level of the jejunojejunostomy, three lumens (side-to-side) or two lumens (end-to-side) can be observed, depending on the reconstruction (see Fig. 11.5E). One lumen leads to the distal jejunum, and the other leads to the limb that contains the hepaticojejunostomy. The third lumen (only if side-to-side) observed along the initial limb occupied by the endoscope ends blindly just beyond the anastomosis. A trial-and-error approach to the first two limbs reveals the one with the hepaticojejunostomy. The end-to-side hepaticojejunostomy is similar to the one described in the Whipple procedure except that here the location is closer to the blind end of the limb. In contrast to the end-to-side hepaticojejunostomy, the side-to-side hepaticojejunostomy preserves the access to the biliary ducts through the major papilla if the distal common bile duct is not obstructed. In this case, a cholangiogram can be obtained with the aid of an occlusion balloon inflated proximal to the hepaticojejunostomy, avoiding the demanding insertion of the endoscope through the Roux-en-Y limb. Air within the intrahepatic ducts is common in bilioenteric anastomosis and may be useful to evaluate patients in whom the hepaticojejunostomy is not reachable with the endoscope (air cholangiogram).4,107
Choledochoduodenostomy
Choledochoduodenostomy is the anastomosis of the bile duct to the second portion of the duodenum, usually performed in a side-to-side fashion (see Fig. 11.14B). Endoscopically, after traversing the pylorus, the choledochoduodenostomy is found at the level of the major papilla on the opposite wall of the duodenum. The anastomosis may be sufficiently wide to allow visualization and partial intubation of the extrahepatic ducts. A side-to-side anastomosis has two lumens. One lumen leads to the proximal biliary tree, and the other leads to the distal common bile duct. Because there is no alimentary diversion from the anastomosis, food impaction may occur in the distal common bile duct causing the sump syndrome, which may be the indication for ERCP in these patients.108 Because the biliary duct can be accessed through the major papilla and through the choledochoduodenostomy, a combination of accesses can be used to manipulate the different portions of the ducts, including anterograde cannulation of the papilla. The approach to the pancreatic duct is the same as for standard ERCP.
1 Max MH, West B, Knutson CO. Evaluation of postoperative gastroduodenal symptoms: endoscopy or upper gastrointestinal roentgenography? Surgery. 1979;86:578-582.
2 Donahue PE, Nyhus LM. Surgeon-endoscopists and the assessment of postoperative patients. South Med J. 1982;75:1570-1575.
3 Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part I. Reconstruction without alteration of pancreaticobiliary anatomy. Gastrointest Endosc. 2001;54:743-749.
4 Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part II. Postsurgical anatomy with alteration of the pancreaticobiliary tree. Gastrointest Endosc. 2002;55:75-79.
5 Little AG. Mechanisms of action of antireflux surgery: Theory and fact. World J Surg. 1992;16:320-325.
6 Nissen R. Eine einfache Operation zur Beeinflussung der Refluxoesophagitis. Schweiz Med Wochenschr. 1956;86:590-592.
7 Nissen R, Rosseti M. Surgery of hiatal hernia and other diaphragmatic hernias. J Int Coll Surg. 1965;43:663-674.
8 DeMeester TR, Bonavina L, Albertucci M. Nissen fundoplication for gastroesophageal reflux disease: evaluation of primary repair in 100 consecutive patients. Ann Surg. 1986;204:9-20.
9 Peters JH, Heimbucher J, Kauer WK, et al. Clinical and physiologic comparison of laparoscopic and open Nissen fundoplication. J Am Coll Surg. 1995;180:385-393.
10 Hinder RA, Filipi CJ, Wetscher G, et al. Laparoscopic Nissen fundoplication is an effective treatment for gastroesophageal reflux disease. Ann Surg. 1994;220:472-481. discussion 481–483
11 Donahue PE, Samelson S, Nyhus LM, et al. The floppy Nissen fundoplication: effective long-term control of pathologic reflux. Arch Surg. 1985;120:663-668.
12 O’Boyle CJ, Watson DI, Jamieson GG, et al. Division of short gastric vessels at laparoscopic Nissen fundoplication: a prospective double-blind randomized trial with 5-year follow-up. Ann Surg. 2002;235:165-170.
13 Chrysos E, Tzortzinis A, Tsiaoussis J, et al. Prospective randomized trial comparing Nissen to Nissen-Rossetti technique for laparoscopic fundoplication. Am J Surg. 2001;182:215-221.
14 Horgan S, Pellegrini CA. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am. 1997;77:1063-1082.
15 Bowrey DJ, Peters JH. Laparoscopic esophageal surgery. Surg Clin North Am. 2000;80:1213-1242.
16 Johnson DA, Younes Z, Hogan WJ. Endoscopic assessment of hiatal hernia repair. Gastrointest Endosc. 2000;52:650-659.
17 Arendt T, Stuber E, Monig H, et al. Dysphagia due to transmural migration of surgical material into the esophagus nine years after Nissen fundoplication. Gastrointest Endosc. 2000;51:607-610.
18 Jailwala J, Massey B, Staff D, et al. Post-fundoplication symptoms: The role for endoscopic assessment of fundoplication integrity. Gastrointest Endosc. 2001;54:351-356.
19 Low DE. Management of the problem patient after antireflux surgery. Gastroenterol Clin North Am. 1994;23:371-389.
20 Malhi-Chowla N, Gorecki P, Bammer T, et al. Dilation after fundoplication: timing, frequency, indications, and outcome. Gastrointest Endosc. 2002;55:219-223.
21 Gadenstatter M, Klingler A, Prommegger R, et al. Laparoscopic partial posterior fundoplication provides excellent intermediate results in GERD patients with impaired esophageal peristalsis. Surgery. 1999;126:548-552.
22 Hunter JG, Richardson WS. Surgical management of achalasia. Surg Clin North Am. 1997;77:993-1015.
23 Vogt D, Curet M, Pitcher D, et al. Successful treatment of esophageal achalasia with laparoscopic Heller myotomy and Toupet fundoplication. Am J Surg. 1997;174:709-714.
24 Mellinger JD, Ponsky JL. Endoscopic evaluation of the postoperative stomach. Gastrointest Endosc Clin N Am. 1996;6:621-639.
25 Trastek VF, Deschamps C, Allen MS, et al. Uncut Collis-Nissen fundoplication: Learning curve and long-term results. Ann Thorac Surg. 1998;66:1739-1744.
26 Horvath KD, Swanstrom LL, Jobe BA. The short esophagus: pathophysiology, incidence, presentation, and treatment in the era of laparoscopic antireflux surgery. Ann Surg. 2000;232:630-640.
27 Sawye JL, Herrington JL. Vagotomy-antrectomy. In: Wastell C, Nyhus LM, Donahue PE, editors. Surgery of the esophagus, stomach and small intestine. New York: Little, Brown; 1994:520-530.
28 Ritchie WPJr. Alkaline reflux gastritis. Gastroenterol Clin North Am. 1994;23:281-294.
29 Thon HJ, Loffler A, Buess G, et al. Is ERCP a reasonable diagnostic method for excluding pancreatic and hepatobiliary disease in patients with a Billroth II resection? Endoscopy. 1983;15:93-95.
30 Jameson LC. Stapling in esophageal and gastric surgery. In: Wastell C, Nyhus LM, Donahue PE, editors. Surgery of the esophagus, stomach and small intestine. New York: Little, Brown; 1994:572-587.
31 Aabakken L, Holthe B, Sandstad O, et al. Endoscopic pancreaticobiliary procedures in patients with a Billroth II resection: A 10-year follow-up study. Ital J Gastroenterol Hepatol. 1998;30:301-305.
32 Bedogni G, Bertoni G, Contini S, et al. Endoscopic sphincterotomy in patients with Billroth II partial gastrectomy: Comparison of three different techniques. Gastrointest Endosc. 1984;30:300-304.
33 Siegel JH, Cohen SA, Kasmin FE, et al. Stent-guided sphincterotomy. Gastrointest Endosc. 1994;40:567-572.
34 Prat F, Fritsch J, Choury AD, et al. Endoscopic sphincteroplasty: A useful therapeutic tool for biliary endoscopy in Billroth II gastrectomy patients. Endoscopy. 1997;29:79-81.
35 Bergman JJ, van Berkel AM, Bruno MJ, et al. A randomized trial of endoscopic balloon dilation and endoscopic sphincterotomy for removal of bile duct stones in patients with a prior Billroth II gastrectomy. Gastrointest Endosc. 2001;53:19-26.
36 van Buuren HR, Boender J, Nix GA, et al. Needle-knife sphincterotomy guided by a biliary endoprosthesis in Billroth II gastrectomy patients. Endoscopy. 1995;27:229-232.
37 Costamagna G, Mutignani M, Perri V, et al. Diagnostic and therapeutic ERCP in patients with Billroth II gastrectomy. Acta Gastroenterol Belg. 1994;57:155-162.
38 Mosca S. How can we reduce complication rates and enhance success rates in Billroth II patients during endoscopic retrograde cholangiopancreatography? Endoscopy. 2000;32:589-590.
39 Lin LF, Siauw CP, Ho KS, et al. ERCP in post-Billroth II gastrectomy patients: emphasis on technique. Am J Gastroenterol. 1999;94:144-148.
40 Hintze RE, Adler A, Veltzke W, et al. Endoscopic access to the papilla of Vater for endoscopic retrograde cholangiopancreatography in patients with Billroth II or Roux-en-Y gastrojejunostomy. Endoscopy. 1997;29:69-73.
41 Olbe L, Becker HD. Partial gastrectomy with Billroth II resection and alternative methods. In: Becker HD, Herfarth CH, Lierse W, Schreiber HW, editors. Surgery of the stomach. Berlin: Springer-Verlag; 1987:50-70.
42 Safrany L, Neuhaus B, Portocarrero G, et al. Endoscopic sphincterotomy in patients with Billroth II gastrectomy. Endoscopy. 1980;12:16-22.
43 Faylona JM, Qadir A, Chan AC, et al. Small-bowel perforations related to endoscopic retrograde cholangiopancreatography (ERCP) in patients with Billroth II gastrectomy. Endoscopy. 1999;31:546-549.
44 Costamagna G. ERCP and endoscopic sphincterotomy in Billroth II patients: a demanding technique for experts only? Ital J Gastroenterol Hepatol. 1998;30:306-309.
45 Kim MH, Lee SK, Lee MH, et al. Endoscopic retrograde cholangiopancreatography and needle-knife sphincterotomy in patients with Billroth II gastrectomy: a comparative study of the forward-viewing endoscope and the side-viewing duodenoscope. Endoscopy. 1997;29:82-85.
46 Delcore R, Cheung LY. Surgical options in postgastrectomy syndromes. Surg Clin North Am. 1991;71:57-75.
47 Haglund UH, Jansson RL, Lindhagen JG, et al. Primary Roux-Y gastrojejunostomy versus gastroduodenostomy after antrectomy and selective vagotomy. Am J Surg. 1990;159:546-549.
48 Donahue PE. Early postoperative and postgastrectomy syndromes: diagnosis, management, and prevention. Gastroenterol Clin North Am. 1994;23:215-226.
49 Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992;72:445-465.
50 Burdick JS, Garza AA, Magee DJ, et al. Endoscopic management of afferent loop syndrome of malignant etiology. Gastrointest Endosc. 2002;55:602-605.
51 Steffes C, Fromm D. Postgastrectomy syndromes. In: Zuidema GD, editor. Shackelford’s surgery of the alimentary tract. 4th ed. Philadelphia: WB Saunders; 1996:166-184.
52 Schirmer BD. Gastric atony and the Roux syndrome. Gastroenterol Clin North Am. 1994;23:327-343.
53 Lehman GA. What are the determinants of success in utilization of ERCP in the setting of pancreatic and biliary diseases? Gastrointest Endosc. 2002;56(6 Suppl 2):S291-S293.
54 Ivatury RR, Nassoura ZE, Simon R, et al. Complex duodenal injuries. Surg Clin North Am. 1996;76:797-812.
55 Lillemoe KD, Barnes SA. Surgical palliation of unresectable pancreatic carcinoma. Surg Clin North Am. 1995;75:953-968.
56 Nyhus LM, Sheaff CM. Recurrent ulcer. In: Wastell C, Nyhus LM, Donahue PE, editors. Surgery of the esophagus, stomach and small intestine. New York: Little, Brown; 1994:531-541.
57 Health implications of obesity. National Institutes of Health Consensus Development Conference Statement. Ann Intern Med. 1985;103:1073-1077.
58 Balsiger BM, Luque de Leon E, Sarr MG. Surgical treatment of obesity: who is an appropriate candidate? Mayo Clin Proc. 1997;72:551-558.
59 Stellato TA, Crouse C, Hallowell PT. Bariatric surgery: creating new challenges for the endoscopist. Gastrointest Endosc. 2003;57:86-94.
60 Byrne TK. Complications of surgery for obesity. Surg Clin North Am. 2001;81:1181-1193.
61 Knol JA. Management of the problem patient after bariatric surgery. Gastroenterol Clin North Am. 1994;23:345-369.
62 Papavramidis ST, Theocharidis AJ, Zaraboukas TG, et al. Upper gastrointestinal endoscopic and histologic findings before and after vertical banded gastroplasty. Surg Endosc. 1996;10:825-830.
63 Balsiger BM, Murr MM, Poggio JL, et al. Bariatric surgery: surgery for weight control in patients with morbid obesity. Med Clin North Am. 2000;84:477-489.
64 Schirmer BD. Laparoscopic bariatric surgery. Surg Clin North Am. 2000;80:1253-1267.
65 Mason EE. Gastric surgery for morbid obesity. Surg Clin North Am. 1992;72:501-513.
66 Needleman BJ, Happel LC. Bariatric surgery: choosing the optimal procedure. Surg Clin North Am. 2008;88:991-1007.
67 Kremen AN, Linner JH, Nelson CH. Experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. 1954;140:439-448.
68 Kaminski DL, Hermann VM, Martin S. Late effects of jejunoileal bypass operation on hepatic inflammation, fibrosis and lipid content. Hepatogastroenterology. 1985;32:159-162.
69 Fisher BL, Barber AE. Gastric bypass procedures. In: Deitel M, Cowan GSJr, editors. Update: surgery for the morbidly obese patient. Toronto: FD-Communications; 2000:139-146.
70 Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515-529.
71 Murr MM, Balsiger BM, Kennedy FP, et al. Malabsorptive procedures for severe obesity: comparison of pancreaticobiliary bypass and very long limb Roux-en-Y gastric bypass. J Gastrointest Surg. 1999;3:607-612.
72 Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin North Am. 2001;81:1145-1179.
73 MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231:524-528.
74 Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279-289. discussion 289–291
75 Capella JF, Capella RF. An assessment of vertical banded gastroplasty-Roux-en-Y gastric bypass for the treatment of morbid obesity. Am J Surg. 2002;183:117-123.
76 Brolin RE. Gastric bypass. Surg Clin North Am. 2001;81:1077-1095.
77 Pope GD, Goodney PP, Burchard KW, et al. Peptic ulcer/stricture after gastric bypass: A comparison of technique and acid suppression variables. Obes Surg. 2002;12:30-33.
78 Schirmer B, Erenoglu C, Miller A. Flexible endoscopy in the management of patients undergoing Roux-en-Y gastric bypass. Obes Surg. 2002;12:634-638.
79 Peters M, Papasavas PK, Caushaj PF, et al. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography for benign common bile duct stricture after Roux-en-Y gastric bypass. Surg Endosc. 2002;16:1106.
80 Silecchia G, Catalano C, Gentileschi P, et al. Virtual gastroduodenoscopy: A new look at the bypassed stomach and duodenum after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2002;12:39-48.
81 Wright BE, Cass OW, Freeman ML. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225-232.
82 Baron TH. Vickers SM: Surgical gastrostomy placement as access for diagnostic and therapeutic ERCP. Gastrointest Endosc. 1998;48:640-641.
83 Sundbom M, Nyman R, Hedenstrom H, et al. Investigation of the excluded stomach after Roux-en-Y gastric bypass. Obes Surg. 2001;11:25-27.
84 Haber GB. Double balloon endoscopy for pancreatic and biliary access in altered anatomy (with videos). Gastrointest Endosc. 2007;66(3 Suppl):S47-S50.
85 Emmett DS, Mallat DB. Double-balloon ERCP in patients who have undergone Roux-en-Y surgery: a case series. Gastrointest Endosc. 2007;66:1038-1041.
86 Chu YC, Yang CC, Yeh YH, et al. Double-balloon enteroscopy application in biliary tract disease—its therapeutic and diagnostic functions. Gastrointest Endosc. 2008;68:585-591.
87 Kuga R, Safatle-Ribeiro AV, Faintuch J, et al. Endoscopic findings in the excluded stomach after Roux-en-Y gastric bypass surgery. Arch Surg. 2007;142:942-946.
88 Marceau P, Hould FS, Lebel S, et al. Malabsorptive obesity surgery. Surg Clin North Am. 2001;81:1113-1127.
89 Scopinaro N, Gianetta E, Pandolfo N, et al. Bilio-pancreatic bypass: proposal and preliminary experimental study of a new type of operation for the functional surgical treatment of obesity [in Italian]. Minerva Chir. 1976;31:560-566.
90 Doherty C. Vertical banded gastroplasty. Surg Clin North Am. 2001;81:1097-1112.
91 Verset D, Houben JJ, Gay F, et al. The place of upper gastrointestinal tract endoscopy before and after vertical banded gastroplasty for morbid obesity. Dig Dis Sci. 1997;42:2333-2337.
92 Nguyen DQ, Buchwald H, Bolman RM3rd, et al. Endoscopic laser treatment of obstructing polypropylene mesh after vertical banded gastroplasty. Gastrointest Endosc. 2000;51:616-617.
93 Pontiroli AE, Pizzocri P, Librenti MC, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab. 2002;87:3555-3561.
94 DeMaria EJ. Laparoscopic adjustable silicone gastric banding. Surg Clin North Am. 2001;81:1129-1144.
95 Baldinger R, Mluench R, Steffen R, et al. Conservative management of intragastric migration of Swedish adjustable gastric band by endoscopic retrieval. Gastrointest Endosc. 2001;53:98-101.
96 Weiner R. A prospective randomized trial of different laparoscopic gastric banding techniques for morbid obesity. Surg Endosc. 2001;15:63-68.
97 Izbicki JR, Bloechle C, Knoefel WT, et al. Surgical treatment of chronic pancreatitis and quality of life after operation. Surg Clin North Am. 1999;79:913-944.
98 Trede M. Technique of Whipple pancreatoduodenectomy. In: Trede M, Carter DC, Longmire WP, editors. Surgery of the pancreas. New York: Churchill Livingstone; 1997:487-498.
99 Farnell MB, Nagorney DM, Sarr MG. The Mayo Clinic approach to the surgical treatment of adenocarcinoma of the pancreas. Surg Clin North Am. 2001;81:611-623.
100 Pitt HA. Curative treatment for pancreatic neoplasms: standard resection. Surg Clin N Am. 1995;75:891-904.
101 Toledo-Pereyra LH. The pancreas: principles of medical and surgical Practice. New York: Wiley; 1985.
102 Braasch JW, Gagner M. Pylorus-preserving pancreatoduodenectomy—technical aspects. Langenbecks Arch Chir. 1991;376:50-58.
103 Pfau PR, Kochman ML, Lewis JD, et al. Endoscopic management of post-operative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63.
104 Elton E, Hanson BL, Qaseem T, et al. Diagnostic and therapeutic ERCP using an enteroscope and a pediatric colonoscope in long-limb surgical bypass patients. Gastrointest Endosc. 1998;47:62-67.
105 Gostout CJ, Bender CE. Cholangiopancreatography, sphincterotomy, and common duct stone removal via Roux-en-Y limb enteroscopy. Gastroenterology. 1988;95:156-163.
106 Chahal P, Baron TH, Poterucha JJ, et al. Endoscopic retrograde cholangiography in post-orthotopic liver transplant population with Roux-en-Y biliary reconstruction. Liver Transpl. 2007;13:1168-1173.
107 Lim PL, Porter KG. Air as contrast for cholangiography in a patient with a history of allergy to radiopaque media. Endoscopy. 1999;31:S9.
108 Caroli-Bosc FX, Demarquay JF, Peten EP, et al. Endoscopic management of sump syndrome after choledochoduodenostomy: retrospective analysis of 30 cases. Gastrointest Endosc. 2000;51:180-183.