Postoperative Care
THE EARLY RECOVERY PERIOD
Most hospitals have a recovery ward (or postanaesthesia care unit, PACU) within, or in close proximity to, the operating theatre suite (see Ch 20). The Association of Anaesthetists of Great Britain and Ireland (AAGBI) recommends that fully staffed recovery facilities must be available at all times in hospitals with an emergency surgical service. Some locations where anaesthesia is provided (e.g. the X-ray department) may not have a recovery ward. This section describes common problems which occur in the immediate postoperative period and refers specifically to their management in a recovery ward; however, the same principles are applicable to recovery in other locations.
Staff, Equipment and Monitoring
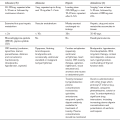
The patient is nursed in a bed if available or if a prolonged stay is anticipated, but sometimes on a trolley (Fig. 40.1). All beds and trolleys must have the facility to be tipped head-down. Suction apparatus, including catheters, an oxygen supply with appropriate face mask, a self-inflating resuscitation bag and anaesthetic mask, a pulse oximeter and an automated non-invasive blood pressure monitor must be available for each patient. In addition, there should be a complete range of resuscitation equipment within the recovery area; this includes an anaesthetic machine, a range of laryngoscopes, tracheal tubes, bougies, intravenous (i.v.) cannulae, fluids, emergency drugs, electrocardiogram (ECG) monitor and defibrillator. Facilities for emergency airway management including surgical airways should also be available (see Ch 22).
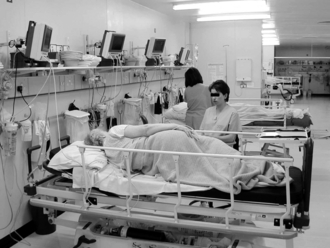
FIGURE 40.1 Part of a recovery ward. Many patients are nursed on a trolley, but a bed is used if available, and particularly for those who have undergone major surgery and those who need to stay for several hours.
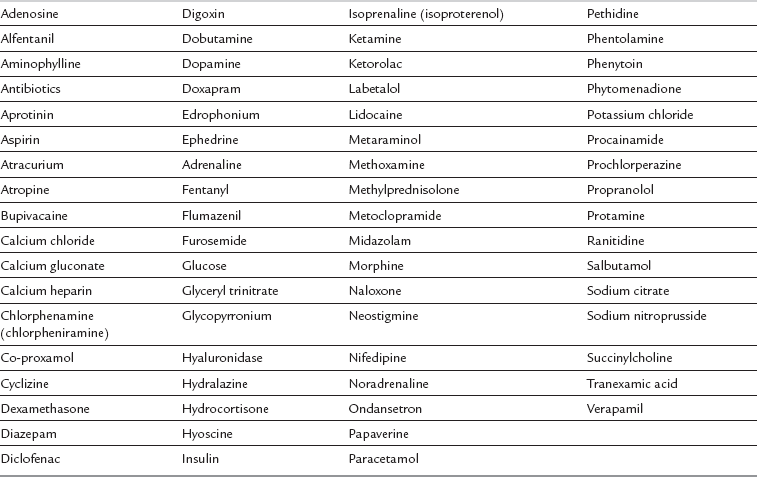
A wide range of drugs should be stored in the recovery area for the treatment of common complications and also emergency events (Table 40.1).
 Consciousness has returned fully, a patent airway can be maintained and protective reflexes are present.
Consciousness has returned fully, a patent airway can be maintained and protective reflexes are present.
 Ventilation and oxygenation are satisfactory.
Ventilation and oxygenation are satisfactory.
 The cardiovascular system is stable with no unexplained cardiac irregularity or persistent bleeding. Consecutive measurements of pulse rate and arterial pressure should approximate to the patient’s normal preoperative values or be at an acceptable level commensurate with the planned postoperative care. Peripheral perfusion should be adequate.
The cardiovascular system is stable with no unexplained cardiac irregularity or persistent bleeding. Consecutive measurements of pulse rate and arterial pressure should approximate to the patient’s normal preoperative values or be at an acceptable level commensurate with the planned postoperative care. Peripheral perfusion should be adequate.
CENTRAL NERVOUS SYSTEM
 The drugs used. Recovery of consciousness may be delayed if the following agents have been used:
The drugs used. Recovery of consciousness may be delayed if the following agents have been used:
 volatile anaesthetics with a high blood/gas solubility coefficient
volatile anaesthetics with a high blood/gas solubility coefficient
 barbiturates, particularly if large total doses have been given
barbiturates, particularly if large total doses have been given
 opioids with a long duration of action, including large doses of fentanyl.
opioids with a long duration of action, including large doses of fentanyl.
 The timing of drug use. Delayed recovery may occur if a long-acting i.v. anaesthetic or analgesic drug has been given towards the end of the procedure, or if the more soluble volatile agents have been continued until the end of surgery.
The timing of drug use. Delayed recovery may occur if a long-acting i.v. anaesthetic or analgesic drug has been given towards the end of the procedure, or if the more soluble volatile agents have been continued until the end of surgery.
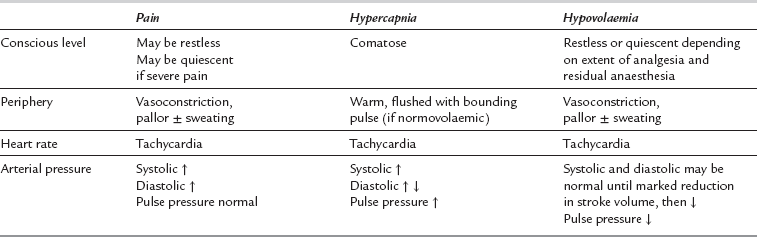
 Pain. The presence of pain speeds recovery of consciousness. Recovery may be delayed after minor procedures or if potent analgesia has been provided by administration of opioids or by regional anaesthesia.
Pain. The presence of pain speeds recovery of consciousness. Recovery may be delayed after minor procedures or if potent analgesia has been provided by administration of opioids or by regional anaesthesia.
 hypoxaemia – in the presence of an adequate circulation, coma occurs only if profound hypoxaemia is present; agitation is a more common sign of hypoxia
hypoxaemia – in the presence of an adequate circulation, coma occurs only if profound hypoxaemia is present; agitation is a more common sign of hypoxia
 hypercapnia – unconsciousness may occur if arterial carbon dioxide tension (PaCO2) exceeds 9–10 kPa
hypercapnia – unconsciousness may occur if arterial carbon dioxide tension (PaCO2) exceeds 9–10 kPa
Hypoglycaemia
This occurs most commonly in diabetic patients treated with oral hypoglycaemic agents or insulin and an inadequate intake of glucose. The perioperative management of the diabetic patient is discussed in Chapter 18.
Cerebral Pathology
Consciousness may be impaired by functional or structural cerebral damage. Possible causes include:
 episodes of cerebral ischaemia (e.g. carotid artery surgery, profound hypotension) or cerebral hypoxia during anaesthesia
episodes of cerebral ischaemia (e.g. carotid artery surgery, profound hypotension) or cerebral hypoxia during anaesthesia
 intracranial haemorrhage, thrombosis or infarction – these may occur coincidentally or may have been associated with intraoperative hypertension, hypotension or arrhythmias
intracranial haemorrhage, thrombosis or infarction – these may occur coincidentally or may have been associated with intraoperative hypertension, hypotension or arrhythmias
 pre-existing cerebral lesions, e.g. tumour, trauma – anaesthetic techniques which increase intracranial pressure are likely to impair cerebral function
pre-existing cerebral lesions, e.g. tumour, trauma – anaesthetic techniques which increase intracranial pressure are likely to impair cerebral function
 epilepsy – convulsions may have been masked by anaesthesia or neuromuscular blocking drugs
epilepsy – convulsions may have been masked by anaesthesia or neuromuscular blocking drugs
 intracranial spread of local anaesthetic solution after subarachnoid injection – introduction into the subarachnoid space may be accidental, e.g. during epidural block or, rarely, interscalene brachial plexus block; unconsciousness is almost always accompanied by apnoea.
intracranial spread of local anaesthetic solution after subarachnoid injection – introduction into the subarachnoid space may be accidental, e.g. during epidural block or, rarely, interscalene brachial plexus block; unconsciousness is almost always accompanied by apnoea.
RESPIRATORY SYSTEM
Common causes of hypoventilation in the immediate postoperative period are listed in Table 40.3. Hypoventilation results in an increase in PaCO2 (Fig. 40.2) and a decrease in alveolar oxygen tension (PAO2), and thus hypoxaemia, which may be corrected by increasing the inspired concentration of oxygen. The risk factors for developing hypoventilation include:
TABLE 40.3
Causes of Postoperative Hypoventilation
| Factors Affecting Airway | Factors Affecting Ventilatory Drive | Peripheral Factors |
| Upper airway obstruction | Respiratory depressant drugs | Muscle weakness |
| Tongue | Preoperative CNS pathology | Residual neuromuscular block |
| Laryngospasm | Intra- or postoperative cerebrovascular accident | Preoperative neuromuscular disease |
| Oedema | Hypothermia | Electrolyte abnormalities |
| Foreign body | Recent hyperventilation (PaCO2 low) | Pain |
| Tumour | Abdominal distension | |
| Bronchospasm | Obesity | |
| Tight dressings | ||
| Pneumo-/haemothorax |
CNS, central nervous system; PaCO2, arterial carbon dioxide tension.
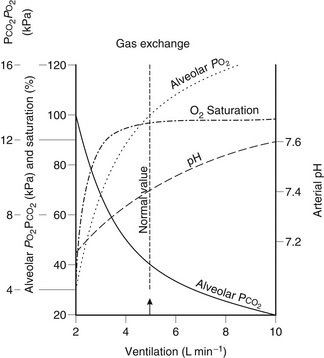
FIGURE 40.2 Gas exchange during hypoventilation. Note the relatively rapid increase in alveolar partial pressure of carbon dioxide (PCO2) compared with the slow decrease in arterial oxygen saturation. PO2, partial pressure of oxygen.
Airway Obstruction
Airway obstruction caused by the tongue, by indrawing of the pharyngeal muscles or by blood or secretions in the pharynx may be ameliorated by placing the patient in the lateral or recovery position (see Fig. 21.7). This position should be used for all unconscious patients who have undergone oral or ear, nose and throat surgery, and for patients at risk of gastric aspiration.
Ventilatory Drive
There are several possible causes of reduced ventilatory drive during recovery from anaesthesia (see Table 40.3). The presence of intracranial pathology, e.g. tumour, trauma or haemorrhage, may affect ventilatory drive in the postoperative period. Ventilation is reduced in the presence of hypothermia, although it is usually appropriate for the metabolic needs of the body. Hypoventilation occurs in the hypocapnic patient, e.g. after a period of hyperventilation until PaCO2 is restored to normal, and in the presence of primary metabolic alkalosis.
Reduced ventilatory drive is easy to diagnose if the ventilatory rate or tidal volume is clearly reduced. However, lesser degrees of hypoventilation may be difficult to detect, and the signs of moderate hypercapnia, e.g. hypertension and tachycardia, may be masked by the residual effects of anaesthetic agents, or misdiagnosed as pain-induced (see Table 40.2).
Peripheral Factors
The commonest peripheral factor associated with hypoventilation is residual neuromuscular blockade. This may be exaggerated by disease of the neuromuscular junction, e.g. myasthenia gravis, or by electrolyte disturbances. Inadequate reversal of neuromuscular blockade is usually associated with uncoordinated, jerky movements, although these may occur occasionally during recovery of consciousness in patients with normal neuromuscular function. Measurement of tidal volume is not a reliable guide to adequacy of reversal of neuromuscular blockade; a normal tidal volume may be achieved with only 20% return of diaphragmatic power, but the ability to cough remains severely impaired. Traditional clinical signs of adequacy of reversal of neuromuscular blockade (such as if the patient is able to lift the head from the trolley for 5 s or maintain a good hand grip) correlate poorly with objective signs of neuromuscular function. Some more objective means of assessment are listed in Table 40.4, but these require the cooperation of the patient. In the unconscious or uncooperative patient, nerve stimulation (see Ch 6) provides the best means of assessing neuromuscular function, although there are differences among the non-depolarizing relaxants in the relationship between their actions in the forearm and diaphragm.
TABLE 40.4
Clinical Assessment of the Adequacy of Antagonism of Neuromuscular Block
Subjective
Grip strength
Adequate cough
Objective
Ability to sustain head lift for at least 5 s
Ability to produce vital capacity of at least 10 mL kg−1
Hypoxaemia
A functional classification of causes of hypoxaemia in the early recovery period is shown in Table 40.5. An inspired oxygen concentration of less than 21% should never occur, although PaO2 is decreased when air is breathed at high altitudes.
TABLE 40.5
Functional Classification of the Causes of Hypoxaemia in the Postoperative Period
Reduced inspired oxygen concentration
Ventilation–perfusion abnormalities
Shunting
Hypoventilation
Diffusion deficits
Diffusion hypoxia after nitrous oxide anaesthesia
Hypoventilation
This has been discussed in detail above. Moderate hypoventilation, with some elevation of PaCO2, leads to a modest reduction in PaO2 (Fig. 40.2). Obstructive sleep apnoea may produce profound transient but repeated decreases in arterial oxygenation. SaO2 may decrease to less than 75%, corresponding to a PaO2 of less than 5 kPa (40 mmHg). These repeated episodes of hypoxaemia cause temporary, and possibly permanent, defects in cognitive function in elderly patients and may contribute to perioperative myocardial infarction. Obstructive sleep apnoea is exacerbated by opioid analgesics, and patients who are known to suffer from this condition should be monitored carefully in the postoperative period, preferably in a high-dependency unit. Patients who normally use a continuous positive airways pressure (CPAP) mask to reduce obstructive sleep apnoeic episodes should use the mask at night throughout the postoperative period.
Pulmonary Changes after Abdominal Surgery
The reduction in FRC may lead to closing capacity impinging upon the tidal breathing range. This results in small airways closure during normal tidal ventilation. Gas trapping occurs in the affected airways and subsequent absorption of air may lead to the development of small, discrete areas of atelectasis which are not visible on chest X-ray. This occurs mainly in the dependent parts of the lung and may be demonstrated by CT scan very soon after induction of anaesthesia. The result is an increase in the number of areas of low ![]() ratio within the lungs. The relationship between changes in FRC and PaO2 postoperatively is shown in Figure 40.3.
ratio within the lungs. The relationship between changes in FRC and PaO2 postoperatively is shown in Figure 40.3.
FIGURE 40.3 Changes in functional residual capacity (FRC) and arterial oxygen tension (PaO2) postoperatively.
 Inability to cough. This results mostly from wound pain. However, excessive sedation may also contribute. Postoperative electrolyte imbalance, especially hypokalaemia or hypophosphataemia, may compound the situation by interfering with muscle function.
Inability to cough. This results mostly from wound pain. However, excessive sedation may also contribute. Postoperative electrolyte imbalance, especially hypokalaemia or hypophosphataemia, may compound the situation by interfering with muscle function.
 Suppression of bronchial mucosal ciliary activity. This results from the use of unhumidified anaesthetic gases.
Suppression of bronchial mucosal ciliary activity. This results from the use of unhumidified anaesthetic gases.
 Antisialagogue drugs. When antisialagogue premedicants have been used, the secretions become more viscid. The dry mucosa itself is more prone to inflammatory reaction. If this occurs, the exudate produced increases the problem still further.
Antisialagogue drugs. When antisialagogue premedicants have been used, the secretions become more viscid. The dry mucosa itself is more prone to inflammatory reaction. If this occurs, the exudate produced increases the problem still further.
 Infection. If pulmonary infection supervenes, impairment of oxygenation may contribute to a lack of cooperation in clearing secretions.
Infection. If pulmonary infection supervenes, impairment of oxygenation may contribute to a lack of cooperation in clearing secretions.
A combination of these factors may result in retention of secretions, leading to areas of visible pulmonary collapse on chest X-ray and an increase in the work of breathing. Ultimately, oxygenation of the blood may become inadequate despite oxygen therapy, or carbon dioxide retention may occur. The sequence of events that culminate in ventilatory failure is shown in Figure 40.4.
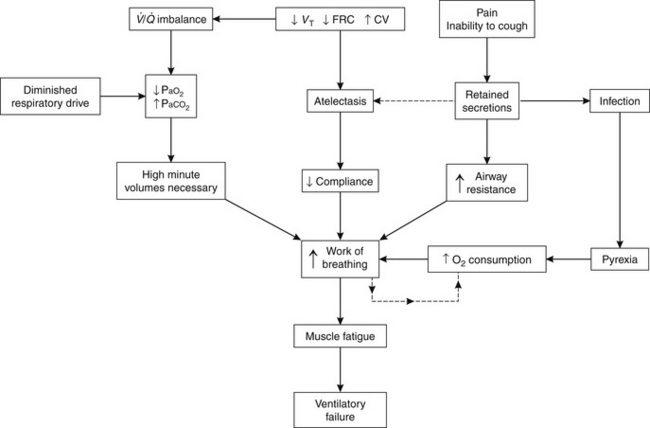
FIGURE 40.4 Diagrammatic representation of events that result in postoperative ventilatory failure. VT, tidal volume; FRC, functional residual capacity; CV, closing volume; ![]() , ventilation/perfusion; PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension.
, ventilation/perfusion; PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension.
Predisposing Factors
 Site of surgery. Pulmonary complications occur more commonly after upper abdominal or thoracic surgery than after lower abdominal operations.
Site of surgery. Pulmonary complications occur more commonly after upper abdominal or thoracic surgery than after lower abdominal operations.
 Pre-existing respiratory disease increases the complication rate. This is particularly so in the presence of concurrent infection or excessive secretions.
Pre-existing respiratory disease increases the complication rate. This is particularly so in the presence of concurrent infection or excessive secretions.
 Smokers have an increased incidence of pulmonary complications compared with non-smokers.
Smokers have an increased incidence of pulmonary complications compared with non-smokers.
 Obesity is associated with a high incidence of pulmonary complications. Obese patients have a low FRC and increased work of breathing postoperatively.
Obesity is associated with a high incidence of pulmonary complications. Obese patients have a low FRC and increased work of breathing postoperatively.
Clinical Findings
Collapse of Lung Units: In patients who develop clinical symptoms, the first signs of atelectasis are usually seen within 24 h of operation. The triad of pyrexia, tachycardia and tachypnoea is often present. Temperature is usually in the range of 38–39 °C. There is often a productive cough. If atelectasis is extensive, the patient is cyanosed. On physical examination, localizing signs are uncommon unless the area of involvement is large. Chest X-ray reveals patchy areas of atelectasis.
Pneumonia: Lobar pneumonia is rarely seen postoperatively. Bronchopneumonia is more common, especially in the elderly. The onset of symptoms is not as rapid as in atelectasis. There is usually fever and associated tachycardia, with an increase in the ventilatory rate. Physical examination usually reveals areas of consolidation, predominantly at the lung bases, which are evident on chest X-ray.
Oxygen Therapy
Hypoxaemia may occur to some degree in any patient during the early recovery period as a result of one or more of the mechanisms described above. Consequently, all patients should receive additional oxygen for the first 10 min after general anaesthesia has been discontinued. Oxygen therapy should be continued for a longer period in the presence of any of the conditions listed in Table 40.6.
TABLE 40.6
Conditions in Which Prolonged Oxygen Therapy is Required After Operation
Hypotension
Ischaemic heart disease
Reduced cardiac output
Anaemia
Obesity
Shivering
Hypothermia
Hyperthermia
Pulmonary oedema
Airway obstruction
After major surgery
Oxygen therapy is particularly beneficial in treating hypoxaemia caused by hypoventilation; PaO2 is substantially increased by a modest increase in FiO2. In contrast, higher concentrations are required in the presence of a shunt fraction in excess of 0.1–0.15 (Fig. 40.5). Known concentrations of oxygen may be administered by a tightly fitting mask supplied with metered flows of air and oxygen via either an anaesthetic breathing system or a CPAP system. In small children, an oxygen tent or headbox may be used. However, oxygen is usually administered by less cumbersome disposable equipment.
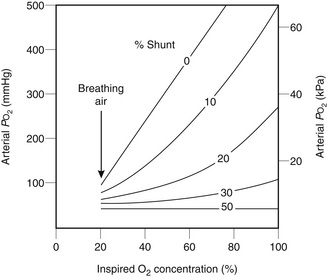
FIGURE 40.5 Response of arterial partial pressure of oxygen (PO2) to increased inspired oxygen concentrations in the presence of various degrees of shunt. Note that, in the presence of shunt, arterial PO2 remains well below the normal value when 100% oxygen is breathed. Nevertheless, useful increases in arterial oxygenation occur with a shunt of up to 30%.
Oxygen Therapy Devices
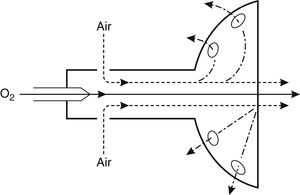
Fixed-Performance Devices: These masks, also termed high air flow oxygen enrichment (HAFOE) devices, provide a constant and predictable inspired oxygen concentration irrespective of the patient’s ventilatory pattern. This is achieved by supplying the mask with oxygen and air at a high total flow rate. Oxygen is passed through a jet which entrains air (Fig. 40.6). The mask is designed in such a way that the total flow rate of gas to the mask exceeds the expected PIFR of most patients who require oxygen therapy. For example, if a jet designed to supply 28% oxygen is supplied with an oxygen flow rate of 4 L min−1, approximately 41 L min−1 of air is entrained and a total flow of 45 L min−1 passes to the patient’s face.
Various types of HAFOE device are available; an example is shown in Figure 40.7. Ventimasks are the most accurate, but a different mask is required for each of the range of oxygen concentrations available. Some manufacturers produce masks in which the jet device can be changed by the user, so that the oxygen concentration may be adjusted as appropriate.
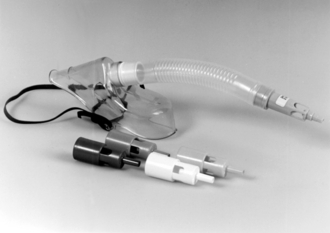
Variable-Performance Devices: All other disposable oxygen masks and nasal cannulae provide an oxygen concentration which varies with the oxygen flow rate and the patient’s ventilatory pattern. Although there is no increase in dead space when nasal cannulae are used, all variable-performance disposable face masks add dead space, the magnitude of which depends on the patient’s pattern of ventilation. Table 40.7 gives an indication of the range of oxygen concentrations achieved with a number of commonly used variable-performance devices; an example is shown in Figure 40.8.
TABLE 40.7
Oxygen Masks, Flow Rates and Approximate Oxygen Concentration Delivered
| Type of mask | Oxygen flow (L min−1) | Oxygen concentration (%) |
| Edinburgh | 1 | 24–29 |
| 2 | 29–36 | |
| 4 | 33–39 | |
| Nasal cannulae | 1 | 25–29 |
| 2 | 29–35 | |
| 4 | 32–39 | |
| Hudson | 2 | 24–38 |
| 4 | 35–45 | |
| 6 | 51–61 | |
| 8 | 57–67 | |
| 10 | 61–73 | |
| MC | 2 | 28–50 |
| 4 | 41–70 | |
| 6 | 53–74 | |
| 8 | 60–77 | |
| 10 | 67–81 |
Oxygen Therapy in the Recovery Ward
The large majority of patients recovering after anaesthesia require only a modest increase in FiO2 to overcome the combined effects of mild hypoventilation, diffusion hypoxia and some degree of increased ![]() scatter. Usually, an inspired concentration of 30% is adequate and this may be achieved in most instances by supplying an oxygen flow rate of 4 L min−1 to any of the variable-performance devices (see Table 40.7). However, in a small proportion of patients, it is necessary to control the FiO2 more strictly.
scatter. Usually, an inspired concentration of 30% is adequate and this may be achieved in most instances by supplying an oxygen flow rate of 4 L min−1 to any of the variable-performance devices (see Table 40.7). However, in a small proportion of patients, it is necessary to control the FiO2 more strictly.
Controlled Oxygen Therapy: This is required in two categories of patient:
 Some patients with chronic bronchitis develop chronic hypercapnia, and ventilatory drive is produced largely by hypoxaemia. If PaO2 increases above the level which stimulates breathing, ventilatory depression may occur. However, these patients may become dangerously hypoxaemic after anaesthesia, and oxygen therapy is required so that adequate oxygenation of the tissues is maintained. The aim of oxygen therapy in these circumstances is to increase arterial oxygen content without an excessive increase in PaO2. This is achieved by a modest increase in FiO2. In the hypoxaemic patient, the relationship between arterial oxygen tension and saturation (and therefore oxygen content) is represented by the steep portion of the oxyhaemoglobin dissociation curve, and a small increase in oxygen tension results in significant increases in saturation and oxygen content (Fig. 40.9). The use of a variable-performance device in these patients is unsatisfactory, as an unacceptably high FiO2 may be delivered. A fixed-performance device delivering 24% oxygen should be used initially, and the response assessed. If the patient remains clinically well, and the PaCO2 does not increase by more than 1–1.5 kPa, 28% oxygen – and subsequently higher concentrations – may be administered if further increases in PaO2 are desirable. The aim is not to achieve ‘normal’ PaO2 or SaO2 but to provide acceptable oxygenation for that patient. Patients whose normal SaO2 is in the low 90s do not need postoperative SaO2 in the high 90s. Most patients with chronic bronchitis do not depend on hypoxaemia for respiratory drive and should not be denied adequate inspired concentrations of oxygen.
Some patients with chronic bronchitis develop chronic hypercapnia, and ventilatory drive is produced largely by hypoxaemia. If PaO2 increases above the level which stimulates breathing, ventilatory depression may occur. However, these patients may become dangerously hypoxaemic after anaesthesia, and oxygen therapy is required so that adequate oxygenation of the tissues is maintained. The aim of oxygen therapy in these circumstances is to increase arterial oxygen content without an excessive increase in PaO2. This is achieved by a modest increase in FiO2. In the hypoxaemic patient, the relationship between arterial oxygen tension and saturation (and therefore oxygen content) is represented by the steep portion of the oxyhaemoglobin dissociation curve, and a small increase in oxygen tension results in significant increases in saturation and oxygen content (Fig. 40.9). The use of a variable-performance device in these patients is unsatisfactory, as an unacceptably high FiO2 may be delivered. A fixed-performance device delivering 24% oxygen should be used initially, and the response assessed. If the patient remains clinically well, and the PaCO2 does not increase by more than 1–1.5 kPa, 28% oxygen – and subsequently higher concentrations – may be administered if further increases in PaO2 are desirable. The aim is not to achieve ‘normal’ PaO2 or SaO2 but to provide acceptable oxygenation for that patient. Patients whose normal SaO2 is in the low 90s do not need postoperative SaO2 in the high 90s. Most patients with chronic bronchitis do not depend on hypoxaemia for respiratory drive and should not be denied adequate inspired concentrations of oxygen.
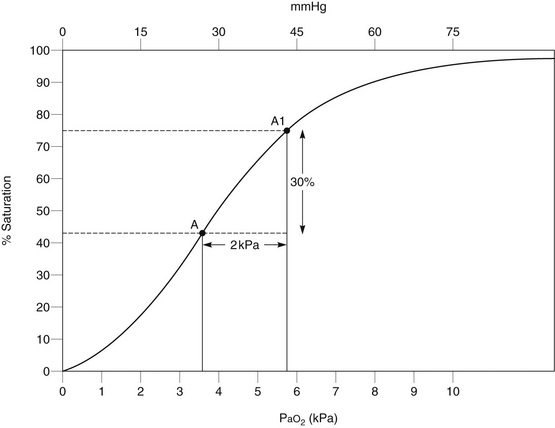
FIGURE 40.9 Effect of controlled oxygen therapy on oxygen saturation in a hypoxaemic chronic bronchitic patient. A small increase in inspired oxygen concentration produces a modest increase in arterial oxygen tension (PaO2) but a substantial increase in arterial oxygen saturation.
 Patients with increased shunt, e.g. those with acute respiratory distress syndrome (ARDS), pulmonary oedema or pulmonary consolidation, may require a high inspired oxygen concentration (see Fig. 40.5), which cannot be guaranteed if a variable-performance device is used. In addition, serial blood gas analysis is normally used to assess improvement or deterioration in their condition. Changes in PaO2 and the degree of shunt may be interpreted accurately only if the FiO2 is known. Thus, controlled oxygen therapy should be employed, using a fixed-performance device which delivers 40% oxygen or more.
Patients with increased shunt, e.g. those with acute respiratory distress syndrome (ARDS), pulmonary oedema or pulmonary consolidation, may require a high inspired oxygen concentration (see Fig. 40.5), which cannot be guaranteed if a variable-performance device is used. In addition, serial blood gas analysis is normally used to assess improvement or deterioration in their condition. Changes in PaO2 and the degree of shunt may be interpreted accurately only if the FiO2 is known. Thus, controlled oxygen therapy should be employed, using a fixed-performance device which delivers 40% oxygen or more.
CARDIOVASCULAR SYSTEM
Hypovolaemia
 after massive blood transfusion, which results in decreased concentrations of clotting factors and reduced platelet numbers
after massive blood transfusion, which results in decreased concentrations of clotting factors and reduced platelet numbers
 pre-existing bleeding tendency, e.g. haemophilia
pre-existing bleeding tendency, e.g. haemophilia
 disseminated intravascular coagulation produced by sepsis, amniotic fluid embolism, etc.
disseminated intravascular coagulation produced by sepsis, amniotic fluid embolism, etc.
A coagulation disorder is frequently associated with prolonged bleeding after venepuncture, oozing from the wound and the development of petechiae or bruises. The investigation and management of coagulation disorders are discussed in Chapter 13.
Hypertension
Arrhythmias
These are common during and immediately after anaesthesia (see also Ch 8). The majority are benign and require no treatment. However, the cause should be sought and its effect on the circulation assessed. Common causes include the following:
 electrolyte or acid–base disturbance
electrolyte or acid–base disturbance
 vagal stimulation, e.g. by tracheal tube or suction catheters
vagal stimulation, e.g. by tracheal tube or suction catheters
Bradycardia may also occur as a result of complete heart block; this may require electrical pacing.
Acute Coronary Syndrome
Several factors which may be detected during preoperative assessment are known to increase the likelihood of perioperative MI (see Ch 17). The most important of these is the time interval between surgery and a previous MI. One extensive study of risk factors which might predict major cardiac complications (including, but not exclusively, MI) showed that preoperative evidence of cardiac failure, arrhythmias (of any type) or aortic stenosis, and age were also associated with a high risk. In addition, there is evidence that pre-existing uncontrolled hypertension is associated with increased risk. These problems are discussed more fully in Chapter 18.
Reduction of Risk
The incidence of perioperative MI may be reduced by the following.
 Identification of patients at risk. Elective surgery should be postponed if possible until at least 3 months after a previous MI.
Identification of patients at risk. Elective surgery should be postponed if possible until at least 3 months after a previous MI.
 Treatment of risk factors. Cardiac failure, hypertension and arrhythmias should be controlled before surgery. If necessary, the operation should be postponed until control is achieved. Coronary artery bypass grafting or aortic valve replacement may be required in patients with severe coronary artery disease or aortic stenosis, respectively, before other major abdominal or thoracic surgery is undertaken.
Treatment of risk factors. Cardiac failure, hypertension and arrhythmias should be controlled before surgery. If necessary, the operation should be postponed until control is achieved. Coronary artery bypass grafting or aortic valve replacement may be required in patients with severe coronary artery disease or aortic stenosis, respectively, before other major abdominal or thoracic surgery is undertaken.
 Avoidance of ischaemia. The anaesthetic technique and postoperative management should ensure adequate oxygenation of the myocardium and should minimize myocardial oxygen demand (see Ch 18).
Avoidance of ischaemia. The anaesthetic technique and postoperative management should ensure adequate oxygenation of the myocardium and should minimize myocardial oxygen demand (see Ch 18).
 Monitoring. ECG must be monitored throughout anaesthesia, including induction, in all patients at risk; the CM5 electrode configuration (see Fig. 16.2) is suitable for detection of ischaemic changes. Arterial pressure should be monitored regularly, and continuously in patients undergoing major surgery.
Monitoring. ECG must be monitored throughout anaesthesia, including induction, in all patients at risk; the CM5 electrode configuration (see Fig. 16.2) is suitable for detection of ischaemic changes. Arterial pressure should be monitored regularly, and continuously in patients undergoing major surgery.
OTHER MAJOR POSTOPERATIVE COMPLICATIONS
The main factors postulated by Virchow as contributing to the formation of venous thrombi are:
Investigations
Prophylaxis
As with most complications, appropriate assessment is a key step in reduction of events. Prophylactic measures to minimize the risk of DVT and PE are detailed in Chapter 13.
Elimination of Stasis: Early ambulation after operation is widely promoted as a method to reduce the incidence of DVT, although the degree of benefit is not clear. Attempts directed at preventing stasis, including physiotherapy, elastic stockings and elevation of the feet, may reduce the incidence of DVT but have not been shown to influence the incidence of pulmonary embolism.
Two methods are currently used for increasing venous return from the lower limbs during surgery.
 Pneumatic compression of the calves. The legs are encased in an envelope of plastic material, which is inflated and deflated rhythmically, thus squeezing the calves intermittently. These devices mimic the effect of the calf muscle pump, promoting venous return, and also stimulate fibrinolytic activity. This technique may be continued postoperatively.
Pneumatic compression of the calves. The legs are encased in an envelope of plastic material, which is inflated and deflated rhythmically, thus squeezing the calves intermittently. These devices mimic the effect of the calf muscle pump, promoting venous return, and also stimulate fibrinolytic activity. This technique may be continued postoperatively.
 Anti-embolism stockings. It is important that these are fitted properly because ill-fitting stockings are at best useless and at worst may cause injury due to local constrictions. There is no good evidence that thigh-length stockings confer benefit over knee-length stockings. They are contraindicated in patients with:
Anti-embolism stockings. It is important that these are fitted properly because ill-fitting stockings are at best useless and at worst may cause injury due to local constrictions. There is no good evidence that thigh-length stockings confer benefit over knee-length stockings. They are contraindicated in patients with:
 suspected or proven peripheral arterial disease
suspected or proven peripheral arterial disease
 peripheral arterial bypass grafting
peripheral arterial bypass grafting
 peripheral neuropathy or other causes of sensory impairment
peripheral neuropathy or other causes of sensory impairment
 any local conditions in which stockings may cause damage, e.g. fragile ‘tissue paper’ skin, dermatitis, gangrene or recent skin graft
any local conditions in which stockings may cause damage, e.g. fragile ‘tissue paper’ skin, dermatitis, gangrene or recent skin graft
 known allergy to material of manufacture
known allergy to material of manufacture
 severe leg oedema or pulmonary oedema from congestive heart failure
severe leg oedema or pulmonary oedema from congestive heart failure
Alteration of Blood Coagulability
Platelet Aggregation: Various drugs which interfere with different aspects of platelet function have been investigated. These include dextran 70, dipyridamole, aspirin and chloroquine. High dose, but not low dose, aspirin reduces the incidence of DVT and PE. Infusion of dextran during and after surgery may reduce the incidence of fatal postoperative pulmonary embolism but its role in the prevention of peripheral venous thrombosis is undetermined.
The Coagulation Mechanism: Low-molecular-weight heparins reduce the rate of DVT and PE, but at the expense of an increase in major bleeding. Pharmacological methods should therefore be used only in patients at risk of major bleeding if the risk of venous thromboembolism (VTE) is felt to outweigh the risk of bleeding. This is described more fully in Chapter 13. Pharmacological prophylaxis is usually with low-molecular-weight heparin. The anaesthetist should be aware of when this is given as it may impact on the use of neuraxial blockade and removal of epidural catheters.
Pulmonary Embolism
Diagnosis
Presenting features: The principal features are circulatory collapse and sudden dyspnoea, often associated with chest pain. If the embolus is large, the pulmonary artery outflow is blocked and sudden death results. If the embolus involves more than 50% of the main pulmonary arteries, or is associated with arterial hypotension or shock, it is termed massive.
Investigations
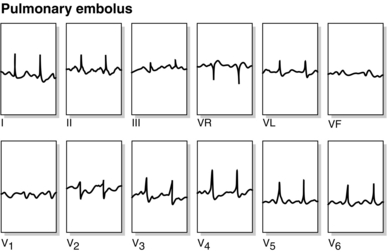
 ECG (Fig. 40.10). This reflects acute right ventricular strain, with features that often include right axis deviation, T-wave inversion in leads V1–V4 and sometimes right bundle branch block. The classic S1–Q3–T3 pattern is less common.
ECG (Fig. 40.10). This reflects acute right ventricular strain, with features that often include right axis deviation, T-wave inversion in leads V1–V4 and sometimes right bundle branch block. The classic S1–Q3–T3 pattern is less common.
 Chest X-ray. This is often unremarkable but may show areas of oligaemia reflecting pulmonary vascular obstruction.
Chest X-ray. This is often unremarkable but may show areas of oligaemia reflecting pulmonary vascular obstruction.
 Arterial blood gases. There is usually hypoxaemia because of ventilation–perfusion imbalance, and hypocapnia resulting from hyperventilation.
Arterial blood gases. There is usually hypoxaemia because of ventilation–perfusion imbalance, and hypocapnia resulting from hyperventilation.
 Pulmonary angiography. This provides a definitive diagnosis of major obstruction in the pulmonary circulation, and is now performed usually by CT scan. This investigation is particularly useful if the patient is critically ill and the diagnosis is in doubt, and is essential if pulmonary embolectomy is planned. However, it requires transfer of the patient to the X-ray department.
Pulmonary angiography. This provides a definitive diagnosis of major obstruction in the pulmonary circulation, and is now performed usually by CT scan. This investigation is particularly useful if the patient is critically ill and the diagnosis is in doubt, and is essential if pulmonary embolectomy is planned. However, it requires transfer of the patient to the X-ray department.
Treatment
Deep Venous Thrombosis: The mainstay of therapy is anticoagulation. Low molecular weight heparin is started at a therapeutic dose. At the same time, oral anticoagulant therapy is started. Warfarin is used most commonly. Heparin is continued until adequate anticoagulation is in place with oral agents (warfarin). Oral anticoagulants are usually continued for at least 3 months depending on the underlying cause.
Pulmonary Embolism: Immediate treatment consists of administration of oxygen in a high concentration and therapeutic doses of low-molecular-weight heparin. Sometimes it is necessary to use additional inotropic support for the circulation. Heparin is continued until adequate anticoagulation is in place with oral agents (warfarin). Oral anticoagulant therapy is started as soon as possible and is continued for several months in consultation with the haematologists.
Postoperative Renal Dysfunction
The kidney is vulnerable to a wide range of drugs (see Ch 10), chemicals and pathological insults. It is particularly susceptible to toxic substances for the following reasons:
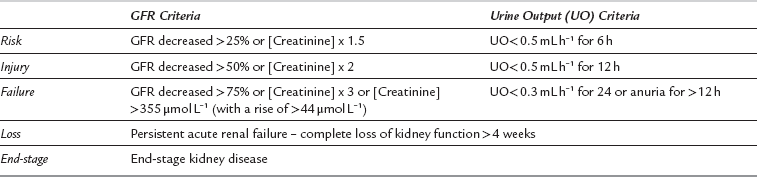
Acute kidney injury (AKI) following surgery is relatively common; almost 25% of elderly patients who died following emergency surgery were found to have evidence of acute kidney injury on admission. There is no single diagnostic criterion for defining acute kidney injury, but a consensus clinical tool is the ‘RIFLE’ criteria (Table 40.8).
Acute kidney injury in the surgical patient is often multifactorial. Key factors are:
Postoperative care should therefore focus on:
 identifying patients at high risk of developing AKI
identifying patients at high risk of developing AKI
 avoiding factors which may increase risk of or from AKI
avoiding factors which may increase risk of or from AKI
 close monitoring and intervention in those patients who develop signs of AKI.
close monitoring and intervention in those patients who develop signs of AKI.
Postoperative Hepatic Dysfunction
There are many causes of postoperative hepatic dysfunction (Table 40.9). Most patients show no evidence of hepatic damage after anaesthesia and surgery. If it occurs, it is usually attributable to one of the causes shown in Table 40.9. However, if other causes are excluded, consideration should be given to the possibility of hepatotoxicity from anaesthetic drugs.
TABLE 40.9
Causes of Postoperative Hepatic Dysfunction
| Increased Bilirubin Load | Hepatocellular Damage | Extrahepatic Biliary Obstruction |
| Blood transfusion Haemolysis and haemolytic disease Abnormalities of bilirubin metabolism |
Pre-existing liver disease Viral hepatitis Sepsis Hypotension/hypoxia Drug-induced hepatitis Congestive heart failure |
Gallstones Ascending cholangitis Pancreatitis Surgical misadventure |
Halothane
 Metabolites of reductive halothane metabolism bind covalently to hepatocyte macromolecules, causing hepatocellular damage.
Metabolites of reductive halothane metabolism bind covalently to hepatocyte macromolecules, causing hepatocellular damage.
 Halothane or more likely its trifluoroacetyl metabolites react with hepatocyte proteins to form antigenic compounds, against which the body mounts an immune response which results in hepatocellular damage. There may be a genetic predisposition to this response.
Halothane or more likely its trifluoroacetyl metabolites react with hepatocyte proteins to form antigenic compounds, against which the body mounts an immune response which results in hepatocellular damage. There may be a genetic predisposition to this response.
OTHER COMPLICATIONS (TABLE 40.10)
Serious complications are described in detail in Chapter 43.
TABLE 40.10
Minor Morbidity Resulting from Anaesthesia
Nausea and vomiting (see Chapter 42)
Related to nature of operation
Females > males
Sore throat
Around 12% of all general anaesthesia
Around 45% of those with tracheal tubes
Hoarseness
Around 50%
Laryngeal granulomata
Headache
Up to 60% of patients
Backache
Discomfort from catheters, drains, nasogastric tubes
Anxiety
Muscle pains
Up to 100% of those who receive succinylcholine
Shivering
Drowsiness
Anorexia
Disorientation
Thrombophlebitis at injection site
Oral trauma
Around 5%
Dental injury
Around 1%; 0.02% require surgical treatment
Corneal abrasions
Around 0.05–0.1%
Nausea and Vomiting
Although often regarded by medical and nursing staff as only a minor complication of anaesthesia and surgery, nausea and vomiting are frequently the cause of great distress to patients. Many studies have been undertaken to investigate nausea and vomiting after anaesthesia and surgery. The incidence varies from 14% to 82%, the wide range resulting partly from differences in design of studies. Many factors contribute to the aetiology of postoperative nausea and vomiting and these are discussed in detail in Chapter 42.
Headache
The reported incidence of severe headache after anaesthesia and surgery ranges from 12% to 35%, but up to 60% of patients complain of some headache. Individuals who are susceptible to headaches caused by stress, etc., are more likely to complain of postoperative headache. Most investigations have failed to identify any single agent as being responsible for postoperative headache after general anaesthesia. Severe postural headache may occur after dural puncture (see p. 529).
Sore Throat
 Trauma during tracheal intubation. Damage to the pharynx and tonsillar fauces may be caused by the laryngoscope blade.
Trauma during tracheal intubation. Damage to the pharynx and tonsillar fauces may be caused by the laryngoscope blade.
 Trauma to the larynx. This is more likely if the tube has been forced through the vocal cords. A poorly stabilized tube causes more frictional damage to the larynx than one which is securely stabilized.
Trauma to the larynx. This is more likely if the tube has been forced through the vocal cords. A poorly stabilized tube causes more frictional damage to the larynx than one which is securely stabilized.
 Trauma to the pharynx. This may occur during passage of a nasogastric tube or insertion of an oropharyngeal or laryngeal mask airway, and is particularly common when a throat pack has been used. Occasionally, the pharynx or upper oesophagus may be perforated during insertion of a nasogastric tube, or during difficult tracheal intubation, and severe pain in the throat is often the first symptom. Sore throat is likely if a nasogastric tube remains in situ during the postoperative period.
Trauma to the pharynx. This may occur during passage of a nasogastric tube or insertion of an oropharyngeal or laryngeal mask airway, and is particularly common when a throat pack has been used. Occasionally, the pharynx or upper oesophagus may be perforated during insertion of a nasogastric tube, or during difficult tracheal intubation, and severe pain in the throat is often the first symptom. Sore throat is likely if a nasogastric tube remains in situ during the postoperative period.
 Other factors. The mucous membranes of the mouth, pharynx and upper airway are sensitive to the effects of unhumidified gases; the drying effect of anaesthetic gases may cause postoperative sore throat. The antisialagogue effect of anticholinergic drugs may also contribute to this symptom.
Other factors. The mucous membranes of the mouth, pharynx and upper airway are sensitive to the effects of unhumidified gases; the drying effect of anaesthetic gases may cause postoperative sore throat. The antisialagogue effect of anticholinergic drugs may also contribute to this symptom.
Muscles
Problems associated with inadequate reversal of neuromuscular blocking drugs are discussed above. The detection and treatment of malignant hyperthermia are described on page 879; it is important to appreciate that this condition may present during recovery.
Succinylcholine Pains
 Age. Succinylcholine pains are unusual in young children and the elderly.
Age. Succinylcholine pains are unusual in young children and the elderly.
 Gender. Women are more susceptible than men. The incidence is reduced during pregnancy.
Gender. Women are more susceptible than men. The incidence is reduced during pregnancy.
 Type of surgery. There is an increased incidence after minor procedures, when early ambulation is likely.
Type of surgery. There is an increased incidence after minor procedures, when early ambulation is likely.
 Physical fitness. The incidence is higher in individuals who are physically fit.
Physical fitness. The incidence is higher in individuals who are physically fit.
 Repeated doses. The incidence is increased if repeated doses of succinylcholine are administered.
Repeated doses. The incidence is increased if repeated doses of succinylcholine are administered.
Association of Anaesthetists of Great Britain and Ireland. Immediate postanaesthetic recovery 2013. London: AAGBI, 2013.
Mecca, R.S. Postanesthesia recovery. In: Kirby R.R., Gravenstein N., Lobato B., Gravenstein N., eds. Clinical anesthesia practice. Philadelphia: WB Saunders; 2002:83–127.
Venkataraman, R., Kellum, J.A. Defining acute renal failure: the RIFLE criteria. J. Intensive Care Med. 2007;22:187–193.