35 Postoperative Respiratory Care
Patients undergoing cardiac surgery experience physiologic stresses from anesthesia, thoracotomy, surgical manipulation, and cardiopulmonary bypass (CPB). Each of these interventions can create transient deleterious effects on pulmonary function even with normal lungs; the effects may be exaggerated in the presence of preexisting pulmonary pathology. Important pulmonary changes after cardiac surgery include diminished functional residual capacity after general anesthesia and muscle relaxants,1 transient reduction in vital capacity (VC) after median sternotomy and intrathoracic manipulation, atelectasis, and increased intravascular lung water.2 Acute functional residual capacity reduction creates arterial hypoxemia because of a mismatch between ventilation and perfusion, and diminishes lung compliance with increased work of breathing. This additional work of breathing, which increases oxygen consumption by up to 20% in spontaneously breathing patients,3 also increases myocardial work at a time when myocardial reserves may be limited. Changes in spirometric measurements and respiratory muscle strength can last up to 8 weeks after surgery.4
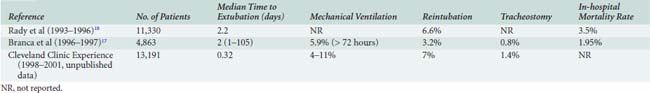
Thus, a sizeable proportion of cardiac surgical patients can be expected to have respiratory complications. In our experience, about a fourth of cardiac surgical patients were extubated in the operating room or within 4 hours of intensive care unit (ICU) arrival, and about half within 8 hours of ICU arrival. Median postprocedure intubation time was 7.6 hours. About 8% of patients experienced prolonged mechanical ventilation (defined as ≥ 72 hours after ICU arrival), and about 7% required reintubation of the trachea either shortly after initial extubation or because of delayed respiratory failure. Acute lung injury (ALI), sometimes progressing to acute respiratory distress syndrome (ARDS), can occur in up to 12% of postoperative cardiac patients.5 Tracheostomy was performed in 1.4% of post-CPB patients to facilitate recovery and weaning from ventilatory support. Although these figures represent the experience of one referral center, others have reported similar results (Table 35-1). A more recent study at this same institution showed a trend toward less ventilator dependency but little change in the rate of tracheostomy.6 In this study, 5.5% experienced ventilator dependency, whereas 1.45% required tracheostomy.
Risk factors for respiratory insufficiency
The lung is especially vulnerable because disturbances may affect it directly (atelectasis, effusions, pneumonia) or indirectly (via fluid overload in heart failure, as the result of mediator release from CPB, shock states, or infection, or via changes in respiratory pump function as with phrenic nerve injury). Postoperative status will be determined, in part, by the patient’s preoperative pulmonary reserve, as well as by the level of stress imposed by the procedure. Thus, a patient with reduced VC caused by restrictive lung disease undergoing minimally invasive surgery may have fewer postoperative pulmonary issues than a relatively healthy patient undergoing simultaneous CABG and valve replacement with its longer accompanying operative/anesthetic and CPB times. Respiratory muscle weakness contributes to postoperative pulmonary dysfunction, and prophylactic inspiratory muscle training has been shown to improve respiratory muscle function, pulmonary function tests, and gas exchange. Training reduces the percentage of patients requiring more than 24 hours of postoperative ventilation support from 26% to 5%.7
Assessing Risk Based on Preoperative Status
A number of robust models are available to stratify mortality outcome by preoperative risk factors in patients undergoing cardiac surgery.8 The independent (predictive) variables and their weighting vary somewhat from model to model and vary between models predicting mortality versus those predicting morbidity or length-of-stay outcomes,9 but the commonalities are greater than the differences. The Society of Thoracic Surgeons National Adult Cardiac Surgery Database is widely used in the United States and offers, in addition to a mortality prediction, a model customized to predict prolonged ventilation.10,11 The EuroSCORE is commonly used in Europe.12 Factors common to outcome risk adjustment models include age, sex, body surface area, presence of diabetes or renal failure, chronic lung disease, peripheral vascular disease, cerebrovascular disease, prior cardiac surgery, and emergency or unstable status.9–11 Chronic obstructive pulmonary disease (COPD) might be expected to be a major risk for postoperative respiratory morbidity and mortality and appears as a factor in many models. However, hospital mortality with mild-to-moderate COPD is not especially high; it is the minority of patients with severe COPD, especially those older than 75 years and receiving steroids, who are at greatest risk.13 Patients with preexisting COPD have greater rates of pulmonary complications (12%), atrial fibrillation (27%), and death (7%).13 Obesity, defined by increased body mass index, does not appear to increase the risk for postoperative respiratory failure.14 In contrast, even modest increases of serum creatinine concentration (> 1.5 mg/dL) are independently associated with greater morbidity and mortality.9,15
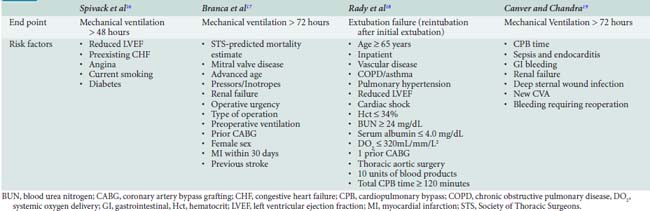
At least four studies have used multivariate regression techniques to elucidate factors specifically associated with postoperative respiratory failure (Table 35-2). The studies differ in their end points for outcome and in their choice of preoperative versus operative versus postoperative variables. Spivack et al16 examined 513 consecutive patients undergoing CABG and identified reduced left ventricular ejection fraction, preexisting congestive heart failure, angina, current smoking, and diabetes mellitus as predictors of mechanical ventilation support beyond 48 hours. In this study, pulmonary diagnosis, lung mechanics, and blood gas parameters were not independently useful in predicting outcome. Branca et al17 found that the mortality rate predicted by the Society of Thoracic Surgeons model10 was the single best predictor of mechanical ventilation support for longer than 72 hours, but also identified mitral valvular disease, age, vasopressor and inotrope use, renal failure, operative urgency, type of operation, preoperative ventilation, prior cardiac surgery, female sex, myocardial infarction within 30 days, and previous stroke as contributors.17 Rady et al18 examined both preoperative and intraoperative factors and noted that transfusion of more than 10 units of blood products or total CPB time in excess of 120 minutes were important operative events in addition to the usual preoperative predictors of extubation failure. Canver and Chandra19 looked only at operative and postoperative predictors versus the end point of mechanical ventilation for more than 72 hours; they found that prolonged CPB time, sepsis and endocarditis, gastrointestinal bleeding, renal failure, deep sternal wound infection, new stroke, and bleeding requiring reoperation were important predictors of prolonged ventilatory support. None of these models, general or specific for respiratory complications, is sufficiently sensitive or specific to prohibit consideration of surgery for an individual patient, but they do provide the clinician with early warning for patients at high risk.
Operating Room Events
Identification of the patient who is difficult to intubate is important for planning extubation for a time when sufficient personnel and equipment are available to deal with a potentially difficult reintubation. Opioids and neuromuscular blocking agents with long half-lives might be expected to influence extubation time. It is not the specific duration of action of these drugs but rather the skill of the anesthesiologist in knowing how to use them well that influences extubation time. Reoperative patients are at risk18–20 partly because of longer CPB time, increased blood transfusion, and the additional likelihood of bleeding in this population. CPB time is repeatedly identified as a risk,18–20 and a correlation between CPB time and inflammatory cytokine release has been demonstrated.21 However, levels of C-reactive protein, an inflammatory marker, do not correlate with outcomes such as time on mechanical ventilation.22 Genetic polymorphisms are associated with respiratory complications,23 suggesting that risk prediction may require more sophisticated understanding of individual patient variables. Recent observations of dose-dependent reductions in adverse events after CABG in patients receiving statins are also intriguing.24,25
Low cardiac output states may be important predictors of prolonged ventilation because prolonged periods of inadequate perfusion result in additional mediator release. Patients maintained on an intra-aortic balloon pump (IABP) or a ventricular assist device may have borderline or insufficient cardiac output; it makes little sense to impose the additional work of breathing3 until their cardiac issues have resolved. Cardiovascular collapse occasionally occurs at the time of chest closure secondary to severe distension or edema of the lungs. Physiologically, this acts much like cardiac tamponade, and the solution is to leave the chest open for 24 to 48 hours. An open chest delays early extubation and also has a potential to produce long-term ventilator dependency should infection or sternal osteomyelitis develop.
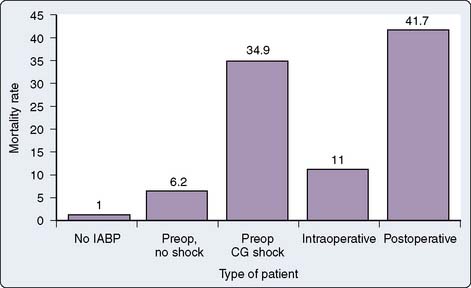
The prognostic and therapeutic implications of an IABP depend on the reasons for which the device was inserted (Figure 35-1). Not surprisingly, mortality and ventilation-dependency rates are lowest in those not requiring any mechanical support. In patients in whom the IABP was placed before surgery for unstable angina, definitive surgery should correct the problem and removal of the IABP and extubation need not be delayed. In all other scenarios, intubation and ventilatory support may be required beyond the time of removal of the IABP because of residual cardiac dysfunction, fluid overload, or associated organ injury. Patients whose IABP was placed for preoperative cardiogenic shock, as an assist to separating from CPB, or for low output states in the postoperative period have a high mortality risk and frequently need prolonged ventilatory support.
Positive end-expiratory pressure (PEEP) while on CPB has been advocated as one method to prevent atelectasis. This turns out to be impractical in patients with COPD because air trapping interferes with surgical exposure. Recruitment maneuvers after CPB have variable impact on intubation time; most studies show it to be ineffective in reducing the need for long-term ventilatory support. Alveolar recruitment maneuvers can be performed without deleterious effects, even in morbidity obese patients, as long as intravascular volume is adequate.26 There are no compelling data that fluid management choices or the use of steroids before CPB have substantial effects on intubation time or respiratory failure. A number of studies27,28 suggest that infusion of small volumes of hypertonic saline prepared in a hydroxyethyl starch solution may reduce total fluid needs, improve cardiac index, and lessen the pulmonary gas exchange compromise, but studies have not examined whether these differences in the operating room translate to improved outcome or shorter length of stay.
Postoperative Events
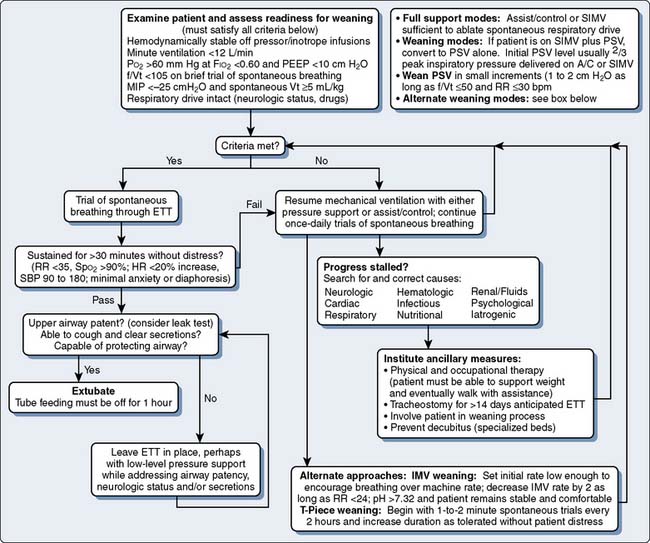
The expected postoperative course is a short period of ventilatory support while the patient is warmed, allowed to awaken, and observed for bleeding or hemodynamic instability. Preoperative risks, issues with difficult intubation, and operating room events should be communicated from the operating room team to the ICU team at the time of ICU admission. Box 35-1 outlines criteria to be met before routine extubation. Reduced cuff leak volume reliably identifies patients at risk for laryngeal edema. Intravenous methylprednisolone can reduce the incidence of postextubation stridor.29 Prophylactic nasal continuous positive airway pressure (CPAP) at 10 cm H2O for a minimum of 6 hours has been shown to reduce hypoxemia, pneumonia, and reintubation rates after elective cardiac surgery.30
BOX 35-1 Criteria to be met Before Early Postoperative Extubation
Although postoperative care of low-risk cardiac surgical patients has come to resemble a recovery room model, high-risk patients benefit from postoperative involvement of anesthesiologists, cardiologists, and critical care specialists.31 Numerous individual studies and systematic reviews32 have confirmed the value of full-time intensive care specialists in a variety of settings, although there is at least one dissenting opinion.33 Wide variation continues in adherence to the Leapfrog group physician staffing standard,34 despite demonstrated financial return on investment.35 It is likely that a robust organizational environment, rather than the mere presence of intensivists, is necessary to achieve the best results.36
Hospital-acquired infections are an important cause of postoperative morbidity and nosocomial pneumonia is common in patients receiving continuous mechanical ventilation. The historic risk for ventilator-associated pneumonia (VAP) appears to be around 1% per day when diagnosed using protected specimen brush and quantitative culture techniques.37 More recent data suggest that VAP rates can be decreased by an order of magnitude with careful attention to patient management.38,39 Strategies believed to be effective at reducing the incidence of VAP include early removal of nasogastric or endotracheal tubes, formal infection control programs, hand washing, semirecumbent positioning of the patient,40 daily sedation “vacation,”41 avoiding unnecessary reintubation, providing adequate nutritional support, avoiding gastric overdistention, use of the oral rather than the nasal route for intubation, scheduled drainage of condensate from ventilator circuits,42 and maintenance of adequate endotracheal tube cuff pressure.43 Strategies that are not considered effective include routine changes of the ventilator circuit, dedicated use of disposable suction catheters, routine changes of in-line suction catheters, daily replacement of heat and moisture exchangers, and chest physiotherapy.44 The literature supports both continuous aspiration of subglottic secretions and use of silver-coated endotracheal tubes to reduce the incidence of VAP.45–47
Diagnosis of acute lung injury and acute respiratory distress syndrome
Intravascular and intra-alveolar fibrin deposition are common. Procoagulant activity becomes enhanced in ARDS, and bronchoalveolar lavage will reveal increased tissue factor levels.48 The clinical presentation is typically an acute onset of severe arterial hypoxemia refractory to oxygen therapy, with a PaO2 to FiO2 (P/F ratio) of less than 200 mm Hg. ARDS is classically diagnosed only in the absence of left ventricular failure, which complicates the diagnosis in the postoperative cardiac patient who may also be in heart failure. Other findings in ARDS include decreased lung compliance (< 80 mL/cm H2O) and bilateral infiltrates on chest radiograph.49 Murray et al50 created a Lung Injury Score that awards points for affected quadrants on chest radiograph, P/F ratio, amount of PEEP applied, and the static compliance of the lung. Scores above zero, but less than 2.5, are considered ALI, and scores greater than 2.5 meet the threshold for ARDS.
The proliferative phase of ARDS occurs on days 3 to 7 as inflammatory cells accumulate as a result of chemoattractants released by the neutrophils. At this stage, the normal repair process would remove debris and begin repair, but a disordered repair process may result in exuberant fibrosis, stiff lungs, and inefficient gas exchange. Evidence suggests that careful fluid and ventilator management may affect this process.51,52 Conventional ventilator support after cardiac surgery is to maintain large tidal volumes (VT; typically 10 mL/kg) to reopen atelectatic but potentially functional alveolae. The problem is that the compromised lung is no longer homogenous, and high pressures can further damage the remaining normal lung. Direct mechanical injury may occur as a result of overdistention (volutrauma), high pressures (barotrauma), or shear injury from repetitive opening and closing. “Biotrauma” also may occur as a result of inflammatory mediator release and impaired antibacterial barriers. Nahum et al53 showed that dissemination of Escherichia coli via bacterial translocation from the lung was highest in dogs ventilated with a high-VT strategy. Thus, current clinical practice with known or suspected lung injury is to limit inflation pressures. The maximal “safe” inflation pressure is not known, but evidence favors keeping peak inspiratory pressures less than 35 cm H2O and restricting VT to ≤6 mL/kg of ideal body weight in patients at risk for ALI.54 The landmark ARDSNet trial randomized patients to 6 versus 12 mL/kg of ideal body weight and demonstrated a significant difference in 28-day survival with the low-VT group.55 The same study showed significant decreases in interleukin-6 (IL-6) release when the low-VT strategy was used. Most recently, ventilation with lower VT has been shown to be beneficial in critically ill patients even without ALI, as measured by plasma IL-6 levels and progression to lung injury,56 but this issue has not yet been studied in the cardiac surgical population. A conservative strategy of fluid administration has been shown to improve oxygenation and shorten the duration of mechanical ventilation.51
Therapy with acute lung injury/acute respiratory distress syndrome
Maintaining a lung protective ventilatory strategy can involve permissive hypercapnia,57 if normal Pco2 levels cannot be achieved with low VT. The acid-base changes must be monitored carefully, especially in patients with reactive pulmonary vasculature. Prone positioning can be useful in achieving oxygenation.58 A short daily turn to the prone position does not appear to improve outcome in ARDS, although one post hoc analysis found lower mortality in the sickest patients.59 Lower VT with increasing amounts of PEEP may increase alveolar recruitment and thus improve oxygenation.60 Taken to an extreme, patients with ALI may be ventilated with high-frequency oscillation, which is essentially high PEEP with tiny (smaller than dead space), frequently delivered VT. Other techniques for patients who did not respond successfully to conventional therapy include extracorporeal CO2 removal,61 extracorporeal membrane oxygenation,62 partial liquid ventilation,63 inhaled nitric oxide,64,65 and inhaled prostacyclin.66 Although clearly beneficial to some individuals in extreme circumstances, prospective controlled trials are lacking. High-dose corticosteroids have been in and then out of fashion for treatment of ARDS. More recently, Miduri et al gave prolonged lower dose methylprednisolone therapy for unresolving ARDS and were able to document improvements in P/F ratio, better ICU survival, and shortened duration of mechanical ventilation.67
In the healthy cardiac surgical population, the use of PEEP usually is not necessary.68,69 Increased levels of PEEP may decrease cardiac output, unless volume loading is used to stabilize preload by maintaining transmural filling pressures.70 The effects of PEEP are most marked in the presence of abnormal right ventricular function, particularly if the right coronary artery is compromised.71 PEEP neither protects against the development of ARDS72 nor reduces the amount of mediastinal bleeding after cardiac surgical procedures involving CPB.73 Most clinicians will routinely use 5 cmH2O of PEEP in ventilated patients. However, greater levels of PEEP (often 8 to 15+ cmH2O) are usually necessary to maintain adequate oxygenation with ALI or developing ARDS; application of PEEP in the postoperative patient usually involves a trade-off between cardiac and pulmonary goals.
Lung Recruitment
In laboratory and clinical models, an important component of a lung protective ventilatory strategy is recruitment of the lung. This is the closed-chest analogy to the open-chest recruitment maneuver typically done at the end of CPB to re-expand the collapsed lung. The goal of opening the lung is to allow ventilation to occur at a point on the pressure-volume curve that avoids repetitive atelectasis (by staying above a critical closing pressure) and at the same time avoids overinflation.74 ARDSNet data suggest that the short-term effects of recruitment maneuvers are highly variable and that further study is necessary to determine the role of recruitment maneuvers in the management of ALI/ARDS.75 With anesthetic techniques geared to early extubation, suboptimal oxygenation in the early postoperative period appears to be more common today. In most instances, impaired oxygenation is due to atelectasis and responds quickly to brief recruitment maneuvers. These should be performed with caution because of the adverse impact of increased airway pressure on venous return and cardiac output if the patient is intravascularly “empty.” Although the benefits of early application of the lung protective ventilatory strategy in the high-risk cardiac surgical patient have not been studied formally, the benefits of lung protective ventilatory strategy in other populations suggest that this strategy should be considered as soon as ALI is identified, and perhaps even prophylactically in high-risk patients.56
Permissive Hypercapnia
Conventional management is to maintain Paco2 within a “normal” or eucapnic range, classically between 35 and 45 mm Hg. A patient who chronically retains CO2 would be considered eucapnic at his or her higher baseline Paco2. The traditional reason for maintaining eucapnia is primarily that acute deviation from a normal or acclimatized Paco2 will result in alkalemia or acidemia to which the kidneys will respond by retaining or excreting bicarbonate ion. Normal kidneys can compensate for a Pco2-induced pH change in 12 to 36 hours.76 If high airway pressures would be required to maintain a “normal” Paco2, then Paco2 values up to 60 mm Hg are acceptable as long as cardiovascular stability is present and the pH remains greater than 7.30. It has been hypothesized that increased Pco2 levels might even be protective and that low levels of Pco2 could play a role in organ injury.77 Permissive hypercapnia should be used judiciously in patients with pulmonary hypertension because acidosis can exacerbate pulmonary vasoconstriction and further impair right ventricular function and cardiac output.78 (See Chapter 24.)
Cardiopulmonary Interactions
An understanding of cardiopulmonary interactions associated with mechanical ventilation is critical to the cardiothoracic intensivist. Hemodynamic changes may occur secondary to changes in lung volume and intrathoracic pressure even when VT remains constant.79 Pulmonary vascular resistance and mechanical heart-lung interactions play prominent roles in determining the hemodynamic response to mechanical ventilation. Because lung inflation alters pulmonary vascular resistance and right ventricular wall tension, there are limits to intrathoracic pressure that a damaged heart will tolerate. High lung volumes also may mechanically limit cardiac volumes. In patients with airflow obstruction, occult PEEP (auto-PEEP) may also contribute to hypotension and low cardiac output.80 Auto-PEEP can be detected by respiratory waveform monitoring or by pressure monitoring with the ventilator’s expiratory port held closed at end-exhalation. Auto-PEEP may respond to bronchodilators and/or increased expiratory time to permit more complete exhalation.
General Support Issues
Patients requiring long-term ventilatory support are prone to a number of complications including venous thromboembolism, central venous catheter–related bloodstream infections, surgical site infections, VAP, pressure ulcers, nutritional depletion, delirium, and gastrointestinal bleeding. The Agency for Healthcare Research and Quality has identified a number of patient safety practices applicable to the ICU patient; high on the list are appropriate venothromboembolism prophylaxis, use of perioperative β-blockers, use of maximum sterile barriers during catheter insertion, and appropriate use of antibiotic prophylaxis.81 Standard practice in long-term ventilator patients includes prophylaxis against gastrointestinal bleeding with histamine blockers or proton pump inhibitors (unless the patient is receiving continuous gastric feedings), head of bed elevation to 30 degrees or more in hemodynamically stable patients,40 a brief daily wake up from sedation,82 use of in-line suction catheters, glucose control,83 and appropriate venothromboembolism prophylaxis. Box 35-2 summarizes these risk-reduction efforts. Ensuring that each of these goals is met on each patient every day requires extra work but can be accomplished with a daily goals form84 or with information technology.85
Impediments to weaning and extubation
Factors that limit the removal of mechanical ventilatory support include delirium, neurologic dysfunction, unstable hemodynamics, respiratory muscle dysfunction, renal failure with fluid overload, and sepsis. Figure 35-2 outlines one approach to identifying readiness to wean and possible alternative approaches to weaning.
Neurological Complications
Delirium is common in long-stay ICU patients and is associated with greater costs in the ICU and for the entire hospital stay.86 Delirium after cardiac surgery is a common complication in cardiovascular ICUs; estimated incidence rates are approximately 30% in the general cardiac surgical population to 83% in mechanically ventilated patients.87,88 Delirium resolves spontaneously or with pharmacologic intervention in almost all patients by postoperative day 6. Evidence from a large trial of mostly medical critical care patients suggests dexmedetomidine is associated with less delirium than midazolam.89 Dexmedetomidine has been shown to be safe and effective in the post-CABG population.90 Alcohol or benzodiazepine withdrawal should be considered in the differential diagnosis of delirium. Recent evidence suggests that ketamine may attenuate delirium in CPB patients, possibly because of anti-inflammatory effects.91 Initial postoperative management of agitation consists of reassurance and orientation of the patient, as well as control of pain with opioids. Agitation accompanied by disorientation may be worsened by benzodiazepines, which should be restricted to treatment of oriented but anxious patients or for prophylaxis of alcohol and benzodiazepine withdrawal. If the patient remains agitated and disoriented, haloperidol is useful.92 Newer agents such as risperidone, olanzapine, and quetiapine also may be useful, but they have not been well studied in the cardiothoracic ICU setting.
Diaphragmatic paralysis may complicate any procedure, but it is more common in patients undergoing reoperation, because of the difficulty in identifying the phrenic nerve in fibrotic pericardial tissue. In our experience, permanent bilateral diaphragmatic paralysis occurs in less than 0.1% of patients after CPB, but temporary diaphragmatic weakness may occur in 4% or more. The diagnosis of diaphragmatic paralysis should be suspected whenever a patient does not successfully wean from mechanical ventilation; it should be documented by observing paradoxic movement of the diaphragm during inspiration, and by comparing VC and VT in the supine and seated positions. Differences in supine and seated VC of more than 10% to 15% should prompt fluoroscopic examination of the diaphragm (“sniff” test). Bilateral paralysis may be missed by this test, because comparison of left and right diaphragmatic excursion has lower specificity when both diaphragms are involved. Transient diaphragmatic paralysis can occur secondary to cold injury to the phrenic nerve.93 Less often, the phrenic nerve is injured or transected during dissection of the internal mammary arteries or during mobilization of the heart in patients undergoing reoperation.
Patients with respiratory failure and systemic inflammatory response syndrome frequently develop critical illness polyneuropathy, the first sign of which may be failure to wean from the ventilator.94 Disuse atrophy95 and steroid administration96 also contribute to muscle weakness. Patients with severe COPD who are dependent on diaphragmatic breathing before surgery are most likely to manifest diaphragmatic weakness as postoperative ventilator dependency. Full recovery of diaphragmatic function may take from 4 months to more than 2 years, and partial recovery is apparent when the patient can lie flat without dyspnea.97 Adjuncts to improving diaphragmatic strength include inspiratory muscle training,7 normalizing calcium and phosphate levels, and possibly the use of aminophylline.98
Cardiac Complications
Acute myocardial dysfunction occurs in almost all post-CPB patients, reaching a nadir about 4 hours (range, 2 to 6) after CPB. Patients with persistent low-output syndrome have an increased risk for cardiac death, as well as complications such as renal failure, respiratory failure, disseminated intravascular coagulation, gastrointestinal bleeding, and neurologic sequelae. Regional differences in blood flow also can occur even in the presence of an adequate overall cardiac output, so a normal whole-body cardiac index does not guarantee adequate perfusion of individual organs. Multiorgan failure may be precipitated by a period of gut ischemia or hyperpermeability followed by translocation of gut bacteria and release of endotoxin and other vasoactive substances, leading to generalized inflammation and organ injury.99
Patients maintained on chronic amiodarone therapy are prone to postoperative respiratory failure, longer intubation times, and longer ICU stays, even with only subclinical evidence of pulmonary amiodarone toxicity.100 Rarely, patients taking amiodarone experience development of life-threatening pulmonary complications, including ARDS. Histologic lung examination of these patients demonstrates marked interstitial fibrosis with enlarged air spaces (“honeycomb” appearance) and hyperplasia of type II pneumocytes.101
Acute left ventricular dysfunction may occur in patients with COPD during the shift from mechanical to spontaneous ventilation.102 Attention to fluid balance and aggressive diuresis or use of ultrafiltration help with weaning. Although it is useful to compare the patient’s current and preoperative body weights, catabolic states arising after surgery, and especially during sepsis, reduce lean body weight. Patients may be “unweanable” until fluid removal reduces body weight to several kilograms less than the preoperative value.
Patients with valvular disease have significantly higher respiratory system and lung elastances and resistances than those undergoing surgery for ischemic heart disease, but these may correct with successful surgery.103 Thus, valve surgery patients have less work of breathing and improved respiratory function after correction of the valvular pathology, but CABG patients are less likely to show dramatic improvement after surgery.
Utility of Echocardiography in the Intensive Care Unit Setting
For many years, echocardiography has been practiced routinely in the operating room to assess ventricular function and valvular pathology. In recent years, transesophageal color-Doppler echocardiography (TEE) or transthoracic echocardiography (TTE) has moved to the bedside.104–106 High-quality images from TEE/TTE can be a valuable diagnostic tool in the management of critically ill patients, as well as in the diagnosis and treatment of respiratory disease related to cardiopulmonary dysfunction.107 Use of a focused cardiovascular examination frequently alters perioperative management.108
Pleural Effusion
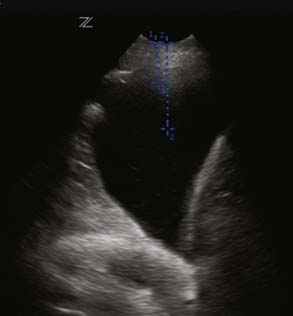
Accumulation of fluid in the pleural space from bleeding or collection of fluid can compress the lung parenchyma and cause basal atelectasis, resulting in impaired gas exchange. An undetected pleural effusion also may act as a potential source of postoperative infection. As with the ultrasound probe used by radiologists, the TTE probe can be used to assess the size of a pleural effusion, mark the skin at the site that is optimal for needle insertion to drain the effusion, and verify that there is no lung tissue in the area where the physician plans to insert the needle (Figure 35-3). Orihashi et al109 have described the TEE appearance of pleural fluid. When the patient is supine, fluid pools in the dorsal and caudal portions of the pleural space. From the four-chamber view, the TEE probe is rotated counterclockwise to obtain the short-axis view of the descending aorta. In the presence of left-side pleural effusion, a crescent shape linked to a tiger’s claw could be noted. The probe is rotated clockwise from the four-chamber view position to examine for a right pleural effusion. A crescent-shaped, echo-free space adjacent to the transducer is noted.
Treatment of pleural effusion is thoracentesis or placement of tube thoracostomy. Talmor et al110 showed that drainage of pleural fluid resulted in a significant improvement in oxygenation in patients with acute respiratory failure with pleural effusions who were refractory to treatment with mechanical ventilation and PEEP.
Patent Foramen Ovale
When a mechanically ventilated patient becomes more hypoxemic despite efforts to improve ventilation, shunt-induced hypoxemia caused by a patent foramen ovale should be suspected, among other causes such as pulmonary embolism. Only echocardiography can identify such specific abnormalities in mechanically ventilated patients, when weaning is difficult or refractory hypoxemia is not explained by pulmonary disease alone.111
Septic Shock
The treatment of critically ill patients frequently requires a comprehensive evaluation of the hemodynamic status. Pulmonary artery catheters are widely used but have not been shown to improve survival112 and have substantial limitations.113 Echocardiography appears to be an alternative modality for assessment of patients with circulatory failure in the ICU,114 allowing rapid assessment and differentiation of the cause of shock.
The diagnostic accuracy of TEE has been superior to that of TTE in identifying the cardiac source of shock, especially in ventilated patients.115 Inotropic support may be necessary to facilitate weaning from mechanical ventilation.116 In the presence of tricuspid regurgitation, the measurement of cardiac output by thermodilutional techniques will underestimate its value. Balik et al117 confirmed that the severity of tricuspid regurgitation, an abnormality frequently observed in mechanically ventilated ICU patients,118 reduces the agreement between thermodilution and TEE for the cardiac output determination.
Inadequate fluid administration can be detrimental, but avoiding inefficient and potentially deleterious volume expansion is equally important. Bedside echocardiography is one option for goal-directed therapy.119 Pulse-pressure variation under volume-controlled ventilation has been used successfully to predict the response to a fluid challenge in patients with sepsis because this variation is mainly based on the respiratory change in the left ventricular stroke volume. Feissel et al120 were able to measure the variation of aortic blood flow velocity by echo Doppler and predict preload responsiveness in septic ventilated patients. Variation of aortic Doppler velocities was calculated as the ratio of the difference between maximal (inspiratory) and minimal (expiratory) velocities to the mean of these two velocities, and responders were defined as patients who increased their cardiac index by at least 15% after fluid challenge. Passive leg raising has been shown, using esophageal Doppler and respiratory variation in pulse pressure, to predict fluid responsiveness.121 Vieillard-Baron et al122 showed that the collapsibility index (maximal diameter on expiration – minimal diameter on inspiration/maximal diameter on expiration expressed as a percentage) of the superior vena cava greater than 36% using TEE accurately distinguished responders from nonresponders to a fluid challenge. The pathophysiology and treatment of ARDS often impose additional strain on the right ventricle. Echocardiography can be useful in helping in the assessment of the management of fluids, mechanical ventilation, and inotropic support on right ventricular function.123 A recent review summarized the advantages and disadvantages of minimally invasive cardiac output monitoring in the perioperative setting (see Chapter 14).124
Renal Failure and Fluid Overload
Significant oliguria or anuric renal failure occurs after 1% to 4% of cardiac surgical procedures, and lesser degrees of renal dysfunction, marked by increased serum creatinine concentration, occur in up to 30%. Univariate predictors of serious acute renal failure include low cardiac output at the end of CPB, advanced age, preoperative heart failure, need for postoperative circulatory support or blood transfusions, and prolonged time on CPB.125
When renal failure occurs, it often follows one of three well-defined patterns.126 Abbreviated acute renal failure occurs after an isolated insult, results in a peak in serum creatinine around the fourth postevent day and generally resolves if no other events occur. The second pattern initially resembles the first, except that the acute insult is accompanied by prolonged circulatory failure. This pattern runs a longer course, with recovery typically occurring in the second or third week after injury, in tandem with improvements in cardiac output. In the third pattern, recovery is complicated by a second insult such as sepsis, massive gastrointestinal bleeding, or myocardial infarction, and permanent renal failure may result. Because fluid overload with renal failure may precipitate respiratory and cardiac failure, early application of hemodialysis and related techniques to remove excess fluid can facilitate separation from ventilator support.127
Infectious Complications
Mediastinitis, sternal dehiscence, or both, are complications of CABG, with an incidence rate of about 1%, a mortality rate of about 13%, and a tendency to prolong ventilator dependency. Predisposing factors for wound complications after cardiac surgery include diabetes, low cardiac output, use of bilateral internal mammary grafts, and reoperation for control of bleeding.128 Keeping the blood glucose less than 200 mg/dL in the perioperative period reduces the sternal wound infection rate from 2.4% to 1.5%.129 Mediastinal infection manifests as unexplained fever, an unstable sternum, and sometimes, failure to wean. In addition to selective antibiotic therapy, surgical debridement and drainage of the wound are usually necessary. Polymicrobial isolates are associated with poor outcome. Additional management of mediastinitis may include primary or delayed sternal closure using pectoralis or omental flaps.
Pneumonia, tracheobronchitis, catheter sepsis, and urinary tract infections are frequent in the ventilator-dependent patient. Continuous lateral rotational therapy reduces the prevalence of pneumonia but may not affect mortality or length of mechanical support.130 The diagnosis of VAP131–157 can be difficult to confirm because upper airway organism can contaminate the sputum specimen. Special suction catheters with a protected tip may be used for a “mini”-bronchoalveolar lavage to improve the yield of sputum cultures. Because typical perioperative antibiotic prophylaxis consists of an antistaphylococcal penicillin or cephalosporin, nosocomial pneumonia is likely to occur with organisms such as Pseudomonas, Klebsiella, Serratia, Acinetobacter, or methicillin-resistant Staphylococcus aureus. Treatment of the secondary infection may be followed by a tertiary infection with more difficult organisms, such as Candida, Torulopsis, or other fungal species.
Modes of ventilator support
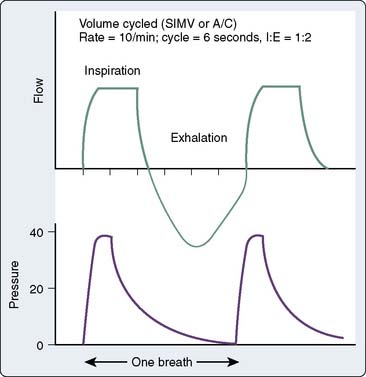
Positive-pressure ventilators used outside the operating room have a nonrebreathing circuit, may be volume or pressure limited, and may be triggered by changes in flow or changes in pressure. All modern ventilators contain multiple modes of ventilatory support that accommodate both mandatory and patient-triggered breaths. The most common modes of positive-pressure ventilation are assist control, synchronized intermittent mandatory ventilation (SIMV), and pressure-support ventilation (PSV). With volume modes, the inspiratory flow rate, targeted volume, and inspiratory time are set by the clinician, and inspiratory peak pressure will vary depending on the patient’s lung compliance and synchrony with the ventilator. Volume cycling ensures consistent delivery of a set VT as long as the pressure limit is not exceeded. With nonhomogenous lung pathology, however, delivered volume tends to flow to areas of low resistance, which may result in overdistension of healthy segments of lung and underinflation of atelectatic segments and consequent ventilation/perfusion (V/Q) mismatching. Figure 35-4 demonstrates pressure and flow tracings with volume ventilation. Volume breaths may be triggered by a timer (control mode ventilation) or by patient effort between the control mode breaths (assist control ventilation). In either case, the VT delivered will be determined by the ventilator settings. This can present a problem in a patient with tachypnea as a response to neurologic injury. If the patient breathes inappropriately in response to normal arterial levels of carbon dioxide, significant respiratory alkalosis will result. Assist-control mode is most appropriate for the patient whose respiratory drive is normal but muscles are weak, or when neuromuscular blockade is used, in which case assist control essentially becomes control-mode ventilation.
Pressure-Controlled Ventilation
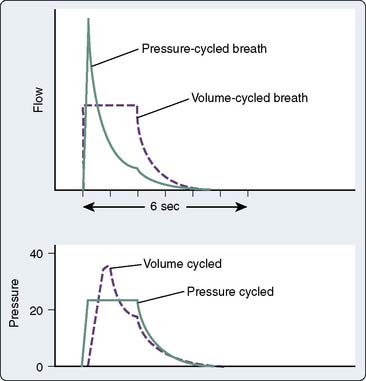
Pressure-controlled ventilation is available on most newer ventilators and allows the clinician to specify a target inspiratory pressure; the ventilator then calculates and delivers the optimal flow rate to achieve the desired VT and inspiratory-to-expiratory ratio. Figure 35-5 demonstrates the difference between pressure- and volume-controlled ventilation with regard to inspiratory flow. Pressure-controlled inverse ratio ventilation (PC-IRV) is pressure-controlled ventilation with an inspiratory time that exceeds expiratory time (I:E ratio > 1.0). Opening alveoli with damaged lungs sometimes requires exceeding a critical opening pressure for an adequate amount of time (Figure 35-6). With standard ventilation, this time above the critical pressure only can be lengthened by increasing the peak inspiratory pressure, which is generally undesirable with a damaged lung. With PC-IRV, the pressure waveform is optimized to allow a long inspiratory time above the critical opening pressure while avoiding high peak pressures. The disadvantage of PC-IRV is that the increased inspiratory time necessarily reduces exhalation time, which may lead to stacking of breaths or auto-PEEP in patients with abnormal lungs. Tharat et al137 demonstrated that PC-IRV allowed a reduction in minute ventilation and reduced peak pressures while increasing mean airway pressures in patients with ARDS. At a given plateau pressure (similar end-inspiratory distention), lower VT and increased PEEP are associated with better recruitment and oxygenation.
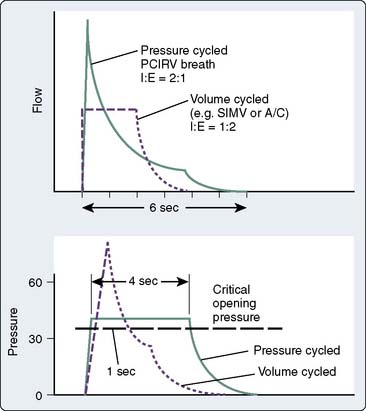
Figure 35-6 Volume- and pressure-cycle modes are compared as in Figure 35-5. If there is a critical opening pressure to recruit atelectatic lung segments, a pressure-controlled ventilation mode, particularly if the inspiration-to-expiration (I:E) ratio is inverted, is far more successful at maintaining an inspiratory pressure above the critical opening pressure for a greater length of time. Attempting to increase the volume-cycled flow would result in unacceptably high peak inspiratory pressures in an attempt to achieve more time above the critical opening pressure. But even in volume control, inspiratory pauses or changes to the flow rate in I:E ratio may be able to achieve longer sustained inspiratory pressures. PCIRV, pressure-controlled inverse ratio ventilation; SIMV, synchronized intermittent mandatory ventilation.
Liberation from mechanical support (weaning)
Objective Measures of Patient Strength and Endurance
Attempts have been made to predict endurance using the resting minute ventilation rate, but using the common value of 10 L/min as the cutoff results in a false-positive rate of 11% and a false-negative rate of 75% in predicting successful extubation. Similarly, the ability to sustain maximum voluntary ventilation twice the minute ventilation correctly predicts in only about 75% of the patients. More sophisticated measurement of endurance can be accomplished using a diaphragmatic or intercostal electromyogram power spectrum, but these are not practical for routine clinical use. Yang and Tobin142 attempted to develop a scoring system for weaning that integrated lung compliance, respiratory rate, oxygenation levels, and maximum inspiratory pressure to predict weaning success. Although all of these elements and the total score have some predictive valve, the best index turned out to be the f/VT. An f/VT greater than 105 has a predictive value of 89% for weaning failure. The tension-time index of the diaphragm (TTdi) integrates the inspiratory pressure with time and is a reliable measurement of endurance, but it is difficult to perform in the clinical setting. If the percentage of inspiratory time over total time in a spontaneously breathing patient is more than 15%, respiratory failure will eventually ensue, probably because of limitations of diaphragmatic blood flow, which occurs only during the expiratory phase. The tension time of the human diaphragm (TTDI) is calculated by plotting the inspiratory time on the y-axis against the ratio of transdiaphragmatic pressure with normal and maximal effort on the x-axis. Fatigue is unlikely with values of TTDI less than 0.15; values greater than 0.18 define the fatigue zone. Commercially available equipment such as the BiCor CP-100 pulmonary monitor can provide Ti/T total, f/VT, and P 0.1, a marker of respiratory drive. Some newer ventilators also incorporate this technology. A problem with all measured respiratory parameters or scoring systems relying on pulmonary function is that they do not include the nonrespiratory parameters affecting weaning (Box 35-3).
Weaning: The Process
Regardless of which weaning method is chosen, it is important to end each weaning trial with success rather than to stress the patient to the point of fatigue. Cohen et al146 identified the clinical sequence of inspiratory muscle fatigue. The earliest sign of inspiratory muscle fatigue was a spectrum shift in the electromyogram power spectrum, which is impractical in the clinical setting. However, the next most sensitive sign was an increase in respiratory rate, which occurred before respiratory alternans, abdominal paradox, and increase in the Paco2 level, or acidemia. Thus, respiratory rate can serve as a sensitive marker of weaning progress.
Specific Impediments to Weaning
Older ventilators with demand-valve systems impose an additional work of breathing, although it would be rare to see such ventilators (Bear I & II, Puritan Bennett MA-II) in clinical use today. Most newer ventilators use computer-assisted demands valve technology to supply a variable flow rate, unlike older ventilators in which a fixed low gas flow rate occasionally resulted in the inability to supply peak flow on demand. Nonetheless, if the patient is demonstrating apparent air hunger during the weaning process, a quick check of the inspiratory flow rates often can solve the problem. Pulmonary effusions and pneumothorax can develop in otherwise stable patients and also may present as stalled weaning. Stacking of breaths or auto-PEEP can occur if the expiratory time is too short for patients, particularly those with obstructive disease, to exhale fully before the next breath is delivered. Brown and Pierson147 demonstrated that the magnitude of auto-PEEP varied between 0 and 16 cm H2O during routine ventilator checks.
Muscle Weakness and Critical Illness Polyneuropathy
Long-term administration of neuromuscular blocking agents, particularly those such as vecuronium with a steroid structure, has been associated with persistent paralysis. One explanation may be the accumulation of the metabolite 3-desacetylvecuronium, which rarely is seen in patients with normal renal function but is quite common in patients with delayed recovery.148 However, prolonged paralysis also can be seen after treatment with other drugs such as pancuronium, metocurine, and cisatracurium, which do not necessarily share the same structure or have persistent metabolism. The suspicion is that neurogenic atrophy occurs with prolonged paralysis resulting in a flaccid quadriplegia or more localized weakness of respiratory muscles.
Tracheostomy
Prolonged endotracheal intubation results in damage to the respiratory epithelium and cilia and may lead to vocal cord damage and airway stenosis.150,151 If mechanical ventilation is anticipated for longer than 14 days, consideration should be given to early tracheostomy.152,153 Other indications for tracheostomy include copious or tenacious secretions in debilitated patients who are unable to clear secretions spontaneously. Tracheostomy is relatively contraindicated with ongoing mediastinitis or local infection at the tracheostomy site because of the potential for mediastinal contamination with respiratory secretions. Tracheostomy is not a risk-free procedure, and complications include pneumothorax, pneumomediastinum, subcutaneous emphysema, incisional hemorrhage, late tracheal stenosis or tracheomalacia, stomal infections, and rarely, tracheoinnominate fistula. Early tracheostomy can be accomplished at the bedside with commercially available kits such as the Cook-Ciaglia or the “Rhino” device.154,155 Swallowing dysfunction may occur after tracheostomy or after prolonged endotracheal intubation, introducing the risk for aspiration pneumonia or respiratory failure. A swallowing evaluation is indicated before allowing a tracheostomy patient to attempt oral feeding. This is usually accomplished with a formal speech pathology consult, but swallowing difficulty also may be noted by the nurses during attempted feedings. The predictors and outcome of cardiac surgical patients requiring tracheotomy were recently studied.6 Hemodynamic status on ICU admission (low cardiac output, vasopressor use, pulmonary hypertension) and early postoperative events (stroke, bacteremia) were more important than preoperative and intraoperative variables in predicting ventilatory dependency. Survival at 30 days, 1 year, and 5 years thereafter was 76%, 49%, and 33%, respectively, and was strongly associated with favorable hemodynamic status.
Inability to Wean
A small percentage of patients will not be able to wean from ventilator support despite all efforts. Predictive models, however, are rarely useful for deciding which individuals will not benefit from further intensive care.5,9,16,20,156
Our experience has been that it is rarely a single problem, but the interaction among multiple morbidities that creates a situation in which the patient may never be able to separate from the ventilator. At this point, a frank discussion with the patient (if he/she has decisional capacity) or the health care proxy can be helpful in defining the benefits and burdens of further therapy and the patient’s desires. Consultation from the hospital’s ethics team may be helpful.157 Patients who remain in a low cardiac output state and who have sustained multiple organ failure rarely, if ever, end their dependence on high-technology support including ventilation and hemodialysis. In contrast, malnutrition and deconditioning in the absence of ongoing sepsis and organ system failure sometimes respond to prolonged rehabilitation, which may be better handled by a long-term ventilation facility than an acute-care hospital. The critical issue is patient reserve, for without adequate cardiac and pulmonary reserve to tolerate stress once all remediable problems have been addressed, a patient is unlikely to remain technology independent.
Conclusions
Success in weaning from mechanical ventilation requires an individualized and holistic approach to the patient. Weaning should be the first priority for the day, and all other demands minimized if possible. If trips to the computed tomography scanner or therapeutic intervention such as wound debridement are anticipated, weaning may not be possible for part of the day. Thus, it is wise to try to minimize interruptions and to group them so as not to interrupt the weaning process. With that goal in mind, it is also essential to avoid disrupting the patient’s nighttime sleep so that the patient can be well rested and ready to participate in the weaning process. Detailed and full instructions need to be given to the patient, and it frequently is helpful to include family members in the discussion so that they can serve as adjunct respiratory coaches. We try to avoid pushing the patient to the point of exhaustion or panic and use a planned, conservative approach such that weaning always ends in a sense of accomplishment for the patient rather than failure. Windows of opportunity for weaning from mechanical support are few and often must be created. Box 35-4 summarizes the recommendations for the difficult-to-wean cardiac surgical patient.
BOX 35-4 The Difficult to wean Patient
1 Westbrook R.R., Stuvs S.E., Sessler A.D., et al. Effects of anesthesia and muscle paralysis on respiratory mechanisms in normal man. J Appl Physiol. 1973;34:81.
2 Sivak E.D., Wiedemann H.P. Clinical measurement of extravascular lung water. Crit Care Clin. 1986;2:511-526.
3 Wilson R.S., Sullivan S.F., Malm J.R., et al. The oxygen cost of breathing following anesthesia and cardiac surgery. Anesthesiology. 1973;99:387.
4 Johnson D., Hurst T., Thompson D., et al. Respiratory function after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:571.
5 Rady M.Y., Ryan T., Starr N.J. Early onset of acute pulmonary dysfunction after cardiovascular surgery: Risk factors and clinical outcome. Crit Care Med. 1997;25(11):1831.
6 Murthy S.C., Arroliga A.C., Walts P.A., et al. Ventilatory dependency after cardiovascular surgery. J Thorac Cardiovasc Surg. 2007;134:484-490.
7 Weiner P., Zeidan F., Zamir D., et al. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J Surg. 1998;22:427-431.
8 Higgins T.L. Quantifying risk in assessing outcome in cardiac surgery. J Cardiothor Vasc Anesth. 1998;12:330-340.
9 Higgins T.L., Estafanous E.G., Loop F.D., et al. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344-2348.
10 Hatler B.G., Armitage J.M., Haristy R.L., et al. Risk stratification using the Society of Thoracic Surgeons Program. Ann Thorac Surg. 1994;58:1348-1352.
11 The Society of Thoracic Surgeons. 30-Day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856-1865.
12 Nashef S.A.M., Roques F., Michel P., et al. EuroSCORE Study Group: European system for cardiac operative risk evalauation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9-13.
13 Samuels L.E., Kaufman M.S., Rohinton B.A., et al. Coronary artery bypass grafting in patients with COPD. Chest. 1998;113:878-882.
14 Rockx M.A., Fox S.A., Stitt L.W., et al. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg. 2004;47:34-38.
15 O’Brien M.M., Gonzales R., Shroyer A.L., et al. Modest serum creatinine elevation affects adverse outcome after general surgery. Kidney Int. 2002;62:585.
16 Spivack S.D., Shinozaki T., Albertini J.J., et al. Preoperative prediction of postoperative respiratory outcome. Coronary artery bypass grafting. Chest. 1996;109:1222-1230.
17 Branca P., McGaw P., Light R.W., et al. Factors associated with prolonged mechanical ventilation following coronary artery bypass surgery. Chest. 2001;119:537-546.
18 Rady M.Y., Ryan T. Perioperative predictors of extubation failure and the effect on clinical outcomes after cardiac surgery. Crit Care Med. 1999;27:340-347.
19 Canver C.C., Chanda J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg. 2003;75:853-857.
20 Higgins T.L., Estafanous F.G., Loop F.D., et al. ICU admission score for predicting morbidity and mortality risk after coronary artery bypass grafting. Ann Thorac Surg. 1997;64:1050-1058.
21 Hall R.I., Smith M.S., Rocker G. The systemic inflammatory response to cardiopulmonary bypass—pathophysiological, therapeutic and pharmacological considerations. Anesth Analg. 1997;85:766.
22 Corral L., Carrio M.L., Ventura J.L., et al. Is C-reactive protein a biomarker for immediate clinical outcome after cardiac surgery? J Cardiothorac Vasc Anesth. 2009;23:166-169.
23 Yende S., Quasney M.W., Tolley E.A., et al. Clinical relevance of angiotensin-converting enzyme gene polymorphisms to predict risk of mechanical ventilation after coronary artery bypass graft surgery. Crit Care Med. 2004;32:922-927.
24 Huffmyer J.L., Mauermann W.J., Thiele R.H., et al. Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:468-473.
25 Ouattara A., Benhaoua H., Le Manach Y., et al. Perioperative statin therapy is associated with a significant and dose-dependent reduction of adverse cardiovascular outcomes after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:633-638.
26 Bohm S.H., Thamm O.C., von Sandersleben A., et al. Alveolar recruitment strategy and high positive end-expiratory pressure levels do not affect hemodynamics in morbidly obese intravascular volume-loaded patients. Anesth Analg. 2009;109:160-163.
27 Boldt J., Kling D., Weidler B., et al. Acute preoperative hemodilution in cardiac surgery: Volume replacement with a hypertonic saline-hydroxyethyl starch solution. J Cardiothorac Vasc Anesth. 1991;5:23-28.
28 Boldt J., Zickman B., Ballesteros M., et al. Cardiorespiratory responses to hypertonic saline solution in cardiac operations. Ann Thorac Surg. 1991;51:610-615.
29 Cheng K.C., Hou C.C., Huang H.C., et al. Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit Care Med. 2006;34:1345-1350.
30 Zarbock A., Mueller E., Netzer S., et al. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications. Chest. 2009;135:1252-1259.
31 Jacobs M.C., Hussain E., Hall M.H., et al. Stranger in a strange land: Internists in cardiothoracic intensive care. New Horizons. 1999;7:562-568.
32 Pronovost P.J., Angus D.C., Dorman T., et al. Physician staffing patterns and clinical outcomes in critically ill patients. A systematic review. JAMA. 2002;288:2151-2162.
33 Levy M.M., Rapoport J., Lemeshow S., et al. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148:801-809.
34 Pronovost P.J., Thompson D.A., Holzmueller C.G., et al. The organization of intensive care unit physician services. Crit Care Med. 2007;25:2256-2261.
35 Pronovost P.J., Needham D.M., Waters H., et al. Intensive care unit physician staffing: Financial modeling of the Leapfrog standard. Crit Care Med. 2004;32:1247-1253.
36 Gajic O., Afessa B. Physician staffing models and patient safety in the ICU. Chest. 2009;135:1038-1044.
37 Fagon J.-Y., Chastre J., Domart Y., et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989;139:877-884.
38 Weireter L.J., Collins J.N., Britt R.C., et al. Impact of a monitored program of care on incidence of ventilator-associated pneumonia: Results of a longterm performance-improvement project. J Am Coll Surg. 2009;208:700-705.
39 Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
40 Drakulovic M.B., Torres A., Bauer T.T., et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomized trial. Lancet. 1999;354:1851-1858.
41 Schweickert W.D., Gehlbach B.K., Pohlman A.S., et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272-1276.
42 Craven D.E., Goularte T.A., Make B.J. Contaminated condensate in mechanical ventilator circuits: A risk factor for nosocomial pneumonia? Am Rev Respir Dis. 1984;129:625-628.
43 Sonora R., Jubert P., Artigas A., et al. Pneumonia in intubated patients: Role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111-115.
44 Kollef M.H. The prevention of ventilator-associated pneumonia. N Engl J Med. 1999;340:627-634.
45 Dezfulian C., Shojania K., Collard H.R., et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am J Med. 2005;118:11-18.
46 Kollef M.H., Afessa B., Anzueto A., et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia. The NASCENT randomized trial. JAMA. 2008;300:805-813.
47 Collard H.R., Sanjay S., Matthay M.A. Prevention of ventilator-associated pneumonia: An evidence-based systemic review. Ann Intern Med. 2003;138:494-501.
48 Ware L.B., Matthay M.A. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L514-L521.
49 Bernard G.R., Artigas A., Brigham K.L., et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824.
50 Murray J.F., Matthay M.A., Luce J.M., et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720-723.
51 The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564-2575.
52 Kallet R.H., Jasmer R.M., Pittet J.-F., et al. Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med. 2005;33:925-929.
53 Nahum A., Hoyt J., Schmitz L., et al. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med. 1997;25:1733-1743.
54 Amato M.B., Barbas C.S., Medeiros D.M., et al. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome: A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med. 1995;152:1835-1846.
55 The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
56 Determan R.M., Royakkers A., Wolthuis E.K., et al. Ventilation with lower tidal volumes as compared to conventional tidal volumes for patients without acute lung injury—a preventive randomized controlled trial. Crit Care. 2010;14:R1.
57 Bidani A., Tzouanakis A.E., Cardenas V.J., et al. Permissive hypercapnea in acute respiratory failure. JAMA. 1994;272:957-962.
58 Tidswell M. Prone ventilation. Clin Intensive Care. 2001;12:193-201.
59 Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568-573.
60 Richard J.-C., Brochard L., Vandelet P., et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med. 2003;31:89-92.
61 Morris A.H., Wallace C.J., Menlove Rl., et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295-305.
62 Pranikoff T., Hirschl R.B., Steimle C.N., et al. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med. 1997;25:28-32.
63 Hirschl R.B., Pranikoff T., Wise C., et al. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA. 1996;275:383-389.
64 Body S.C., Shernan S.K. The utility of nitric oxide in the postoperative period. Semin Cardiothorac Vasc Anesth. 1998;2:4-30.
65 Ullrich R., Lorber C., Röder G., et al. Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS. Anesthesiology. 1999;91:1577-1586.
66 Walmrath D., Schneider T., Schermuly R., et al. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:991-996.
67 Miduri G.U., Headley A.S., Golden E., et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA. 1998;280:159-165.
68 Michalopoulos A., Anthi A., Rellos K., et al. Effects of positive end-expiratory pressure (PEEP) in cardiac surgery patients. Respir Med. 1998;92:858-862.
69 Calzia E., Lindner K.H., Radermacher P., et al. Effects of continuous positive airway pressure on respiratory mechanics and work of breathing after cardiac surgery. Clin Intensive Care. 1998;9:105-110.
70 Guyton R.A., Chiavarelli M., Padgett C.A., et al. The influence of positive-end expiratory pressure on intrapericardial pressure and cardiac function after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1987;1:98.
71 Boldt J., Kling D., Bormann B.V., et al. Influence of PEEP ventilation immediately after cardiopulmonary bypass on right ventricular function. Chest. 1988;94:566.
72 Pepe P.E., Hudson L.D., Carrico C.J. Early application of positive end-expiratory pressure in patients at risk for the adult respiratory distress syndrome. N Engl J Med. 1984;311:281.
73 Zurick A.M., Urzua J., Ghattas M., et al. Failure of positive end-expiratory pressure to decrease postoperative bleeding after cardiac surgery. Ann Thorac Surg. 1982;34:608.
74 Rimensberger P.C., Pristine G., Mullen B.M., et al. Lung recruitment during small tidal volume ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med. 1999;27:1940-1944.
75 The ARDS Clinical Trials Network, National Heart, Lung and Blood Institute, National Institutes of Health. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 2003;31:2592-2597.
76 Hood V.L., Tannen R.L. Protection of acid-base balance by pH regulation of acid production. N Engl J Med. 1998;339:819-826.
77 Laffrey J.G., Kavanagh B.P. Carbon dioxide and the critically ill—too little of a good thing? Lancet. 1999;354:1283-1286.
78 Mekontso D.A., Charron C., Devaquet J., et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;11:1850-1858.
79 Steingrub J.S., Tidswell M.A., Higgins T.L. Hemodynamic consequences of heart-lung interactions. J Intensive Care Med. 2003;18:92-99.
80 Pepe P.E., Marini J.J. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction. Am Rev Respir Dis. 1982;126:166.
81 Agency for Healthcare Research and Quality, Available at: http://www.AHRQ.gov/clinic/ptsafety/addend.htm Accessed May 20, 2004
82 Kress J.P., Pohlman A.S., O’Connor M.F., et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
83 Van den Berghe G., Wouters P., Welkes F., et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359-1367.
84 Pronovost P., Berenholtz S., Dorman T., et al. Improving communication in the ICU using daily goals. J Crit Care. 2003;18:71-75.
85 Bates D.W., Gawande A.A. Improving safety with information technology. N Engl J Med. 2003;348:2526-2534.
86 Milbrandt E.B., Deppen S., Harrison P.L., et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;31:955-962.
87 Smith L.W., Dimsdale J.E. Postcardiotomy delirium: Conclusions after 25 years. Am J Psychiatry. 1989;146:452-458.
88 Eli E.W., Inouye S.K., Bernard G.R., et al. Delirium in mechanically ventilated patients. Validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286:2703-2710.
89 Riker R.R., Shehabi Y., Bokesch P.M., et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients. JAMA. 2009;301:489-499.
90 Herr D.L., Sum-Ping St, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576-584.
91 Hudetz J.A., Patterson K.M., Iqbal Z., et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:651-657.
92 Tesar G.E., Murray G.B., Cassem N.H. Use of high-dose intravenous haloperidol in the treatment of agitated cardiac patients. J Clin Psychopharmacol. 1985;5:344.
93 Wilcox P., Baile E.M., Hards J., et al. Phrenic nerve function and its relationship to atelectasis after coronary artery bypass surgery. Chest. 1988;93:693.
94 Lorin S., Sivak M., Nierman D.M. Critical illness polyneuropathy: What to look for in at-risk patients. Diagnosis requires a high index of suspicion. J Crit Illness. 1998;13:608.
95 Ibebunjo C., Martyn J.A.JA. Fiber atrophy, but not changes in acetylcholine receptor expression, contributes to the muscle dysfunction after immobilization. Crit Care Med. 1999;27:275.
96 Van Balkom R.H.H., Dekhuijzen R., Folgering H.T.M., et al. Effects of long-term low-dose methylprednisone on rat diaphragm function and structure. Muscle Nerve. 1997;20:983.
97 Abd A.G., Braun N.M.T., Baskin M.I., et al. Diaphragmatic dysfunction after open heart surgery; treatment with a rocking bed. Ann Intern Med. 1989;111:881.
98 Aubier M., Detroyer A., Sampson M., et al. Aminophylline improves diaphragmatic contractility. N Engl J Med. 1981;305:249.
99 Riddington D.W., Venkatesh B., Boivin C.M., et al. Intestinal permeability, gastric intramucosal pH and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007.
100 Tuzcu E.M., Maloney J.D., Sangani B.H., et al. Cardiopulmonary effects of chronic amiodarone therapy in the early postoperative course of cardiac surgery patients. Cleve Clin J Med. 1987;54:491.
101 Nalos P.C., Kass R.M., Gang E.S., et al. Life-threatening postoperative pulmonary complications in patients with previous amiodarone pulmonary toxicity undergoing cardiothoracic operations. J Thorac Cardiovasc Surg. 1987;93:904.
102 Manthous C.A., Zarich S. Myocardial ischemia during weaning from mechanical ventilation. Semin Cardiothorac Vasc Anesth. 1998;2:78.
103 Zin W.A., Caldeira M.P.R., Cardoso W.V., et al. Expiratory mechanics before and after uncomplicated heart surgery. Chest. 1989;1:21.
104 Guillory R.K., Gunter O.L. Ultrasound in the surgical intensive care unit. Curr Opin Crit Care. 2008;14:415-422.
105 Melamed R., Sprenkle M.D., Ulstad V.K., et al. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest. 2009;135:1416-1420.
106 Beaulieu Y., Marik P.E. Bedside ultrasonography in the ICU: Part 2. Chest. 2005;128:1766-1781.
107 Omoto R., Kyo S., Matsumara M., et al. Evaluation of biplane color Doppler Transesophageal echocardiography in 200 consecutive patients. Circulation. 1992;85:1237-1247.
108 Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: Feasible and alters patient management. J Cardiothorac Vasc Anesth. 2009;23:450-456.
109 Orihashi K., Hong Y.W., Chung G., et al. New application of two-dimensional echocardiography in cardiac surgery. J Cardiothorac Vasc Anesth. 1991;5:33-39.
110 Talmor M., Hydo L., Gershenwald J.G., et al. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery. 1998;123:137-143.
111 Seward J.B., Khanderia B.K., Edwards W.D., et al. Biplanar transesophageal echocardiography: Anatomic correlation, image orientation and clinical application. Mayo Clin Proc. 1990;65:1193-1213.
112 American Society of Anesthesiologists Task Force on Pulmonary artery Catheterization. Practice guidelines for pulmonary artery catheterization. Anesthesiology. 2003;99:988-1014.
113 Jardin F., Bourdarias J.P. Right heart catheterization at bedside: A critical view. Intensive Care Med. 1995;21:291-295.
114 Joseph M.X., Disney P.J.S., Da Costa R., et al. Transthoracic echocardiography to identify or exclude cardiac cause of shock. Chest. 2004;126:1592-1597.
115 Subramaniam B., Talmor D. Echocardiography for management of hypotension in the intensive care unit. Crit Care Med. 2007;35(8 Suppl):S401-S407.
116 Sterba M., Banerjee A., Mudaliar Y. Prospective observational study of levosimendan and weaning of difficult-to-wean ventilator dependent intensive care patients. Crit Care Resusc. 2008;10:182-186.
117 Balik M., Pachl J., Hendl J. Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med. 2002;28:1117-1121.
118 Jullien T., Valtier B., Hongnat J.M., et al. Incidence of tricuspid regurgitation and vena cava backward flow in mechanically ventilated patients. A color Doppler and contrast echocardiography study. Chest. 1995;107:488-493.
119 Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007;35:S235-S249.
120 Feissel M., Teboul J.L., Merlani P., et al. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med. 2007;33:993-999.
121 Monnet X., Rienzo M., Osman D., et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402-1407.
122 Vieillard-Baron A., Charron C. Preload responsiveness or right ventricular dysfunction? Crit Care Med. 2009;37:2662-2663.
123 Vieillard-Baron A.I. Is right ventricular function the one that matters in ARDS patients? Definitely yes. Intensive Care Med. 2009;35:4-6.
124 Funk D.J., Moretti E.W., Gan T.J. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887-897.
125 Koning H.M., Koning A.J., Leusink J.A. Serious acute renal failure following open heart surgery. J Thorac Cardiovasc Surg. 1985;33:283.
126 Myers B.D., Moran S.M. Hemodynamically mediated acute renal failure. N Engl J Med. 1987;314:97.
127 Joy M.S., Matske G.R., Armstrong D.K., et al. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32:362.
128 Loop F.D., Lytle B.W., Cosgrove D.M., et al. Sternal wound complications after isolated coronary artery bypass grafting: Early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49:179.
129 Zerr K.J., Furnary A.P., Grunkemeier G.L., et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356.
130 Kirschenbaum L., Azzi E., Sfeir T., et al. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory care. Crit Care Med. 2002;30:1983-1986.
131 Chastre J., Fagon J.-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
132 Krasna M.J., Flanchbaum L., Trooskin Z.S., et al. Gastrointestinal complications after cardiac surgery. Surgery. 1988;104:733.
133 Ephgrave K.S., Kleinman-Wexler R.L., Adar C.G. Enteral nutrients prevent stress ulceration and increase intragastric volume. Crit Care Med. 1990;18:621.
134 Zilberberg M.D., Nathanson B., Higgins T.L., et al. Epidemiology and outcomes of Clostridium difficile-associated disease among patients on prolonged acute mechanical ventilation. Chest. 2009;136:752-758.
135 Bassilli H.R., Deitel M. Effect of nutritional support on weaning patients off mechanical ventilators. J Parenter Enteral Nutr. 1981;5:161.
136 Larca L., Greenbaum D.M. Effectiveness of intensive nutritional regimes in patients who fail to wean from mechanical ventilation. Crit Care Med. 1982;10:297-300.
137 Tharratt R.S., Allen R.P., Albertson T.E. Pressure controlled inverse ratio ventilation in severe adult respiratory failure. Chest. 1988;94:755-762.
138 Richard J.-C., Brochard L., Vandelet P., et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med. 2003;31:89-92.
139 Stock A.C., Downs J.B., Frolicher D.A. Airway pressure release ventilation. Crit Care Med. 1987;15:462-466.
140 Dongelmans D.A., Veelo D.P., Bindels A., et al. Determinants of tidal volumes with adaptive support ventilation: A multicenter observational study. Anesth Analg. 2008;107:932-937.
141 Millbern S.M., Downs J.B., Jumper L.C., et al. Evaluation of criteria for discontinuing mechanical ventilator. Arch Surg. 1978;113:1441-1443.
142 Yang K.L., Tobin M.J. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445-1450.
143 Bellemare F., Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol. 1982;53:1190-1195.
144 Grassino A., Bellemare F., Laporta D. Diaphragm fatigue and the strategy of breathing in COPD. Chest. 1984;85:515-535.
145 Ceriana P., Carlucci A., Navalesi P., et al. Physiological responses during a T-piece weaning trial with a deflated tube. Intensive Care Med. 2006;32:1399-1403.
146 Cohen C.A., Zagelbaum G., Cross D., et al. Clinical manifestations of inspiratory muscle fatigue. Am J Med. 1982;73:308-316.
147 Brown D.G., Pierson D.J. Auto-PEEP is common in mechanically ventilated patients: A study of incidence, severity, and detection. Respir Care. 1986;31:1069-1074.
148 Segredo V., Caldwell J.E., Matthay M.A., et al. Persistent paralysis in critically ill patients after long-term administration of Vecuronium. N Engl J Med. 1992;327:524-528.
149 Rosenthal L.J., Kim V., Kim D. Weaning from prolonged mechanical ventilation using an antipsychotic agent in a patient with acute stress disorder. Crit Care Med. 2007;35:2417-2419.
150 Kastanos N., Estopa R., Peez A.M., et al. Laryngotracheal injury due to endotracheal intubation: Incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med. 1983;11:362-367.
151 Norwood S., Vallina V.L., Short K., et al. Incidence of tracheal stenosis and other late complications after percutaneous tracheostomy. Ann Surg. 2000;232:233-241.
152 Gibbons K.J. Tracheostomy: Timing is everything. Crit Care Med. 2000;28:1663-1664.
153 Brook A.D., Sherman G., Malen J., et al. Early versus late tracheostomy in patients who require prolonged mechanical ventilation. Am J Crit Care. 2000;9:352-359.
154 Westphal K., Byhahn C., Rinne T., et al. Tracheostomy in cardiosurgical patients: Surgical tracheostomy versus Ciaglia and Fantoni methods. Ann Thorac Surg. 1999;68:486-492.
155 Freeman B.D., Isabella K., Cobb P., et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001;29:926-930.
156 Holmes L., Loughead K., Treasure T., et al. Which patients will not benefit from further intensive care after cardiac surgery. Lancet. 1994;344:1200-1202.
157 Baldyga A.P. Ethical issues in the postoperative intensive care unit. Semin Cardiothorac Vasc Anesth. 1998;2:90.