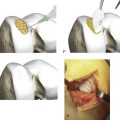
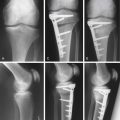
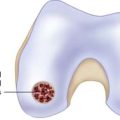
Chapter 16 Postoperative Cartilage Repair Rehabilitation
Introduction
Participation in physical activity is at the forefront of international health promotion agendas, and there is increasing encouragement for the maintenance of participation in sports and exercise activities throughout an individual’s life span.1 Moderate recreational physical exercise is associated with a decrease in the risk of knee osteoarthritis.2 However, participation in high-impact sports can increase the risk of developing articular cartilage lesions,3,4 and athletes are at a higher risk of developing knee osteoarthritis.5 There is increasing evidence that excessive stress on a joint with an articular cartilage lesion may accelerate further degenerative changes6 and that, if unaddressed, small cartilage defects progress to osteoarthritis.7 Messner and Maletius established that 75% of young athletes demonstrated radiographic joint space reduction an average of 14 years after sustaining chondral damage and returning to their preinjury sport levels.8 Consequently, it is not surprising that individuals are seeking out elective surgical procedures more frequently in order to address cartilage defects with the aim of maintaining or regaining their ability to participate in sports and exercise activities.
The ultimate goal for surgical intervention on chondral defects is the restoration of the joint surface to restore “normal” hyaline articular cartilage. Since the late 1980s, orthopaedic surgeons and tissue engineers have immersed themselves in this quest, resulting in the development of a new range of surgical procedures to restore the structure of normal articular cartilage.9 The treatment of articular cartilage defects has undergone a rapid and exciting evolution in recent years, most notably in the field of advanced cell-based orthobiological technologies. However, although some of these surgical interventions have demonstrated great promise, the new tissue in its current state does not precisely replicate natural articular cartilage in terms of form and function. Consequently, these interventions can only be considered reparative rather than the desired restorative.
To maximize the benefits of ACI surgery, it is essential for patients to be well informed and educated in order for them to comply to a specific rehabilitation program.9–11 Patient education, the management of patient expectations, and clear goal setting are indispensable within ACI rehabilitation. These values are reliant on a collaborative environment, with thorough communication between the surgeon, therapist, and patient. In addition, patient selection and the patients’ respective characteristics can affect the functional outcome in an inordinate way. deWindt et al. published a study examining the prognostic factors related to cartilage implantation and found that in lesions smaller than 3 cm2, defect location and defect age were statistically linked to better outcome scores on the knee injury and osteoarthritis outcome score (KOOS) at three years after ACI surgery.12 We cannot underestimate the notion of patient selection and the characteristics of the defect itself.
The two primary goals for an ACI rehabilitation program are as follows:
The three main components of the rehabilitation program are as follows:
Rehabilitation following an ACI procedure typically begins the day after the surgery, beginning with continuous passive motion (CPM). The frequency, duration, and ROM will depend on the location and size of the lesion. Active and passive motion is also utilized to facilitate the integration of the graft into the surrounding articular cartilage and subchondral bone. CPM is clinically recommended for 2 to 8 hours per day (depending on the site of the lesion) at one cycle per minute during the early phase of rehabilitation.
The contact area (distribution and magnitude), contact load, and contact pressure during rehabilitation should be considered to minimize the danger of damaging the graft and to support the healing process by stimulating the graft physiologically in harmless positions. The articulation and contact area at various degrees of knee flexion are of crucial importance to ACI rehabilitation in relationship to the graft location (Table 16-1).
TABLE 16-1 Summary of Patellar Articulation During Knee Flexion and Extension
| Articulation | Contact Area | |
|---|---|---|
| Full extension | Patella sits above femoral articular surface and rests on supratrochlear fat pad. | No patellofemoral contact with femur. |
| 10°–20° | Initial contact occurs between inferior patella and trochlea. | Joint contact area increases steadily with flexion. Mean contact area at 10° = 126 mm2; mean contact area at 60° = 560 mm2. |
| 30°–60° | Middle surface of patella makes contact with middle third of trochlea. | |
| 60°–90° | Superior patella makes contact with trochlea. | Contact area remains constant. |
| 90°–135° | Superior patella contact area splits into medial and lateral contact areas that articulate with the opposing femoral condyles. | Controversial—research differs, with contact area either leveling off after 90° or continuing to increase with increasing flexion. |
| 135° | Odd facet of patella contacts medial femoral condyle. | |
| Full flexion | Lateral femoral condyle is fully covered by patella, and medial femoral condyle is nearly completely exposed. |
The patella has a large articulating surface, presenting with the thickest layer of articular cartilage in order to optimize the distribution of forces and stresses.13–15 The patellar cartilage presents with multiple facets in a pattern that is unique to each individual, and it does not follow the contour of the underlying subchondral bone.13 The articular surface of the joint is congruent in the axial plane but not in the sagittal plane, and the material properties of the patellar cartilage differ from those in the cartilage of the articulating trochlea.5,13
The kinematics of the tibiofemoral joint is initiated, guided, and limited mainly by the cruciate ligaments, muscles, and capsular structures. Injury or loss of function to one of these structures leads to altered arthrokinematics, which may be deleterious to the menisci and cartilage.16
During normal activities, the joint contact forces (shear and compressive forces) that are produced are attenuated by several structures of the joint. Shear forces are primarily restrained by the cruciate ligaments. Compressive forces are mostly attenuated by the menisci and the articular cartilage.5,17 Excessive shear and compressive forces can be deleterious to the menisci and the cartilage. Numerous studies have measured these forces;16 the exact level of musculoskeletal loading is influenced by a number of individual factors such as weight, gender, movement coordination, and the activity being undertaken.
To develop a safe and effective ACI rehabilitation program, shear forces have to be minimized, and the size and location of the defect have to be known because during several activities only parts of the femur/tibia are articulating.5,18–21 For example, the posterior aspect of the medial femur condyle contacts the tibia between 90° and 120°; therefore, loading in positions between 0° and 80° of knee flexion are unlikely to be injurious to an ACI in this area.
Neuromuscular reeducation is a critical component in the restoration of functional joint stability. Neuromuscular function broadly involves the detection of afferent input via mechanoreceptors locally in the joint, the processing of a motor response to the stimulus in the central nervous system, and the initiation of an efferent reaction to maintain balance, stability, and mobility.22 Rehabilitation can assist in the restoration of proprioception, but high-level studies are scarce.23–25
The exercises should be performed throughout the full available ROM and should be performed on both the involved and the uninvolved limbs because of the likelihood that proprioception deficits are also present in the contralateral limb.13,26–29 Specific exercises for neuromuscular rehabilitation after ACI should be addressed on an individual basis in line with any weight bearing or ROM restrictions that may be in place.
Hydrotherapy
Exercises in water allow early active mobilization and early loading and improve neuromuscular performance, especially during the initial phase of a rehabilitation program.15,25,30,31
The reduction in gravity under water decreases the deleterious effects of weight bearing and dissipates the impact forces on joint structures during movement,13,32 enabling ROM exercises to be performed in a functional position with a reduced risk of high shear forces under compression. Factors such as water depth and flow will also influence the loading demands on the knee joint, so it is important to base the rehabilitation program on the general principles of hydrotherapy.
Exercises under water produce lower electromyography (EMG) activity during isometric and dynamic conditions when compared to similar exercises on dry land,33 thereby leading to lower joint forces. Research has shown that an early and intensive application of hydrotherapy for improving coordination and strength during rehabilitation is advisable.34 In addition, moving in water endows patients with a “feeling of freedom,” as they can walk without assistive devices and move around without restriction. This is an important psychological advantage.
Manual Therapy After ACI
The ability to define passive movement disorders in a joint, the localization of swelling, the involvement of anatomical structures, and temperature, are necessary for good clinical practice and for a comprehensive tailoring of the rehabilitation.5,35,36
Manual therapy as an independent application of manual techniques for general knee disorders is questionable. However, the combination of manual therapy with exercises and specific manual techniques for the enhancement of ROM prove to be more effective than exercises alone.2,37,38
Therapeutic Ultrasound and Laser
Low-intensity pulsed ultrasound (LIPUS)28,34,39 and low-level laser therapy17,25,40,41 have been proposed as providing appropriate stimuli for the acceleration of chondrogenesis. Naito et al. studied the effect of LIPUS on cartilage in a rat osteoarthritis (OA) model using serum biomarkers such as CTX-II (type II collagen degradation) and CPII (type II collagen synthesis). CPII was significantly increased in +LIPUS group compared to −LIPUS and the sham control group. In addition, the histological damage on the cartilage (Mankin score) was ameliorated by LIPUS, and type II collagen was immunohistochemically increased by LIPUS in the cartilage of an OA model. Of interest, mRNA expression of type II collagen was enhanced by LIPUS in chondrocytes. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro.32
Chondrocytes were exposed to LIPUS (30 mW/cm2; 20 min/day, 6 days). Stimulation of [35S]-sulphate incorporation into proteoglycans by LIPUS was 1.3-fold higher in degenerative than in collateral monolayers and 1.9-fold higher in explants. LIPUS increased the number of nests containing four to six chondrocytes by 4.8-fold in collateral and by 3.9-fold in degenerative explants. This suggests that LIPUS stimulates chondrocyte proliferation and matrix production in chondrocytes of human articular cartilage in vitro.42
Interferential Therapy
Interferential therapy (IFT) has been shown to have significant effects in reducing postoperative pain, increasing ROM, and reducing edema after knee surgery.20
Transcutaneous Electrical Nerve Stimulation
The effectiveness of transcutaneous electrical nerve stimulation (TENS) as a pain-relieving modality has been studied in a range of populations with variable outcomes. On one hand, several studies have found TENS to be effective in decreasing pain after knee surgery,35,43 but other studies have found no significant benefit in pain reduction.44
A review of the role of TENS concluded that it had no place in the treatment of acute postoperative pain, as it was not an effective analgesic.25 Bjordal et al. found that transcutaneous electrical nerve stimulation (TENS, including interferential currents), electroacupuncture (EA), and low-level laser therapy (LLLT) offered clinically relevant pain relieving effects of 18.8 mm [95% CI: 9.6 to 28.1] (n = 414), 21.9 mm [95% CI: 17.3 to 26.5] (n = 73), and 17.7 mm [95% CI: 8.1 to 27.3] (n = 343) on the visual analog scale (VAS), respectively, versus placebo control.45
Neuromuscular Electrical Stimulation
An alternative strategy to address arthrogenic muscle inhibition (AMI) utilizes the production of involuntary muscle contractions by neuromuscular electrical stimulation (NMES). Neuromuscular electrical stimulation has been found to be effective in reducing quadriceps extensor lag46 and in strengthening the quadriceps after knee arthroplasty and anterior cruciate ligament (ACL) reconstruction.39 However, it is important to note that voluntary muscle strengthening has been found to be just as effective as NMES.47
Therapeutic Exercise
Recent studies have advocated the avoidance of certain ranges of knee movement, for example, active knee flexion between 40° and 70° in the early stages after patellofemoral ACI.48 However, virtually all exercise modalities, including common activities such as walking, cycling, and rowing, involve a knee flexion/extension pattern within this range.
Cycling
In comparison with other activities of daily living such as walking or stair climbing, the maximum load-moments on the knee joint in cycling are small.42
Increases in the cycling workload result in a significant increase in knee load-moments and compressive and shear forces, but increases in the pedaling rate do not appear to affect the maximum knee load-moment.42 It is therefore possible to introduce stationary cycling at an early stage as long as resistance is minimal and there is sufficient ROM to allow a complete pedal revolution.
Along with the correct selection of resistance, another important factor in cycling that needs to be considered is saddle height because of its direct influence on knee flexion angles (Table 16-1).42 If the saddle height is too low, increased patellofemoral joint reaction forces (PFJRFs) occur,49 especially if combined with too high a gearing; tibiofemoral joint (TFJ) load-moments decrease with increasing saddle height.37 Too high a saddle height, often as a consequence of insufficient available range of knee flexion, results in frontal plane rocking from the pelvis and hip, which is unfavorable for rehabilitation in terms of control and muscle activation patterns.
High saddle heights are a predisposing factor for an increased risk of developing iliotibial band friction syndrome (ITBFS), especially if knee ROM is not full.2 An increase in saddle height for a short postoperative period is unlikely to significantly predispose a patient to ITBFS because the condition is predominantly due to overuse.
Analysis of the effect that changing the direction of pedaling has on knee joint biomechanics has shown that reverse pedaling requires quadriceps muscle activity in ranges of greater knee flexion compared with forward pedaling50 and that the vastus medialis is more active in reverse pedaling.50
Tibiofemoral compressive loads have been shown to be lower in reverse pedaling, especially near peak extension of the knee.47 However, PFJRFs have been found to be significantly higher in reverse pedaling compared with forward pedaling.51
Other Exercise Modalities
Other low-impact exercise modalities commonly available in fitness centers include elliptical trainers, cross-trainers, ski trainers, and stair climbers. These modalities have the advantage of being closed kinetic chain activities; however, clinical biomechanical data are limited, and the implications for loading on the knee joint are not fully understood.
Whole-body vibration (WBV), in which the patient undergoes a sensory bombardment, has recently become a popular training modality for gaining strength.40,44,50,52
Liphardt and associates researched the effects of WBV following 14 days of immobilization of young healthy subjects to see if it would reduce cartilage thickness in the knee and serum cartilage oligomeric matrix protein (COMP) concentration. The control intervention resulted in an overall loss in average cartilage thickness of −8% (pre: 3.08 mm ±0.6 mm, post: 2.82 mm ±0.6 mm) in the weight-bearing regions of the tibia. The average cartilage thickness increased by 21.9% (pre: 2.66 mm ±0.45 mm, post: 3.24 mm ±0.63 mm) with the vibration intervention. No significant differences were found in the weight-bearing regions of the femur. During both interventions, reduced serum COMP concentrations were observed (control intervention: −13.6 ±8.4%, vibration intervention: −9.9 ±3.3%). The results of this study suggest that articular cartilage thickness is sensitive to unloading and that vibration training may be a viable countermeasure against these effects.53 There is a need for further research concerning cartilage tissue repair, the overload in a sustained exercise position, and the exact effect of different training parameters are all reasons for not implementing whole-body vibration in the early stages of rehabilitation after ACI at this time.
Return to Sport After ACI
Rehabilitation after ACI is widely recognized as being lengthy, with maximum improvement in knee symptoms taking up to 3 years postoperatively.20 This is crucial to consider because of the level of impact that the duration of the rehabilitation has on the time out of sport. Only one multicenter study to date has researched return to sport after ACI.15,24,54
Mithöfer et al. studied the ability of 45 soccer players to return to soccer in a 40-month (± 4 months) follow-up period after ACI. They found that despite 72% of players reporting good to excellent knee function, only 33% were able to return to soccer.5,9,39,45
What is unclear is whether the two thirds of players who did not return to soccer were clinically unable to return to play or whether they either chose to switch to a lower-impact activity or opted not to return to sport at all. The definition of “ability to return to sport” and the relevance of current outcome measures to sporting participation require further exploration and clarification. Younger age and shorter preoperative duration of symptoms were also shown to significantly improve the ability to return to soccer.55,56
Wondrasch and associates published a study examining the effects of accelerated weight bearing after a matrix associated autologous chrondrocyte implantation (MACI). Thirty-one patients (22 male, 9 female) after MACI on the femoral condyle were randomly assigned to the accelerated weight-bearing group (group A) or the delayed weight-bearing group (group B). In both groups, there were no differences with regard to the clinical outcome. For the radiological outcome, group A showed a higher prevalence of bone marrow edema after 6 months without correlation to the clinical outcome (P=0.06-0.1). However, after 104 weeks, there were no differences in the radiological outcome between group A and group B. A rehabilitation protocol with accelerated weight bearing leads to good clinical and functional outcome after 2 years without jeopardizing the healing graft.36
Della Villa et al. also recognized the challenge of developing a timely postoperative rehabilitation that would optimize a return to preinjury activities without jeopardizing the integrity of the newly implanted graft. Thirty-one athletic males with a grade 3-4 cartilage lesion of the medial or lateral femoral condyle or trochlea were evaluated at 1-, 2-, and 5-year follow-up. The athletic cohort was compared with a similar control cohort of 34 nonathletic patients who were treated with autologous chondrocyte implantation. A greater improvement in the group of athletes was achieved at 5-year follow-up (P=0.037) in the self-assessment of quality of life and International Knee Documentation Committee (IKDC) subjective evaluation at 12 months and at 5 years of follow-up (P=0.001 and P=0.002, respectively). When analyzing the return to sports activity, 80.6% of the athletes returned to their previous activity level in 12.4±1.6 months; athletes treated with the on-field rehabilitation and isokinetic exercise program had faster recovery and an even earlier return to competition (10.6±2.0 months). For optimal results, autologous chondrocyte implantation rehabilitation should not only follow but also facilitate the process of graft maturation. Intensive rehabilitation may safely allow a faster return to competition and also influence positively the clinical outcome at medium-term follow-up.17
With the uncertainties that surround ACI rehabilitation at present, the general consensus of opinion among cartilage repair centers appears to be that ACI surgery should be targeted on the reduction of symptoms and on improving functional daily activities rather than as a method of returning to high-level sports participation for competitive athletes with chondral damage.
General recommendations are that low-impact sports and exercise such as swimming, cycling, and golf can usually be resumed within 6 months.1,34,42,52,57–59 Recommendations for timescales for a return to higher-impact activities such as racquet sports, team sports, martial arts, and running range from an earliest postoperative return at 12 months up to 18 months. However, there is considerable variation between people, so the return to sports after ACI should be based on the key criteria that address the following factors:
Discussion
Cartilage repair rehabilitation is lengthy,27,60,61 and autologous chondrocyte implantation (ACI) has one of the longest rehabilitation processes in the field of elective orthopaedic surgery. Numerous papers have been published since the late 1990s documenting surgical techniques and clinical outcomes of articular cartilage repair procedures.1,43,62–65
The quality of these cartilage repair studies has been variable, and follow-up time periods are generally short.40,66,67 Time frames have been indicated, but we do not recommend the adoption of a rigid timetable, as the proposed phases are not mutually exclusive and considerable variation exists between people. Modifications to the rehabilitation program may be necessary based on defect size, location, age, previous activity level, concomitant surgical procedures, and individual patient demands.57,68
Summary
Osteoarthritis (OA) is a degenerative disease with a tremendously detrimental impact on an individual’s quality of life. It is the most common form of arthritis, and it accounts for more pathology during walking, stair climbing, and other lower-extremity activities of daily living than any other disease, especially in the elderly.50 Its economic impact is truly remarkable. Yelin completed a meta-analysis to determine that the estimated cost of treating OA in the United States is $15.5 billion, roughly three times the cost of rheumatoid arthritis.31
Indications
Articular cartilage defects are not life threatening, but they can, and frequently do, threaten a person’s quality of life, especially in an active population.10,28 Internationally, thousands of people each year experience symptoms related to chondral defects, with the knee being the most prevalent joint affected.25 Articular cartilage lesions of the knee are relatively common, one study found that of more than 31,000 arthroscopies, up to 63% of patients had articular cartilage lesions.1 More recently, in 993 arthroscopies, 11% of patients demonstrated a localized full-thickness cartilage lesion,69 and in 1000 arthroscopies, 19% of patients had a focal chondral or osteochondral defect.70 It has been estimated that between 4% and 11% of cartilage lesions may be suitable for cartilage repair procedures.58,69
Results
There is a distinct paucity of research regarding the optimal rehabilitation following an articular cartilage repair procedure. Many research studies published in peer review journals do not specifically detail the nuances of the rehabilitation program, the compliance to such a program, and the functional outcome of the patient longitudinally. To date, many of the standardized protocols have been conservative because of the lack of level I research in this area. Recent studies by Wondrasch and Della Villa have reported on the role of accelerated weight bearing after an articular cartilage repair.33,36
1. Curl W.W., Krome J., Gordon E.S., et al. Cartilage injuries: a review of 31,516 arthroscopies. Arthroscopy. 1997;13:456-460.
2. Lingard E.A., Sledge C.B., Learmonth I.D. Kinemax Outcomes Group. Patient expectations regarding total knee arthroplasty: differences among the United States, United Kingdom, and Australia. J Bone Joint Surg Am. 2006;88:1201-1207.
3. Bryman A., Burgess R.C. Analyzing qualitative data. London: Routledge; 2001.
4. Pimm T., Weinman J. Applying Leventhal’s self regulation model to adaptation and intervention in rheumatic disease. Clin Psychol Psychother. 1998;5:62-75.
5. Sherer M., Maddux J. The self-efficacy scale: construction and validation. Psychol Rep. 1982;51:663-671.
6. Hawker G.A. Who, when, and why total joint replacement surgery? The patient’s perspective. Curr Opin Rheumatol. 2006;18:526-530.
7. Tegner Y., Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985 Sep;198:43-49.
8. Marx R.G., Stump T.J., Jones E.C., Wickiewicz T.L., et al. Development and Evaluation of an Activity Rating Scale for Disorders of the Knee. Am J Sports Med. 2001;29:213-218.
9. Alford J.W., Cole B.J. Cartilage restoration, Part 1: Basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306.
10. Ajzen I. From intentions to actions: A theory of planned behaviour. In: Kuhl J., Beckman J., editors. Action control: from cognition to behaviour. New York: Springer; 1985:11-39.
11. Moon L.B., Backer J. Relationships among self-efficacy, outcome expectancy, and postoperative behaviors in total joint replacement patients. Orthop Nurs. 2000;19:77-85.
12. de Windt T.S., Bekkers J.E., Creemers L.B., et al. Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am J Sports Med. 2009 Nov;37(suppl 1):S58-S62.
13. Orbell S., Johnstone M., Rowley D., Espley A., et al. Cognitive representations of illness and functional and affective adjustment following surgery for osteoarthritis. Soc Sci Med. 1998;47:93-102.
14. Weinman J. The Illness Perception Questionnaire: a new method for assessing the cognitive representation of illness. Psychol Health. 1996;11:431-445.
15. Wilson W.J., Jacobs J.E. Patellar graft for the severely depressed comminuted fractures of the lateral tibial condyle. J Bone Joint Surg Am. 1952;34:436-442.
16. Mancuso C.A., Sculco T.P., Wickiewicz T.L., Jones E.C., et al. Patients’ expectations of knee surgery. J Bone Joint Surg Am. 2001;83(A):1005-1012.
17. Jakobsen R.B., Engebretsen L., Slauterbeck J.R. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-2239.
18. Pelletier L.G., Tuson K.M., Fortier M.S., Vallerand R.J., et al. Toward a new measure of intrinsic motivation, extrinsic motivation, and amotivation in Sports: The sport motivation scale (SMS). J Sport Ex Psych. 1995;17:35-53.
19. Rosenberger P.H., Jokl P., Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14:397-405.
20. Sangha O., Stucki G., Liang M.H., Fossel A.H., et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156-163.
21. Saxon L., Finch C., Bass S. Sports participation, sports injuries and osteoarthritis implications for prevention. Sports Medi. 1999;28:123-135.
22. Thomeé P., Währborg P., Börjesson M., Thomeé R., et al. Self-efficacy, symptoms and physical activity in patients with an anterior cruciate ligament injury: a prospective study. Scand J Med Sci Sports. 2006;17:238-245.
23. Robinson M.E., Riley J.L.3rd, Myers C.D., Papas R.K., et al. Gender role expectations of pain: relationship to sex differences in pain. J Pain. 2001;2:251-257.
24. Ware J.E.Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
25. Willers C., Wood D.J., Zheng M.H. A current review on the biology and treatment of articular cartilage defects (part I and part II). J Musculoskelet Res. 2003;7:157-181.
26. Mancuso C.A., Sculco T.P., Salvati E.A. Patients with poor preoperative functional status have high expectations of total hip arthroplasty. J Arthroplasty. 2003;18:872-878.
27. Manninen P., Riihimaki H., Heliovaara M., Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford). 2001;40:432-437.
28. Mithoefer K., Gill T.J., Giza E., Mandelbaum B., et al. Treatment of full-thickness articular cartilage lesions of the knee in high-demand athletes with autologous chondrocyte transplantation. Orthop J Harvard Medical School. 2002;4:77-79.
29. Vlaeyen J., Kole-Snijders A., Boeren R., van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363-372.
30. Vad V.B., Bhat A.L. The athlete with early knee arthritis. Phys Med Rehabil Clin N Am. 2000;11:881-894.
31. Yelin E. The economics of osteoarthritis. In: Brandt K., Doherty M., Lohmander L.S., editors. Osteoarthritis, 2 ed. New York: Oxford University Press; 1998:23-30.
32. Naito K., Watari T., Muta T., et al. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res. 2010 Mar;28(3):361-369.
33. Della Villa S., Kon E., Filardo G., et al. Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes? Am J Sports Med. 2010;38:68-77.
34. Lorig K., Chastain R.L., Ung E., Shoor S., et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37-44.
35. Stevens M., van den Akker-Scheek I., van Horn J.R. A Dutch translation of the Self-efficacy for rehabilitation outcome scale (SER): a first impression on reliability and validity. Patient Educ Couns. 2005;58:121-126.
36. Wondrasch B., Zak L., Welsch G.H., Marlovits S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle on radiographic and clinical outcome after 2 years: a prospective, randomized controlled pilot study. Am J Sports Med. 2009 Nov;37(suppl 1):S88-S96.
37. Leventhal H., Benyamini Y., Brownlee S., Diefenbach M., et al. Illness representations: theoretical foundations. In: Petrie K.J., Weinman J., editors. Perceptions of health and illness. The Netherlands: Harwood; 1997:19-45.
38. Leventhal H., Nerenz D., Steele D., Illness perceptions and coping with health threat, Baum A., Taylor S., Singer J., editors. Handbook of psychology and health: social psychological aspects of health, Vol. IV, Erlbaum, Hillsdale, NJ, 219-252, 1984.
39. Mithöfer K., Peterson L., Mandelbaum B.R., Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33:1639-1646.
40. Joshy S., Datta A., Perera A., Thomas B., et al. Ethnic differences in preoperative function of patients undergoing total knee arthroplasty. Int Orthop. 2006 Oct;30(5):426-428.
41. Mahomed N.N., Liang M.H., Cook E.F., Daltroy L.H., et al. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J Rheumatol. 2002;29:1273-1279.
42. Korstjens C.M., van der Rijt R.H., Albers G.H., et al. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008 Dec;46:1263-1270.
43. Bandura A. Self-efficacy: Toward a unifying theory of behavioural change. Psychological Review. 1977;84:85-100.
44. Hampson S.E., Glasgow R.E., Zeiss A.M. Personal models of osteoarthritis and their relation to self-management activities and quality of life. J Behav Med. 1994;17:143-158.
45. Bjordal J.M., Johnson M.I., Lopes-Martins R.A., et al. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;22(8):51.
46. Moss-Morris R., Weinman J., Petrie K., Horne H., et al. The revised illness perception questionnaire (IPQ-R). Psychol Health. 2002;17:1-6.
47. Yamashita F., Sakakida K., Suzu F., Takai S., The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee Clin Orthop Relat Res 1985(201) Dec:43-50.
48. Hambly K., Bobic V., Wondrasch B., Van Assche D., et al. Autologous Chondrocyte Implantation Postoperative Care and Rehabilitation: Science and Practice. Am J Sports Med. 2006;34(6):1020-1038.
49. Kvist J., Ek A., Sporrstedt K., Good L. Fear of re-injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2005;13:393-397.
50. Guccione A.A., Felson D.T., Anderson J.J., Anthony J.M., Zhang Y., Wilson P.W., et al. The effects of specific medical conditions on functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351-358.
51. Farley F.A., Weinstein S.L. The case for patient-centered care in orthopaedics. J Am Acad Orthop Surg. 2006;14:447-451.
52. Ibrahim S.A., Siminoff L.A., Burant C.J., Kwoh C.K. Differences in expectations of outcome mediate African American/white patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 2002;46:2429-2435.
53. Liphardt A.M., Mündermann A., Koo S., Bäcker N., Andriacchi T.P., Zange J., Mester J., Heer M. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligomeric matrix protein (COMP) during immobilization. Osteoarthritis Cartilage. 2009;17:1598-1603:2009.
54. Wang Y., Ding C., Wluka A.E., Davis S., et al. Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology (Oxford). 2006;45:79-84.
55. Pellino T., Tluczek A., Collins M., Trimborn S., et al. Increasing self-efficacy through empowerment: preoperative education for orthopaedic patients. Orthop Nurs. 1998;17:48-51. 54–49
56. Robinson M.E., Gagnon C.M., Dannecker E.A., Brown J.L., et al. Sex differences in common pain events: expectations and anchors. J Pain. 2003;4:40-45.
57. Bayley K.B., London M.R., Grunkemeier G.L., Lansky D.J. Measuring the success of treatment in patient terms. Med Care. 1995;33:AS226-AS235.
58. Carlisle A.C., John A.M., Fife-Schaw C., Lloyd M. The self-regulatory model in women with rheumatoid arthritis: relationships between illness representations, coping strategies, and illness outcome. Br J Health Psychol. 2005;10(Pt 4):571-587.
59. Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., et al. Knee injury and osteoarthritis outcome score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88-96.
60. Irrgang J.J., Anderson A.F., Boland A.L., Harner C.D., et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-613.
61. King P.J., Bryant T., Minas T. Autologous chondrocyte implantation for chondral defects of the knee: indications and technique. J Knee Surg. 2002;15:177-184.
62. Alford J.W., Cole B.J. Cartilage restoration, Part 2: Techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443-460.
63. Botha-Scheepers S., Riyazi N., Kroon H.M., Scharloo M., et al. Activity limitations in the lower extremities in patients with osteoarthritis: the modifying effects of illness perceptions and mental health. Osteoarthritis Cartilage. 2006;14:1104-1110.
64. Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631-637.
65. Buckwalter J.A. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33:578-588.
66. Hawker G.A., Wright J.G., Glazier R.H., Coyte P.C., et al. The effect of education and income on need and willingness to undergo total joint arthroplasty. Arthritis Rheum. 2002;46:3331-3339.
67. Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86A:455-464.
68. Thomas J.R., Nelson J.K. Research methods in physical activity, 4th ed. Champaign, IL: Human Kinetics; 2001.
69. Arøen A., Løken S., Heir S., Alvik E., et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215.
70. Hjelle K., Solheim E., Strand T., Muri R., et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-734.
1. Anderson A.F., Irrgang J.J., Kocher M.S., Mann B.J., et al. The International Knee Documentation Committee subjective knee evaluation form: Normative data. Am J Sports Med. 2006;34:128-135.
2. Bandura A., Locke E.A. Negative self-efficacy and goal effects revisited. J Appl Psychol. 2003;88:87-99.
3. Cain E.L., Clancy W.G. Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med. 2001;20:321-342.
4. Chen G., Gully S.M., Eden D. Validation of a new general self-efficacy scale. Organizational Research Methods. 2001;4:62-83.
5. Department of Health. Choosing activity: a physical activity action plan. London, UK: Crown, 2005.
6. Hambly K. Cartilage repair rehabilitation: The human factor. Paper presented at the 6th International Cartilage Repair Society Congress. CA: San Diego, 2006.
7. Irrgang J.J., Pezzullo D. Rehabilitation following surgical procedures to address articular cartilage lesions in the knee. J Orthop Sports Phys Ther. 1998;28:232-240.
8. Jenkinson C., Stewart-Brown S., Petersen S., Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health. 1999;53:46-50.
9. Leventhal H., Meyer D., Nerenz D., The common sense model of illness dange, Rachman S., editor. Contributions to medical psychology, Vol. 2, Pergamon, New York, 27-30, 1980.
10. Lundberg M., Styf J., Carlsson S. A psychometric evaluation of the Tampa scale for kinesiophobia—from a physiotherapeutic perspective. Physiother Theory Pract. 2004;20:121-133.
11. Messner K., Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996;67:165-168.
12. Moran M., Khan A., Sochart D.H., Andrew G. Expect the best, prepare for the worst: surgeon and patient expectation of the outcome of primary total hip and knee replacement. Ann R Coll Surg Engl. 2003;85:204-206.
13. Noble P.C., Conditt M.A., Cook K.F., Mathis K.B. The John Insall Award: patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35-43.
14. Podlog L., Eklund R.C. Return to sport after serious injury: A retrospective examination of motivation and psychological outcomes. J Sport Rehabil. 2005;14:20-34.
15. Rosenberger P.H., Jokl P., Cameron A., Ickovics J.R. Shared decision making, preoperative expectations, and postoperative reality: Differences in physician and patient predictions and ratings of knee surgery outcomes. Arthroscopy. 2005;21:562-569.
16. Strauss A., Corbin J. Grounded theory in practice. Thousand Oaks, CA: Sage Publications; 1997.
17. Thomeé P., Währborg P., Börjesson M., Thomeé R., et al. A new instrument for measuring self-efficacy in patients with an anterior cruciate ligament injury. Scand J Med Sci Sports. 2006;16:181-187.
18. Uhlmann R.F., Inui T.S., Carter W.B. Patient requests and expectations. Definitions and clinical applications. Med Care. 1984;22:681-685.
19. Waldrop D., Lightsey O., Ethington C., Woemmel C., et al. Self-efficacy, optimism, health competence and recovery from orthopaedic surgery. J Couns Psychol. 2001;48:233-238.
20. Weinman J., Petrie K.J. Illness perceptions: a new paradigm for psychosomatics? J Psychosom Res. 1997;42:113-116.