CHAPTER 105 Postoperative Pseudomeningocele, Hematoma, and Seroma
PSEUDOMENINGOCELE
A pseudomeningocele is an extradural collection of cerebrospinal fluid (CSF) that has extravasated through a dural or arachnoid tear.1–3 Other terms used to describe this condition are ‘meningeal pseudocysts’ and ‘meningeal spurius’.4 Pseudomeningocele was first reported in the literature in 1946 following laminectomy for removal of a neoplasm.5
Epidemiology
Among patients undergoing primary decompressive lumbar spine surgery, incidental durotomy occurs at an incidence of 9–12%, making it the most frequently reported complication of lumbar spine surgery.6,7 After cauda equina syndrome, dural tears are the most frequent underlying diagnosis in malpractice litigation involving lumbar spine surgery.8
Pseudomeningoceles are a rare consequence of dural injury. In a review of 1700 laminectomies, Swanson and Fincher reported pseudomeningocele formation in four patients.9 In a group of 400 postlaminectomy patients with persistent back pain and/or radicular symptoms, Teplick et al. found pseudomeningoceles in 8 patients (2%).3
Factors that predispose to a dural tear in surgery are prior surgery or irradiation, congenital spinal malformations, unrecognized dural adhesions, dural calcification contiguous with overlying lamina, and the absence of epidural fat.8,10,11 Severe spinal stenosis and large herniated discs make root dissection and dural retraction more difficult and can predispose to dural tears. Dural injuries and pseudomeningoceles are understandably more frequent with posterior than anterior approaches.
Pathogenesis
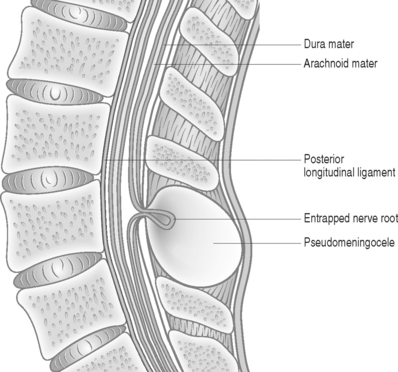
Pseudomeningoceles generally develop following an intraoperative rent in the dura and arachnoid, but can occur following dural needle puncture procedures, especially after multiple punctures.9 The cerebrospinal fluid leaks dorsal to the lamina, into a space artificially created by dissection of the paravertebral musculature. The fluid cavity is lined by flattened connective tissue cells resting on loose, areolar connective tissue.2,4 An encysted pseudomeningocele refers to a CSF-filled herniation of an arachnoid lined cyst through a small rent in the dura. The narrow opening in the dura acts like a one-way valve leading to an increase in size of the CSF collection and formation of a pseudomembrane.2
The size of the dural defect, pressure of the inflowing CSF, and the resistance of the surrounding soft tissues all influence the size of a pseudomeningocele. Postoperative weakness of paraspinal muscles may be a reason contributing to expansion of the pseudomeningocele.12 Spinal fluid pressure keeps the dural defect open and prevents healing. Herniation of nerve filaments into the original dural defect may be another factor responsible for keeping the dural opening patent (Fig. 105.1).4
Puncture of the dura can result in escape of large volumes of CSF, leading to intracranial hypotension and a demonstrable reduction in CSF volume. The adult subarachnoid pressure of 15 cmH2O can be reduced to 4 cmH2O or less. The rate of loss of CSF through a dural perforation following puncture with a 25-gauge or larger needle is generally greater than the rate of CSF production.13
Clinical features
Cerebrospinal fluid leaks after needle procedures in the lumbar spine classically present with headache. There is evidence that CSF leaks may be inevitable following needle puncture of the thecal sac,14 and it is unclear why only some of these patients are symptomatic. The size of the dural leak has no direct relationship with the occurrence of headache.14 The actual mechanism of headache is also unclear. Some authors suggest that the lowering of CSF pressure following a leak causes traction on the intracranial structures in the upright position. These structures are pain sensitive, leading to the characteristic headache.13 Others authors report that the drop in intracranial pressure causes a compensatory intracranial venodilation, which causes the headache. The headache is generally localized to the frontal and occipital regions, with occasional radiation to the neck and shoulders. Other symptoms may include nausea, vomiting, tinnitus, and vertigo.
While pseudomeningoceles may be asymptomatic, many present with local swelling, symptoms of CSF leak, or nerve root impingement. Local swelling15 may occasionally be the only suggestion of a pseudomeningocele.12 The swelling may vary in size, depending on whether the patient is standing or recumbent. There is no apparent correlation between the size of the pseudomeningocele, the size of the defect in the dura, and the degree of symptoms.4,16 Postural headache frequently occurs in patients with pseudomeningocele. Miller and Elder4 reported one patient whose postural headache could be increased by manual pressure directly over a large fluctuant meningocele.
Back pain, with or without radicular leg symptoms, is commonly associated with pseudomeningocele. In a review of 10 patients with postoperative pseudomeningocele, Miller and Elder found that back pain was a common feature in all patients, with nine having radicular pain radiating into the lower limbs.4 Manual pressure over the paraspinal musculature on the side of the cyst may exacerbate the back pain.16 Coexisting worker’s compensation issues can lead to doubts being expressed as to the authenticity of such complaints in the postoperative patient.16
Radicular findings in patients with pseudomeningocele result from herniation and kinking of the nerve roots in the dural defect,17 adhesion of the nerve roots to the edges of the sac,18 or direct pressure of the pseudomeningocele on an adjacent nerve root. Eismont et al.19 reported a case of persistent postoperative radicular pain along with focal deficits following lumbar disc excision. Myelography revealed a pseudomeningocele involving the left fourth and fifth lumbar nerve roots. Intraoperatively, it was found that the L4 root had buttonholed through the site of the dural tear, while the pseudomeningocele was found to be compressing the L5 root. Ossification of a pseudomeningocele can rarely cause neural compression.20 In many patients an obvious site of nerve root entrapment is not identifiable.4 Asymptomatic meningoceles can become symptomatic after a precipitating event leading to nerve entrapment.21 Radicular pain in a previously asymptomatic patient can be precipitated by maneuvers that increase intracranial and intraspinal pressure, such as coughing, sneezing, or jugular compression.16 Unusual presentations associated with pseudomeningoceles include bone erosion of the posterior vertebral elements22 and chronic meningitis.23
Investigations
Myelography shows a characteristic pattern of dye extruding through the stalk of the pseudomeningocele, and a fluid level may be visible when the opening in the dura is large. Percutaneous injection of contrast material directly into the cyst cavity has been carried out when a palpable or visible mass is present at a surgical site.16 CT-myelography can effectively demonstrate the neck and outline of the pseudomeningocele although nerve root entrapment is not well visualized.18
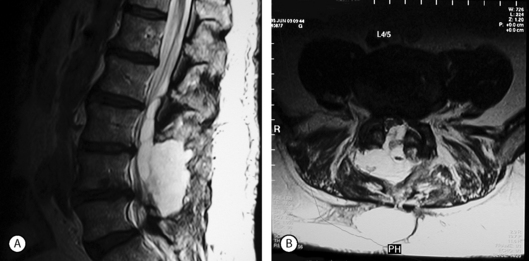
MRI scans are very sensitive for CSF collections and pseudomeningoceles (Fig. 105.2). Some authors recommend a routine postoperative MRI scan following any repair of a durotomy, even in the absence of symptoms of a pseudomeningocele, in order to ensure that no further leakage is present.24 Digital subtraction myelography may occasionally reveal a pseudomeningocele when plain myelography, CT scan, and MRI show a fluid collection posterior to the dural sac, but no obvious connection between the two.25 In radionuclide cisternography, radionuclide contrast material is injected into the subarachnoid space followed by MRI evaluation. Areas filled with CSF show up as high-signal regions.26
Management of dural tear and pseudomeningocele
In an attempt to reduce the incidence of postdural puncture CSF leak, numerous modifications have been made to spinal needles. Newer needle designs with narrow cutting tips and atraumatic bevels have resulted in a lower incidence of postspinal headache, neural irritation,13,27 and CSF leak.28
Spontaneous recovery from postdural puncture headache (PDPH) occurs in over 85% of patients within 6 weeks. Many forms of conservative management have been suggested and practiced, mostly without any scientific evidence to support their efficacy. Bed rest has been historically recommended, but does not lower the incidence of PDPH although it may delay its onset and decrease its intensity.29 Some authors suggest that bed rest may prevent further complications from traction and rupture of meningeal veins caused by intracranial hypotension.30 For severe and persistent headaches epidural blood patch (EBP) injections have been successfully used. Autologous blood is injected into the epidural space, where it forms a clot and seals the dural hole. Although over 90% of PDPH respond initially to EBP injections, a sustained and durable response has been identified in only 61–75%.31
Intraoperative durotomy is managed by primary dural repair, with a watertight suture of the dural defect. The key to prevention of postoperative pseudomeningocele is a meticulous primary repair of dural tears detected intraoperatively. Fine suture material such as 5-0 or 6-0 monofilament, braided or coated synthetic is suitable, although leakage of CSF can occur through the needle holes in the dura. A continuous running suture is quickest to perform, but may result in unraveling of the entire suture line if the tension is not perfect or the suture breaks. The use of magnification and coaxial illumination is essential for adequate repair. Local application of fibrin glue greatly reinforces a repair site.26,32
When a CSF leak is noticed postoperatively, surgical treatment is generally recommended for the immediate and definitive management of the lesion.19 Some authors recommend nonoperative management in a patient with a well-healed incision presenting with a soft subcutaneous bulge and no postural headache.33 Waisman and Schweppe treated seven patients by reinforcing the skin suture line, bed rest in the Trendelenburg position, and repeated puncture and drainage of the subcutaneous CSF collection. The CSF leakage stopped immediately after the incision was resutured, the subcutaneous fluctuation disappeared in 10–28 days, and an 8-year follow-up (clinical and ultrasound examination) revealed no pseudocyst formation.34 Eismont et al. reported one case where resuturing the skin stopped the CSF leakage through the incision. A symptomatic pseudomeningocele subsequently developed, and the authors recommends surgical repair of the dura for all dural leaks noted postoperatively.19
Closed subarachnoid drainage is frequently used in the management of CSF leaks33,35 and occasionally for established pseudomeningocele.36 The catheter is positioned in the subarachnoid space using fluoroscopic technique. Epidural catheter placement may be used for drainage of a pseudomeningocele.33 The proximal end of the catheter is connected to a sterile drainage system. The basis of this external CSF drainage is that a reduction of CSF pressure and preferential escape of the CSF through the catheter for 4–5 days generally allows the dural fistula to heal itself.
Lumbar peritoneal shunting may play a role in the management of refractory CSF leaks.33,37 The dural opening is surgically repaired when feasible. A catheter is then introduced into the subarachnoid space, and a second catheter left in the meningocele cavity; both catheters are subcutaneously tunneled into the peritoneal cavity, where they are positioned under the diaphragm using laparoscopic assistance. The advantages of this technique including immediate mobilization of the patient, avoidance of complications of external drains and repeated aspirations, and a high success rate. The procedure is not in widespread use due to technically simpler and less invasive options being available.
In previously irradiated tissue a vascularized myocutaneous flap can be rotated from an uninvolved area to the poorly vascularized defect and sutured to well-vascularized surrounding tissue. Newer techniques such as laser tissue welding are being investigated. Preliminary results show that primary repair combined with laser welding produces a higher leak pressure and tensile tissue strength than either technique used alone, with no evidence of underlying thermal tissue injury.38
Patients with established pseudomeningoceles who are not significantly disabled by their symptoms may be treated with observation alone. Follow-up MRI scans occasionally show complete resolution of small pseudomeningoceles.2 Sustained local pressure has been found to help control some symptoms of pseudomeningoceles. Leis et al.15 reported this form of treatment in a patient who was advised surgery but declined. The patient used a wide belt worn tightly around the waist and a folded towel under the belt so that it applied direct pressure over the swelling. This relieved the postural headache symptoms immediately. Repeat MRI scans done 8 weeks and 6 months after surgery showed progressive decrease in the size of the pseudomeningocele, till there was only a tiny focus of fluid left. The authors suggest a trial of focal compression for symptomatic relief of postural headache from pseudomeningocele. If symptomatic improvement is obtained, a more prolonged trial of mechanical compression may promote dural closure.15
Symptomatic pseudomeningoceles generally require surgical intervention.2,21 The operative procedure usually involves opening of the pseudomeningocele, followed by identification and closure of the dural defect with flaps from the cyst wall or a free graft. Augmentation of the repair with fibrin glue, muscle, fat, and fascial grafts is frequently necessary. Resection of overlying lamina is generally necessary for adequate visualization of the dural defect, and spinal fusion is indicated when excessive bone resection renders the spine unstable.4 Miller and Elder4 reported on the surgical treatment of 10 symptomatic pseudomeningoceles. The authors reported good results in seven patients, satisfactory results in two, and a poor result in one patient.
Apart from surgical repair, the only other surgical option for pseudomeningocele is CSF diversion, a technique that is not always successful, especially when spinal fusion implants prevent the normal reapproximation of paraspinal tissues around the collection. In addition, a ball-valve mechanism at the dural fistula site may prevent complete drainage of CSF from the pseudomeningocele. The cavity could persist and reaccumulate CSF after the drain is removed.39
Complications of untreated pseudomeningocele include recurrence of radicular symptoms, the possibility of infection with resultant meningitis,23 ossification of the cyst wall producing canal stenosis and claudication,40 and rarely erosion of the bony vertebral elements from an expansile cyst.22
SPINAL HEMATOMA
Introduction
The term ‘hematoma’ when used in postoperative spine patients generally refers to a subcutaneous or subfascial incisional hematoma, and must be distinguished from a true spinal hematoma. The frequency of incisional hematomas depends on many factors, including the type and extent of surgery. An incidence of 0.08% (8.7 in 10 000) incisional hematomas was reported among 28 395 patients undergoing lumbar laminectomy,41 whereas rates of up to 12% have been reported following scoliosis surgery.42
True spinal hematomas occur most frequently in the epidural space (Table 105.1). The overall incidence of symptomatic spinal hematomas is very low, with a recent meta-analysis finding only 613 symptomatic patients over the past 170 years.43 Lawton et al.44 reported a 0.1% incidence (12 in 10 500) over a 14-year period comprising 10 500 spine surgery cases at one institution. Smaller asymptomatic hematomas occur more frequently than recognized in postoperative patients. When postoperative MRI is obtained, the reported incidence is 100%, even with procedures such as microdiscectomies.45
Table 105.1 Distribution of spinal hematomas according to anatomic localization43
| Location | Number (n = 604) | % |
|---|---|---|
| Epidural | 461 | 76.3 |
| Subarachnoid | 96 | 15.9 |
| Subdural | 25 | 4.1 |
| Intramedullary | 5 | 0.8 |
| Combinations | 17 | 2.8 |
The incidence of spinal hematoma following epidural/subarachnoid injection procedures is low. In a meta-analysis of 613 patients with spinal hematoma formation, Kreppel et al. reported that 10.3% (63 in 613) of these incidents occurred following lumbar puncture or spinal anesthesia.43 In a literature review of epidural hematomas after needle procedures, Adler et al. reported that 12 of 15 reported cases either had an abnormal bleeding profile or were on anticoagulant therapy.46 A review of subdural hematomas after lumbar injections showed similar results, with 17 of 19 reported patients receiving some form of anticoagulant therapy.46
Etiology
In an extensive search of the literature on spinal hematomas, Kreppel et al. found a total 613 case studies reported over the last 170 years. Most cases had a multifactorial etiology, and no etiologic factor could be pinpointed in 29.7% of patients. Idiopathic spinal hematomas represented the most common category among all different age groups analyzed.43 Spontaneous epidural bleeds can arise following fibrinolytic therapy for coronary heart disease.47 Spinal hematomas also spontaneously develop from the lumbar facet joint48 and ligamentum flavum.49 The mechanism in these cases is presumed to be minor injury in the setting of degenerative changes. Arteriovenous malformations are another reported cause of spontaneous hematomas.50 Groen and Ponssen51 reported a spontaneous spinal epidural hematoma following rupture of the internal vertebral venous plexus. Spontaneous hematomas have also been reported in patients with liver disease and autoimmune conditions.52 Spinal hematomas occur rarely following spinal fractures, but are more prevalent with fractures in patients with ankylosing spondylitis.53 Spinal manipulation therapy is known to produce hematomas with resultant neurological deficits.54
Spinal hematoma is a recognized cause of postoperative worsening of neurological status in the first 24–48 hours following surgery. Kou et al.52 reviewed factors that predisposed to epidural hematoma formation in 12 patients who underwent lumbar laminectomy over a 10-year period. Multilevel procedures (p=0.037) and a preoperative coagulopathy (p=0.001) were significant risk factors. Age, body mass index, durotomy, and the use of postoperative drains were not statistically significant risk factors. In multilevel laminectomies, the increased exposure needed may increase the risk for bleeding from the paravertebral muscles. The larger exposures of the epidural space also increase the risk of insidious bleeding from the prominent internal vertebral venous plexus.52 The lordotic posture of the spine postoperatively may have a role in accentuating the pressure effects of a spinal hematoma. Ko and Kakiuchi55 reported on a patient who developed paraparesis 3 hours following decompressive surgery for lumbar spinal stenosis. Flexion of the spine was noticed to relieve the patient’s symptoms. The patient was positioned with the lumbar spine in sustained lumbar flexion and the deficits resolved within 5 days.
Clinical features
The presentation of spinal hematomas varies depending on the precipitating event, size of hematoma, canal dimensions, and location of the hematoma within the spinal column. Small hematomas are generally not evident clinically, and may be a universal phenomenon in the immediate postoperative period. Montaldi et al.56 found computed tomographic changes within the spinal canal suggestive of postoperative spinal hematoma or scar tissue in 84% of asymptomatic patients 1 week after lumbar discectomy. Kotilainen found MRI changes representing hematoma in 100% of asymptomatic patients following lumbar microdiscectomy on the first postoperative day.45
Neurologic deficits from larger spinal hematomas are infrequent. Patients with incomplete deficits fare better than those with complete paraplegia, and the location of the hematoma has a bearing on the clinical presentation as well as outcome. Spinal epidural hematomas at the lumbar cauda equina level usually fare better than hematomas at the spinal cord level. Spontaneous remission has been observed in hematomas at the cauda equina level with minor neurologic deficits.57 Spontaneous resolution has also been reported in two cases where the presentation was rapidly progressive over minutes.58 The symptoms resolved while investigations were being carried out over the next few hours. The authors suggested that while urgent decompression remains the treatment of choice in a symptomatic spinal epidural hematoma, conservative management may be indicated where there are definite signs of neurological improvement in the initial few hours.
In patients who develop spontaneous spinal epidural hematomas without preceding trauma, an acute onset of local pain may be followed by radicular paresthesias. Within hours, signs of spinal cord compression can appear, presenting as progressive paraplegia and loss of sensory function. In 61 patients who developed spinal epidural hematomas after epidural puncture for anesthesia, and had been on anticoagulants, Vandermeulen et al. noted muscle weakness was the first sign in 28 of 61 patients, back pain in 23, and sensory deficit in 9.59 Paraplegia was recorded to occur within 14.5±3.7 hours after ending of the anesthetic. The most successful recovery following surgical decompression occurred in patients in whom surgical intervention was performed in less than 8 hours. In another review of five patients with spinal hematomas the authors found that four of five patients complained of acute back pain with paresthesias in both legs, followed by rapidly progressive paraplegia.50 The fifth patient had an incomplete cauda equina syndrome. Slowly accumulating hematomas may result in delayed presentation up to a week following surgery. Long tract symptoms and findings may be seen if the hematoma impinges on the cervical spinal cord, following spinal manipulation or cervical epidural steroid injections. Although the clinical presentation of spinal epidural hematomas is relatively characteristic, other diagnoses need to be considered (Table 105.2)
Table 105.2 Differential diagnosis of spinal epidural haematomas
| Intradural haemorrhage |
| Spondylosis/disc herniation |
| Infections |
| Inflammatory pathologies |
| Spinal cord infarction |
| Spinal arterio-venous malformation |
| Epidural varices |
Investigations
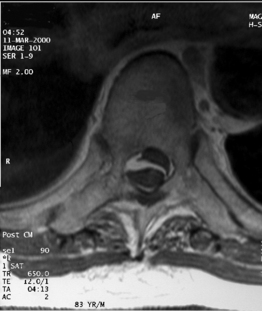
Magnetic resonance imaging scanning is the diagnostic method of choice for evaluation of spinal hematomas (Fig. 105.3). MRI scans allow rapid evaluation of large sections of the spinal column and provide accurate information about the size and longitudinal extent of the lesion. Epidural hematomas have an isointense signal on T1-weighted images. On T2-weighted images, acute hematomas show low-intensity signal in the periphery with a central high-intensity signal region. Beginning 4–14 days after the hematoma, the signal intensity increases from the periphery to the center due to the presence of methemoglobin derived from erythrocytes. Subdural hematomas can mimic epidural hematomas on MRI, but generally show high-intensity signals on both T1- and T2-weighted images. Preservation of the posterior epidural fat and visualization of the dural outline on axial images provide further clues to the subdural location of the hematoma. Intraspinal compressive lesions such as tumors or abscesses may be differentiated from subdural hematomas by virtue of a rim or uniform enhancement on MRI, whereas subdural hematomas rarely enhance. Some authors describe a 3-pointed star or ‘inverted Mercedes star’ appearance in lumbar subdural hematomas.60 CT scanning, myelography, and ultrasound have all been used to detect spinal hematomas, but do not have the sensitivity or specificity of MRI scanning.
Management
An awareness of predisposing factors may substantially lower the incidence of spinal hematoma formation. For patients on anticoagulant therapy and undergoing spinal/epidural anesthetic procedures, the following guidelines may help reduce the occurrence of spinal hematoma:61 (1) atraumatic puncture, (2) a time interval of at least 60 minutes between spinal anesthesia and heparinization (based on the half-life period of heparin), and (3) close monitoring of coagulation parameters. Since the removal of epidural catheters is associated with a high incidence of bleeding complications, Sage62 recommended against the removal of spinal/epidural catheters in the first 2 hours following heparin administration, as many patients develop temporary blood concentration of heparin at this time. Following spine surgery, most authors recommend at least 12 hours before restarting anticoagulant therapy.63
Although the use of postoperative suction drains following spine surgery is widespread, there is surprisingly limited evidence supporting this practice. In a recent Cochrane review of 21 studies in orthopedic literature on this subject, the authors found no difference in the rate of hematoma formation or other wound complications whether drains were used or not.64 Similar results were noted in a randomized trial on the efficacy of postoperative lumbar drainage on 200 patients after single-level nonfusion lumbar surgery. Patients were randomized into two groups of either receiving postoperative drains for 48 hours, or having no drain inserted. The authors found that no patient in either group developed a significant hematoma/seroma requiring surgical drainage.65
Most spinal hematomas presenting with acute neurological deficits are treated by emergency surgical decompression. The need for decompression of extrinsic cord compression has been demonstrated in experimental as well as clinical studies. Using an animal model, Delamarter et al.66 studied the effects of duration of spinal cord compression. Persistent pressure on the cord for 6 hours led to progressive necrosis within the cord, and neurologic recovery did not occur in this group following decompression. In a clinical review of 55 patients who developed spinal hematomas after spinal or epidural anesthesia and who were treated with emergency decompressive laminectomies, Vandermeulen et al found ‘good’ or ‘partial’ neurological recovery in 77% (10/13) of patients operated within 8 hours, 37.5% (3/8) of those treated within 8–24 hours, and only 17% (2/12) of those decompressed after 24 hours.59 Exceptions to the rule of urgent decompression may be made when: (1) a patient presents 48–72 hours after onset of neurologic involvement, and (2) significant recovery is noted in the initial few hours after onset of the deficit.
Operative decompression should be carried out along the entire length of a hematoma to ensure adequate evacuation and maximize chances of neurologic recovery. In some cases of extensive spinal hematoma, a limited decompression may obtain similar results without the morbidity of an extensive decompression. Osmani et al. reported limited decompression for a patient who had a hematoma extending from T4 to L5. The authors performed laminectomies from T11 to L5, and then used an arterial embolectomy balloon catheter to extract the more proximally located epidural blood clots. The patient reportedly recovered ‘significant motor power’ at 2 months.67 Another option that may play a role in the future in extensive spinal hematomas is the use of thrombolytic agents locally. Thrombolysis and evacuation of hematoma is achieved by intermittent irrigation of the subdural space with recombinant tissue plasminogen activator (rt-PA), followed by saline lavage.68
SEROMA
Introduction
The incidence of seromas occurring after lumbar spine procedures varies from 2% to 16%, with higher rates after surgery in the elderly population.69,70 Seromas develop after various posterior spinal procedures, including scoliosis/deformity correction surgery, posterior lumbar interbody fusion, instrumented lumbar fusion procedures, and after paraspinal procedures for the treatment of CSF fistulas. Seroma also occur after implantation of spinal cord stimulators and pain pumps.
Management
Attention to surgical technique with prevention of dead spaces during closure is of primary importance in the prevention of seromas. Particular attention needs to be paid to closure of the subcutaneous layer. Interrupted sutures are preferred to a running closure, to avoid the entire closure coming apart if integrity is lost at one site. The skin should not be unnecessarily undermined during the approach, in order to avoid a potential seroma cavity. A postoperative suction drain does not appear to affect the incidence of postoperative seromas.65 Wearing an abdominal binder may help prevent seroma formation after implantation of spinal cord stimulators or pain pumps.71
Seromas are frequently successfully managed by one or two percutaneous needle aspirations. Sterile technique needs to be used, and the risk of bacterial contamination of the surgical incision makes this option unattractive. Gram-staining of the aspirate is recommended to rule out the possibility of infection, and antibiotic therapy instituted if infection is detected. In situations where an infected seroma develops in the subcutaneous pocket housing a pain pump/spinal cord stimulator, antibiotic irrigation of the cavity is advised.71 Open debridement and implant removal need to be considered in all cases with recognized infection.
1 Barron JT. Radiologic case study. Lumbar pseudomeningocele. Orthopedics. 1990;13:603. 608–609
2 Kumar AJ, Nambiar CS, Kanse P. Spontaneous resolution of lumbar pseudomeningocoele. Spinal Cord. 2003;41:470-472.
3 Teplick JG, Peyster RG, Teplick SK, et al. CT identification of postlaminectomy pseudomeningocele. Am J Roentgenol. 1983;140:1203-1206.
4 Miller PR, Elder FWJr. Meningeal pseudocysts (meningocele spurius) following laminectomy. Report of ten cases. J Bone Joint Surg [Am]. 1968;50A:268-276.
5 Hyndman OR, Gerber WF. Spinal extradural cysts, congenital and acquired. Report of cases. J Neurosurg. 1946;3:474-486.
6 Benz RJ, Ibrahim ZG, Afshar P, et al. Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res. 2001;384:116-121.
7 Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg. 1993;78:695-701.
8 Goodkin R, Laska LL. Unintended ‘incidental’ durotomy during surgery of the lumbar spine: medicolegal implications [comment]. Surg Neurol. 1995;43:4-12. discussion 12–14
9 Swanson HS, Fincher EF. Extradural arachnoid cysts of traumatic origin. J Neurosurg. 1947;4:530-538.
10 Cammisa FPJr, Girardi FP, Sangani PK, et al. Incidental durotomy in spine surgery. Spine. 2000;25:2663-2667.
11 Wiesel SW. The multiply operated lumbar spine. Instr Course Lect. 1985;34:68-77.
12 Rinaldi I, Peach WF. Postoperative lumbar pseudomeningocoele. Report of 2 cases. J Neurosurg. 1969;30:504-506.
13 Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718-729.
14 Iqbal J, Davis LE, Orrison WWJr. An MRI study of lumbar puncture headaches. Headache. 1995;35:420-422.
15 Leis AA, Leis JM, Leis JR. Pseudomeningoceles: a role for mechanical compression in the treatment of dural tears. Neurology. 2001;56:1116-1117.
16 Rinaldi I, Hodges TO. Iatrogenic lumbar meningocoele: report of three cases. J Neurol Neurosurg Psychiatry. 1970;33:484-492.
17 O’Connor D, Maskery N, Griffiths WE. Pseudomeningocele nerve root entrapment after lumbar discectomy. Spine. 1998;23:1501-1502.
18 Hadani M, Findler G, Knoler N, et al. Entrapped lumbar nerve root in pseudomeningocele after laminectomy: report of three cases. Neurosurgery. 1986;19:405-407.
19 Eismont FJ, Wiesel SW, Rothman RH. Treatment of dural tears associated with spinal surgery. J Bone Joint Surg [Am]. 1981;63A:1132-1136.
20 Ishaque MA, Crockard HA, Stevens JM. Ossified pseudomeningocoele following laminectomy: case reports and review of the literature. Eur Spine J. 1997;6:430-432.
21 Nash LJr, Kaufman B, Frankel VH. Postsurgical meningeal pseudocysts of the lumbar spine. Clin Orthop. 1971;75:167-178.
22 Lau KK, Stebnyckyj M, McKenzie A. Post-laminectomy pseudomeningocele: an unusual cause of bone erosion. Australas Radiol. 1992;36:262-264.
23 Koo J, Adamson R, Wagner FCJr, et al. A new cause of chronic meningitis: infected lumbar pseudomeningocele. Am J Med. 1989;86:103-104.
24 Jamjoom AB, Tan JB. Subarachnoid haemorrhage related to a lumbosacral fusion: a case report. J Neurol Neurosurg Psychiatry. 1990;53:174-175.
25 Phillips CD, Kaptain GJ, Razack N. Depiction of a postoperative pseudomeningocele with digital subtraction myelography. Am J Neuroradiol. 2002;23:337-338.
26 Bosacco SJ, Gardner MJ, Guille JT. Evaluation and treatment of dural tears in lumbar spine surgery: a review. Clin Orthop Relat Res. 2001;389:238-247.
27 Sharma SK, Gambling DR, Joshi GP, et al. Comparison of 26-gauge Atraucan and 25-gauge Whitacre needles: insertion characteristics and complications. Can J Anaesth. 1995;42:706-710.
28 Cruickshank RH, Hopkinson JM. Fluid flow through dural puncture sites. An in vitro comparison of needle point types. Anaesthesia. 1989;44:415-418.
29 Chordas C. Post-dural puncture headache and other complications after lumbar puncture. J Pediatr Oncol Nurs. 2001;18:244-259.
30 Spencer HC. Postdural puncture headache: what matters in technique. Reg Anesth Pain Med. 1998;23:374-379. discussion 384–387
31 Duffy PJ, Crosby ET. The epidural blood patch. Resolving the controversies. Can J Anaesth. 1999;46:878-886.
32 Shaffrey CI, Spotnitz WD, Shaffrey ME, et al. Neurosurgical applications of fibrin glue: augmentation of dural closure in 134 patients. Neurosurgery. 1990;26:207-210.
33 Freidberg SR. Surgical management of cerebrospinal fluid leakage after spinal surgery. In: Schmidek HH, Sweet WH, editors. Operative neurosurgical techniques – indications, techniques and results. Philadelphia: WB Saunders; 1995:2049-2054.
34 Waisman M, Schweppe Y. Postoperative cerebrospinal fluid leakage after lumbar spine operations. Conservative treatment. Spine. 1991;16:52-53.
35 Kitchel SH, Eismont FJ, Green BA. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg [Am]. 1989;71A:984-987.
36 Stambough JL, Templin CR, Collins J. Subarachnoid drainage of an established or chronic pseudomeningocele. J Spinal Disord. 2000;13:39-41.
37 Deen HG, Pettit PD, Sevin BU, et al. Lumbar peritoneal shunting with video-laparoscopic assistance: a useful technique for the management of refractory postoperative lumbar CSF leaks. Surg Neurol. 2003;59:473-477. discussion 477–478
38 Foyt D, Johnson JP, Kirsch AJ, et al. Dural closure with laser tissue welding. Otolaryngol Head Neck Surg. 1996;115:513-518.
39 McCormack BM, Taylor SL, Heath S, et al. Pseudomeningocele/CSF fistula in a patient with lumbar spinal implants treated with epidural blood patch and a brief course of closed subarachnoid drainage. A case report. Spine. 1996;21:2273-2276.
40 Shimazaki K, Nishida H, Harada Y, et al. Late recurrence of spinal stenosis and claudication after laminectomy due to an ossified extradural pseudocyst. Spine. 1991;16:221-224.
41 Ramirez LF, Thisted R. Complications and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25:226-230. discussion 230–231
42 Cummine JL, Lonstein JE, Moe JH, et al. Reconstructive surgery in the adult for failed scoliosis fusion. J Bone Joint Surg [Am]. 1979;61A:1151-1161.
43 Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:1-49.
44 Lawton MT, Porter RW, Heiserman JE, et al. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome [comment]. J Neurosurg. 1995;83:1-7.
45 Kotilainen E. Microinvasive lumbar disc surgery. A study on patients treated with microdiscectomy or percutaneous nucleotomy for disc herniation. Ann Chir Gynaecol Suppl. 1994;209:1-50.
46 Adler MD, Comi AE, Walker AR. Acute hemorrhagic complication of diagnostic lumbar puncture. Pediatr Emerg Care. 2001;17:184-188.
47 Harik SI, Raichle ME, Reis DJ. Spontaneously remitting spinal epidural hematoma in a patient on anticoagulants. N Engl J Med. 1971;284:1355-1357.
48 Nishida K, Iguchi T, Kurihara A, et al. Symptomatic hematoma of lumbar facet joint: joint apoplexy of the spine? Spine. 2003;28:E206-E208.
49 Hirakawa K, Hanakita J, Suwa H, et al. A post-traumatic ligamentum flavum progressive hematoma: a case report. Spine. 2000;25:1182-1184.
50 Alexiadou-Rudolf C, Ernestus RI, Nanassis K, et al. Acute nontraumatic spinal epidural hematomas. An important differential diagnosis in spinal emergencies [comment]. Spine. 1998;23:1810-1813.
51 Groen RJ, Ponssen H. The spontaneous spinal epidural hematoma. A study of the etiology. J Neurolog Sci. 1990;98:121-138.
52 Kou J, Fischgrund J, Biddinger A, et al. Risk factors for spinal epidural hematoma after spinal surgery. Spine. 2002;27:1670-1673.
53 Taggard DA, Traynelis VC. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine. 2000;25:2035-2039.
54 Tseng SH, Chen Y, Lin SM, et al. Cervical epidural hematoma after spinal manipulation therapy: case report. J Trauma. 2002;52:585-586.
55 Ko Y, Kakiuchi M. Extended posture of lumbar spine precipitating cauda equina compression arising from a postoperative epidural clot. J Orthop Sci. 2001;6:88-91.
56 Montaldi S, Fankhauser H, Schnyder P, et al. Computed tomography of the postoperative intervertebral disc and lumbar spinal canal: investigation of twenty-five patients after successful operation for lumbar disc herniation. Neurosurgery. 1988;22:1014-1022.
57 Egido Herrero JA, Saldana C, Jimenez A, et al. Spontaneous cervical epidural hematoma with Brown-Sequard syndrome and spontaneous resolution. Case report. J Neurosurg Sci. 1992;36:117-119.
58 Hentschel SJ, Woolfenden AR, Fairholm DJ. Resolution of spontaneous spinal epidural hematoma without surgery: report of two cases. Spine. 2001;26:E525-E537.
59 Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia [comment]. Anesthes Analges. 1994;79:1165-1177.
60 Johnson PJ, Hahn F, McConnell J, et al. The importance of MRI findings for the diagnosis of nontraumatic lumbar subacute subdural haematomas. Acta Neurochir (Wien). 1991;113:186-188.
61 Vandermeulen EP, Vermylen G, Van Aken H. Epidural and spinal anaesthesia in patients receiving anticoagulant therapy. Baillieres Clin Anaesthesiol. 1993;7:663-689.
62 Sage DJ. Epidurals, spinals and bleeding disorders in pregnancy: a review. Anaesth Intensive Care. 1990;18:319-326.
63 Uribe J, Moza K, Jimenez O, et al. Delayed postoperative spinal epidural hematomas. Spine J. 2003;3:125-129.
64 Parker MJ, Roberts C. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2001. CD001825
65 Payne DH, Fischgrund JS, Herkowitz HN, et al. Efficacy of closed wound suction drainage after single-level lumbar laminectomy. J Spinal Disord. 1996;9:401-403.
66 Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77A:1042-1049.
67 Osmani O, Afeiche N, Lakkis S. Paraplegia after epidural anesthesia in a patient with peripheral vascular disease: case report and review of the literature with a description of an original technique for hematoma evacuation. J Spinal Disord. 2000;13:85-87.
68 Little CP, Patel N, Nagaria J, et al. Use of topically applied rt-PA in the evacuation of extensive acute spinal subdural haematoma. Eur Spine J. 2003;12:12.
69 Greenfield RT3rd, Capen DA, Thomas JCJr, et al. Pedicle screw fixation for arthrodesis of the lumbosacral spine in the elderly. An outcome study. Spine. 1998;23:1470-1475.
70 Rhee JM, Bridwell KH, Lenke LG, et al. Staged posterior surgery for severe adult spinal deformity. Spine. 2003;28:2116-2121.
71 Prager JP. Neuraxial medication delivery: the development and maturity of a concept for treating chronic pain of spinal origin. Spine. 2002;27:2593-2605. discussion 2606