CHAPTER 59 Postoperative Imaging of Ischemic Cardiac Disease
Congestive heart failure, the most common admitting diagnosis for patients older than 65 years in the United States,1 continues to increase in incidence and prevalence, with more than 500,000 new cases of chronic heart failure diagnosed yearly. The most common cause of heart failure is ischemic cardiomyopathy; the second most common cause is dilated cardiomyopathy. The diagnosis, management, treatment, and rehabilitation of patients suffering from ischemic heart disease represent a large percentage of health care costs. Current interventions for management and treatment of end-stage ischemic heart disease include aggressive medical management, extracorporeal circulatory support, percutaneous left ventricular assist device placement, implantable ventricular assist device placement, coronary artery revascularization, mitral valve repair or replacement, scar ablation, passive epicardial restraint, surgical ventricular restoration, and heart transplantation. Combinations of these surgical interventions are sometimes used, depending on the patient’s needs.
PREVALENCE, EPIDEMIOLOGY, AND BACKGROUND
For patients obtaining ventricular assist devices as a bridge to transplantation or destination therapy, 1-year survival rates have been reported to be 52% compared with 25% for a medically treated cohort of patients; the 2-year survival rate is 23% compared with 8% for a medically treated cohort of patients.2
In one study including 1198 patients, 5-year survival rates of patients undergoing surgical ventricular restoration with concomitant coronary artery bypass graft or mitral valve surgery were 69.9% ± 4.7% for New York Heart Association (NYHA) class III patients.3 Results of the STICH (Surgical Treatment for Ischemic Heart Failure) trial, a recently completed randomized surgical versus medical trial, will help clarify indications for coronary revascularization, surgical ventricular restoration, and medical therapy. Alternative therapies to heart transplantation will continue to be important because of the yearly increasing gap between demand and supply of hearts.
Surgical Ventricular Restoration: Dor Procedure
The Dor procedure involves excision of akinetic or dyskinetic and nonviable myocardium of the left ventricle and patch repair of the distal left ventricular cavity, thus restoring the normal elliptical shape of the left ventricle from a spherical dilated heart. The opening of the ventricle is closed by Dacron patch or stitches.4 The restoration of ventricular volume reduces the stress on the ventricular wall, reduces myocardial oxygen consumption, and increases wall contractility and compliance.5 Associated mitral valve regurgitation or intraventricular thrombi are corrected simultaneously. Before surgery, appropriate coronary revascularization procedures, including grafting of the left anterior descending coronary artery, which supplies a high portion of the septum, should be performed.5 Perioperatively, appropriate ventricular volume is restored in the septal and anterior wall without deforming the chamber that will result in neither restrictive nor dilated cardiomyopathy. Care is also taken to achieve an optimal postoperative ventricular short-axis/long-axis ratio; otherwise, mitral regurgitation can result.
The first reported surgery to treat left ventricle aneurysms by Cooley and colleagues involved excision of the thinned segment with linear closure of the free edges.6 Alternative approaches were developed by Dor and Jatene; an intraventricular patch was placed to exclude akinetic and nonresectable areas. The Dor procedure, or endoventricular circular patch plasty, was first performed in 1985.4 Some of the criteria for the Dor procedure are ischemic dilated cardiomyopathy involving one third or more of the ventricular perimeter that causes a spherical dilated left ventricle with akinetic or dyskinetic portions of the septum and anterior wall with end-diastolic volume above 100 mL/m2, reduced ejection fraction (<20%), left ventricular regional asynergy (>35%), and symptomatic patient (angina, heart failure, arrhythmias, and inducible ischemia). Contraindications to the procedure are systolic pulmonary artery pressure above 60 mm Hg without associated mitral regurgitation, severe right ventricular dysfunction, and regional asynergy without ventricular dilation.
Cardiac Transplantation
Cardiac transplantation has been established as the most reliable permanent treatment option for patients with deteriorating heart failure due to ischemic dilated cardiomyopathy despite maximum medical therapy and other revascularization techniques. The most commonly performed type is orthotopic cardiac transplantation, in which the recipient heart is removed except for the posterior aspect of the atrial cuffs. The donor heart is then attached to the recipient’s atria, and the donor’s ascending aorta and main pulmonary artery are anastomosed end to end to the severed ends of the recipient’s ascending aorta and main pulmonary artery, respectively.7
Patients with nonischemic causes of heart failure, such as hypertensive heart disease, myocarditis, idiopathic cardiomyopathy, valvular heart disease, congenital heart disease, and peripartum cardiomyopathy, can also benefit from cardiac transplantation.8 Contraindications to heart transplantation may include AIDS, active systemic infection, malignant disease, irreversible pulmonary hypertension, irreversible secondary organ failure, comorbid life-threatening conditions, active substance abuse, psychiatric history likely to result in noncompliance, cachexia or obesity, chronic obstructive pulmonary disease, renal insufficiency, continued smoking, and severe osteoporosis.
Heterotopic cardiac transplantation is performed in patients with potentially reversible cardiac dysfunction, high pulmonary vascular resistance, or small donor hearts.9 The donor heart is placed anterior to the right lung along the right side of the native heart, and the two left atria are anastomosed, resulting in a common left atrium. The orifices of the donor inferior vena cava and right pulmonary veins are closed. The donor ascending aorta is anastomosed to the recipient aorta in end-to-side fashion, and the donor main pulmonary artery is combined with a Dacron graft, resulting in an end-to-side anastomosis with the recipient main pulmonary artery. The donor superior vena cava and right atrium are connected to the native right atrium, allowing systemic venous return to pass into either the native or the donor right ventricle. Chambers involved in functioning include the right ventricle of the recipient and the left ventricle of the donor.
POSTOPERATIVE ASSESSMENT
General Surgery-Related Complications
Mediastinal hematoma, pericardial hematoma, pleural effusions, pneumothorax, hemothorax, pneumoperitoneum and hemoperitoneum, basal lung atelectasis, aspiration, and infectious pneumonia are common complications related to these procedures in the immediate postoperative period. It is not uncommon to identify malpositioning of support tubes and catheters or a foreign body such as a surgical sponge on the portable radiographs. Median sternotomy complications, such as mediastinitis and sternal wound infections, may occur. These patients are prone to pulmonary embolism during the perioperative and immediate postoperative period. Perioperative bleeding is a result of prolonged surgical time under extracorporeal circulation and hypothermia, use of anticoagulants and antiplatelets drugs, vascular injury to the surgical bed, associated malnourishment, and hepatic dysfunction due to low-flow state and congestive hepatopathy.1
Specific Complications
Ventricular Assist Device
Right-sided heart failure may be due to adverse effects of the LVAD on the interventricular septum, increased pulmonary pressure due cardiopulmonary bypass or massive blood transfusions, and right coronary artery disease.1 After the perioperative period, infection related to the VAD, thromboembolism with infarction, and limited reliability of the VAD remain the most important concerns. Patients with a VAD are more susceptible to infection because of tracking along subcutaneous drivelines for connecting batteries and controllers, leading to entry and exit site infection, driveline infection, or pump infection. Pump infection may require removal of the device and hence is of the greatest concern.1 Thrombus can sometimes be seen in the cardiac chambers or inflow and outflow cannulas. Thromboembolism and resultant infarction of lung, brain, or systemic organs may occur after VAD placement, although the HeartMate device is believed to have less chance for this development because of the textured surface of the blood-containing chamber by a polyurethane diaphragm, which leads to pseudointima formation.10 Aortic dissection at the level of the ascending aorta can result from high-velocity blood injected against the aortic wall. Device reliability depends on the type of device; it is 1 to 3 years for pulsatile pumps and about 5 years for miniaturized axial flow pumps. However, because the VAD does not fail catastrophically, further treatment by device exchange or transplantation can be warranted.1
Dor Procedure
Specific complications associated with surgical ventricular restoration are suture line or patch dehiscence, excessive reduction in left ventricular volume resulting in mitral regurgitation, and restrictive or constrictive physiology.11 Intracardiac thrombus that is adherent to the patch at the left ventricle apex is a noted complication of this procedure.
Cardiac Transplantation
Early postoperative complications (between 0 and 30 days) are related to surgical complications, including cardiac ischemia, pulmonary edema, and anoxic brain injury and thromboembolic events. Intermediate postoperative complications (between 1 month and 12 months) mainly include acute allograft rejection and infection. Allograft rejection is manifested usually between 2 and 12 weeks after transplantation.9 The time of greatest immunosuppression is during the first 3 months after transplantation. Bacterial (predominantly aerobic gram-negative rods), viral, and fungal infections occur within the first month and often affect the lungs.
Mediastinal infection can lead to weakening of suture lines and cause aortic dissection and pseudoaneurysm formation. Late complications (after 12 months) resulting in death include transplant-associated coronary artery accelerated graft atherosclerosis, malignant disease, infection, transplant rejection, aortic allograft rejection, and cerebral infarctions.12
Coronary allograft vasculopathy or atherosclerosis is caused by immune-mediated and nonimmunologic injury,7 which affects half of patients within 5 years of transplantation and is characterized by diffuse concentric intimal thickening of both proximal and distal coronary arteries. The major risk factors for late mortality from coronary allograft vasculopathy include ischemic heart disease, younger recipient age (but older than 20 years), black race, cigarette use within 6 months of listing for transplantation, older donor age, and development of coronary artery disease during the first post-transplant year.13 Malignant neoplasms are likely to be secondary to long-term immunosuppression and include lymphomas, acute leukemia, visceral tumors, Kaposi sarcoma, gynecologic cancers, primary lung carcinoma, skin cancer (predominantly squamous cell cancer), and post-transplant lymphoproliferative disorder.9
Other complications are related to long-term corticosteroid use and immunosuppression. These include osteoporosis, vertebral insufficiency fractures, and lipomatosis.8
CLINICAL PRESENTATION
Patients with mediastinitis are often unwell and may have fever, tachycardia, chest discomfort, sternal tenderness, and leukocytosis. Discharge from the sternotomy wound with delayed healing may be indirect evidence of ongoing sternal osteomyelitis or a mediastinal infection or collection.9
Fever or lethargy may be the first indicator of internal systemic infection. If it is associated with breathlessness, productive cough, and pleuritic chest pain, it can be a warning symptom of community-acquired or opportunistic pulmonary infections, which are quite common in the postoperative period because of immunosuppression. Patients with infective (mycotic) pseudoaneurysms may be asymptomatic or may present with fever, lethargy, and chest pain.9 Pulmonary embolism is typically manifested with sudden-onset breathlessness, chest pain, tachycardia, and hypoxia.
In heart transplantation, a common complication in the postoperative period is impending rejection, which may be asymptomatic or can be manifested by dyspnea, palpitation, fatigability, weakness, syncope and signs suggestive of hypotension, worsening of cardiac function, and increasing heart failure.9
Clinical manifestations of coronary vasculopathy include myocardial infarction, graft failure, arrhythmias, and sudden death. It starts distally in small coronaries and progresses proximally to epicardial vessels without formation of collaterals.7 Because of the difficulty in preventing this complication and the clinically silent presentation, routine surveillance has been advocated for detection and early intervention by revascularization procedures.7
New-onset back pain in patients receiving corticosteroids should suggest osteoporosis, vertebral fractures, intervertebral diskitis, or internal malignant disease with bone metastases. Gastrointestinal complications, such as peptic ulcer, diverticulitis, and organ perforation, may go unnoticed because of the effect of corticosteroids, which may mask signs and symptoms.8 A high index of clinical suspicion and appropriate timely imaging would help in identifying these serious pathologic processes.
IMAGING INDICATIONS AND ALGORITHM
Magnetic Resonance Imaging
The high spatial and temporal resolution of MRI allows better morphologic assessment than with two-dimensional echocardiography. Postoperative complications of suture dehiscence and myocardial infarction are better diagnosed on MRI. VADs present challenges to MRI systems because of their ferromagnetic properties. A prototype cannula that replaces the stainless steel wire-reinforced cannula has been tested in vitro on the 3T MRI system.14
Nuclear Medicine/Positron Emission Tomography
Nuclear medicine imaging studies are used in assessing cardiac function after transplantation and related complications such as rejection. Chronic rejection, or cardiac graft vasculopathy, can be diagnosed with serial myocardial perfusion scans. The added advantage of a stress-rest perfusion study includes determination of reversible ischemia. Identification of regional perfusion defects is often difficult because of the balanced ischemia patterns related to the diffuse nature of the distal arterial narrowings. Gated studies can reveal abnormal diastolic function. Dobutamine single photon emission computed tomography (SPECT) thallium 201 study has sensitivity, specificity, and negative predictive value of 89%, 71%, and 96%, respectively.15 The positron emission tomography (PET) scan, like the gated SPECT study, can show perfusion defects indicative of vasculopathy, and PET also quantifies myocardial blood flow.
Other tracers are also used. Indium In 111 pentetreotide uptake may predict impending rejection 1 week before endomyocardial biopsy. Technetium Tc 99m annexin V uptake correlates with areas of myocyte apoptosis, or programmed cell death.16 Gallium Ga 67, 18FDG-PET, and 111In-labeled white blood cell examinations are used to assess complications such as fevers of unknown origin, post-transplantation lymphoproliferative disorder, and infections.
Angiography
Because cardiac allograft vasculopathy is the major cause of long-term mortality after cardiac transplantation, and because of the lack of symptoms such as chest pain secondary to denervation of the transplanted heart, yearly coronary angiography has been the diagnostic study of choice to evaluate for this serious complication. This is often combined with intravascular ultrasound as well.7
IMAGING TECHNIQUE AND FINDINGS
Ventricular Assist Device
Postoperative Appearance of VAD
Plain radiography and CT can be used for proper visualization of the VADs (Figs. 59-1 and 59-2). Normal appearance of VADs depends on the type of device and the components of the device. The HeartMate LVAD pump is placed preperitoneally or in an intra-abdominal location and is seen on plain abdominal radiographs in the left upper quadrant.10 The inflow cannula contains a porcine bioprosthetic valve and is seen in the left ventricle apex aligned with the mitral valve. The outflow cannula also contains a porcine bioprosthetic valve and is attached to a Dacron patch of approximately 12 to 15 cm, which in turn inserts into the ascending aorta. The device is connected to an external portable console that provides pneumatic or electric power through a driveline in the fascial tunnel in the left lower quadrant. The Pierce-Donachy Thoratec VAD is placed external to the patient.10 In a right VAD, the inflow cannula is inserted in the right atrium or ventricle, and the outflow cannula along with a Dacron graft is placed into the pulmonary artery.
Imaging of Postoperative Complications of VAD
Perioperative bleeding in the operative field as well as in potential spaces such as pleura, pericardium, and peritoneum is best seen on CT as a hypodense collection with the attenuation value of blood (30 to 50 HU) (Fig. 59-3). Right-sided heart failure1 is manifested as cardiomegaly and enlarged azygos vein on chest radiography. Echocardiography and CT are more diagnostic and show dilated right atrium, right ventricle, and systemic veins with decreased right ventricular function. Arterial phase contrast-enhanced CT may show reflux of contrast material into enlarged hepatic veins and dilated suprahepatic and hepatic inferior vena cava. On plain radiographs, pneumothorax and pneumomediastinum appear as radiolucencies in the pleural space or around the cardiac shadow in the mediastinum, respectively, and there may be associated surgical emphysema. CT can demarcate air better as very low density of air attenuation. Infection related to the VAD can be seen on contrast-enhanced CT as mixed-density collections with peripheral wall enhancement tracking along subcutaneous drivelines leading to entry and exit sites. Thrombus can be seen as a nonenhancing low-density mass on contrast-enhanced CT in the cardiac chambers and inflow and outflow cannulas (Fig. 59-4B). Thromboemboli can result in segmental infarcts, which are seen as peripheral wedge-shaped low densities without enhancement on CT in lung, brain, or systemic organs. Pulmonary emboli are seen as hypovascular intraluminal filling defects within the branch pulmonary arteries.
CT has high sensitivity and specificity for identification of intramural hematoma and aortic dissection (Fig. 59-4A). On contrast-enhanced CT and contrast-enhanced MRI, aortic dissection appears as two contrast-filled channels separated by a hypodense or hypointense intimal flap in the aorta. Intramural hematoma is seen as mural high density on non–contrast-enhanced CT and low density on contrast-enhanced CTA, but the perfused lumen has a smooth margin with no intimal flap.
Focal accumulation of blood around the left VAD outflow or right VAD inflow can be seen as a focal bulge on the postoperative chest radiograph or CT scan.10 Active bleeding can be seen as an extravascular collection of contrast material.
Ventricular Restoration Procedure (Dor Procedure)
Postoperative Appearance
Radiography may show decreased cardiac size after the surgery due to resection of the left ventricle aneurysm and resection of akinetic and dyskinetic myocardium. Cardiac CT or chest CT with contrast may show surgical material at the newly created left ventricle apex, normal elliptical shape of the left ventricle, interval decrease in size of the cardiac silhouette, and patch repair of aneurysm at the left ventricle apex (Fig. 59-5).
Cardiac Transplantation
Postoperative Appearance
Radiographic findings after orthotopic cardiac transplantation include enlarged cardiac silhouette (Figs. 59-6 to 59-8); double right atrial contour; and resolving findings of pneumomediastinum, pneumothorax, pneumopericardium, subcutaneous emphysema, and mediastinal widening up to the first 20 days after surgery.9 Mediastinal lipomatosis due to steroid therapy can lead to delayed mediastinal widening of relatively low density.9 Pericardial effusions are rarely seen because of the disproportionately larger pericardial sac or cyclosporine immunotherapy.
CT findings include high and redundant main pulmonary artery17 due to size discrepancy of the recipient and donor pulmonary arteries, size discrepancy between the recipient and donor ascending aorta anastomoses, large space between recipient superior vena cava and donor ascending aorta, space between donor ascending aorta and main pulmonary artery, and prominent left atrium with waist or indentation at atrial anastomoses. The remnant donor superior vena cava is usually placed medial to the recipient’s superior vena cava and posterior to the donor ascending aorta.9 If the size discrepancies between donor and recipient aorta and pulmonary arteries are significant, a radiosynthetic patch is used to bridge the difference; this can be imaged as radiodense material encircling the aorta or pulmonary artery on CT.
Imaging of Postoperative Complications
The most definitive diagnosis of acute allograft rejection is made with endomyocardial biopsy, with its associated risks of bleeding, pneumothorax, hemothorax, vascular access complications, right ventricular wall perforation, coronary artery fistula, cardiac arrhythmias, and pseudoaneurysm formation. Noninvasive diagnostic imaging findings include cardiomegaly on chest radiography and CT and increased 67Ga uptake on scintigraphy. Positive MRI findings of rejection include increased left ventricle muscle mass attributed to edema; high T2 myocardial signal,18 especially in the interventricular septum; and marked reductions in the ratio of phosphocreatine and adenosine triphosphate on MR spectroscopy. Functional CT and MRI can show decreased ventricular function. Echocardiography is useful in the diagnosis of moderate rejection. Echocardiography findings include increased wall thickness (sum of interventricular septum and left ventricle posterior wall) and echogenicity, pericardial effusion, more than 20-ms decrease in pressure half-time and isovolumetric relaxation time, more than 10% decrease in left ventricular ejection fraction, and decreased ejection fractions, with reported sensitivity and specificity of 80% and 98.6%.19 Follow-up 111In studies are of value after the first few postoperative months. Heart-to-lung count density ratio is calculated and correlates with severity of damage seen on endomyocardial biopsy. Chronic rejection can be diagnosed with serial myocardial perfusion scans.
Infections can be manifested as pneumonia, empyema, mediastinitis, abdominal abscess, acute diverticulitis with rupture, sinusitis or mastoiditis, and intracranial abscesses. Infections caused by Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae can proceed to abscesses and cavitations recognized on radiographs and CT scan. Aspergillus infections can be manifested radiographically as isolated or multiple pulmonary nodules with cavitation, predominantly in the upper lobe, or as an invasive type with larger nodular consolidation and a rim of ground-glass opacity (CT halo sign) due to hemorrhage, with or without cavitation (Fig. 59-9).20 Cerebral aspergillosis can produce bifrontal hypodense cerebral lesions with rim enhancement, sometimes with intralesional hemorrhage.20 Recipients are susceptible to other infections, such as Legionella, Pneumocystis carinii, and Nocardia infections. Nocardia can produce extensive soft tissue abscesses with an enhancing rim on contrast-enhanced CT. Viral infection with cytomegalovirus results in cytomegaloviral pneumonitis in 9% to 11% of cardiac transplant recipients; death occurs in 14% of affected patients.9,17 Cytomegalovirus can also cause myocarditis, hepatitis, and gastrointestinal ulceration. Pulmonary radiographic and CT findings include diffuse pulmonary airspace disease, consolidation with multiple nodules smaller than 5 mm, bilateral patchy ground-glass opacities, and multiple centrilobular or subpleural nodules.
Mediastinal infection can lead to weakening of suture lines and aortic dissection and pseudoaneurysm formation.9 On chest radiography, it is manifested as widening of the mediastinum and an anterior mediastinal mass. Contrast-enhanced CT demonstrates the contrast-filled focal outpouching from the ascending aorta with or without thrombus or intramural hematoma, usually at the cannulation site for cardiopulmonary bypass.9 Mycotic pseudoaneurysms are manifested with signs of infection and are fatal because of rapid growth. Cerebral infarction or abscess formation can occur, and cerebral infarction will be in respect to vascular territory and will produce less contrast enhancement than abscesses do. Radiologic modalities for diagnosis of coronary allograft vasculopathy or atherosclerosis include invasive coronary angiography, intracoronary ultrasonography, coronary artery CTA with coronary artery calcium screening, and cardiac function MRI with coronary MR angiography. Angiographic findings are typically characterized by diffuse concentric narrowing in the middle to distal coronary arteries with occasional distal vessel obliteration.21 Specific types of lesions are described as focal and discrete, tubular, multiple, abrupt or gradually occurring long smooth narrowings with obliterated distal vessels, or abruptly terminating narrowed and irregular distal vessels (pruned tree effect). Late enhancement patterns that are seen on magnitude and phase-sensitive inversion recovery MRI sequences include infarct-typical transmural patterns and infarct-atypical patterns (diffuse, spotted, intramural, and localized without association with specific coronary artery vascular territory). Infarct-typical patterns are more common in the mid ventricle and apical levels; infarct-atypical patterns are more common in the basal and mid ventricle levels.22
Dobutamine SPECT thallium study can show elevated lung-to-heart uptake. Contrast-enhanced CT can provide a complete survey of malignant neoplasms associated with heart transplantations, such as lymphomas, acute leukemia, skin cancer, visceral tumors, Kaposi sarcoma, gynecologic cancers, and primary lung carcinoma.9 Squamous cell cancer accounts for most skin cancers. Post-transplantation lymphoproliferative disorders are commonly of B-cell origin and are associated with Epstein-Barr virus.9 Lymphomas are usually extramedullary and may be localized or disseminated. They can involve any organ, including lung, liver, kidneys, spleen, gastrointestinal tract, and lymph nodes. Pulmonary involvement is manifested radiographically as solitary or multiple noncavitating nodules with or without adenopathy. Hepatic and splenic findings can include multiple hypoattenuating masses. Gastrointestinal tract involvement can be manifested as bowel wall thickening and intestinal obstruction.20
Aortic allograft rejection can produce aortic dehiscence with severe periaortic hematoma. Leukoencephalopathy produces bilateral white matter hypodensities on CT.20
Postoperative Management
VAD failure, right-sided heart failure, mediastinal bleeding, infection, and thromboembolism are all considered during postoperative management of LVAD patients. Right ventricular failure is supported by phosphodiesterase inhibitors and nitric oxide. Afterload reduction is also important, especially with axial flow devices. Diuresis is necessary because of chronic volume overload status at clinical presentation. Early extubation, enteral feeding, and rehabilitation are desired. Because mediastinal bleeding is not uncommon and often requires reoperation, anticoagulation can be initiated usually by 24 to 36 hours postoperatively after bleeding has subsided with platelet transfusions, fresh frozen plasma, and cryoprecipitate. Aspirin is liberally used for its antiplatelet and anti-inflammatory effects, and the international normalized ratio is maintained between 2.5 and 3.5 with heparin and warfarin; dipyridamole and clopidogrel are also often used to prevent pulmonary and cerebral thromboembolism. Some common infectious agents include Staphylococcus, Pseudomonas, Enterococcus, and Candida; antibiotics, drainage and débridement, device exchanges, and device removal with transplantation are all considered. Urgent reoperation and parts replacement may be required for device failure. Some devices may have backup support, for example, a pneumatic console on the HeartMate LVAD in case of electrical failure. Patients with VADs have higher frequencies of circulating antiphospholipids and anti–human leukocyte antigen antibodies; these patients are at higher risk for post-transplantation acute rejections. Early intravenous immune globulin therapy and cyclophosphamide therapy are used to reduce alloreactivity before transplantation.23
Operative and perioperative mortality, although relatively low, can be encountered in patients undergoing surgical ventricular restoration.24,25 Mortality is most often related to urgency of surgery and cardiogenic shock. These sick patients are likely being treated with inotropic agents, pressors, and short-term mechanical support, such as intra-aortic balloon pump. Stroke is an adverse outcome, sometimes related to unexpected intraventricular soft thrombus that is undetected preoperatively. Refractory postoperative biventricular failure can be addressed by VADs.24,25 A common nonfatal morbidity is residual inducible arrhythmia, such as ventricular tachycardia. If intraoperative scar ablation is not done or is unsuccessful, postoperative cardiac electrophysiology consultation with possible implantation of an implantable cardioverter-defibrillator is an option. Postoperative mitral regurgitation can occur with continued ventricular remodeling, despite effective ventricular restoration. An akinetic or poorly functioning inferobasal ventricular wall can contribute to mitral insufficiency. Preoperative mitral insufficiency can be addressed intraoperatively with annuloplasty at the time of a Dor procedure or with a more complex repair if there is progression of postoperative mitral regurgitation. Long-term postoperative care includes maintenance of combinations of diuretics, β blockers, and angiotensin-converting enzyme inhibitors for patients in NYHA class I.24,25
Allograft failure and opportunistic infections are the most serious complications in the early postoperative period (Fig. 59-10). Infection is the most common cause of death in the early postoperative period.26 Transplant rejection may be caused by both cellular and antibody-mediated processes. Antibody-mediated rejection is associated with greater risk of allograft vasculopathy (Fig. 59-11) and more severe hemodynamic compromise at presentation. Cellular rejection involves myocardial infiltration with mononuclear cells. Induction therapy provides more intensive immunosuppression in the initial days after transplantation for highly sensitized patients and renal failure patients.26 Maintenance immunosuppression includes corticosteroids, calcineurin inhibitors, and an antiproliferative agent.27 Prednisone, tacrolimus, and mycophenolate mofetil are commonly used agents. Strategies to reduce post-transplantation infections include bacterial and viral prophylaxis, early corticosteroid withdrawal, and use of more effective antifungal agents. Survival of patients with invasive aspergillosis is improved with medications such as caspofungin, voriconazole, and posaconazole. Post-transplantation progression and regression of malignant neoplasms may be pursued with proliferation signal inhibitors such as sirolimus. Management options for transplant graft vasculopathy include statin therapy, sirolimus therapy, percutaneous revascularization, and retransplantation, the only definitive therapy.27
KEY POINTS
 Common non–coronary artery bypass graft surgical options for ischemic heart disease include cardiac assist device, ventricular restoration procedure (Dor procedure), and heart transplantation.
Common non–coronary artery bypass graft surgical options for ischemic heart disease include cardiac assist device, ventricular restoration procedure (Dor procedure), and heart transplantation. The ventricular assist device can provide electromechanical circulatory support for heart failure patients despite maximum medical therapy, and it serves as a bridge to transplantation and also as destination therapy. Imaging, especially contrast-enhanced CT, helps identify the normal course of inflow and outflow conduits and cannulas and complications such as perigraft hematoma, conduit thrombosis, perigraft infection, iatrogenic aortic dissection, or intramural hematoma and pulmonary embolus.
The ventricular assist device can provide electromechanical circulatory support for heart failure patients despite maximum medical therapy, and it serves as a bridge to transplantation and also as destination therapy. Imaging, especially contrast-enhanced CT, helps identify the normal course of inflow and outflow conduits and cannulas and complications such as perigraft hematoma, conduit thrombosis, perigraft infection, iatrogenic aortic dissection, or intramural hematoma and pulmonary embolus. Surgical left ventricular restoration repair or the Dor procedure may be an option for cardiac failure patients with left ventricle aneurysm and akinetic or dyskinetic myocardial segments. This procedure reduces left ventricular volume and restores the elliptical shape of the ventricle as well. Echocardiography, CT, and MRI are useful tools to evaluate for an expected elliptical shape of the left ventricle, quantification of left ventricular volumes, and intracardiac thrombus.
Surgical left ventricular restoration repair or the Dor procedure may be an option for cardiac failure patients with left ventricle aneurysm and akinetic or dyskinetic myocardial segments. This procedure reduces left ventricular volume and restores the elliptical shape of the ventricle as well. Echocardiography, CT, and MRI are useful tools to evaluate for an expected elliptical shape of the left ventricle, quantification of left ventricular volumes, and intracardiac thrombus. Orthotopic cardiac transplantation has emerged as the most reliable long-term treatment option for patients with end-stage heart failure, despite maximum revascularization and medical therapies.
Orthotopic cardiac transplantation has emerged as the most reliable long-term treatment option for patients with end-stage heart failure, despite maximum revascularization and medical therapies.Bogot NR, Durst R, Shaham D, Admon D. Cardiac CT of the transplanted heart: indications, technique, appearance, and complications. Radiographics. 2007;27:1297-1309.
Rose EA, Gelijns AC, Moskowitz AJ. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-1443.
Sartipy U, Albage A, Lindblom D. The Dor procedure for left ventricular reconstruction. Ten-year clinical experience. Eur J Cardiothorac Surg. 2005;27:1005-1010.
1 Goldstein DJ, Smego D, Michler RE. Surgical aspects of congestive heart failure. Heart Fail Rev. 2006;11:171-192.
2 Rose EA, Gelijns AC, Moskowitz AJ. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-1443.
3 Athanasuleas CL, Buckberg GD, Stanley AWH. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439-1445.
4 Sartipy U, Albage A, Lindblom D. The Dor procedure for left ventricular reconstruction. Ten-year clinical experience. Eur J Cardiothorac Surg. 2005;27:1005-1010.
5 Menicanti L, Donato M. The Dor procedure: what has changed after fifteen years of clinical practice? J Thorac Cardiovasc Surg. 2002;124:886-890.
6 Blom AS, Acker MA. The surgical treatment of end-stage heart failure. Curr Probl Cardiol. 2007;32:553-599.
7 Bogot NR, Durst R, Shaham D, Admon D. Cardiac CT of the transplanted heart: indications, technique, appearance, and complications. Radiographics. 2007;27:1297-1309.
8 Kuhlman JE. Thoracic imaging in heart transplantation. J Thorac Imaging. 2002;17:113-121.
9 Knisely BL, Mastey LA, Collins J, et al. Imaging of cardiac transplantation complications. Radiographics. 1999;19:321-339.
10 Knisely BL, Collins J, Jahania SA, et al. Imaging of ventricular assist devices and their complications. AJR Am J Roentgenol. 1997;169:385-391.
11 Carmichael BB, Setser RM, Stillman AE, et al. Effects of surgical ventricular restoration on left ventricular function: dynamic MR imaging. Radiology. 2006;241:710-717.
12 Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:50-59.
13 Naftel DC, Brown RN. Survival after heart transplantation. In: Kirklin JK, Young JB, McGiffin DC, editors. Heart Transplantation. Philadelphia: Churchill Livingstone; 2002:587-614.
14 Shellock FG, Valencerina S. Ventricular assist device implant (AB 5000) prototype cannula: in vitro assessment of MRI issues at 3-Tesla. J Cardiovasc Magn Reson. 2008;10:23.
15 Wu YW, Yen RF, Lee CM, et al. Diagnostic and prognostic value of dobutamine thallium-201 single-photon emission computed tomography after transplantation. J Heart Lung Transplant. 2005;24:544-550.
16 Flotats A, Carrio I. Value of radionuclide studies in cardiac transplantation. Ann Nucl Med. 2006;20:13-21.
17 Henry DA, Corcoran HL, Lewis TD, et al. Orthotopic cardiac transplantation: evaluation with CT. Radiology. 1989;170:343-350.
18 Marie PY, Angioi M, Carteaux JP, et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol. 2001;37:825-831.
19 Ciliberto GR, Mascarello M, Gronda E, et al. Acute rejection after heart transplantation: noninvasive echocardiographic evaluation. J Am Coll Cardiol. 1994;23:1156-1161.
20 Knollmann FD, Hummel M, Hetzer R, et al. CT of heart transplant recipients: spectrum of disease. Radiographics. 2000;20:1637-1648.
21 Benza RL, Tallaj J. Cardiac allograft vasculopathy (chronic rejection. In: Kirklin JK, Young JB, McGiffin DC, editors. Heart Transplantation. Philadelphia: Churchill Livingstone; 2002:615-665.
22 Steen H, Merten C, Refle S, et al. Prevalence of different gadolinium enhancement patterns after heart transplantation. J Am Coll Cardiol. 2008;52:1160-1167.
23 Aggarwal S, Cheema F, Oz M, et al. Long-term mechanical circulatory support. In: Cohn LH, editor. Cardiac Surgery in the Adult. 3rd ed. New York: McGraw-Hill; 2008:1609-1628.
24 Raman J, Dixit A, Bolotin G, et al. Failure modes of left ventricular reconstruction or the Dor procedure: a multi-institutional perspective. Eur J Cardiothorac Surg. 2006;30:347-352.
25 Dor V. Left ventricular reconstruction for ischemic heart failure. In: Fang JC, Couper GS, editors. Surgical Management of Congestive Heart Failure. Totowa, NJ: Humana Press; 2005:279-298.
26 Abbara S, Walker G, editors. Diagnostic Imaging: Cardiovascular. 2008. Amirsys. Salt Lake City, Utah. I-7 8-11
27 Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol. 2008;52:587-598.

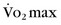 of less than 14 mL/kg per minute,
of less than 14 mL/kg per minute,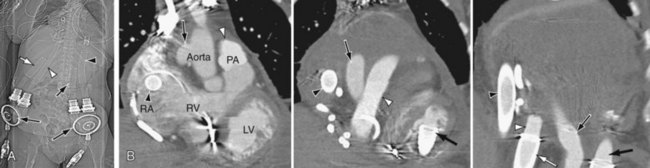
 FIGURE 59-1
FIGURE 59-1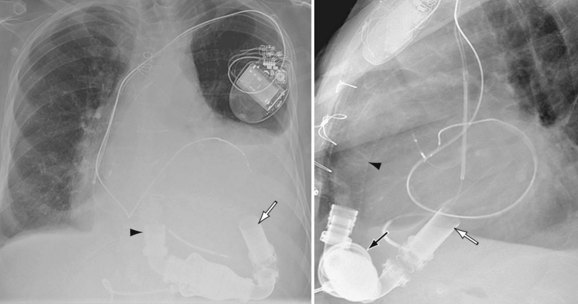
 FIGURE 59-2
FIGURE 59-2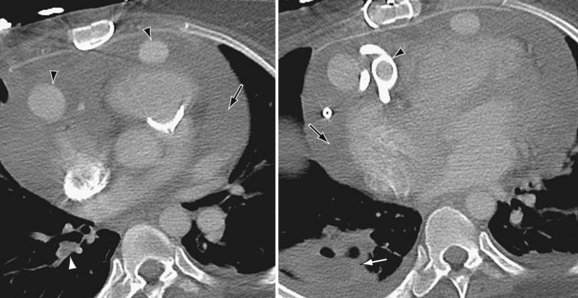
 FIGURE 59-3
FIGURE 59-3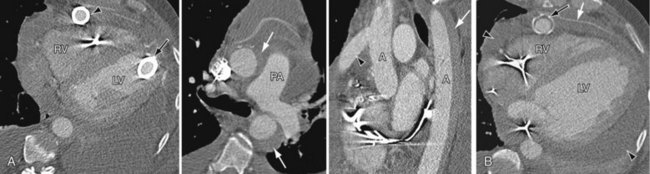
 FIGURE 59-4
FIGURE 59-4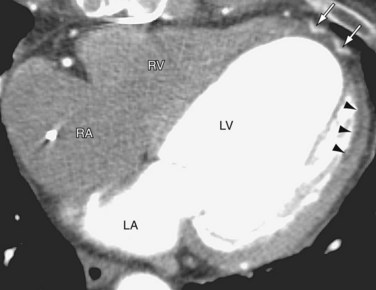
 FIGURE 59-5
FIGURE 59-5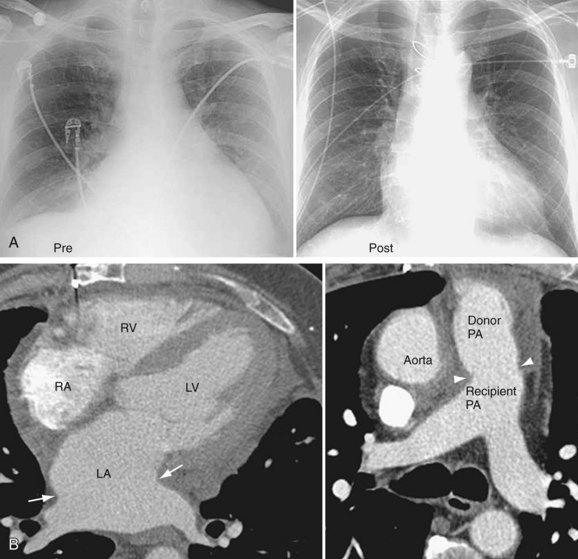
 FIGURE 59-6
FIGURE 59-6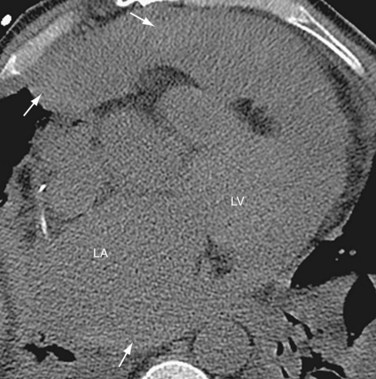
 FIGURE 59-7
FIGURE 59-7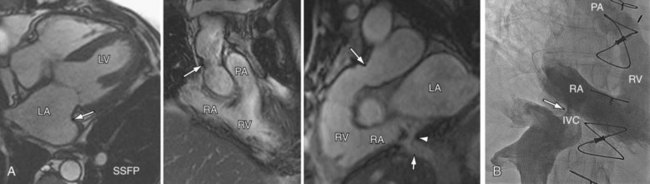
 FIGURE 59-8
FIGURE 59-8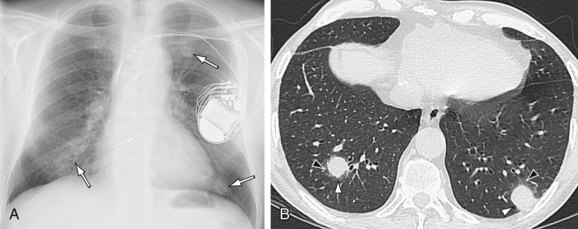
 FIGURE 59-9
FIGURE 59-9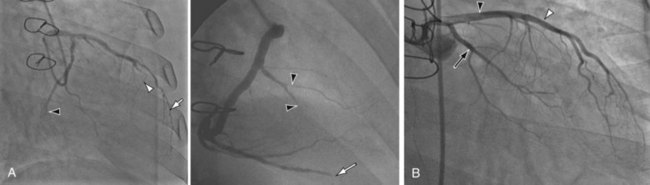
 FIGURE 59-10
FIGURE 59-10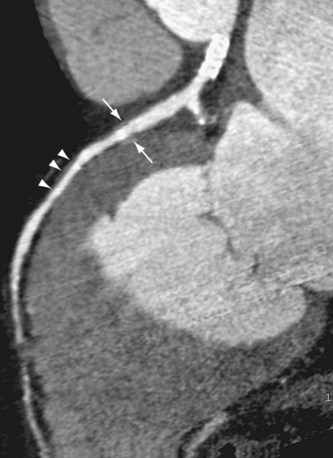
 FIGURE 59-11
FIGURE 59-11

