33 Postoperative Cardiac Recovery and Outcomes
Fast-track cardiac surgery care
Anesthetic Techniques
Few trials have compared inhalation agents for FTCA. A single trial comparing sevoflurane and isoflurane in patients undergoing valve surgery was unable to demonstrate reductions in tracheal extubation times.1 Several studies have examined the effectiveness of propofol versus inhalation agent, which demonstrated reductions in myocardial enzyme release (creatine kinase-MB, troponin I) and preservation of myocardial function in patients receiving inhalation agents.2–5 Although this end point is a surrogate for myocardial damage and does not show improved outcome per se, creatine kinase-MB release post-CABG may be associated with poor outcome6 (Box 33-1).
The choice of muscle relaxant in FTCA is important to reduce the incidence of muscle weakness in the cardiac recovery area (CRA), which may delay tracheal extubation.7 Several randomized trials have compared rocuronium (0.5 to 1 mg/kg) versus pancuronium (0.1 mg/kg) and found significant differences in residual paralysis in the ICU.8–11 Two studies found statistically significant delays in the time to extubation in the pancuronium group.9,10 None of the trials used reversal agents, so the use of pancuronium appears acceptable as long as neostigmine or edrophonium is administered to patients with residual neuromuscular weakness.
Several trials have examined the use of different short-acting narcotic agents during FTCA. In these trials, fentanyl, remifentanil, and sufentanil all were found to be efficacious for early tracheal extubation.12–14 The anesthetic drugs and their suggested dosages are listed in Table 33-1.
TABLE 33-1 Suggested Dosages for Fast-Track Cardiac Anesthesia
| Induction |
| Narcotic |
| Fentanyl, 5–10 μg/kg |
| Sufentanil, 1–3 μg/kg |
| Remifentanil infusions of 0.5–1.0 μg/kg/min |
| Muscle relaxant |
| Rocuronium, 0.5–1 mg/kg |
| Vecuronium, 1–1.5 mg/kg |
| Hypnotic |
| Midazolam, 0.05–0.1 mg/kg |
| Propofol, 0.5–1.5 mg/kg |
| Maintenance |
| Narcotic |
| Fentanyl, 1–5 μg/kg |
| Sufentanil, 1–1.5 μg/kg |
| Remifentanil infusions of 0.5–1.0 μg/kg/min |
| Hypnotic |
| Inhalational 0.5–1 MAC |
| Propofol, 50–100 μg/kg/min |
| Transfer to CRA |
| Narcotic |
| Morphine, 0.1–0.2 mg/kg |
| Hypnotic |
| Propofol, 25–75 μg/kg/min |
CRA, cardiac recovery area; MAC, minimum alveolar concentration.
From Mollhoff T, Herregods L, Moerman A, et al: Comparative efficacy and safety of remifentanil and fentanyl in ‘fast track’ coronary artery bypass graft surgery: A randomized, double-blind study. Br J Anaesth 87:718, 2001; Engoren M, Luther G, Fenn-Buderer N: A comparison of fentanyl, sufentanil, and remifentanil for fast-track cardiac anesthesia. Anesth Analg 93:859, 2001; and Cheng DC, Newman MF, Duke P, et al: The efficacy and resource utilization of remifentanil and fentanyl in fast-track coronary artery bypass graft surgery: A prospective randomized, double-blinded controlled, multi-center trial. Anesth Analg 92:1094, 2001.
Evidence Supporting Fast-Track Cardiac Recovery
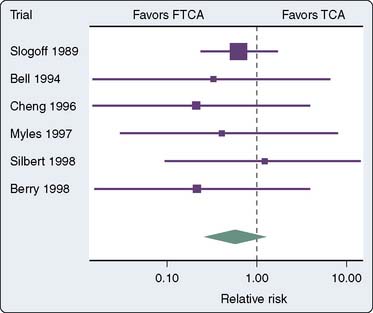
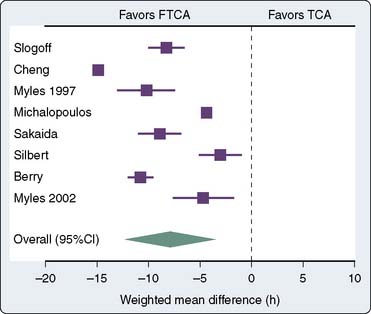
Several randomized trials and one meta-analysis of randomized trials have addressed the question of safety of FTCA.15–21 None of the trials was able to demonstrate differences in outcomes between the fast-track group and the conventional anesthesia group (Figure 33-1). The meta-analysis of randomized trials demonstrated a reduction in the duration of intubation by 8 hours (Figure 33-2) and the ICU length of stay (LOS) by 5 hours in favor of the fast-track group. However, the length of hospital stay was not statistically different.
One concern with FTCA is the potential for an increase in the incidence of adverse events, notably awareness. Awareness in patients undergoing FTCA was systematically investigated in a single trial, a prospective observational study of 617 FTCA patients. The reported incidence rate of explicit intraoperative awareness was 0.3% (2/608).22 This is comparable with the reported incidence during conventional cardiac surgery.23 This suggests that FTCA does not increase the incidence of awareness compared with conventional cardiac surgery.
FTCA appears safe in comparison with conventional high-dose narcotic anesthesia. It reduces the duration of ventilation and ICU LOS considerably without increasing the incidence of awareness or other adverse events.20,21 It appears effective at reducing costs and resource utilization.24 As such, it is becoming the standard of care in many cardiac centers. The usual practice at many institutions is to treat all patients as fast-track candidates with the goal of allowing early tracheal extubation for every patient. However, if complications occur that would prevent early tracheal extubation, then the management strategy is modified accordingly. It has been demonstrated that the risk factors for delayed tracheal extubation (> 10 hours) are increased by age, female sex, postoperative use of intra-aortic balloon pump (IABP), inotropes, bleeding, and atrial arrhythmia. The risk factors for prolonged ICU LOS (> 48 hours) are those of delayed tracheal extubation plus preoperative MI and postoperative renal insufficiency.25 Care should be taken to avoid excess bleeding (antifibrinolytics) and to treat arrhythmias either prophylactically or on occurrence (β-blockers, amiodarone).
Postcardiac Surgical Recovery Models
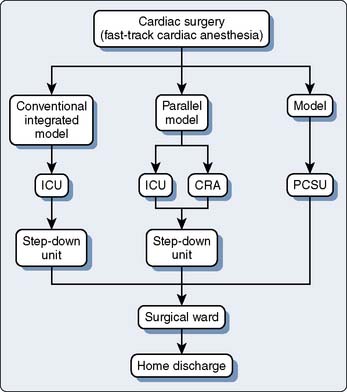
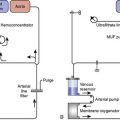
The failure of many randomized FTCA trials to show reductions in resource utilization likely stems from the traditional ICU models used by these centers during the study period. Even when trials were combined in a meta-analysis, the ICU LOS was reduced only by 5 hours despite patients being extubated a mean of 8 hours earlier.21 Typically, patients who are extubated within the first 24 hours of ICU admission are transferred to the ward on postoperative day 1 in the morning or early afternoon. This allows the following daytime cardiac cases to have available ICU beds but prevents patient transfers during nighttime hours. Two models have been proposed to deal with this issue: the parallel model and the integrated model (Figure 33-3). In the parallel model, patients are admitted directly to a CRA, where they are monitored with 1:1 nursing care until tracheal extubation. After this, the level of care is reduced to reflect reduced nursing requirements with ratios of 1:2 or 1:3. Any patients requiring overnight ventilation are transferred to the ICU for continuation of care. The primary drawback with the parallel model is the physical separation of the CRA and ICU, which leads to two separate units and, thus, does not eliminate the requirement to transfer patients. The integrated model overcomes these limitations because all patients are admitted to the same physical area, but postoperative management such as nursing-to-patient ratio is variable based on patient requirements.26–28 Because nursing care accounts for 45% to 50% of ICU costs, reducing the nursing requirements where possible creates the greatest saving. Other cost savings from reductions in arterial blood gases (ABGs) measurement, use of sedative drugs, and ventilator maintenance are small. The goal is a postoperative unit that allows variable levels of monitoring and care based on patient need.28 Furthermore, FTCA has been demonstrated to be a safe and cost-effective practice that decreases resource utilization after patient discharge from the index hospitalization up to 1-year follow-up.29
Initial management of fast-track cardiac anesthesia patients: the first 24 hours
On arrival in the CRA, initial management of cardiac patients consists of ensuring an efficient transfer of care from operating room (OR) staff to CRA staff, while at the same time maintaining stable patient vital signs. The anesthesiologist should relay important clinical parameters to the CRA team. To accomplish this, many centers have devised hand-off sheets to aid in the transfer of care. Initial laboratory work should be sent (Table 33-2). An electrocardiogram should be ordered, but a chest radiograph is required only in certain circumstances (Table 33-3). The patient’s temperature should be recorded, and if low, active rewarming measures should be initiated with the goal of rewarming the patient to 36.5°C. Shivering may be treated with low doses of meperidine (12.5 to 25 mg intravenously). Hyperthermia, however, is common within the first 24 hours after cardiac surgery and may be associated with an increase in neurocognitive dysfunction, possibly a result of hyperthermia exacerbating cardiopulmonary bypass (CPB)–induced neurologic injury30,31 (Box 33-2).
TABLE 33-2 Suggested Initial Laboratory Work in Routine Cases, with Additional Laboratory Work to Be Ordered Where Indicated
| Routine |
| CBC |
| Electrolytes |
| BUN/creatinine |
| aPTT/INR |
| ABGs |
| As indicated |
| Fibrinogen |
| LFTs (AST/ALT) |
| Calcium |
| Magnesium |
| Cardiac enzymes (CK-MB, CK, troponin I) |
ABG, arterial blood gas; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CBC, complete blood count; CK, creatine kinase; CK-MB, creatine kinase myocardium band; INR, international normalized ratio; LFT, liver function test.
TABLE 33-3 Suggested Indications for Ordering a Chest Radiograph
| Respiratory |
| Pao2/Fio2 ratio > 200 |
| Peak pressure > 30 cm H2O |
| Asymmetric air entry |
| Circulatory |
| Uncertainty of pulmonary artery catheter position (poor trace, unable to wedge) |
| Hypotension resistant to treatment |
| Excessive bleeding |
| Gastrointestinal |
| Nasogastric/orogastric tube feeding |
Ventilation Management: Admission to Tracheal Extubation
Ventilatory requirements should be managed with the goal of early tracheal extubation in patients (Table 33-4). ABGs are initially drawn within 1/2 hour after admission and then repeated as needed. Patients should be awake and cooperative, be hemodynamically stable, and have no active bleeding with coagulopathy. Respiratory strength should be assessed by hand grip or head lift to ensure complete reversal of neuromuscular blockade. The patient’s temperature should be more than 36° C, preferably normothermic. When these conditions are met and ABG results are within the reference range, tracheal extubation may take place. ABGs should be drawn about 30 minutes after tracheal extubation to ensure adequate ventilation with maintenance of Pao2 and Paco2. Inability to extubate patients as a result of respiratory failure, hemodynamic instability, or large amounts of mediastinal drainage will necessitate more complex weaning strategies (see Chapter 35). Some patients may arrive after extubation in the OR. Careful attention should be paid to these patients because they may subsequently develop respiratory failure. The patient’s respiratory rate should be monitored every 5 minutes during the first several hours. An ABG should be drawn on admission and 30 minutes later to ensure the patient is not retaining carbon dioxide. If the patient’s respirations become compromised, ventilatory support should be provided. Simple measures such as reminders to breathe may be effective in the narcotized/anesthetized patient. Low doses of naloxone (0.04 mg intravenously) also may be beneficial. Trials of continuous positive airway pressure or bilevel positive airway pressure may provide enough support to allow adequate ventilation. Reintubation should be avoided because it may delay recovery; however, it may become necessary if the earlier mentioned measures fail, resulting in hypoxemia, hypercarbia, and a declining level of consciousness.
TABLE 33-4 Ventilation Management Goals during the Initial Trial of Weaning from Extubation
| Initial ventilation parameters |
| A/C at 10–12 beats/min |
| TV 8 –10 mL/kg |
| PEEP 5 cm H2O |
| Maintain ABGs |
| pH 7.35–7.45 |
| Paco2 35–45 |
| Pao2 > 90 |
| Saturations > 95% |
| Extubation criteria |
| ABGs as above |
| Awake and alert |
| Hemodynamically stable |
| No active bleeding (< 400 mL/2 hr) |
| Temperature > 36°C |
| Return of muscle strength (> 5 seconds, head lift/strong hand grip) |
ABG, arterial blood gas; A/C, assist-controlled ventilation; PEEP, positive end-expiratory pressure; TV, tidal volume.
Regulation of Hemoglobin Level
Anemia is common during and after cardiac surgery as a result of both dilutional changes and bleeding. Although a hemoglobin transfusion threshold of 10 g% was once common, increasing evidence suggests that a threshold of 7 g% is reasonably safe.32 However, in the post-CPB period, patients with incomplete revascularization or with poor target vessels may require a higher transfusion threshold.32 As a result, blood transfusions should be individualized for each patient but certainly should be used to maintain a minimal hemoglobin level of 7 g%.
Management of Bleeding
Chest tube drainage should be checked every 15 minutes after ICU admission to assess a patient’s coagulation status. Although blood loss is commonly divided into two types, surgical or medical, determining the cause of bleeding is often difficult. When bleeding exceeds 400 mL/hr during the first hour, 200 mL/hr for each of the first 2 hours, or 100 mL/hr over the first 4 hours, returning to the OR for chest reexploration should be considered. The clinical situation must be individualized for each patient, however, and in the face of a known coagulopathy, more liberal blood loss before chest re-exploration may be acceptable. There are numerous medical causes for bleeding after cardiac surgery. Platelet dysfunction after cardiac surgery is common. The CPB circuit itself leads to contact activation and degranulation of platelets, resulting in their dysfunction. Residual heparinization is common postcardiac surgery and frequently occurs when either heparinized pump blood is transfused after CPB or insufficient protamine is administered. Fibrinolysis is also common after CPB, predominantly caused by a host of activated inflammatory and coagulation pathways. Coagulation factors may decrease from activation at air–blood interfaces or from dilution with the CPB pump-priming solution. Hypothermia also may aggravate the coagulation cascade and lead to further bleeding. Conventional coagulation tests are helpful to identify the coagulation abnormality contributing to the bleeding. Common laboratory testing includes activated partial thromboplastin time, international normalized ratio (INR), platelet count, fibrinogen level, and d-dimers. Unfortunately, most conventional measures take 20 to 40 minutes before results are available. This has led to the development of new methods to help guide treatment. These bedside point-of-care tests are providing more rapid, clinically relevant results compared with laboratory testing. The use of point-of-care testing such as thromboelastography has been demonstrated to reduce transfusion requirements without increasing blood loss and is commonly used, especially following difficult cardiac cases33,34 (see Chapters 17 and 28 to 31).
Initial medical treatment of excessive blood loss consists of 50 to 100 mg intravenous protamine to ensure complete heparin reversal. This may need to be repeated if heparinized CPB pump blood has been administered after protamine reversal. Although the reinfusion of chest tube blood was common to avoid exposure to donor packed red blood cells, it is no longer used routinely in practice because this blood is known to contain activated coagulation and inflammatory mediators that may predispose to an increased risk for infection.35
Fresh-frozen plasma usually is given in the setting of an increased INR (> 1.5). Platelet levels of less than 100,000/mm3 may warrant platelet transfusion, but caution must be exercised when considering this course. Platelet transfusions carry the greatest risk for transfusion-related complications of any blood component, typically sepsis from bacterial contamination. Platelets should be used only when platelet counts are low or the patient has a known platelet dysfunction, secondary to the use of acetylsalicylic acid, glycoprotein IIb/IIIa inhibitors, or clopidogrel.36 Certain physical measures should be instituted, including warming of the hypothermic patient. The benefit of positive end-expiratory pressure on postoperative bleeding is equivocal and likely has little benefit in the face of surgical bleeding or in patients who are coagulopathic.37,38 The use of antifibrinolytics after cardiac surgery is likely of little benefit because several randomized trials were unable to demonstrate the efficacy of antifibrinolytics used after surgery39,40 (Box 33-3).
BOX 33-3 Management of the Bleeding Patient
Factor VIIa recently has become available and initially was introduced for treatment of hemophiliacs who present with bleeding. It was introduced in cardiac surgery as rescue therapy in patients with uncontrolled bleeding, usually in the presence of normal coagulation results and no surgical evidence of bleeding.41 Although frequently used in the OR before returning to the ICU, it is still given frequently in the ICU setting. Doses initially were in the range of 75 to 100 μg/kg, but concern over thrombotic complications has led to dosage reductions ranging down to as little as 17 μg/kg.41–43
Electrolyte Management
Hypokalemia is common after cardiac surgery, especially if diuretics were given intraoperatively. Hypokalemia contributes to increased automaticity and may lead to ventricular arrhythmias, ventricular tachycardia, or ventricular fibrillation. Treatment consists of potassium infusions (20 mEq potassium in 50 mL D5W infused over 1 hour) until the potassium exceeds 3.5 mEq/mL. In patients with frequent premature ventricular contractions caused by increased automaticity, 5.0 mEq/mL potassium may be desirable. Hypomagnesemia contributes to ventricular pre-excitation and may contribute to atrial fibrillation (AF). It is common in malnourished and sick patients, a frequent occurrence in the cardiac surgical setting. Management consists of intermittent boluses of magnesium—1 to 2 g over 15 minutes. Hypocalcemia also is frequent during cardiac surgery and may reduce cardiac contractility. Intermittent boluses of calcium chloride or calcium gluconate (1 g) may be required (Table 33-5).
TABLE 33-5 Common Electrolyte Abnormalities and Possible Treatment Options
| Hypokalemia (K+ < 3.5 mmol/L) |
| SSx: muscle weakness, ST-segment depression, “u” wave, T-wave flat, ventricular pre-excitation |
| Rx: IV KCl at 10–20 mEq/hr via central catheter |
| Hyperkalemia (K+ > 5.2 mmol/L) |
| SSx: muscle weakness, peaked T wave, loss of P wave, prolonged PR/QRS |
| Rx: CaCl2 1 g, insulin/glucose, HCO3–, diuretics, hyperventilation, dialysis |
| Hypocalcemia (ionized Ca2+ < 1.1 mmol/L) |
| SSx: hypotension, heart failure, prolonged QT interval |
| Rx: CaCl2 or Ca gluconate |
| Hypercalcemia (Ionized Ca2+ > 1.3 mmol/L) |
| SSx: altered mental state, coma, ileus |
| Rx: dialysis, diuretics, mithramycin, calcitonin |
| Hypermagnesemia (Mg2+ > 0.7 mmol/L) |
| SSx: weakness, absent reflexes |
| Rx: stop Mg infusion, diuresis |
| Hypomagnesemia (Mg2+ < 0.5 mmol/L) |
| SSx: arrhythmia, prolonged PR and QT intervals |
| Rx: Mg infusion 1 to 2 g |
IV, intravenous; Rx, treatment; SSx, signs and symptoms.
Glucose Management
Diabetes is a common comorbidity (up to 30%) and is a known risk factor for adverse outcome in patients presenting for cardiac surgery.44–46 Hyperglycemia itself is common during CPB. The risk factors for hyperglycemia include diabetes, administration of steroids before CPB, volume of glucose-containing solutions administered, and use of epinephrine infusions.47 Poor perioperative glucose control is associated with increases in mortality and morbidity, including an increased risk for infection and a prolonged duration of ventilation.48–52 In a large prospective, randomized, controlled trial of tight glucose control (blood glucose levels of 4.1 to 6.5 mmol/L) during postoperative ICU stay, reductions in mortality were shown by the authors compared with more liberal glucose control (blood glucose levels of 12 mmol/L).52 This trial enrolled both diabetic and nondiabetic hyperglycemic patients who underwent cardiothoracic surgery and demonstrated that tight management of glucose is beneficial in the CRA. However, another recent multicenter trial, as well as a meta-analysis of tight glucose control in the ICU, suggest an increase in harm, likely related to an increase in episodic hypoglycemia.53,54 Therefore, it may be prudent to accept a more liberal blood sugar level (< 10.0 mmol/L) to reduce hypoglycemic episodes.
Pain Control
Pain control after cardiac surgery has become a concern as narcotic doses have been reduced to facilitate fast-track protocols. Intravenous morphine is still the mainstay of treatment for postcardiac surgery patients. The most common approach is patient-demanded, nurse-delivered intravenous morphine, and this treatment remains popular because of 1:1 to 1:2 nursing typically provided during cardiac recovery. However, with a change to more flexible nurse coverage and, therefore, higher nurse-to-patient ratios, patient-controlled analgesia morphine is becoming increasingly popular. Several studies have examined patient-controlled analgesia morphine use in patients after cardiac surgery.55–61 A meta-analysis looking at patient-controlled analgesia morphine for postoperative pain showed small incremental benefits. However, young patients, those who use narcotics before surgery or are transferred to a regular ward within 24 hours, may benefit from patient-controlled analgesia for pain management62 (Table 33-6; see Chapter 38).
| Patient-Controlled Analgesia |
| May be of benefit in a stepdown unit |
| Reduced 24-hour morphine consumption demonstrated in 2 of 7 randomized trials |
| Intrathecal Morphine |
| Doses studied: 500 μg to 4 mg |
| May be of benefit to reduce IV morphine use |
| May be of benefit in reducing VAS pain scores |
| *Potential for respiratory depression |
| Ideal dosing not ascertained; range, 250–400 μg |
| Thoracic Epidurals |
| Common dosages from literature |
| Ropivacaine 1% with 5 μg/mL fentanyl at 3–5 mL/hr |
| Bupivacaine 0.5% with 25 μg/mL morphine at 3–10 mL/hr |
| Bupivacaine 0.5% to 0.75% at 2–5 mL/hr |
| Reduced pain scores |
| Shorter duration of intubation |
| *Risk for epidural hematoma difficult to quantify |
| Nonsteroidal Anti-inflammatory Drugs |
| Common dosages from literature |
| Indomethacin 50–100 mg PR BID |
| Diclofenac 50–75 mg PO/PR q8h |
| Ketorolac 10–30 mg IM/IV q8h |
| Reduces narcotic utilization |
| Many different drugs studied; difficult to determine superiority of a given agent |
| *May increase serious adverse events (one trial using cyclooxygenase-2–specific inhibitors) |
BID, twice daily; IM, intramuscular; IV, intravenous; PO, orally; PR, rectally; VAS, visual analogue scale.
Regional Analgesia Techniques
Intrathecal Morphine
ITM has been investigated in randomized trials as an adjuvant for pain control in cardiac surgical patients, with doses ranging from 500 μg to 4 mg.63–72 A meta-analysis of 17 randomized, controlled trials compared ITM with standard treatment. There was no difference in mortality, MI, or time to extubation. There were modest reductions in morphine use and pain scores, whereas the incidence of pruritus was increased.
Thoracic Epidural Analgesia
Thoracic epidural analgesia has gained some popularity as a method of providing intraoperative and postoperative pain control in cardiac surgery (see Table 33-6). The best evidence for benefit comes from a meta-analysis of 15 randomized, controlled trials.73 Thoracic epidural analgesia did not significantly affect the incidence of mortality or MI. It did significantly reduce arrhythmias, pulmonary complications, and time to tracheal extubation. All the randomized trials were performed in CABG patients. There were no reported complications as a result of epidural insertion, specifically epidural hematoma; however, all trials were inadequately powered to detect this complication. Attempts have been made to calculate the risk for epidural hematoma using available published series, with estimates of maximum risk ranging from 1:1000 to 1:3500 depending on the confidence limits chosen (99% vs. 95%).74 A large retrospective review reported no epidural hematomas in 727 patients undergoing cardiac surgery with CPB receiving thoracic epidural analgesia the day of surgery (on entrance into the OR).75 At least 1 hour elapsed between the insertion of the epidural catheter and heparin administration. There were 9 failed catheter insertions and 4 failed analgesia blocks with 11 bloody taps in this study.75 Unfortunately, the population of cardiac surgical patients is increasingly on antiplatelet medication, such as clopidogrel or prasugrel, which increase the risk for epidural hematoma.76 The risk for epidural hematoma and the potential delay of surgery from a bloody tap have limited the widespread adoption of thoracic epidural analgesia for cardiac surgery, especially in the United States (see Chapter 38).
Nonsteroidal Anti-inflammatory Drugs
The use of NSAIDs has gained popularity in a multimodal approach, allowing reductions in both pain levels and narcotic side effects (see Table 33-6). The conventional NSAIDs, which nonselectively block the cyclooxygenase-2 (COX-2) isoenzyme, reduce inflammation, fever, and pain, and also block the COX-1 isoenzyme resulting in the side effects of gastrointestinal toxicity and platelet dysfunction.77 Numerous randomized trials have examined the benefit of NSAID use for postoperative pain control.61,78–88 In addition, a meta-analysis looking at the benefit of NSAIDs in the setting of cardiac and thoracic surgery demonstrated reductions in narcotic consumption in patients given NSAIDs.89 Most patients were younger than 70 years and had no coexisting renal dysfunction. The NSAIDs used in this meta-analysis were nonselective COX inhibitors. Several trials have suggested increased adverse events, especially in patients with coronary artery disease, who receive the COX-2 selective NSAIDs both in the perioperative cardiac setting and in ambulatory patients. For this reason, COX-2 selective NSAIDs are no longer used in most cardiac centers.90 Therefore, although NSAIDs have theoretic side effects, the benefit in reduced narcotic consumption and improved visual analogue scale pain scores is well demonstrated; many centers continue to use nonselective NSAIDs as analgesia adjuvants in cardiac surgery.91 However, NSAIDs should be avoided in patients with renal insufficiency, a history of gastritis, or peptic ulcer disease. Adjuvant ranitidine treatment should be considered to prevent gastric irritation.
Medications for Risk Reduction after Coronary Artery Bypass Graft Surgery
CABG surgery itself reduces the risk for mortality and angina recurrence, but several medical management issues may help maintain the long-term benefit after CABG surgery. Specifically, the use of aspirin, β-blockers, and lipid-lowering agents has been demonstrated to prolong survival or reduce graft restenosis, or both (Box 33-4).
BOX 33-4 Medications for Cardiac Risk Reduction After Coronary Artery Bypass Grafting Surgery
Aspirin
Several studies have demonstrated the efficacy of aspirin (acetylsalicylic acid) use on graft patency and reductions in MI and mortality after CABG surgery.92–96 A large observational study showed a reduction in mortality of nearly 3% and a reduction in MI rate of 48% with the early use of aspirin after surgery (within 48 hours).96 Acetylsalicylic acid dosages have ranged from 100 mg once daily to 325 mg three times daily orally or by suppository up to 48 hours after ICU admission. There was no additional benefit from the use of aspirin before surgery.97 The beneficial effect on saphenous vein graft patency appears to be lost after 1 year, with prolonged use of aspirin having no further benefit.98 However, because aspirin, in dosages of 75 to 325 mg/day, reduces mortality and morbidity in patients at risk for cardiovascular disease, its continued long-term use is clearly warranted.99 Ticlopidine, clopidogrel, or prasugrel may be suitable alternatives in patients who are allergic to aspirin. Clopidogrel, through reductions in all-cause mortality, stroke, and MI, may be superior to acetylsalicylic acid in patients who return with recurrent ischemic events after cardiac surgery.100 Ticlopidine, however, should be used with caution because it may cause neutropenia (necessitating white blood cell counts to be monitored during initial use). Clopidogrel has a lower incidence of adverse reactions compared with ticlopidine and is, therefore, preferred as a second-line agent when aspirin is contraindicated.
β-Blockers
The use of β-blockers in patients after CABG surgery has not been shown to improve mortality.101 They also have failed to reduce myocardial ischemia rate, unlike the angiotensin-converting enzyme inhibitors, which have, in a single study, demonstrated efficacy at reducing ischemic events after CABG.102 However, patients who received β-blockers after perioperative MI had reductions in mortality at 1 year.103 Patients with a previous history of MI should be continued on β-blocker therapy.
Statins
Statin use in the cardiac surgical population has focused on its ability to prolong the patency of SVG grafts and, more recently, its possible role in reducing the incidence of AF. Statin use has been shown to reduce the amount and speed of atherosclerotic plaque formation within saphenous vein grafts. This resulted in reductions in the need for subsequent revascularization in one trial.104,105 It was recently suggested that statin use before surgery may reduce the incidence of AF in the postoperative period.105–107
Anticoagulation for Valve Surgery
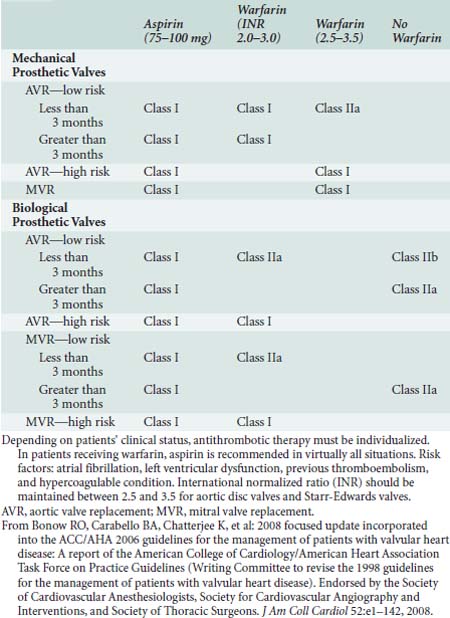
Anticoagulation should be started in the early postoperative period for patients who have undergone valve replacement, with either a mechanical or bioprosthesis and also should be considered when AF complicates the postoperative course.108 The recommended prophylactic regimens for patients with both mechanical and bioprosthetic heart valves are shown in Table 33-7. AF management is discussed later.
Management of complications
Complications are frequent after cardiac surgery. Although many are short-lived, some complications, like stroke, are long-term catastrophic events that seriously affect a patient’s functional status (see Chapters 36 and 37). The incidence and predisposing risk factors are well studied for many of the complications (Table 33-8). Many of these complications have specific management issues, which may improve recovery after surgery (Box 33-5).
| Complication | Incidence Rate | Risk Factors |
|---|---|---|
| Stroke | 2–4% | Age |
| Previous stroke/TIA | ||
| PVD | ||
| Diabetes | ||
| Unstable angina | ||
| Delirium | 8–15% | Age |
| Previous stroke | ||
| Duration of surgery | ||
| Duration of aortic cross-clamp | ||
| AF | ||
| Blood transfusion | ||
| Atrial fibrillation | Up to 35% | Age |
| Male sex | ||
| Previous AF | ||
| Mitral valve surgery | ||
| Previous CHF | ||
| Renal failure | 1% | Low postoperative CO |
| Repeat cardiac surgery | ||
| Valve surgery | ||
| Age | ||
| Diabetes |
AF, atrial fibrillation; CHF, congestive heart failure; CO, cardiac output; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Stroke
Stroke after cardiac surgery occurs in 2% to 4% of patients and carries a very high 1-year mortality rate of 15% to 30%.45,109–111 Known risk factors for stroke include age, diabetes, previous history of stroke or transient ischemic attack, peripheral vascular disease, and unstable angina.45,110 The most common cause is emboli sheared off the aorta during aortic manipulation (proximal anastomosis of the vein grafts, clamping and unclamping of the aorta). However, hemorrhagic and watershed infarcts do occur secondary to the use of large doses of heparin and the frequent occurrence of hypotension during surgery, respectively. AF in the postoperative period also appears to be an important cause of strokes in cardiac patients.112 Resource utilization is increased in patients who sustained a stroke, with prolonged ICU and hospital stays.110 There are numerous proposed methods of preventing neurologic injury during the intraoperative period including epiaortic scanning, alpha-stat pH management during CABG with CPB, and OPCAB surgery with no-touch surgical techniques.113–117 Prevention of postoperative neurologic injury may be possible with the aggressive treatment of AF (antiarrhythmics, anticoagulants, early cardioversion) to prevent thromboembolism (see Atrial Fibrillation section later in this chapter). Patients who sustain an intraoperative stroke should be managed with the goal of preventing further brain injury. Hyperglycemia should be avoided because it is associated with poor outcome in brain injury patients.118–120 Hyperthermia also is known to exacerbate brain injury and should be avoided.121 Hemoglobin concentrations certainly should be maintained above 7 g%. Whether there is benefit to maintaining hemoglobin levels greater than 10 g% is uncertain, but this may be prudent in patients with perioperative stroke.
Delirium
Delirium is defined generally as an acute transient neurologic condition with impairment of cognitive function, attention abnormalities, and altered psychomotor activity. It often includes a disorder with the sleep/wake cycle. It is fairly common after cardiac surgery, with a prevalence rate of 8% to 15%.122,123 Risk factors associated with delirium include age, previous history of stroke, duration of surgery, duration of aortic cross-clamp, AF, and blood transfusion.122–125 Interestingly, delirium is self-limited and does not adversely affect patient outcome or hospital LOS.124,125 Treatment is supportive, involving close observation of patients and sedatives (midazolam, diazepam) or antipsychotics (haloperidol) as required.
Atrial Fibrillation
AF after cardiac surgery is common and occurs in up to 35% of patients.126 Although the cause of AF is not completely understood, it is associated with increases in mortality, stroke, and prolonged hospital stay.110,112,127 Known risk factors include age, male sex, previous AF, mitral valve surgery, and a history of congestive heart failure.110,128 Prevention and treatment of AF can be achieved effectively with amiodarone, sotalol, magnesium, or β-blockers.129 Biatrial pacing also may be effective prophylaxis.129,130 Management of AF consists of rate control with conversion to sinus rhythm or anticoagulation. Several studies conducted to determine which strategy was superior were unable to find a difference between treatment strategies (AFFIRM [Atrial Fibrillation Follow-up Investigation of Rhythm Management] and RACE [Rate Control Versus Electrical Cardioversion] trials).131–133
Rate control may be achieved with a β-blocker or a calcium channel blocker. Digoxin also may be effective, but it is difficult to achieve therapeutic levels quickly. An observational review of the AFFIRM trial suggests that β-blockers are superior to either calcium channel blockers or digoxin for rate control in AF.134 Conversion to sinus rhythm in the stable patient may be achieved with amiodarone, sotalol, or procainamide. Amiodarone is more commonly used in acute management of postoperative AF (150 to 300 mg intravenously) than other antiarrhythmics, particularly in patients with compromised ventricular function, because it causes little cardiac depression. Finally, persistent AF over 48 to 72 hours requires thromboembolic prophylaxis. Warfarin is recommended for patients at high risk of thromboembolic complications. Patients at high risk include those with previous stroke or TIA or thromboembolism. Moderate risk factors include congestive heart failure, ejection fraction less than 35%, diabetes mellitus, or age 75 or older. Patients with one high-risk factor or two or more moderate-risk factors should be treated with warfarin with an INR between 2 and 3 considered therapeutic.135 In a meta-analysis of randomized trials, warfarin reduced the risk for stroke by 67% and 32% compared with placebo or acetylsalicylic acid, respectively. However, the incidence of hemorrhagic complications also was greater with warfarin (0.3% absolute risk rate increase per year).136 Aspirin, 325 mg once daily, may be sufficient for patients with a low thromboembolic risk135,137,138 (Table 33-9).
TABLE 33-9 Suggested Atrial Fibrillation Thromboembolic Prophylaxis
| Risk Category | Recommended Therapy |
|---|---|
| No risk factors | Aspirin, 81–325 mg daily |
| One moderate-risk factor | Aspirin, 81–325 mg daily or warfarin (INR 2.0–3.0, target 2.5) |
| Any high-risk factor or more than one moderate-risk factor | Warfarin (INR 2.0–3.0, target 2.5) |
INR, international normalized ratio.
From Estes NA 3rd, Halperin JL, Calkins H, et al: ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): Developed in collaboration with the Heart Rhythm Society. Circulation 117:1101–1120, 2008.
Left Ventricular Dysfunction
Patients with poor left ventricular (LV) function commonly require inotropes or mechanical support after cardiac surgery. Preoperative factors that predict inotrope use in patients undergoing cardiac surgery include age, underlying LV dysfunction, and female sex.139,140 The significance of inotrope use on postoperative outcome is uncertain because some centers routinely use these drugs after CPB.139 Although pulmonary artery (PA) catheters are useful for monitoring trends in cardiac function, transesophageal echocardiography (TEE) provides more detailed information for diagnosing the cause of acute hypotensive episodes and cardiac function. TEE is used commonly in the ICU to assess patients after cardiac surgery and has demonstrated efficacy for diagnosing cardiac tamponade, cardiac ischemia, and valve dysfunction, resulting in improvements in the postoperative course for these patients.141–144 TEE also provides information on the filling volumes after CPB.145–147
Patients who have an unstable intraoperative course should have PA filling pressures correlated to TEE findings and the results then passed to the recovery unit to allow for optimal initial management in the recovery unit. If the patient remains unstable in the ICU, then TEE is used and cardiac function is reassessed. When hypovolemia is thought to be the underlying cause of hypotension/low cardiac output, then colloids (Pentaspan, Voluven) initially may be used to optimize filling, because third spacing of fluids is common after CPB. The intravascular hypovolemia is best treated with the use of small intermittent boluses of colloid with continuous reassessment of central venous pressure (CVP), PA pressures, systemic pressures, or LV end-diastolic area.145
If ventricular dysfunction is the main cause of hypotension/low cardiac output state, then inotropes and vasopressors should be added (see Chapter 34). Unfortunately, few articles have been published examining the superiority of one inotrope over another. Epinephrine (0.02 to 0.04 μg/kg/min) or dopamine (3 to 5 μg/kg/min) is commonly used to support patients coming off CPB and is usually continued into the ICU. If systolic pressure remains low, then the epinephrine infusion is usually increased to allow for greater α-receptor action (vasoconstriction). For patients with a low cardiac output or poor myocardial function on TEE, milrinone commonly is used (with or without a full loading dose). Milrinone has the advantage of bypassing β-adrenergic receptors, which are downregulated after cardiac surgery.148 Phosphodiesterase inhibitors appear to improve myocardial performance even with the concomitant use of epinephrine.149 If despite this blood pressure remains low, then a vasopressin infusion may be started with doses ranging from 1 to 10 U/hr. When volume and medical strategies are insufficient, especially in the presence of ischemic heart disease, mechanical support is added (see Chapters 27 and 32). IABPs are used in approximately 3% of cardiac surgical patients. The IABP can be placed before surgery in patients with unstable angina unresponsive to medical treatment, intraoperatively in high-risk patients (redo-sternotomy with poor LV function), in patients who fail to wean from CPB, or in patients on maximal inotropic support after CPB.
For patients who do not successfully wean from CPB, one retrospective study found 85% success in weaning with the institution of an IABP; however, the overall mortality rate was 35%.150 Several studies have looked at the timing of IABP insertion and found reductions in mortality in patients who received the IABP before initiation of CPB.151–154 Complications from the use of IABP are numerous and include wound site infections, leg ischemia, and renal dysfunction. Several retrospective reviews have examined the outcomes after IABP insertion; again, mortality rates were high in patients who received an IABP in the OR or recovery unit (35%).155,156 Finally, in patients who require long-term inotropic support and use of an IABP, angiotensin-converting enzyme inhibitors may help to reduce mortality. One small trial investigated the use of captopril in patients who required more than two inotropes and/or IABP placement and showed reductions in mortality in patients randomized to receive captopril.157
Right Ventricular Dysfunction
RV dysfunction presents with features of peripheral edema, hypotension, confusion, and abdominal pain or cramping. Liver function tests may be elevated, including the INR, aspartate aminotransferase, and alanine aminotransferase. Thus, the differential diagnosis frequently includes renal failure, sepsis, bowel ischemia, and liver failure.158,159
In patients with invasive monitoring, assessment of RV function may be done indirectly through measurement of CVP, cardiac output, and PA pressures. Unless there is direct myocardial dysfunction, PA pressures are almost always increased. Echocardiography also is useful in assessing patients with suspected RV failure. RV volume overload presents with an enlarged RV and an associated small and underfilled LV (because of both poor RV output and ventricular interdependence). Tricuspid regurgitation is also frequently present. If the RV also is pressure overloaded, the interventricular septum shifts to the left and the LV is said to have a D shape. Tricuspid annular plane systolic excursion (TAPSE) may be helpful in measuring the degree of RV failure.158–162
Management of RV failure consists of reducing afterload, increasing systemic pressures to prevent RV ischemia, and ensuring adequate RV filling. Volume, although often useful in LV failure and in cases of RV failure associated with normal PA pressures, often is detrimental in high-pressure RV failure; caution must be exercised to prevent overloading patients. Inotropes, often in combination with afterload reduction, will increase both blood pressure and cardiac output. Norepinephrine, phenylephrine, and vasopressin may all help to increase systemic pressures and, thus, reduce RV ischemia. Afterload reduction with agents specific to the pulmonary vascular tree also may be beneficial. Nitric oxide and inhaled prostaglandin may be selective for pulmonary vasodilation. Milrinone (0.125 to 0.5 μg/kg/hr) or sildenafil (up to 25 mg orally three times a day) also may be of benefit to reduce pulmonary vascular resistance and improve cardiac output (see Chapters 24, 27, 32, and 34).
Renal Insufficiency
Renal failure in the postoperative period is rare, occurring in approximately 1% of patients after cardiac surgery. Not surprisingly, when it does occur, it prolongs CRA LOS and hospital LOS and increases mortality.46,163 Unfortunately, no clear definition exists as to what constitutes renal impairment or failure after CPB. Although the need for dialysis is a straightforward and easily measured outcome, unfortunately, it ignores patients who have reductions in creatinine clearance but do not require dialysis.46,163 Change in calculated creatinine clearance, which is predictive of need for dialysis, prolonged hospitalization, and mortality may be a more suitable outcome measurement of renal failure.164 There are several risk factors for postoperative renal failure, including postoperative low cardiac output, repeat cardiac surgery, valve surgery, age older than 65, and diabetes.46,163,165 Management of these patients consists of supportive treatment, determining the primary cause, and then directing specific treatment as needed. Supportive treatment consists of ensuring adequate cardiac output, perfusion pressure, and intravascular volume. The cause of renal failure is broadly defined as prerenal, renal, or postrenal failure. Prerenal causes commonly are related to poor cardiac output or low systemic pressure and may be associated with the use of angiotensin-converting enzyme inhibitors and NSAIDs. Renal causes include acute tubular necrosis from an ischemic insult or interstitial nephritis caused by a host of medications including NSAIDs and antibiotics.
Although uncommon in the face of bladder catheterization, the potential for postrenal obstruction is possible. Management of renal failure usually requires correction of the underlying problem, which may include improving renal blood flow (volume, inotropes) or discontinuing the offending agent (NSAIDs, antibiotic). To date, there is no specific treatment to prevent acute tubular necrosis. Although dopamine and diuretics were once both thought to be renoprotective, neither has demonstrated efficacy to prevent renal failure.166 Fenoldopam, a D1-receptor agonist, may improve renal function in cardiac surgical patients.167 There is a suggestion that diuretics may be potentially harmful in patients experiencing development of renal failure.168 If patients do require dialysis, continuous dialysis may be better than intermittent dialysis.169 N-acetylcysteine has demonstrated efficacy in preventing further renal failure from radiocontrast agent in patients with chronic renal insufficiency.170 This, however, does not appear to translate to cardiac surgery because several meta-analyses of randomized trials have shown little benefit to N-acetylcysteine.170–172
Postoperative Outcomes
Treatment Options for Coronary Artery Disease
Until the advent of CABG surgery, medical management was the only treatment option for patients with coronary artery disease. Today, treatment can be broadly categorized as medical or invasive management, with invasive management divided geographically into interventions performed in the cardiac catheterization laboratory or in the OR. Catheterization procedures commonly performed include balloon angioplasty, cardiac stenting, and drug-eluting stents, which release drugs capable of preventing restenosis (see Chapter 3). OR procedures include conventional CABG (with the use of the CPB machine) and OPCAB (without the use of the CPB machine). OPCAB surgery may include full sternotomy, thoracotomy (minimally invasive direct coronary artery bypass), or robotically assisted thoracotomy surgery. With the ever-increasing number of available options, it becomes crucial to establish which option is superior with regard to angina recurrence, graft patency, and long-term survival with the least morbidity (MI, stroke, AF) at the lowest costs (hospital LOS, ICU LOS, blood transfusions).
Medical Treatment versus Surgical Management
Several large randomized trials have examined the efficacy of medical versus surgical management in patients with symptomatic coronary artery disease (Table 33-10). Most trials were conducted between 1974 and 1984. A meta-analysis was published in 1994 that incorporated seven trials addressing medical versus surgical management with a 10-year follow-up.173 Although surgical and medical management have advanced since then (i.e., only 9% of patients received IMA grafts), the findings clearly support the benefits of surgery in high-risk patients. This study reviewed 2600 patients and observed an absolute risk reduction in mortality for patients undergoing CABG of 5.6% at 5 years, 5.9% at 7 years, and 4.1% at 10 years. This improvement was most marked in patients with left main disease, proximal left anterior descending coronary artery disease, or triple-vessel disease. The results tended to underestimate the benefits of surgical treatment because 37.4% of medically treated patients eventually underwent surgery.
TABLE 33-10 Treatment Options and Outcomes for Coronary Artery Disease
| Comparison | Outcome | Revascularization |
|---|---|---|
| Medical vs. surgical management | Absolute risk reduction in mortality | 37% of medically treated patients converted to surgery |
| 5.6% at 5 years | ||
| 5.9% at 7 years | ||
| 4.1% at 10 years | ||
| Benefit of surgery greatest in LM, three-vessel disease | ||
| Angioplasty vs. surgical management | Absolute risk reduction in mortality | 50% rate of revascularization at 5 years in angioplasty group |
| 1.9% at 5 years | ||
| Rates of in-hospital MI and stroke significantly lower in angioplasty group | ||
| Benefit of surgery greatest in diabetics, multivessel revascularization | ||
| Stent vs. surgical management | Mortality mixed results, relative reduction ranged from a 50% reduction in favor of CABG to a 75% reduction in favor of stenting | 15–25% rate of revascularization in stent group |
| MI rates at 1-year equivalent | ||
| OPCAB vs. conventional surgical management | No difference in mortality | Most OPCAB patients received fewer grafts than CCAB group (0.2 fewer grafts per patient) |
| No difference for in-hospital stroke | ||
| No difference for in-hospital MI |
CABG, coronary artery bypass grafting; CCAB, conventional coronary artery bypass; LM, left main disease; MI, myocardial infarction; OPCAB, off-pump coronary artery bypass.
Balloon Angioplasty versus Conventional Coronary Artery Bypass Graft Surgery
A number of randomized trials comparing percutaneous transluminal coronary angioplasty (PTCA) with CABG have been performed (see Chapters 3 and 18). One of the largest trials was the Bypass Angioplasty Revascularization Investigation (BARI), which enrolled 1829 patients who were randomized to undergo either PTCA or CABG.174 It found no significant differences in survival at both 1 and 5 years, with the 5-year survival rates of 89.3% in the CABG group and 86.3% in PTCA group. The rates of in-hospital MI and stroke were greater in the CABG patients compared with the PTCA group (4.6% and 2.1% for Q-wave MI, P < 0.01; 0.8% and 0.2% for stroke). For patients with diabetes, 5-year survival rate was 80.6% in the CABG group compared with 65.5% in the PTCA group (P = 0.003). The need for repeated revascularization after initial intervention was greater in the PTCA group; at 5 years, only 8% of the patients assigned to CABG had undergone additional revascularization procedures, compared with 54% of those assigned to PTCA (31% of PTCA patients eventually underwent CABG). Several smaller randomized trials found similar results.175,176
A meta-analysis of randomized trials was recently published comparing PTCA with CABG for the management of symptomatic coronary artery disease. A total of 13 trials involving 7964 patients were included.177 They reported a 1.9% absolute survival advantage favoring CABG over PTCA at 5 years (P < 0.02). There was no significant difference at 1, 3, or 8 years. In subgroup analysis of patients with multivessel disease, CABG provided a significant survival advantage at both 5 and 8 years. Patients randomized to PTCA had more repeat revascularizations at all time points. This meta-analysis included some coronary stent trials, in which angina recurrence was reduced by 50% at 1 and 3 years, and the stent cohort had a significant decrease in nonfatal MI at 3 years compared with CABG.
These trials focused primarily on patients with multivessel disease. In patients with single-vessel left anterior descending coronary artery disease, few randomized trials have been conducted. One trial enrolled 134 patients randomized to PTCA or CABG. After 5 years, six patients (9%) had died in the PTCA group versus two (3%) in the CABG group (not significant). MI was more frequent after PTCA (15% vs. 4%, P = 0.0001), but there were no differences in the rates of Q-wave infarction (6% in the PTCA group vs. 3% in the CABG group, not significant). Repeat revascularization was required in significantly more patients assigned to the PTCA group (38% vs. 9%, P = 0.0001).178
Stenting versus Conventional Coronary Artery Bypass Graft Surgery
Surprisingly similar results have been seen when stent implantation (bare metal stents) was compared with CABG surgery179–181 (see Chapters 3 and 18). Two trials found no difference in the mortality rate at 1 year.180,181 One trial found a greater mortality rate in the percutaneous coronary intervention (PCI) patients (5% PCI vs. 2% CABG, P = 0.01), whereas another trial found reduced mortality in the PCI group (0.9% vs. 5.7%, P < 0.013).179,182 For Q-wave MI, the studies demonstrated equivalent or slightly greater rates with CABG at 1 year. The requirement for repeat revascularizations was still high in all the stent trials, ranging from approximately 15% to 25% (over 1 to 2 years). The costs associated with both PTCA and PCI stents are lower than the cost of CABG. The greatest cost difference is seen at 1 year and decreases after this, because of the high rate of repeat revascularization in the PCI and PTCA groups. Although drug-eluting stents offer the promise of reductions in restenosis, too few trials are published to make a meaningful comment on the effect of this new technology.
A meta-analysis comparing off-pump CABG with PTCA for predominantly single-vessel left internal mammary artery to left anterior descending coronary artery also supported the superiority of surgery. Disease-free survival and need for reintervention were reduced in the surgical group at a cost of longer hospital stay. Most studies used bare metal stents, and thus the impact of drug eluting stents remains to be investigated.183
Off-Pump Coronary Artery Bypass Surgery versus Coronary Artery Bypass Graft Surgery
The use of OPCAB techniques has increased in popularity, and its greatest benefit will likely be in patients at greatest risk for stroke (e.g., elderly patients, patients with high-grade aortic atheromatous disease).184–186 A number of randomized trials have investigated the benefits of OPCAB surgery.187–190 A comprehensive meta-analysis of randomized trials was published demonstrating that OPCAB reduces the rate of blood transfusion, AF, infections, and resource utilization (hospital LOS, ICU LOS, and ventilation time). OPCAB surgery may minimize midterm cognitive dysfunction compared with CABG.191 OPCAB should be considered a safe alternative to CABG with respect to risk for mortality. However, most trials to date have focused on relatively healthy patients and have been underpowered to determine benefits in the high-risk cohort group purported to benefit the most by OPCAB surgery.184–186 A large randomized trial, involving more than 2200 patients, drew similar conclusions. It found no difference in stroke, mortality, or MI outcomes, while suggesting that blood transfusions were reduced. Again, however, this was not a high-risk cohort.192
The number of coronary vessels bypassed was usually lower in the OPCAB group for all randomized trials. Whether this will translate into worsened outcomes at 1 or 5 years is unknown. Finally, concern has been raised over graft patency.190 Reports on graft patency after OPCAB are conflicting, with some studies suggesting reduced patency and others suggesting no difference.192,193 Ultimately, long-term outcomes are needed to assess whether important direct outcomes like mortality, angina recurrence, MI, and reoperation differ in patients undergoing OPCAB versus CABG. In healthy patients, OPCAB has similar rates of mortality and major morbidity compared with CABG, while reducing the rate of AF and blood transfusions.
Echocardiography cases
Data Collection
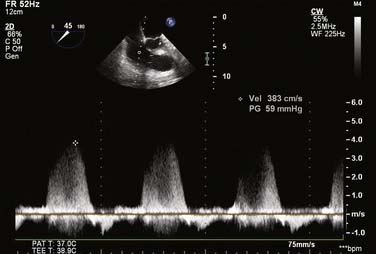
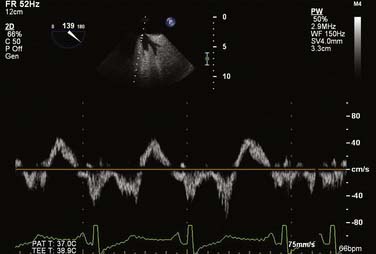
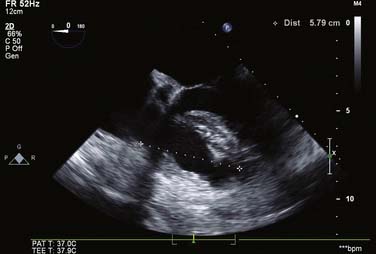
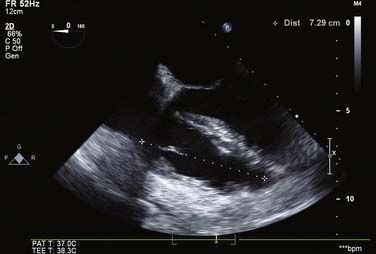
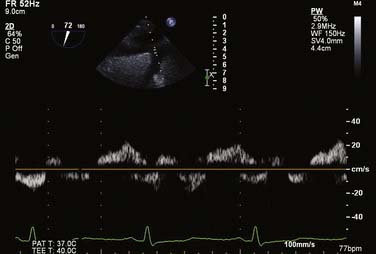
For all cases of hemodynamic deterioration in the ICU, data from the arterial catheter, electrocardiogram, CVP (or PA catheter), ABGs (lactate, pH, Po2), and indicators of organ perfusion (urine output, central nervous system function) need to be assessed to determine whether the patient is in failure and whether this failure is biventricular, left-sided, or right-sided predominantly. In the case presented, there was a suggestion of biventricular failure; because the cause of the problem was unclear, it was decided to proceed with TEE. Assessment consisted of LV assessment (wall motion, ejection fraction estimation, mitral valve function) and RV assessment (RV TAPSE, wall motion, and tricuspid valve function). After this, a comprehensive examination was completed. In this case, the TEE showed an empty LV with a normal ejection fraction of 50% to 60%, and no regional wall motion abnormalities were noted. The RV TAPSE was depressed at 0.9 cm. Tricuspid regurgitation (TR) was severe and the RV was dilated. This was in keeping with a previous transthoracic echocardiogram from 3 days prior that suggested pulmonary hypertension (Figures 33-4 through 33-8; also see Videos 1 through 3, available online).
1 Bennett S.R., Griffin S.C. Sevoflurane versus isoflurane in patients undergoing valvular cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:175-178.
2 De Hert S.G., Cromheecke S., ten Broecke P.W., et al. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99:314-323.
3 De Hert S.G., ten Broecke P.W., Mertens E., et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97:42-49.
4 Julier K., Da Silva R., Garcia C., et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. Anesthesiology. 2003;98:1315-1327.
5 Belhomme D., Peynet J., Louzy M., et al. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation. 1999;100:II340-II344.
6 Boyce S.W., Bartels C., Bolli R., et al. Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: Results of the CABG surgery cohort of the GUARDIAN study. J Thorac Cardiovasc Surg. 2003;126:420-427.
7 Van Oldenbeek C., Knowles P., Harper N.J. Residual neuromuscular block caused by pancuronium after cardiac surgery. Br J Anaesth. 1999;83:338-339.
8 McEwin L., Merrick P.M., Bevan D.R. Residual neuromuscular blockade after cardiac surgery: Pancuronium vs rocuronium. Can J Anaesth. 1997;44:891-895.
9 Murphy G.S., Szokol J.W., Marymont J.H., et al. Impact of shorter-acting neuromuscular blocking agents on fast-track recovery of the cardiac surgical patient. Anesthesiology. 2002;96:600-606.
10 Thomas R., Smith D., Strike P. Prospective randomised double-blind comparative study of rocuronium and pancuronium in adult patients scheduled for elective ‘fast-track’ cardiac surgery involving hypothermic cardiopulmonary bypass. Anaesthesia. 2003;58:265-271.
11 Murphy G.S., Szokol J.W., Marymont J.H., et al. Recovery of neuromuscular function after cardiac surgery: Pancuronium versus rocuronium. Anesth Analg. 2003,;96:1301-1307. table of contents
12 Mollhoff T., Herregods L., Moerman A., et al. Comparative efficacy and safety of remifentanil and fentanyl in ‘fast track’ coronary artery bypass graft surgery: A randomized, double-blind study. Br J Anaesth. 2001;87:718-726.
13 Engoren M., Luther G., Fenn-Buderer N. A comparison of fentanyl, sufentanil, and remifentanil for fast-track cardiac anesthesia. Anesth Analg. 2001;93:859-864.
14 Cheng D.C., Newman M.F., Duke P., et al. The efficacy and resource utilization of remifentanil and fentanyl in fast-track coronary artery bypass graft surgery: A prospective randomized, double-blinded controlled, multi-center trial. Anesth Analg. 2001;92:1094-1102.
15 Quasha A.L., Loeber N., Feeley T.W., et al. Postoperative respiratory care: A controlled trial of early and late extubation following coronary-artery bypass grafting. Anesthesiology. 1980;52:135-141.
16 Cheng D.C., Karski J., Peniston C., et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: A prospective randomized controlled trial. J Thorac Cardiovasc Surg. 1996;112:755-764.
17 Berry P.D., Thomas S.D., Mahon S.P., et al. Myocardial ischaemia after coronary artery bypass grafting: Early vs late extubation. Br J Anaesth. 1998;80:20-25.
18 Silbert B.S., Santamaria J.D., O’Brien J.L., et al. Early extubation following coronary artery bypass surgery: A prospective randomized controlled trial. The Fast Track Cardiac Care Team. Chest. 1998;113:1481-1488.
19 Ovrum E., Tangen G., Schiott C., et al. Rapid recovery protocol applied to 5,658 consecutive “on-pump” coronary bypass patients. Ann Thorac Surg. 2000;70:2008-2012.
20 Meade M.O., Guyatt G., Butler R., et al. Trials comparing early vs late extubation following cardiovascular surgery. Chest. 2001;120:445S-453S.
21 Myles P.S., Daly D.J., Djaiani G., et al. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99:982-987.
22 Dowd N.P., Cheng D.C., Karski J.M., et al. Intraoperative awareness in fast-track cardiac anesthesia. Anesthesiology. 1998,;89:1068-1073. discussion 9A
23 Liu W.H., Thorp T.A., Graham S.G., et al. Incidence of awareness with recall during general anaesthesia. Anaesthesia. 1991;46:435-437.
24 Cheng D.C., Karski J., Peniston C., et al. Early tracheal extubation after coronary artery bypass graft surgery reduces costs and improves resource use. A prospective, randomized, controlled trial. Anesthesiology. 1996;85:1300-1310.
25 Wong D.T., Cheng D.C., Kustra R., et al. Risk factors of delayed extubation, prolonged length of stay in the intensive care unit, and mortality in patients undergoing coronary artery bypass graft with fast-track cardiac anesthesia: A new cardiac risk score. Anesthesiology. 1999;91:936-944.
26 Brown M.M. Implementation strategy: One-stop recovery for cardiac surgical patients. AACN Clin Issues. 2000;11:412-423.
27 Joyce L., Pandolph P. One Stop Post Op cardiac surgery recovery—a proven success. J Cardiovasc Manag. 2001;12:16-18.
28 Cheng D.C., Byrick R.J., Knobel E. Structural models for intermediate care areas. Crit Care Med. 1999;27:2266-2271.
29 Cheng D.C., Wall C., Djaiani G., et al. Randomized assessment of resource use in fast-track cardiac surgery 1-year after hospital discharge. Anesthesiology. 2003;98:651-657.
30 Grocott H.P., Mackensen G.B., Grigore A.M., et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537-541.
31 Nathan H.J., Wells G.A., Munson J.L., et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: A randomized trial. Circulation. 2001;104:I85-I91.
32 Hebert P.C. Transfusion requirements in critical care (TRICC): A multicentre, randomized, controlled clinical study. Transfusion Requirements in Critical Care Investigators and the Canadian Critical Care Trials Group. Br J Anaesth. 1998;81(Suppl 1):25-33.
33 Shore-Lesserson L., Manspeizer H.E., DePerio M., et al. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312-319.
34 Spiess B.D., Gillies B.S., Chandler W., et al. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth. 1995;9:168-173.
35 Body S.C., Birmingham J., Parks R., et al. Safety and efficacy of shed mediastinal blood transfusion after cardiac surgery: A multicenter observational study. Multicenter Study of Perioperative Ischemia Research Group. J Cardiothorac Vasc Anesth. 1999;13:410-416.
36 Hillyer C.D., Josephson C.D., Blajchman M.A., et al. Bacterial contamination of blood components: Risks, strategies, and regulation: Joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program. 2003:575-589.
37 Zurick A.M., Urzua J., Ghattas M., et al. Failure of positive end-expiratory pressure to decrease postoperative bleeding after cardiac surgery. Ann Thorac Surg. 1982;34:608-611.
38 Collier B., Kolff J., Devineni R., et al. Prophylactic positive end-expiratory pressure and reduction of postoperative blood loss in open-heart surgery. Ann Thorac Surg. 2002;74:1191-1194.
39 Forestier F., Belisle S., Robitaille D., et al. Low-dose aprotinin is ineffective to treat excessive bleeding after cardiopulmonary bypass. Ann Thorac Surg. 2000;69:452-456.
40 Ray M.J., Hales M.M., Brown L., et al. Postoperatively administered aprotinin or epsilon aminocaproic acid after cardiopulmonary bypass has limited benefit. Ann Thorac Surg. 2001;72:521-526.
41 Hardy J.F., Belisle S., Van der Linden P. Efficacy and safety of activated recombinant factor VII in cardiac surgical patients. Curr Opin Anaesthesiol. 2009;22:95-99.
42 Gill R., Herbertson M., Vuylsteke A., et al. Safety and efficacy of recombinant activated factor VII: A randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21-27.
43 Diprose P., Herbertson M.J., O’Shaughnessy D., et al. Activated recombinant factor VII after cardiopulmonary bypass reduces allogeneic transfusion in complex non-coronary cardiac surgery: Randomized double-blind placebo-controlled pilot study. Br J Anaesth. 2005;95:596-602.
44 BARI. Seven-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) by treatment and diabetic status. J Am Coll Cardiol. 2000;35:1122-1129.
45 Newman M.F., Wolman R., Kanchuger M., et al. Multicenter preoperative stroke risk index for patients undergoing coronary artery bypass graft surgery. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Circulation. 1996;94:II74-II80.
46 Conlon P.J., Stafford-Smith M., White W.D., et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158-1162.
47 London M.J., Grunwald G.K., Shroyer A.L., et al. Association of fast-track cardiac management and low-dose to moderate-dose glucocorticoid administration with perioperative hyperglycemia. J Cardiothorac Vasc Anesth. 2000;14:631-638.
48 Golden S.H., Peart-Vigilance C., Kao W.H., et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
49 Guvener M., Pasaoglu I., Demircin M., et al. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49:531-537.
50 McAlister F.A., Man J., Bistritz L., et al. Diabetes and coronary artery bypass surgery: An examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26:1518-1524.
51 Latham R., Lancaster A.D., Covington J.F., et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607-612.
52 van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
53 Griesdale D.E., de Souza R.J., van Dam R.M., et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821-827.
54 Finfer S., Chittock D.R., Su S.Y., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297.
55 Munro A.J., Long G.T., Sleigh J.W. Nurse-administered subcutaneous morphine is a satisfactory alternative to intravenous patient-controlled analgesia morphine after cardiac surgery. Anesth Analg. 1998;87:11-15.
56 Tsang J., Brush B. Patient-controlled analgesia in postoperative cardiac surgery. Anaesth Intensive Care. 1999;27:464-470.
57 O’Halloran P., Brown R. Patient-controlled analgesia compared with nurse-controlled infusion analgesia after heart surgery. Intensive Crit Care Nurs. 1997;13:126-129.
58 Myles P.S., Buckland M.R., Cannon G.B., et al. Comparison of patient-controlled analgesia and nurse-controlled infusion analgesia after cardiac surgery. Anaesth Intensive Care. 1994;22:672-678.
59 Searle N.R., Roy M., Bergeron G., et al. Hydromorphone patient-controlled analgesia (PCA) after coronary artery bypass surgery. Can J Anaesth. 1994;41:198-205.
60 Boldt J., Thaler E., Lehmann A., et al. Pain management in cardiac surgery patients: Comparison between standard therapy and patient-controlled analgesia regimen. J Cardiothorac Vasc Anesth. 1998;12:654-658.
61 Gust R., Pecher S., Gust A., et al. Effect of patient-controlled analgesia on pulmonary complications after coronary artery bypass grafting. Crit Care Med. 1999;27:2218-2223.
62 Bainbridge D., Martin J.E., Cheng D.C. Patient-controlled versus nurse-controlled analgesia after cardiac surgery—a meta-analysis. Can J Anaesth. 2006;53:492-499.
63 Chaney M.A., Smith K.R., Barclay J.C., et al. Large-dose intrathecal morphine for coronary artery bypass grafting. Anesth Analg. 1996;83:215-222.
64 Bettex D.A., Schmidlin D., Chassot P.G., et al. Intrathecal sufentanil-morphine shortens the duration of intubation and improves analgesia in fast-track cardiac surgery. Can J Anaesth. 2002;49:711-717.
65 Boulanger A., Perreault S., Choiniere M., et al. Intrathecal morphine after cardiac surgery. Ann Pharmacother. 2002;36:1337-1343.
66 Zarate E., Latham P., White P.F., et al. Fast-track cardiac anesthesia: Use of remifentanil combined with intrathecal morphine as an alternative to sufentanil during desflurane anesthesia. Anesth Analg. 2000;91:283-287.
67 Fitzpatrick G.J., Moriarty D.C. Intrathecal morphine in the management of pain following cardiac surgery. A comparison with morphine i.v. Br J Anaesth. 1988;60:639-644.
68 Aun C., Thomas D., St John-Jones L., et al. Intrathecal morphine in cardiac surgery. Eur J Anaesthesiol. 1985;2:419-426.
69 Shroff A., Rooke G.A., Bishop M.J. Effects of intrathecal opioid on extubation time, analgesia, and intensive care unit stay following coronary artery bypass grafting. J Clin Anesth. 1997;9:415-419.
70 Lena P., Balarac N., Arnulf J.J., et al. Intrathecal morphine and clonidine for coronary artery bypass grafting. Br J Anaesth. 2003;90:300-303.
71 Chaney M.A., Nikolov M.P., Blakeman B.P., et al. Intrathecal morphine for coronary artery bypass graft procedure and early extubation revisited. J Cardiothorac Vasc Anesth. 1999;13:574-578.
72 Chaney M.A., Furry P.A., Fluder E.M., et al. Intrathecal morphine for coronary artery bypass grafting and early extubation. Anesth Analg. 1997;84:241-248.
73 Liu S.S., Block B.M., Wu C.L. Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: A meta-analysis. Anesthesiology. 2004;101:153-161.
74 Ho A.M., Chung D.C., Joynt G.M. Neuraxial blockade and hematoma in cardiac surgery: Estimating the risk of a rare adverse event that has not (yet) occurred. Chest. 2000;117:551-555.
75 Pastor M.C., Sanchez M.J., Casas M.A., et al. Thoracic epidural analgesia in coronary artery bypass graft surgery: Seven years’ experience. J Cardiothorac Vasc Anesth. 2003;17:154-159.
76 Horlocker T.T., Wedel D.J., Benzon H., et al. Regional anesthesia in the anticoagulated patient: Defining the risks. Reg Anesth Pain Med. 2004;29:1-12.
77 Lipsky P.E. Defining COX-2 inhibitors. J Rheumatol Suppl. 2000;60:13-16.
78 Bigler D., Moller J., Kamp-Jensen M., et al. Effect of piroxicam in addition to continuous thoracic epidural bupivacaine and morphine on postoperative pain and lung function after thoracotomy. Acta Anaesthesiol Scand. 1992;36:647-650.
79 Carretta A., Zannini P., Chiesa G., et al. Efficacy of ketorolac tromethamine and extrapleural intercostal nerve block on post-thoracotomy pain. A prospective, randomized study. Int Surg. 1996;81:224-228.
80 Fayaz K., Abel R., Pugh S., et al. Opioid sparing and side effect profile of three different analgesic techniques for cardiac surgery. Eur J Anesth. 2003;20:A6.
81 Hynninen M.S., Cheng D.C., Hossain I., et al. Non-steroidal anti-inflammatory drugs in treatment of postoperative pain after cardiac surgery. Can J Anaesth. 2000;47:1182-1187.
82 Immer F.F., Immer-Bansi A.S., Trachsel N., et al. Pain treatment with a COX-2 inhibitor after coronary artery bypass operation: A randomized trial. Ann Thorac Surg. 2003;75:490-495.
83 Jones R.M., Cashman J.N., Foster J.M., et al. Comparison of infusions of morphine and lysine acetyl salicylate for the relief of pain following thoracic surgery. Br J Anaesth. 1985;57:259-263.
84 Kavanagh B.P., Katz J., Sandler A.N., et al. Multimodal analgesia before thoracic surgery does not reduce postoperative pain. Br J Anaesth. 1994;73:184-189.
85 Keenan D.J., Cave K., Langdon L., et al. Comparative trial of rectal indomethacin and cryoanalgesia for control of early postthoracotomy pain. Br Med J (Clin Res Ed). 1983;287:1335-1337.
86 McCrory C., Diviney D., Moriarty J., et al. Comparison between repeat bolus intrathecal morphine and an epidurally delivered bupivacaine and fentanyl combination in the management of post-thoracotomy pain with or without cyclooxygenase inhibition. J Cardiothorac Vasc Anesth. 2002;16:607-611.
87 Merry A.F., Wardall G.J., Cameron R.J., et al. Prospective, controlled, double-blind study of i.v. tenoxicam for analgesia after thoracotomy. Br J Anaesth. 1992;69:92-94.
88 Ott E., Nussmeier N.A., Duke P.C., et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481-1492.
89 Bainbridge D., Cheng D.C., Martin J.E., et al. NSAID-analgesia, pain control and morbidity in cardiothoracic surgery: [L’analgesie avec des AINS, le controle de la douleur et la morbidite en chirurgie cardiothoracique]. Can J Anaesth. 2006;53:46-59.
90 Nussmeier N.A., Whelton A.A., Brown M.T., et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
91 Camu F., Beecher T., Recker D.P., et al. Valdecoxib, a COX-2-specific inhibitor, is an efficacious, opioid-sparing analgesic in patients undergoing hip arthroplasty. Am J Ther. 2002;9:43-51.
92 Collaborative overview of randomised trials of antiplatelet therapy—II. Maintenance of vascular graft or arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:159-168.
93 Weber M., von Schacky C., Lorenz R., et al. [Low-dose acetylsalicylic acid (100 mg/day) following aortocoronary bypass operation]. Klin Wochenschr. 1984;62:458-464.
94 Goldman S., Copeland J., Moritz T., et al. Starting aspirin therapy after operation. Effects on early graft patency. Department of Veterans Affairs Cooperative Study Group. Circulation. 1991;84:520-526.
95 Goldman S., Copeland J., Moritz T., et al. Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study. Circulation. 1989;80:1190-1197.
96 Mangano D.T. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309-1317.
97 Sethi G.K., Copeland J.G., Goldman S., et al. Implications of preoperative administration of aspirin in patients undergoing coronary artery bypass grafting. Department of Veterans Affairs Cooperative Study on Antiplatelet Therapy. J Am Coll Cardiol. 1990;15:15-20.
98 Goldman S., Zadina K., Krasnicka B., et al. Predictors of graft patency 3 years after coronary artery bypass graft surgery. Department of Veterans Affairs Cooperative Study Group No. 297. J Am Coll Cardiol. 1997;29:1563-1568.
99 Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81-106.
100 Bhatt D.L., Chew D.P., Hirsch A.T., et al. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001;103:363-368.
101 Effect of metoprolol on death and cardiac events during a 2-year period after coronary artery bypass grafting. The MACB Study Group. Eur Heart J. 1995;16:1825-1832.
102 Oosterga M., Voors A.A., Pinto Y.M., et al. Effects of quinapril on clinical outcome after coronary artery bypass grafting (The QUO VADIS Study). QUinapril on Vascular Ace and Determinants of Ischemia. Am J Cardiol. 2001;87:542-546.
103 Chen J., Radford M.J., Wang Y., et al. Are beta-blockers effective in elderly patients who undergo coronary revascularization after acute myocardial infarction? Arch Intern Med. 2000;160:947-952.
104 The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336:153-162.
105 Ouattara A., Benhaoua H., Le Manach Y., et al. Perioperative statin therapy is associated with a significant and dose-dependent reduction of adverse cardiovascular outcomes after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:633-638.
106 Miceli A., Fino C., Fiorani B., et al. Effects of preoperative statin treatment on the incidence of postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2009;87:1853-1858.
107 Liakopoulos O.J., Choi Y.H., Kuhn E.W., et al. Statins for prevention of atrial fibrillation after cardiac surgery: A systematic literature review. J Thorac Cardiovasc Surg. 2009;138:678-686e1.
108 Bonow R.O., Carabello B.A., Chatterjee K., et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1-e142.
109 Naylor A.R., Mehta Z., Rothwell P.M., et al. Carotid artery disease and stroke during coronary artery bypass: A critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23:283-294.
110 Stamou S.C., Hill P.C., Dangas G., et al. Stroke after coronary artery bypass: Incidence, predictors, and clinical outcome. Stroke. 2001;32:1508-1513.
111 Salazar J.D., Wityk R.J., Grega M.A., et al. Stroke after cardiac surgery: Short- and long-term outcomes. Ann Thorac Surg. 2001;72:1195-1201. discussion 201–202
112 Lahtinen J., Biancari F., Salmela E., et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241-1244.
113 Murkin J.M., Martzke J.S., Buchan A.M., et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II. Neurologic and cognitive outcomes. J Thorac Cardiovasc Surg. 1995;110:349-362.
114 Murkin J.M. Attenuation of neurologic injury during cardiac surgery. Ann Thorac Surg. 2001;72:S1838-S1844.
115 Royse A.G., Royse C.F., Ajani A.E., et al. Reduced neuropsychological dysfunction using epiaortic echocardiography and the exclusive Y graft. Ann Thorac Surg. 2000;69:1431-1438.
116 Sharony R., Bizekis C.S., Kanchuger M., et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: A case control study. Circulation. 2003;108(Suppl 1):II15-II20.
117 Leacche M., Carrier M., Bouchard D., et al. Improving neurologic outcome in off-pump surgery: The “no touch” technique. Heart Surg Forum. 2003;6:169-175.
118 Bruno A., Levine S.R., Frankel M.R., et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669-674.
119 Williams L.S., Rotich J., Qi R., et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67-71.
120 Quast M.J., Wei J., Huang N.C., et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17:553-559.
121 Dietrich W.D., Busto R., Valdes I., et al. Effects of normothermic versus mild hyperthermic forebrain ischemia in rats. Stroke. 1990;21:1318-1325.
122 Bucerius J., Gummert J.F., Borger M.A., et al. Predictors of delirium after cardiac surgery delirium: Effect of beating-heart (off-pump) surgery. J Thorac Cardiovasc Surg. 2004;127:57-64.
123 van der Mast R.C., van den Broek W.W., Fekkes D., et al. Incidence of and preoperative predictors for delirium after cardiac surgery. J Psychosom Res. 1999;46:479-483.
124 Gokgoz L., Gunaydin S., Sinci V., et al. Psychiatric complications of cardiac surgery postoperative delirium syndrome. Scand Cardiovasc J. 1997;31:217-222.
125 Rolfson D.B., McElhaney J.E., Rockwood K., et al. Incidence and risk factors for delirium and other adverse outcomes in older adults after coronary artery bypass graft surgery. Can J Cardiol. 1999;15:771-776.
126 Ommen S.R., Odell J.A., Stanton M.S. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429-1434.
127 Zimmer J., Pezzullo J., Choucair W., et al. Meta-analysis of antiarrhythmic therapy in the prevention of postoperative atrial fibrillation and the effect on hospital length of stay, costs, cerebrovascular accidents, and mortality in patients undergoing cardiac surgery. Am J Cardiol. 2003;91:1137-1140.
128 Mathew J.P., Fontes M.L., Tudor I.C., et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729.
129 Crystal E., Connolly S.J., Sleik K., et al. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: A meta-analysis. Circulation. 2002;106:75-80.
130 Toraman F., Karabulut E.H., Alhan H.C., et al. Magnesium infusion dramatically decreases the incidence of atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2001;72:1256-1261. discussion 1261–1262
131 Blackshear J.L., Safford R.E. AFFIRM and RACE trials: Implications for the management of atrial fibrillation. Card Electrophysiol Rev. 2003;7:366-369.
132 Wyse D.G., Waldo A.L., DiMarco J.P., et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
133 Hagens V.E., Ranchor A.V., Van Sonderen E., et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241-247.
134 Olshansky B., Rosenfeld L.E., Warner A.L., et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: Approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201-1208.
135 Estes N.A.3rd, Halperin J.L., Calkins H., et al. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): Developed in collaboration with the Heart Rhythm Society. Circulation. 2008;117:1101-1120.
136 Hart R.G., Benavente O., McBride R., et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: A meta-analysis. Ann Intern Med. 1999;131:492-501.
137 Prystowsky E.N., Benson D.W.Jr, Fuster V., et al. Management of patients with atrial fibrillation. A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996;93:1262-1277.
138 Fuster V., Ryden L.E., Asinger R.W., et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: Executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): Developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231-1266.
139 Butterworth J.F.t., Legault C., Royster R.L., et al. Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg. 1998;86:461-467.
140 Royster R.L., Butterworth JFt, Prough D.S., et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729-736.
141 Krivec B., Voga G., Zuran I., et al. Diagnosis and treatment of shock due to massive pulmonary embolism: Approach with transesophageal echocardiography and intrapulmonary thrombolysis. Chest. 1997;112:1310-1316.
142 van der Wouw P.A., Koster R.W., Delemarre B.J., et al. Diagnostic accuracy of transesophageal echocardiography during cardiopulmonary resuscitation. J Am Coll Cardiol. 1997;30:780-783.
143 Cicek S., Demirilic U., Kuralay E., et al. Transesophageal echocardiography in cardiac surgical emergencies. J Card Surg. 1995;10:236-244.
144 Wake P.J., Ali M., Carroll J., et al. Clinical and echocardiographic diagnoses disagree in patients with unexplained hemodynamic instability after cardiac surgery. Can J Anaesth. 2001;48:778-783.
145 Tousignant C.P., Walsh F., Mazer C.D. The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg. 2000;90:351-355.
146 Swenson J.D., Bull D., Stringham J. Subjective assessment of left ventricular preload using transesophageal echocardiography: Corresponding pulmonary artery occlusion pressures. J Cardiothorac Vasc Anesth. 2001;15:580-583.
147 Fontes M.L., Bellows W., Ngo L., et al. Assessment of ventricular function in critically ill patients: Limitations of pulmonary artery catheterization. Institutions of the McSPI Research Group. J Cardiothorac Vasc Anesth. 1999;13:521-527.
148 Schwinn D.A., Leone B.J., Spahn D.R., et al. Desensitization of myocardial beta-adrenergic receptors during cardiopulmonary bypass. Evidence for early uncoupling and late downregulation. Circulation. 1991;84:2559-2567.
149 Royster R.L., Butterworth JFt, Prielipp R.C., et al. Combined inotropic effects of amrinone and epinephrine after cardiopulmonary bypass in humans. Anesth Analg. 1993;77:662-672.
150 Tokmakoglu H., Farsak B., Gunaydin S., et al. Effectiveness of intraaortic balloon pumping in patients who were not able to be weaned from cardiopulmonary bypass after coronary artery bypass surgery and mortality predictors in the perioperative and early postoperative period. Anadolu Kardiyol Derg. 2003;3:124-128.
151 Marra C., De Santo L.S., Amarelli C., et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction: A prospective randomized study on the timing of perioperative intraaortic balloon pump support. Int J Artif Organs. 2002;25:141-146.
152 Christenson J.T., Badel P., Simonet F., et al. Preoperative intraaortic balloon pump enhances cardiac performance and improves the outcome of redo CABG. Ann Thorac Surg. 1997;64:1237-1244.
153 Christenson J.T., Simonet F., Badel P., et al. Optimal timing of preoperative intraaortic balloon pump support in high-risk coronary patients. Ann Thorac Surg. 1999;68:934-939.
154 Christenson J.T., Schmuziger M., Simonet F. Effective surgical management of high-risk coronary patients using preoperative intra-aortic balloon counterpulsation therapy. Cardiovasc Surg. 2001;9:383-390.
155 Torchiana D.F., Hirsch G., Buckley M.J., et al. Intraaortic balloon pumping for cardiac support: Trends in practice and outcome, 1968 to 1995. J Thorac Cardiovasc Surg. 1997;113:758-764. discussion 764–769
156 Castelli P., Condemi A., Munari M., et al. Intra-aortic balloon counterpulsation: Outcome in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15:700-703.
157 Sirivella S., Gielchinsky I., Parsonnet V. Angiotensin converting enzyme inhibitor therapy in severe postcardiotomy dysfunction: A prospective randomized study. J Card Surg. 1998;13:11-17.
158 Haddad F., Couture P., Tousignant C., et al. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg. 2009;108:422-433.
159 Haddad F., Couture P., Tousignant C., et al. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108:407-421.
160 Haddad F., Doyle R., Murphy D.J., et al. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717-1731.
161 Haddad F., Hunt S.A., Rosenthal D.N., et al. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436-1448.
162 Cecconi M., Johnston E., Rhodes A. What role does the right side of the heart play in circulation? Crit Care. 2006;10(Suppl 3):S5.
163 Abrahamov D., Tamariz M., Fremes S., et al. Renal dysfunction after cardiac surgery. Can J Cardiol. 2001;17:565-570.
164 Wijeysundera D.N., Rao V., Beattie W.S., et al. Evaluating surrogate measures of renal dysfunction after cardiac surgery. Anesth Analg. 2003;96:1265-1273. table of contents
165 Wang F., Dupuis J.Y., Nathan H., et al. An analysis of the association between preoperative renal dysfunction and outcome in cardiac surgery: Estimated creatinine clearance or plasma creatinine level as measures of renal function. Chest. 2003;124:1852-1862.
166 Kellum J.A. The use of diuretics and dopamine in acute renal failure: A systematic review of the evidence. Crit Care. 1997;1:53-59.
167 Landoni G., Biondi-Zoccai G.G., Tumlin J.A., et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: A meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56-68.
168 Mehta R.L., Pascual M.T., Soroko S., et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547-2553.
169 Kellum J.A., Angus D.C., Johnson J.P., et al. Continuous versus intermittent renal replacement therapy: A meta-analysis. Intensive Care Med. 2002;28:29-37.
170 Adabag A.S., Ishani A., Bloomfield H.E., et al. Efficacy of N-acetylcysteine in preventing renal injury after heart surgery: A systematic review of randomized trials. Eur Heart J. 2009;30:1910-1917.
171 Naughton F., Wijeysundera D., Karkouti K., et al. N-acetylcysteine to reduce renal failure after cardiac surgery: A systematic review and meta-analysis. Can J Anaesth. 2008;55:827-835.
172 Misra D., Leibowitz K., Gowda R.M., et al. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: A meta-analysis. Clin Cardiol. 2004;27:607-610.
173 Yusuf S., Zucker D., Peduzzi P., et al. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
174 BARI. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217-225.
175 Carrie D., Elbaz M., Puel J., et al. Five-year outcome after coronary angioplasty versus bypass surgery in multivessel coronary artery disease: Results from the French Monocentric Study. Circulation. 1997;96:II-1-II-6.
176 Hamm C.W., Reimers J., Ischinger T., et al. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med. 1994;331:1037-1043.
177 Hoffman S.N., TenBrook J.A., Wolf M.P., et al. A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: One- to eight-year outcomes. J Am Coll Cardiol. 2003;41:1293-1304.
178 Goy J.J., Eeckhout E., Moret C., et al. Five-year outcome in patients with isolated proximal left anterior descending coronary artery stenosis treated by angioplasty or left internal mammary artery grafting. A prospective trial. Circulation. 1999;99:3255-3259.
179 Rodriguez A., Bernardi V., Navia J., et al. Argentine Randomized Study: Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in patients with Multiple-Vessel Disease (ERACI II): 30-day and one-year follow-up results. ERACI II Investigators. J Am Coll Cardiol. 2001;37:51-58.
180 Serruys P.W., Unger F., Sousa J.E., et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117-1124.
181 Legrand V.M., Serruys P.W., Unger F., et al. Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation. 2004;109:1114-1120.
182 SOS. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): A randomised controlled trial. Lancet. 2002;360:965-970.
183 Bainbridge D., Cheng D., Martin J., et al. Does off-pump or minimally invasive coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with percutaneous coronary intervention? A meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2007;133:623-631.
184 D’Ancona G., Karamanoukian H., Kawaguchi A.T., et al. Myocardial revascularization of the beating heart in high-risk patients. J Card Surg. 2001;16:132-139.
185 Hoff S.J., Ball S.K., Coltharp W.H., et al. Coronary artery bypass in patients 80 years and over: Is off-pump the operation of choice? Ann Thorac Surg. 2002;74:S1340-S1343.
186 Yokoyama T., Baumgartner F.J., Gheissari A., et al. Off-pump versus on-pump coronary bypass in high-risk subgroups. Ann Thorac Surg. 2000;70:1546-1550.
187 Lee J.D., Lee S.J., Tsushima W.T., et al. Benefits of off-pump bypass on neurologic and clinical morbidity: A prospective randomized trial. Ann Thorac Surg. 2003;76:18-26.
188 Puskas J., Sharoni E., Williams W., et al. Is routine use of temporary epicardial pacing wires necessary after either OPCAB or conventional CABG/CPB? Heart Surg Forum. 2003;6(Suppl 1):s47.
189 Angelini G.D., Taylor F.C., Reeves B.C., et al. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): A pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194-1199.
190 Khan N.E., De Souza A., Mister R., et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21-28.
191 Cheng D.C., Bainbridge D., Martin J.E., et al. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology. 2005;102:188-203.
192 Shroyer A.L., Grover F.L., Hattler B., et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361:1827-1837.
193 Puskas J.D., Williams W.H., Mahoney E.M., et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA. 2004;291:1841-1849.