CHAPTER 98 Postoperative Spinal Infections
Incidence/Epidemiology
Postoperative wound infections are the most common complication following spinal surgery. The incidence documented in the literature has historically been quite variable with reported ranges from 0.5% to 20%.1–7 This discrepancy is in part to significant variation in case complexity, use of instrumentation, and surgical approach in the reported cases. In general, increasing the complexity and invasiveness of the surgery correlates with a higher incidence of infection.
Historically, lower-risk spinal surgeries include those that do not require instrumentation. Discectomy and laminectomy have reported incidences of infection less than 3%. With the addition of instrumentation, the incidence of postoperative infection increases to greater than 12% in some studies.8–18 Specifically, lumbar discectomy has had a reported incidence of 0.7%, and using a microscope for the procedure increases the incidence to 1.4%. In the United States, the National Nosocomial Infections Surveillance (NNIS) System, a Centers for Disease Control and Prevention (CDC) orchestrated voluntary performance-measurement system, has reported a 1.25% rate of surgical site infection following laminectomy and a 2.1% rate following laminectomy with noninstrumented fusion.19
Consistently throughout the literature, cases that require more extensive soft tissue dissection, longer operative time, greater blood loss, more significant soft tissue devitalization, or the creation of dead space have an increased infection rate. One study comparing infection rates in patients undergoing discectomy alone and those undergoing discectomy and fusion showed infection rates of 1% versus 6%, respectively. In other reports fusion without instrumentation has been associated with an infection rate ranging from 0.4% to 4.3%.20 The use of devitalized bone graft material in fusions results in an infection rate from 1% to 5%.
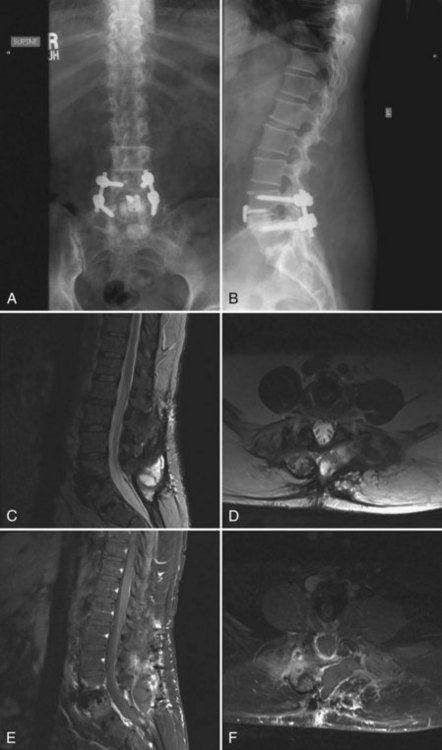
As the use of instrumentation has become more commonplace, attention must be paid to a possible associated increased infection risk. Colonization of implanted devices occurs in upwards of 50% of patients, although most do not display clinical symptoms of infection. Although implants rarely act as the initial source of infection, they may become a nidus for inoculation and subclinical growth of infectious organisms. The implant provides an avascular surface on which bacteria can create a glycocalyx, which serves as a barrier to the host immune response and antibiotic treatment. In addition, micromotion can create metallosis and subsequent granulomas, which may become a potential site for bacterial colonization. Other theories postulate that local soft tissue inflammation and postoperative seromas may serve as a potential cause for the increased infection risk seen with instrumented fusions (Fig. 98–1). Due to these unique risk factors, instrumented fusions have reported postoperative infection rates of up to 20%8,21 with dramatic variation in the reported literature. Moe reported a postoperative infection rate of 7% in cases with Harrington instrumentation.22 More recent literature reports infection rates in elective instrumented surgical cases between 2.8% and 6%.8,23,24 Many authors feel that the actual infection risk with the use of spinal instrumentation is between 5% and 6%.16–18,23,25
Spinal trauma patients represent a unique population that has an increased risk for developing postoperative infections. The significant soft tissue devitalization and devascularization caused by the traumatic event results in local hypoxia leading to tissue necrosis, edema, acidosis, and hematoma formation. This combination results in a media optimal for bacterial proliferation isolated from the host defenses.26 Systemically, the patient sustaining major trauma shows a hyperinflammatory state with alterations in the normally tightly controlled homeostasis of pro- and anti-inflammatory cytokine levels. The resultant imbalance leads to a state of immunosuppression that is thought to increase susceptibility to infection. In addition, comorbid factors such as age, medical conditions, poor nutritional status, and body habitus cannot be controlled in the same manners that they are in elective surgery. The presence of complete neurologic injury significantly increases the risk of postoperative infection. In the largest clinical series investigating 256 surgically treated traumatic spinal injuries, the rate of postoperative wound infections was 9.4% compared with an infection rate of 3.7% seen in patients undergoing elective spinal surgery during the same time period at the same hospital.27 Similar reviews have found postoperative infection rates in spinal trauma patients ranging from 9% to 15%,8,28,29 which is greater than the previously discussed average infection rate seen in elective spinal surgeries.
Anterior spinal procedures appear to be less susceptible to infection than posterior procedures. Infection rates following anterior cervical spinal surgery have been reported in the literature to be as low as 0% to 1%.30–32 Anterior thoracic and lumbar surgery also display significantly less infection risk than their posterior counterparts, with rates 50% lower than those occurring after posterior surgery. The infection rates for anterior approaches are likely decreased by multiple factors including better vascularity of the spinal column and decreased dead space creation. Furthermore, the incidence of infection in combined anterior and posterior procedures does not appear to be greater than posterior surgery alone.27
Risk Factors
Obese patients are also considered at high risk for developing postoperative infections.33–35 Overweight patients often require more extensive dissection through poorly vascularized adipose tissue. The resulting tissue devitalization and fat necrosis result in an environment favoring bacterial growth and proliferation. In addition, the increased operative time and blood loss necessary with obese patients increase their risk of infection. Obesity in itself is a risk factor for malnutrition, diabetes, and other medical comorbidities, further contributing to a poor healing environment with diminished immunogenic potential.
Nonmodifiable risk factors that may increase the susceptibility to infection must also be evaluated before surgery. Thorough assessment of a patient’s medical history during the preoperative evaluation may reveal systemic comorbidities that should be identified and optimized before surgery. In all patients, preoperative infections, whether in the spine or elsewhere, should be addressed and treated before undergoing elective surgery.10,23,30,36 Conditions such as rheumatoid arthritis, acquired immunodeficiency syndrome (AIDS), adrenocortical insufficiency, long-term corticosteroid use, and malignancy may pose significant risk for developing postoperative infections.37–39 A thorough discussion of potential complications associated with these confounding medical conditions is important during preoperative counseling. Medical optimization of these conditions may limit potential postoperative complications. Although age is not considered an independent risk factor, older patients are more likely to have comorbidities associated with an increased risk of postoperative infection.
Microbiology
Three potential sources are hypothesized to be responsible for postoperative infections: (1) direct inoculation during the operative procedure, (2) contamination during the early postoperative period, and (3) hematogenous seeding.21,40–43 Of these, direct inoculation during the surgery is the most common, making aseptic technique and the appropriate use of prophylactic antibiotics of paramount importance.
Gram-positive cocci are the most common pathogens responsible for acute postoperative infections. The most commonly reported organism in the literature is Staphylococcus aureus, which causes greater than 50% of the infections in some reports.23,31,34 Other common gram-positive species that cause postsurgical infections include Staphylococcus epidermidis and β-hemolytic streptococci. Common gram-negative organisms cultured from infected surgical sites include Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter cloacae, Bacteroides, and Proteus species.
Infections that present greater than 1 year after surgery are generally caused by low-virulence organisms such as coagulase-negative Staphylococcus, Propionibacterium acnes, and diphtheroids.30,41 These organisms can be present as normal skin flora, and it is hypothesized that prolonged surgical wound drainage and inflammation may result in these infections. These low-virulent organisms are usually rapidly cleared by the host immune response with appropriate treatment and generally do not result in a clinical sepsis. In a retrospective review of postoperative infections presenting more than 1 year after surgery, 10 of 11 patients with cultures incubated for longer than 1 week grew low-virulence skin organisms.44
Hematogenous spread can also cause surgical site infections. These blood-borne infections are usually due to highly virulent organisms including gram negative bacteria. These infections are often associated with systemic illness and sometimes have grave consequences such as multisystem organ failure. Due to repeated cannulization of the venous system, intravenous drug users have a higher incidence of gram-negative infections, as do patients who have prolonged hospital admissions.34
Diagnostic Modalities
Laboratory Testing
ESR elevates following spinal surgery and may not normalize until several weeks postoperatively. Variations in this time period to normalization have been reported. In one study, ESR rarely elevated to levels greater than 25 mm/hour and returned to baseline levels by the third postoperative week.45 Another study showed that ESR elevation was prolonged and lasted up to 6 weeks postoperatively.46 Peak ESR levels have been shown to correlate with the degree of invasiveness of the surgery, with more extensive surgeries causing higher ESR elevations than less invasive procedures.47
As with ESR, CRP values rise sharply during the initial postoperative period. Unlike ESR, however, CRP decreases to baseline levels more rapidly. CRP levels generally peak on the third day postoperatively and return to baseline within 10 to 14 days. This rapid normalization makes CRP a more sensitive indicator of infection and a more useful diagnostic tool when determining the presence of infection, especially in the acute and subacute postoperative period.46–50 Elevated ESR or CRP outside of this postoperative period can indicate a developing infection and can be used to monitor the efficacy of treatment.
The precise and accurate identification of the culprit organism is a critical step in the treatment of a postoperative spinal infection. Cultures obtained from the superficial wound are often contaminated with skin flora and can confuse the diagnostic workup. Some authors suggest early aspiration of a suspicious wound in order to attempt to isolate the infectious organism.21 If there is no fluctuant mass to aspirate, as is often the case, computed tomography (CT) or fluoroscopic guidance can be used to accurately obtain a deep culture from the affected area. Frequently, fine-needle aspiration of the affected region does not provide ample tissue for an accurate diagnosis. We prefer to obtain a core biopsy specimen in order to ensure that an adequate sample is provided to the laboratory. Blood cultures can reveal the responsible organism if taken in a septic individual before the initiation of antibiotics. If the blood cultures are positive and provide identification of an organism, it can be presumed that the same organism is the cause of the spinal infection and a biopsy of the spinal infection site is not necessary. The most accurate cultures are those obtained during the surgical débridement before the administration of antibiotics. In many cases, however, such a surgical intervention is not necessary and these surgical cultures are not obtained.
Imaging
Plain radiographs are often the first imaging modality obtained during workup of a suspected infection. Findings on plain radiographs frequently can be quite subtle, and up to 4 weeks are often required to pass before radiographs show evidence of infection.50 Inspection of the instrumentation for loosening or adjacent bony lysis may be clues of an infection. In cases of postoperative discitis, disc space narrowing is the first radiographic finding. This change generally occurs 4 to 6 weeks postoperatively. Early infectious disc space changes, however, may be difficult to distinguish from degenerative changes. Paravertebral soft tissue swelling may indicate the presence of an abscess, especially in the retropharyngeal space or paraspinal musculature. More significant radiographic findings such as reactive bone formation, endplate destruction, osteolysis, and deformity indicate a more significant infectious process and usually require at least 2 months to develop.
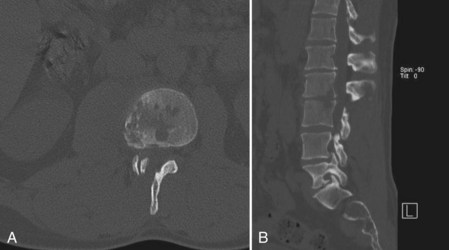
CT provides a more detailed view of spinal anatomy and may allow for earlier detection of postoperative infections than plain radiographs. Endplate changes, bony lysis, and/or soft tissue fluid collections can indicate early infection (Fig. 98–2). As the infection progresses, more significant bony and intervertebral disc destruction may be seen. Implant-related artifact may distort the detail and limit the usefulness of CT scans in patients with spinal instrumentation. CT-guided biopsies can also be used to provide an aspirate for culture or tissue biopsy from infected soft tissue or bone as noted earlier.
Nuclear medicine studies are sometimes used to supplement other radiographic methods when working up a postoperative infection. Unlike magnetic resonance imaging (MRI) and CT, nuclear medicine studies are not limited by the implant-associated artifacts. Bone scans are often nonspecific and may show generalized uptake around the surgical site in a postoperative spinal infection.50 Although gallium-67 and technetium-99m scans provide early evidence of postoperative infections, their diagnostic value is somewhat compromised relative to studies evaluating the appendicular skeleton. Diagnosis of early postoperative disc space infection is better achieved with gallium-67 relative to technetium-99.51,52 Indium 111-labeled WBC scans will often have limited usefulness because of their poor specificity, particularly in the early postoperative period. Technetium-labeled ciprofloxacin, when combined with single photon emission computed tomography (SPECT), has been shown to have improved sensitivity and specificity over other nuclear medicine modalities, particularly if performed more than 6 months after surgery.53
MRI is the most important imaging modality when evaluating postoperative spinal infections. Because of its high contrast resolution, MRI with and without intravenous gadolinium contrast is the most effective imaging technique available. Relative to other imaging modalities, MRI is both highly sensitive (93%) and specific (96%) when evaluating spinal infections.54–56 As with other imaging techniques, it may be difficult to distinguish nonpathologic postoperative changes from infections on MRI scans obtained in the early postoperative period. Thus accuracy and reliability of the study is dependent on the elapsed time from the date of surgery, the level of clinical suspicion, and correlation with other diagnostic tools. Spinal instrumentation, particularly when composed of stainless steel, can cause significant MRI artifact and severely limit the diagnostic utility of the study.
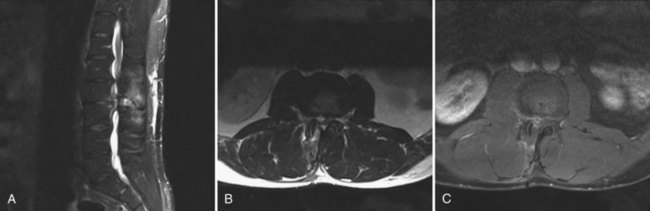
MRI can identify, with high sensitivity and specificity, postoperative osteomyelitis, discitis, and epidural abscesses. An epidural abscess will display a T1 isointense fluid collection with potential obliteration of the otherwise well-defined neural elements, and the T2-weighted images show significant increased intensity. Abscesses will display ring enhancement on T1 images following the addition of IV gadolinium (Fig. 98–3). Osteomyelitis appears as areas of vertebral body and disc space hypointensity on T1-weighted images and hyperintensity on T2 images. In addition, there is a loss of definition between the vertebral bodies and the intervertebral disc space.55–57
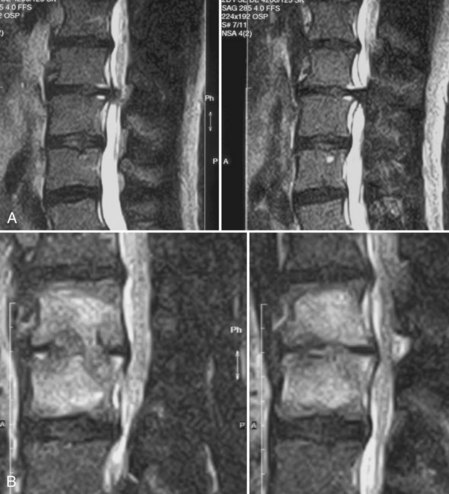
Boden and colleagues58 compared the early postoperative MRI changes in noninfected individuals with those with confirmed discitis. The most reliable difference seen in the discitis group was increased signal intensity of the adjacent vertebral body on T1-weighted images that was not present on preoperative studies. In addition, T2-weighted images showed increased signal intensity in the disc and adjacent marrow (Fig. 98–4). Only 1 of 17 control group patients displayed these signal intensity changes in the adjacent vertebral marrow. All cases of discitis displayed intervertebral disc signal enhancement with the addition of a contrast agent.58
Classification
A classification scheme proposed by Thalgott and colleagues59 was adapted from the previously described Cierny classification for the diagnosis and management of osteomyelitis. Thalgott’s classification scheme is based on the severity of infection and ability for host response. The severity of infection is divided into three groups: (1) superficial or deep infection with a single organism, (2) deep infection with multiple organisms, and (3) deep infection and myonecrosis with multiple or resistant organisms. Host response was divided into three physiologic classes: (1) normal systemic defenses, metabolic capabilities, and vascularity; (2) local or multiple systemic diseases including cigarette smoking; and (3) immunocompromised or severely malnourished. Using this classification, Thalgott studied 32 patients with postoperative infections following fusion with instrumentation. Patients with group 1 infections were successfully treated with simple irrigation, débridement, and primary closure over a closed suction drainage tube without an irrigant system. Patients with group 2 infections required an average of three irrigations and débridements and displayed greater success when treated with closed inflow-outflow suction irrigation systems when compared with simple suction drainage systems without constant inflow irrigation. Group 3 patients were difficult to manage and had poor overall outcomes. In addition, cigarette smoking was found to be a significant risk factor for developing postoperative infection.59
Prevention
The use of preoperative prophylactic antibiotics is routine for most spine surgeons. Although there is an increasing body of evidence-based medicine that supports the use of prophylactic antibiotics in spinal surgery, there is still no level I evidence clearly defining their efficacy. In a meta-analysis pooling data from six separate randomized controlled trials (RCTs), Barker and colleagues60 found an infection rate of 2.2% following the administration of antibiotics and 5.9% if antibiotics were not administered. In a prospective RCT, Pavel and colleagues61 compared infection rates following orthopedic procedures with and without preoperative antibiotic prophylaxis with cephalozidine. In a subgroup analysis of spinal procedures, infection rates were 3% in patients treated with antibiotics and 9.2% in those without.61 In another RCT comparing the efficacy of cefazolin relative to placebo in 141 patients, Rubinstein’s group showed that the use of preoperative antibiotics decreased the incidence of postoperative infection from 12.7% in untreated controls to 4.3% in those receiving prophylactic antibiotics.62
In order for antibiotics to be effective prophylactic agents, they must have antimicrobial action against the most common bacteria encountered and must be present in tissues adjacent to the surgical site in sufficient concentrations. Current recommendations suggested that antibiotic administration should begin 30 minutes to 1 hour preoperatively to ensure adequate antibiotic levels at the surgical site at the time of skin incision. With prolonged operative time, serum and tissue antibiotic levels decrease.63,64 Some authors recommend repeating the dose of antibiotics after 4 hours of surgery, and our group follows this protocol. The limited data regarding redosing patients intraoperatively and postoperatively does not show any significant differences in infection rates, however.65–67 In a retrospective cohort, Dobzyniak and colleagues65 compared infection rates in 433 patients who received preoperative and postoperative doses of antibiotics to 201 patients who received only a single dose of preoperative antibiotics. No significant differences in infection rates were detected in this study.65
The human spine is a unique environment because of its combination of bony structures, intervertebral discs, and adjacent soft tissue musculature. Antibiotics used for the treatment of spinal infections need to be able to penetrate the involved structures to be maximally effective. Studies have documented the half-life and penetration of antibiotics into bone and intervertebral discs. Cefazolin displays a longer half-life in the serum and bone relative to other cephalosporins.62,68,69 Human and animal studies evaluating the penetration of antibiotics into intervertebral discs have provided mixed results.68–76 With increased age and decreased disc vascularity, systemic antibiotic penetration into the disc is impeded. In addition, the environment within a degenerating disc likely presents a barrier to obtaining consistent inhibitory levels of antibiotics. Penetration of antibiotics into the adult intervertebral disc depends on passive diffusion from adjacent bony structures and cartilaginous endplates, as well as from the annulus fibrosis.77 Studies have demonstrated the ability of cefazolin to penetrate the disc, although concentrations are higher in the annulus fibrosis relative to the nucleus pulposus.68,70,71 Evidence in the literature suggests that the molecular charge of an antibiotic is an important determinant of its ability to diffuse into the disc. Studies have evaluated the penetration of negatively charged antibiotics into the positively charged disc when adequate serum concentrations are maintained.68,70,78 Positively charged antibiotics such as gentamicin and vancomycin have been shown to freely penetrate the annulus fibrosis and nucleus pulposus, whereas negatively charged antibiotics such as penicillin are found in the annulus fibrosis but not the nucleus pulposus. The use of antibiotic delivery systems to circumvent this problem has been studied. In a prospective cohort study, Rohde evaluated the infection rate following discectomy in 1712 patients, 1134 of whom received prophylactic gentamicin-containing collagenous sponges at the cleared disc space. Patients receiving the prophylactic antibiotic showed a 0% infection rate relative to a 3.7% infection rate in those that did not.79 Gentamicin-associated toxicity, however, makes it a suboptimal choice for routine surgical prophylaxis.
As a result of significant soft tissue disruption and the creation of a potential space, hematoma formation following spinal surgery is a frequent occurrence. Hematomas provide an excellent milieu for bacteria proliferation. In an attempt to address this potential complication, many surgeons use closed suction devices during the postoperative course. The effectiveness of postoperative suction drains to prevent infection has not been clearly demonstrated in the literature. In a prospective randomized study examining 83 patients undergoing extensive lumbar spine surgery, no infections were reported in those who received and those who did not receive closed suction drainage postoperatively.80 Similar results are reported elsewhere in the literature.81,82
Irrigation solutions are often used intraoperatively in an effort to prevent or treat infections. Commonly used irrigants include solutions of bacitracin, iodine, chlorhexidine, neomycin, or a combination of triple antibiotics including neomycin, bacitracin, and polymyxin. No significant clinical data clearly support the use of these antibiotic irrigants in spinal surgery. In vitro studies, however, have shown a significant reduction in bacterial counts with use of the additives.83–85 Intraoperative anaphylactic reactions have been reported with the use of bacitracin irrigation, especially when combined with cellsaver blood recycling devices, although these reactions are extremely rare. Pulsatile lavage has improved the removal of contaminants from bone and soft tissue. In vitro effects have shown significant reduction in bacterial colony counts, but the reported toxic effects on osteoblasts and osteoclast by the pulsatile irrigation must be taken into account when using this technique.
Appropriate maintenance of the operative room environment is important to reduce the potential for contamination. The use of methods of decreasing airborne bacteria can be implemented in an attempt to decrease infection rates. Operating in a vertical laminar flow operating room has been suggested in the literature as a method of reducing infection rates and is used by some institutions. Gruenberg and colleagues86 described a significant reduction in infection rates in a retrospective study comparing conventional and vertical laminar flow operating rooms. Basic principles of sterile technique should be adapted for every spinal procedure. Maintenance of a sterile field and double gloving with intermittent glove changes decrease the potential of surgeon contamination of the wound.87 The use of an iodine-impregnated adherent plastic barrier over the operative field has been suggested by some authors as an additional method to decrease inoculation of the wound.17 Limiting blood loss with meticulous attention to hemostasis, débridement of necrotic tissue, and periodic release of retractors helps to minimize possible sites of infection.88 In addition, flash sterilization of implantable devices is discouraged unless absolutely necessary.
Management
The timing of infection in the postoperative spine is significantly influenced by the mode of inoculation and virulence of the organism. Early presenting infections are generally a result of more virulent organisms such as MRSA and gram-negative bacteria89 and are the result of intraoperative seeding. Delayed infections are typically caused by low-virulence organisms such as skin flora, which may be introduced intraoperatively and subsequently adhere to instrumentation while encased in a glycocalyx biofilm.
The fundamental principles involved in the management of a postoperative infection include prompt diagnosis with isolation of a specific organism, if possible, and initiation of appropriate medical and surgical management. As previously discussed, image-guided biopsy can be used to provide specimens to accurately identify the offending organism. Our group generally performs a CT or fluoroscopically guided biopsy before proceeding with an open surgical biopsy (Fig. 98–5). Additionally, a minimum of two sets of blood cultures are sent on all patients with a suspected spinal infection. If the blood cultures identify an organism, then that same organism can be presumed to be the cause of the spinal infection and invasive biopsies are not necessary. After an organism has been identified, appropriate antibiotic therapy should be initiated. Extremely superficial infections such as a stitch abscess can be treated with 2 weeks of oral antibiotics and close clinical follow-up. For any significant infection, however, a minimum of 6 weeks of IV antibiotics followed by 6 weeks of oral antibiotics are used. ESR and CRP measurements are used to monitor the response to treatment.
Most significant superficial and deep infections will require surgical débridement in addition to IV antibiotic therapy. When surgical débridement is used, a meticulous approach must be taken to thoroughly eradicate the infection. The surgical treatment of a postoperative infection includes débridement of each layer of the wound. At each layer, assessment of tissue devitalization and possible communication with underlying planes must be assessed. In addition, appropriate specimens for staining and cultures should be taken from each layer before the initiation of intraoperative antibiotics. We routinely send all specimens for bacterial studies including aerobic, anaerobic, fungal, and acid-fast studies. The surgical incision should excise all dermal margins that appear infected. In the underlying subcutaneous layers, infectious material may be encountered and must be appropriately removed along with all necrotic tissue. If the underlying deep fascial layers appear to be intact and the infection limited to the subcutaneous planes, some have advocated aspiration of the deep wound and exploration only if the Gram stain results are positive.16 Other authors, however, recommend routine exploration and débridement of the deep layers to prevent missing a potentially disastrous deep infection. We follow this latter recommendation. In our experience, there is usually some communication between the superficial and deep surgical planes.
When involved with the infection, the deep fascial layers should be opened and all loose tissue and foreign material should be removed. After specimens for microbiologic studies have been obtained, broad-spectrum antibiotics can be initiated. Bone graft that appears to be significantly infected or is loosened by the débridement should also be removed.17,18,59,88 If not obviously necrotic or infected, the bone graft should be left in place.
Following sufficient débridement and irrigation of the wound, assessment of the wound and a plan for closure must be devised. There remains debate in the literature as to whether infected surgical wounds should be closed primarily after the initial surgical débridement versus a delayed staged closure with intermittent débridements.17,44,90 We prefer a primary layered closure because wounds that remain open are susceptible to superinfection and wound contracture. In cases with significant myonecrosis that require multiple repeat débridements, primary closure may not be possible. Use of a closed suction drain for a few days postoperatively is generally advocated. Closure over a suction irrigation system or packing the wound with a vacuum-assisted sponge system has been described.30,91,92 In patients with significant infections or soft tissue involvement, the use of reconstructive soft tissue techniques including flap coverage may be necessary.
Spinal Instrumentation and Infections
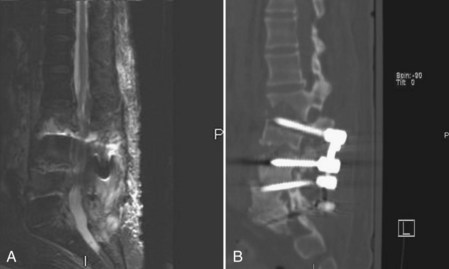
The use of instrumentation in spinal procedures results in a greater risk of developing a postoperative infection. Reported infection rates following instrumented posterior lumbar fusion range from 2.8% to 20%, with most authors suggesting a true infection rate of approximately 5% to 6%. In patients with extensive infections in the presence of instrumentation, even repeated surgical débridements along with intravenous antibiotics may fail to resolve the infection. The metallic implants provide a surface for the formation of a glycocalyx biofilm that is difficult to penetrate with systemic antibiotics. During the débridement of infections with instrumentation, the implants should be routinely inspected. If the implants show obvious signs of loosening, they should be removed and possibly replaced with new instrumentation if necessary. Removal of infected instrumentation that remains well fixed has long been debated in the literature. Some authors advocate complete removal of all instrumentation, independent of fixation and fusion status, because of the difficulty of eliminating the infection without removal.17,40,44,93–95 Retention of spinal instrumentation, however, at the time of débridement has been recommended by many. Authors have described leaving well-fixed instrumentation in place following thorough débridement to prevent possibly catastrophic spinal instability.* Additionally, these authors have shown successful eradication of both anterior and posterior spinal infections with retained instrumentation. Vertebral column malalignment, spinal cord compression, and paralysis are potential complications associated with instability if fusion is not complete at the time of instrumentation removal (Fig. 98–6). An approach taken by many is to administer long-term antibiotics to suppress the infection until a solid fusion is obtained. If the infection persists, the instrumentation can be removed after the arthrodesis has been achieved. One potential complication with this method is subsequent instability following instrumentation removal if the patient has an undetected pseudarthrosis. Titanium implants are less adherent to the bacterial glycocalyx and are hence favored over stainless steel implants if instrumentation needs to be reimplanted in an infected site.104 If loose instrumentation is encountered during the débridement and there is a potential for spinal instability, the instrumentation should be replaced.
Complex Wound Closure
Severe postoperative spinal infections may result in significant soft tissue defects that require complex wound management. The goals of treating these complex wounds include obliteration of dead space, creation of adequate coverage for prominent bone or instrumentation, and enhancing vascularity for improved healing. Methods that may be used to treat these wounds include flap coverage and healing by secondary intention. Local, rotational, and free flaps are often used for coverage and have shown successful results. Even in complex wounds with deficient local tissue, muscle flaps can bring increased vascularity and adequate soft tissue coverage from distal sites to allow for healing while protecting instrumentation and bone graft.105,106 Healing of complex wounds by secondary intention using vacuum-assisted closure devices has become popular. These devices create negative pressure in the wound, which results in increased vascularity and cellular proliferation. They help form granulation tissue over bone and instrumentation and have shown success in healing large open wounds.107,108 Before surgical intervention of patients who may require soft tissue reconstruction, it is critical that patients are optimized medically and nutritionally and plastic and reconstructive surgeons should be involved before the surgical intervention.
Length of Antibiotic Treatment
Appropriate antibiotic treatment is critical to the successful eradication of any postoperative infection. Consultation with an infection disease specialist is usually obtained to assist in formulating a treatment plan. Once antibiotics have been initiated, close follow-up of the culture results is required to appropriately tailor the subsequent treatment regimen. During the treatment period, adjustment of the antibiotic might be necessary depending on the patient’s response to treatment. Intravenous antibiotics are usually administered for approximately 6 weeks followed by approximately 6 weeks, or longer, of oral antibiotics. The specific time for treatment depends on the virulence of the infectious organism and its sensitivity to the antibiotics. Regular follow-up of infectious laboratory markers including WBC, ESR, and CRP assists in determining the response to treatment. Postsurgical spine infections with antibiotic-resistant organisms require special considerations including patient isolation and treatment with appropriate antibiotics. Fortunately, new classes of antibiotics have been developed to supplement the previously limited options for treatment of infections such as MRSA and vancomycin-resistant enterococci (VRE).109 Occasionally, prolonged antibiotic treatment may be necessary for suppression of infection in patients with retained instrumentation or recalcitrant infections.
Summary
Pearls
Pitfalls
Key Points
1 Riley LH, Banovac K, Martinez OV, Eismont FJ. Tissue distribution of antibiotics in the intervertebral disc. Spine. 1994;19:2619-2625.
2 Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine. 2004;29:1066-1068.
3 Boden SD, Davis DO, Dina TS, et al. Postoperative diskitis: distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184:765-771.
4 Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 1992;17:400-404.
5 Weiss LE, Vaccaro AR, Scuderi G, et al. Pseudarthrosis after postoperative wound infection in the lumbar spine. J Spinal Disord. 1997;10:482-487.
1 El-Gindi S, Aref S, Salama M, et al. Infection of intervertebral discs after operation. J Bone Joint Surg Br. 1976;58:114-116.
2 Ford LT, Key JA. Postoperative infection of intervertebral disc space. South Med J. 1955;48:1295-1303.
3 Lindholm TS, Pylkkanen P. Discitis following removal of intervertebral disc. Spine. 1982;7:618-622.
4 Stolke D, Sollmann WP, Seifert V. Intra- and postoperative complications in lumbar disc surgery. Spine. 1989;14:56-59.
5 Pilgaard S. Discitis (closed space infection) following removal of lumbar inervertebral disc. J Bone Joint Surg Am. 1969;51:713-716.
6 Wright TE, Orr RJ, Haberkern CM, Walbergh EJ. Complications during spinal anesthesia in infants: high spinal blockade. Anesthesiology. 1990;73:1290-1292.
7 Whitecloud TS3rd, Butler JC, Cohen JL, Candelora PD. Complications with the variable spinal plating system. Spine. 1989;14:472-476.
8 Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566-569.
9 Brown EM, Pople IK, de Louvois J, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29:938-945.
10 Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine. 1996;21:2163-2169.
11 Graziano GP, Sidhu KS. Salvage reconstruction in acute and late sequelae from pyogenic thoracolumbar infection. J Spinal Disord. 1993;6:199-207.
12 Karlsson MK, Hasserius R, Olerud C, Ohlin A. Posterior transpedicular stabilisation of the infected spine. Arch Orthop Trauma Surg. 2002;122:522-525.
13 Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114-121.
14 Labbe AC, Demers AM, Rodrigues R, et al. Surgical-site infection following spinal fusion: a case-control study in a children’s hospital. Infect Control Hosp Epidemiol. 2003;24:591-595.
15 Li S, Zhang J, Li J, et al. Wound infection after scoliosis surgery: an analysis of 15 cases. Chin Med Sci J. 2002;17:193-198.
16 Perry JW, Montgomerie JZ, Swank S, et al. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis. 1997;24:558-561.
17 Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine. 2001;26:1990-1996.
18 Richards BS. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:524-529.
19 National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470-485.
20 Li YZ. [Wound infection after spinal surgery: analysis of 15 cases]. Zhonghua Wai Ke Za Zhi. 1991;29:484-486. 524-525
21 Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine. 2000;25:2461-2466.
22 Moe JH. Complications of scoliosis treatment. Clin Orthop Relat Res. 1967;53:21-30.
23 Massie JB, Heller JG, Abitbol JJ, et al. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99-108.
24 Hodges SD, Humphreys SC, Eck JC, et al. Low postoperative infection rates with instrumented lumbar fusion. South Med J. 1998;91:1132-1136.
25 Roberts FJ, Walsh A, Wing P, et al. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366-370.
26 Kalicke T, Schlegel U, Printzen G, et al. Influence of a standardized closed soft tissue trauma on resistance to local infection. An experimental study in rats. J Orthop Res. 2003;21:373-378.
27 Blam OG, Vaccaro AR, Vanichkachorn JS, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine. 2003;28:1475-1480.
28 Kornberg M, Rechtine GR, Herndon WA, et al. Surgical stabilization of thoracic and lumbar spine fractures: a retrospective study in a military population. J Trauma. 1984;24:140-146.
29 McAfee PC, Bohlman HH. Complications following Harrington instrumentation for fractures of the thoracolumbar spine. J Bone Joint Surg Am. 1985;67:672-686.
30 Weinstein MA, McCabe JP, Cammisa FPJr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422-426.
31 Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975-980.
32 Zeidman SM, Ducker TB, Raycroft J. Trends and complications in cervical spine surgery:1989-1993. J Spinal Disord. 1997;10:523-526.
33 Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-210.
34 Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124-128.
35 Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27:83-86.
36 Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277-285.
37 Cruse PJ, Foord R. The epidemiology of wound infection. A v10-year prospective study of 62,939 wounds. Surg Clin North Am. 1980;60:27-40.
38 Calderone RR, Thomas JCJr, Haye W, Abeles D. Outcome assessment in spinal infections. Orthop Clin North Am. 1996;27:201-205.
39 Mishriki SF, Law DJ, Jeffery PJ. Factors affecting the incidence of postoperative wound infection. J Hosp Infect. 1990;16:223-230.
40 de Jonge T, Slullitel H, Dubousset J, Miladi L, Wicart P, Illes T. Late-onset spinal deformities in children treated by laminectomy and radiation therapy for malignant tumours. Eur Spine J. 2005;14:765-771.
41 Richards BS, Herring JA, Johnston CE, et al. Treatment of adolescent idiopathic scoliosis using Texas Scottish Rite Hospital instrumentation. Spine. 1994;19:1598-1605.
42 Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 1997;22(20):2444-2450. discussion 2450-2451
43 Heggeness MH, Esses SI, Errico T, Yuan HA. Late infection of spinal instrumentation by hematogenous seeding. Spine. 1993;18:492-496.
44 Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909-1912.
45 Kapp JP, Sybers WA. Erythrocyte sedimentation rate following uncomplicated lumbar disc operations. Surg Neurol. 1979;12:329-330.
46 Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 1992;17:400-404.
47 Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine. 1991;16:1049-1050.
48 Fouquet B, Goupille P, Jattiot F, et al. Discitis after lumbar disc surgery. Features of “aseptic” and “septic” forms. Spine. 1992;17:356-358.
49 Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wien). 1995;136:145-150.
50 Silber JS, Anderson DG, Vaccaro AR, et al. Management of postprocedural discitis. Spine J. 2002;2:279-287.
51 Bruschwein DA, Brown ML, McLeod RA. Gallium scintigraphy in the evaluation of disk-space infections: concise communication. J Nucl Med. 1980;21:925-927.
52 Norris S, Ehrlich MG, Keim DE, et al. Early diagnosis of disc-space infection using Gallium-67. J Nucl Med. 1978;19:384-386.
53 De Winter F, Gemmel F, Van Laere K, et al. 99mTc-ciprofloxacin planar and tomographic imaging for the diagnosis of infection in the postoperative spine: experience in 48 patients. Eur J Nucl Med Mol Imaging. 2004;31:233-239.
54 Vaccaro AR, Shah SH, Schweitzer ME, et al. MRI description of vertebral osteomyelitis, neoplasm, and compression fracture. Orthopedics. 1999;22:67-73. quiz 74-75
55 Djukic S, Genant HK, Helms CA, Holt RG. Magnetic resonance imaging of the postoperative lumbar spine. Radiol Clin North Am. 1990;28:341-360.
56 Djukic S, Lang P, Morris J, et al. The postoperative spine. Magnetic resonance imaging. Orthop Clin North Am. 1990;21:603-624.
57 Djukic S, Vahlensieck M, Resendes M, Genant HK. The lumbar spine: postoperative magnetic resonance imaging. Bildgebung. 1992;59:136-146.
58 Boden SD, Davis DO, Dina TS, et al. Postoperative diskitis: distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184:765-771.
59 Thalgott JS, Cotler HB, Sasso RC, et al. Postoperative infections in spinal implants. Classification and analysis—a multicenter study. Spine. 1991;16:981-984.
60 Barker FG. 2nd: Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002;51:391-400. discussion 400-1
61 Pavel A, Smith RL, Ballard A, Larson IJ. Prophylactic antibiotics in elective orthopedic surgery: a prospective study of 1,591 cases. South Med J. 1977;70(Suppl 1)):50-55.
62 Rubinstein E, Findler G, Amit P, Shaked I. Perioperative prophylactic cephazolin in spinal surgery. A double-blind placebo-controlled trial. J Bone Joint Surg Br. 1994;76:99-102.
63 Swoboda SM, Merz C, Kostuik J, et al. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg. 1996;131:1165-1171. discussion 1171-1172
64 Polly DWJr, Meter JJ, Brueckner R, et al. The effect of intraoperative blood loss on serum cefazolin level in patients undergoing instrumented spinal fusion. A prospective, controlled study. Spine. 1996;21:2363-2367.
65 Dobzyniak MA, Fischgrund JS, Hankins S, Herkowitz HN. Single versus multiple dose antibiotic prophylaxis in lumbar disc surgery. Spine. 2003;28:E453-E455.
66 Mastronardi L, Tatta C. Intraoperative antibiotic prophylaxis in clean spinal surgery: a retrospective analysis in a consecutive series of 973 cases. Surg Neurol. 2004;61:129-135. discussion 135
67 Riley LH. 3rd: Prophylactic antibiotics for spine surgery: description of a regimen and its rationale. J South Orthop Assoc. 1998;7:212-217.
68 Boscardin JB, Ringus JC, Feingold DJ, Ruda SC. Human intradiscal levels with cefazolin. Spine. 1992;17(6 Suppl):S145-S148.
69 Guiboux JP, Cantor JB, Small SD, et al. The effect of prophylactic antibiotics on iatrogenic intervertebral disc infections. A rabbit model. Spine. 1995;20:685-688.
70 Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025-1032.
71 Rhoten RL, Murphy MA, Kalfas IH, et al. Antibiotic penetration into cervical discs. Neurosurgery. 1995;37:418-421.
72 Scuderi GJ, Greenberg SS, Banovac K, et al. Penetration of glycopeptide antibiotics in nucleus pulposus. Spine. 1993;18:2039-2042.
73 Gibson MJ, Karpinski MR, Slack RC, et al. The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br. 1987;69:784-786.
74 Eismont FJ, Wiesel SW, Brighton CT, Rothman RH. Antibiotic penetration into rabbit nucleus pulposus. Spine. 1987;12:254-256.
75 Riley LH3rd, Banovac K, Martinez OV, Eismont FJ. Tissue distribution of antibiotics in the intervertebral disc. Spine. 1994;19:2619-2625.
76 Currier BL, Banovac K, Eismont FJ. Gentamicin penetration into normal rabbit nucleus pulposus. Spine. 1994;19:2614-2618.
77 Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res. 1977;129:101-114.
78 Walters R, Rahmat R, Fraser R, Moore R. Preventing and treating discitis: cephazolin penetration in ovine lumbar intervertebral disc. Eur Spine J. 2006;15:1397-1403.
79 Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine. 1998;23:615-620.
80 Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine. 2004;29:1066-1068.
81 Scuderi GJ, Brusovanik GV, Fitzhenry LN, Vaccaro AR. Is wound drainage necessary after lumbar spinal fusion surgery? Med Sci Monit. 2005;11:CR64-CR66.
82 Sasso RC, Williams JI, Dimasi N, Meyer PRJr. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J Bone Joint Surg Am. 1998;80:631-635.
83 Dire DJ, Coppola M, Dwyer DA, et al. Prospective evaluation of topical antibiotics for preventing infections in uncomplicated soft-tissue wounds repaired in the ED. Acad Emerg Med. 1995;2:4-10.
84 Rosenstein BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to prevent infection in postoperative skeletal wounds. An experimental study. J Bone Joint Surg Am. 1989;71:427-430.
85 Benjamin JB, Volz RG. Efficacy of a topical antibiotic irrigant in decreasing or eliminating bacterial contamination in surgical wounds. Clin Orthop Relat Res. 1984:114-117.
86 Gruenberg MF, Campaner GL, Sola CA, Ortolan EG. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: a comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine. 2004;29:2330-2334.
87 Short LJ, Bell DM. Risk of occupational infection with blood-borne pathogens in operating and delivery room settings. Am J Infect Control. 1993;21:343-350.
88 Smilanich RP, Bonnet I, Kirkpatrick JR. Contaminated wounds: the effect of initial management on outcome. Am Surg. 1995;61:427-430.
89 Bose B. Delayed infection after instrumented spine surgery: case reports and review of the literature. Spine J. 2003;3:394-399.
90 Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005;14:783-788.
91 Page CP, Bohnen JM, Fletcher JR, et al. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128:79-88.
92 Bhandari M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from bone. J Bone Joint Surg Am. 2001;83-A:412-419.
93 Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine. 2008;33:289-294.
94 McCarthy RE, Peek RD, Morrissy RT, Hough AJJr. Allograft bone in spinal fusion for paralytic scoliosis. J Bone Joint Surg Am. 1986;68:370-375.
95 Stevens DB, Beard C. Segmental spinal instrumentation for neuromuscular spinal deformity. Clin Orthop Relat Res. 1989;242:164-168.
96 Lonstein J, Winter R, Moe J, Gaines D. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop Relat Res. 1973;96:222-233.
97 Deckey JE, Court C, Bradford DS. Loss of sagittal plane correction after removal of spinal implants. Spine. 2000;25:2453-2460.
98 Wenger DR, Mubarak SJ, Leach J. Managing complications of posterior spinal instrumentation and fusion. Clin Orthop Relat Res. 1992;284:24-33.
99 Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthop Clin North Am. 1972;3:99-111.
100 Benli IT, Acaroglu E, Akalin S, et al. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12:224-234.
101 Abbey DM, Turner DM, Warson JS, et al. Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Disord. 1995;8:278-283.
102 Ha KY, Kim YH. Postoperative spondylitis after posterior lumbar interbody fusion using cages. Eur Spine J. 2004;13:419-424.
103 Picada R, Winter RB, Lonstein JE, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord. 2000;13:42-45.
104 Sheehan E, McKenna J, Mulhall KJ, et al. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004;22:39-43.
105 Mitra A, Harlin S. Treatment of massive thoracolumbar wounds and vertebral osteomyelitis following scoliosis surgery. Plast Reconstr Surg. 2004;113:206-213.
106 Dumanian GA, Ondra SL, Liu J, et al. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine. 2003;28:1203-1211.
107 Yuan-Innes MJ, Temple CL, Lacey MS. Vacuum-assisted wound closure: a new approach to spinal wounds with exposed hardware. Spine. 2001;26:E30-E33.
108 Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563-576. discussion 577
109 Clark NM, Hershberger E, Zervosc MJ, Lynch JP3rd. Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr Opin Crit Care. 2003;9:403-412.