CHAPTER 59 Posterolateral Endoscopic Lumbar Discectomy
Posterolateral endoscopic lumbar surgery is a less invasive surgical procedure to address lumbar pathology in the disc and boney foramen. Like any surgical procedure, it is based on visual identification and exposure of the target pathology and adequate surgical tools to address the offending pathology. Modern day endoscopic technology allows for visualized discectomy and decompression of the traversing and exiting nerve roots from a percutaneous posterolateral/transforaminal approach. This is safe and equally efficacious to microscopic discectomy in properly selected patients.1–4 Recent advances also allow for bony decompression of foraminal stenosis.5,6
Numerous other nonvisualized percutaneous techniques often get categorized and confused with posterolateral endoscopic lumbar discectomy. These include automated percutaneous lumbar discectomy (APLD), percutaneous laser discectomy, and percutaneous discectomy with the Dekompressor or Arthrocare wand (Coblation). These are all fluoroscopically guided nonvisualized procedures that access the disc via the same posterolateral approach as endoscopic lumbar surgery. The underlying principle of these procedures is that through central nucleus removal or ablation, intradiscal pressure can be substantially lowered. This was based on the work of Hirsh and his postulated relationship between intradiscal pressure, disc herniation, and low back pain. He hypothesized that lowering this pressure in an injured disc could be efficacious in the relief of sciatica.7 Multiple studies described decreases in intradiscal pressures of 50% or greater.8–10
The results of these types of indirect decompressive procedures have been similar with initial favorable reports. However, subsequent studies have shown varying degrees of success. The inability to consistently see the decompressed nerve or the targeted patho-anatomy has limited the use of these nonvisualized decompressive procedures.11–14 It is unfortunate that these procedures, and their results, are mistaken for posterolateral endoscopic discectomy.
History
The basis for percutaneous lumbar disc procedures came from accepted posterolateral percutaneous biopsy techniques of the lumbar vertebrae. These procedures were initially performed with the use of a Craig needle to perform a posterolateral biopsy for neoplastic conditions.15,16
Minimal access surgery for lumbar disc herniation was first independently reported by Kambin and colleagues17 and Hijikata18 in 1975. The technique used a posterolateral approach to the foraminal zone of the disc bordered by the traversing nerve dorsally, the exiting nerve ventrally, and the endplate of the inferior vertebra caudally. The goal was to decompress nerve roots secondary to lumbar disc herniation by the “inside-out technique” of central and posterior nuclectomy and fragmentectomy. Advances in technique and instrumentation since Kambin, however, have allowed the surgeon to also enlarge the medial or lateral foramen by decompression of the lateral and ventral portion of the facet joint complex to reach the midline of the disc and the epidural space. Thus it is feasible to treat the full spectrum of disc herniations with advanced endoscopic instrumentation and techniques that can either target the extruded fragment directly or with a combination of the “inside-out technique.”19
The early efforts were limited to a nonvisualized central discectomy to achieve an indirect decompression of the nerve roots,17,18,20 but improvements in surgical equipment and technique evolved gradually over the next 30 years. In the past 10 years, the important major equipment improvements have included various-sized high-resolution rod lens operating endoscopes with variable-size working channels, beveled and slotted cannulas, flexible shavers and pituitary forceps, a bipolar flexible high-frequency/low-temperature radiofrequency (RF) electrode, multidirectional Holmium Yttrium-Aluminum-Garnet lasers, and high-speed diamond burrs and motorized shavers to decompress the foramen.19 An improved fluoroscopically guided approach method introduced by Yeung and reported by Tsou21–23 outlined a consistent and safe technique for entry into all lumbar posterior disc spaces including the L5-S1 level. This specific technique has been termed selective endoscopic discectomy (SED) but can be classified under the more general descriptive term of posterolateral endoscopic lumbar discectomy (PELD) that other authors describe.
These refinements have enhanced the capabilities of foraminal endoscopic discectomy to deliver surgical results similar to the results obtainable by traditional transcanal approaches for treating common lumbar disc herniations.1–4
Anatomy
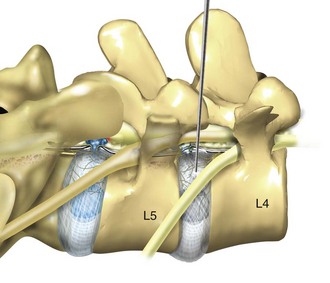
Posterolateral endoscopic lumbar surgery is performed through what has been named the triangular working zone, or Kambin’s triangle (Fig. 59–1). This triangular zone is defined as a safe zone in the posterolateral annulus between the exiting and traversing nerve roots. The exiting nerve root forms the anterior border of the triangular zone as it exits under the cephalad pedicle. The superior endplate of the caudal vertebral body forms the inferior border and the articular process and superior articulating facet of the caudal vertebra form the posterior border. The working zone is bordered medially by the traversing nerve root and dura. From cadaveric measurements it was determined that cannulas ranging from 4 mm to 10 mm could be safely used in the triangular working zone.24–27 A thorough understanding of the three-dimensional anatomy is necessary to understand and perform posterior percutaneous lumbar surgery.
Endoscopic Lumbar Discectomy
Endoscopic surgery developed out of fluoroscopically guided percutaneous procedures that initially used a working cannula with modified instruments designed for disc removal. The first surgeon credited with percutaneous nucleotomy was Hijikata in 1975.18 The evolution of endoscopic techniques followed a series of transitions. Initially, an arthroscope was used to inspect the disc and annulus intermittently through the cannula while the mechanical nuclectomy was done under fluoroscopic guidance. The introduction of a biportal approach allowed for direct visualization of instruments introduced through a cannula inserted into the disc from the opposite posterolateral portal. The later development of an operating spine scope with a working channel allowed for surgical removal of disc material and visualization of foraminal anatomy under direct visualization via a uniportal approach.
Parviz Kambin performed the first true endoscopic lumbar procedures. The arthroscope was at first used intermittently through the working cannula. At certain stages of the procedure such as perforating the disc in the triangular working zone, the arthroscope would be placed in the cannula. The nonworking channel scope was used for identification of the annulus and periannular structures. The basis was to see that the nerve was not in the way before advancing the cannula. Once the cannula was safely within the disc, the nucleotome, an arthroscopic shaver, and pituitary rongeurs were passed through the cannula to perform mechanical disc removal. The majority of the procedure was only fluoroscopically visualized.17 Kambin reported an 88% success rate in his first 100 patients.28,29
The early endoscopic procedures were limited by the absence of a working channel arthroscope. This led Kambin to the development of a biportal technique in which the scope was inserted on one side and the working cannula on the opposite side. Kambin’s indications for a biportal approach included large subligamentous herniations, extraligamentous herniations, and arthroscopic interbody fusion.26 In later studies Kambin reports results from both uniportal and biportal procedures together. Overall results ranged from 85% to 92% satisfactory results at a minimum 2-year follow-up. There was no differentiation made between the results of uniportal versus biportal approaches.30–32
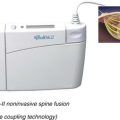
Kambin’s first prototype of the working channel scope was not fully developed and was not successfully marketed. The problems with the initial scope included fragility, limited degree of angulation for the working instruments, and the inability to establish sufficient inflow or outflow for adequate visualization.33 Anthony Yeung developed the first working channel endoscope to become widely available. The scope was developed in 1997 and was approved for use by the U.S. Food and Drug Administration in March 1998. The Yeung Endoscopic Spine Surgery (YESS) system (Richard Wolf Surgical Instruments, Vernon Hills, Ill.) modified the scope by adding multichannel integrated irrigation, specialized beveled cannulas, a two-hole obturator, and newly designed discectomy tools that allowed for constant real-time visualization with a uniportal technique.34 (Fig. 59–2)
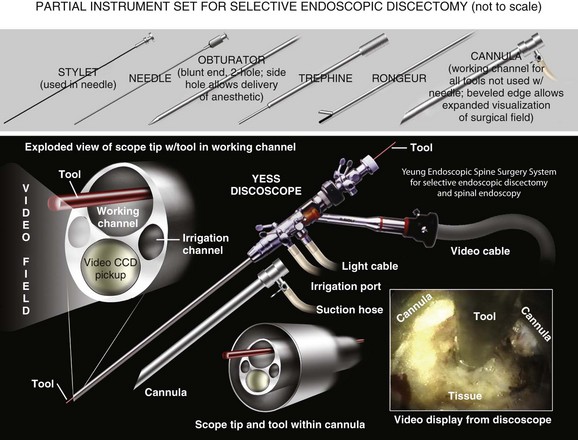
FIGURE 59–2 The Yeung Endoscopic Spine Surgery system.
(Courtesy Richard Wolf Surgical Instruments, Vernon Hills, Ill.)
Another major change, which allowed for advancement in the field of endoscopic spinal surgery, was the emphasis on placement of the cannula closer to the epidural space and the base of the targeted disc herniation. This enabled surgeons to target extruded herniations in addition to contained herniations. Previous percutaneous modalities all focused on entry through Kambin’s triangle and working within the center of the disc with the cannula anchored inside the annulus. The cannula was advanced past the annulus and remained there under fluoroscopic control. Mathews’ transforaminal approach for microdiscectomy allowed for routine visualization of the epidural space and greater access to the traversing nerve root.35
The development of a working channel scope and use of the transforaminal approach using beveled and slotted cannulas enhanced endoscopic lumbar surgery. Using this approach, surgeons can operate under full visualization throughout most of the procedure and follow the neural structures into the epidural space. The specialized cannulas provide greater access to pathology and help protect and retract sensitive anatomy such as the exiting nerve and dorsal root ganglion. The working channel also allowed the passage of high-speed burrs for bone removal and direct foraminal enlargement and decompression of foraminal stenosis (foraminoplasty) (Fig. 59–3).
Indications/Contraindications
Radiofrequency energy can be applied to the annular tears under direct visualization to contract the collagen and ablate ingrown granulation tissue, neoangiogenesis, and sensitized nociceptors.36 Frequently interpositional nuclear tissue is seen within the fibers of the annular tear, preventing the tear from healing. This tissue can then be removed to allow the tear to heal.
Endoscopic foraminoplasty can be readily achieved with bone trephines/rasps, the side-firing Holmium-YAG laser, and endoscopic high-speed drills.5,6 The roof of the foramen is formed by the undersurface of the superior articular facet. This is easily visualized and accessed via the endoscope, and the previously mentioned tools are used to remove bone and enlarge the foraminal opening. Synovial cysts can also be visualized and removed.
In cases of discitis the posterolateral endoscopic approach will provide a robust biopsy for culture diagnosis, and the infected/necrotic disc tissue can be thoroughly débrided to reduce the bacterial load and accelerate healing.37
Contraindications include any pathology not accessible from the posterolateral endoscopic approach. This may include some extruded sequestered disc herniations, extruded migrated disc herniations (migrated extent greater than the measured height of the posterior marginal disc space on T2 sagittal magnetic resonance imaging [MRI]), larger herniations occupying greater than 50% of the spinal canal,4 recurrent or virgin disc herniations with associated epidural scarring, moderate-severe central canal stenosis, and hard calcified herniations. These contraindications are considered relative contraindications dependent on the surgeons’ technical experience and comfort level. More experienced endoscopic surgeons can gain greater access to pathology using advanced techniques for bone removal of osteophytes, stenosis, and the posterolateral corner of the vertebral body before addressing the pathology. Other relative contraindications include inadequate support staff or equipment to successfully perform procedure and uncooperative patients.
Step-by-Step Operative Technique
Needle Placement
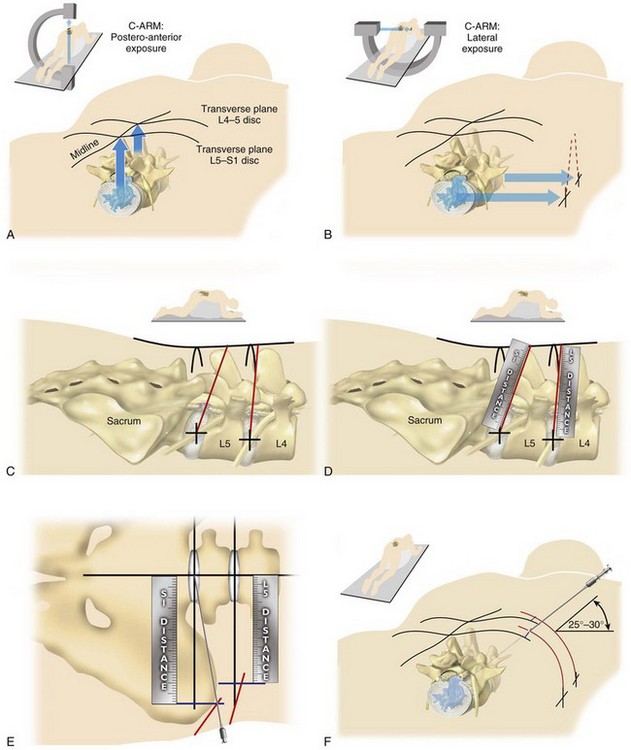
Optimal needle placement is the most crucial step of the procedure and is based on the type of pathology being addressed. The specific protocol21 is demonstrated in the accompanying DVD. The skin window (needle insertion site) is determined using this protocol. This will typically be about 12 to 15 cm lateral to the midline aligned parallel to the disc inclination (Fig. 59–3).
Instrument Placement
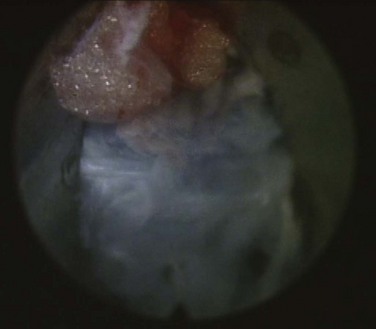
The next step is the through-and-through fenestration of the annular window by advancing the bluntly tapered obturator with a mallet. Annular fenestration is the most painful step of the entire procedure. Advise the anesthesiologist to heighten the sedation level just before annular fenestration. Advance the obturator tip deep into the annulus and confirm on the C-arm views. Now slide the beveled access cannula over the obturator toward the disc. Advance the cannula until the beveled tip is deep in the annular window with the beveled opening facing dorsally. Remove the obturator and insert the endoscope to get a view of the disc nucleus and annulus (Fig. 59–4). The subsequent steps depend on the goal of the procedure and pathology being addressed. The basic endoscopic method to excise a noncontained paramedian extruded lumbar herniated disc via a uniportal technique is described here. Different steps are used for other pathology and are beyond the scope of this chapter.
Performing the Discectomy
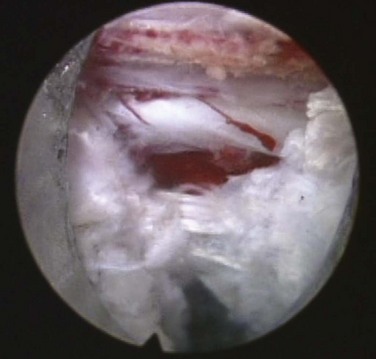
First enlarge the annulotomy medially to the base of the herniation with a cutting forcep. The side-firing Holmium-YAG laser can also be used to enlarge and widen the annulotomy. This is performed to release the annular fibers at the herniation site that may pinch off or prevent the extruded portion of the herniation from being extracted. Directly under the herniation apex a large amount of blue stained nucleus is usually present, likened to the submerged portion of an iceberg. The nucleus here represents a migrated and unstable nucleus. The endoscopic rongeurs are used to extract the blue-stained nucleus pulposus under direct visualization. The larger straight and hinged rongeurs are used directly through the cannula after the endoscope is removed. Fluoroscopy and surgeon feel guide this step. By grabbing the base of the herniated fragment, one can usually extract the extruded portion of the herniation. Initial medialization and widening of the annulotomy reduce the prospect of breaking off the herniated nucleus and retaining the apex of the herniation in the spinal canal. The traversing nerve root is readily visualized after removal of the extruded herniation (Fig. 59–5).
Clinical Outcomes
Yeung has reported his initial results using the YESS system in his first 307 patients with disc herniations who were candidates for open microdiscectomy. The study included intracanal and extracanal herniations. Recurrent herniations and patients with previous surgery at the same level were not excluded. Results were reported with 1-year follow-up. Overall patient satisfaction was found to be 91%. The same percentage of patients said they would undergo the procedure again if faced with the same diagnosis. The overall complication rate was 3.5%.22 Tsou and Yeung separated out a subgroup of 219 patients with noncontained herniations and reported results at 1 year. Patient satisfaction was 91%.23 These initial results demonstrated that endoscopic surgery could provide equivalent results to reported results of open microdiscectomy, even with noncontained herniations.
There are three prospective randomized studies comparing traditional microdiscectomy and percutaneous endoscopic discectomy. Hermantin performed a prospective randomized study with 30 patients in each group. The mean duration of follow-up was 31 months. Patient satisfaction was 93% in the open surgical group and 97% in the endoscopic group. The endoscopic group had shorter duration of narcotic use and shorter time out of work compared with open discectomy.1 Mayer performed a randomized prospective study in 1993 with 20 patients in each group. He chose return to previous occupation as his measurement of success. This study showed a significant difference in this outcome measure. In the percutaneous group 95% of patients returned to their previous profession, whereas only 72% of the microdiscectomy group returned to a previous profession.2 In 2008 Ruetten compared traditional microdiscectomy with full endoscopic discectomy via either the transforaminal or interlaminar route. There were 178 patients (87 microdiscectomy and 91 endoscopic) with 2-year follow-up. The microdiscectomy group had a 79% success rate and the full endoscopic group had an 85% success rate with no leg pain at all.3 It is noteworthy that all three of these prospective randomized studies showed a trend toward better outcomes with the endoscopic procedure, but statistically they were comparable.
Kambin reported an 82% success rate for the treatment of lateral recess stenosis and foraminal herniations using an oval cannula with two portals and the transforaminal approach. Even though they were working next to the exiting nerve root, they reported no neurovascular complications in their series.38 Successful posterolateral endoscopic treatment of foraminal and extraforaminal herniations has been described by many authors. Lew reported an 85% success rate in 47 patients,39 Choi reported a 92% success rate in 41 patients,40 Jang reported an 85% success rate in 35 patients,41 and Sassani reported an 89% success rate in 66 patients.42
Knight and Goswami6 have reported on the use of the endoscope in foraminal decompressions for isthmic spondylolisthesis. In 79% of patients a good or excellent outcome was obtained with an average follow-up of 34 months. Of the initial group only two went on to have spinal fusion.6
Ahn and colleagues43 reported an 81% success rate with PELD on 43 patients with recurrent disc herniations after a posterior microdiscectomy. Hoogland also had good success (85%) using endoscopic transforaminal discectomy for recurrent herniations in 262 consecutive cases from 1994 to 2002. Of the 262 patients, 194 had a previous posterior microdiscectomy and 68 had a prior endoscopic discectomy.44 Both studies pointed out the advantage of avoiding the posterior scar tissue.
The ability to effectively remove pathology using endoscopic surgery has been validated by postprocedure imaging studies. Casey and colleagues45 looked at a group of patients who had immediate postoperative computed tomography scans. The imaging studies demonstrated 88.9% of patients undergoing biportal endoscopy had significant reduction in the amount of neural compromise. The results of uniportal, extraforaminal, and foraminal herniations showed only mild to moderate change in canal diameter. They concluded that arthroscopic discectomy had a high rate of canal clearance and removal of disc fragments.45
Lee and colleagues4 reported on a matched cohort comparing radiographic changes 3 years postsurgery in PELD versus posterior microdiscectomy. They revealed less degenerative progression in the PELD group with loss of disc height and foraminal height being statistically significant. Clinical success rates were 96% in the PELD group and 93% in the microdiscectomy group. The authors conclude that PELD is a less invasive procedure that causes less approach related damage and less damage to the targeted disc.4
Complications and Avoidance
The risks of serious complications or injury are low—approximately 3%. The usual risks of infection, nerve injury, dural tears, bleeding, and scar tissue formation are present, as with any surgery. Because the transforaminal endoscopic approach passes adjacent to the exiting spinal nerve root and dorsal root ganglion, there is potential for nerve irritation (dysesthesia) or overt nerve damage. Dysesthesia occurrence is 5% to 15% and is almost always transient.22,23 This rate of occurrence is similar to dysesthesia rates in posterior open discectomy, but in the latter situation, because the dysesthesia affects the retracted traversing nerve root that was already the source of radiculopathy, the transient persistent or increased postoperative dysesthesia is generally not considered a complication after posterior discectomy. Both situations, however, are transient the vast majority of the time. Routine injection of steroid medication at the conclusion of the endoscopic discectomy has reduced the rates of dysesthesia significantly.
Future Considerations
Pearls
Pitfalls
Key Points
1 Hermantin FU, Peters T, Quartararo L, et al. A prospective randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg. 1999;81-A:958-965.
2 Ahn Y, Lee SH, Park WM, et al. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29:326-332.
3 Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome and complications in 307 consecutive cases. Spine. 2002;27:722-731.
4 Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal Lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33:930-939.
5 Lee SH, Kang B, Ahn Y, et al. Operative failure of percutaneous endoscopic lumbar discectomy: a radiologic analysis of 55 cases. Spine. 2006;31:E285-E290.
1 Hermantin FU, Peters T, Quartararo L, et al. A prospective randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg. 1999;81-A:958-965.
2 Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78:216-225.
3 Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33:930-939.
4 Lee SH, Chung SE, Ahn Y, et al. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med. 2006;73:795-801.
5 Knight MTN, Goswami AKD. Endoscopic laser foraminoplasty. In: Savitz MH, Chiu JC, Yeung AT, editors. The Practice of Minimally Invasive Spinal Technique. Richmond, VA: AAMISMS Education, LLC; 2000:337-340.
6 Knight M, Goswami A. Management of isthmic spondylolisthesis with posterolateral endoscopic foraminal decompression. Spine. 2003;28:573-581.
7 Hirsh C, Ingelmark B, Miller M. The anatomic basis for low back pain. Acta Orthop Scand. 1963;33:1-17.
8 Choy DS, Altman P. Fall of intradiscal pressure with laser ablation. J Clin Laser Med Surg. 1995;13:149-151.
9 Prodoehl JA, Lane GJ, Black J, et al. The effects of lasers on intervertebral disc pressures. Spine: State of the Art Reviews. 1993;7:17-21.
10 Nerubay J, Caspi I, Levinkopf M, et al. Percutaneous laser nucleolysis of the intervertebral lumbar disc. Clin Orthop. 1997;337:42-44.
11 Chatterjee S, Foy PM, Findlay GF. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20:734738.
12 Grevitt MP, McLaren A, Shakelford IM, et al. Automated percutaneous lumbar discectomy—an outcome study. J Bone Joint Surg (Br). 1995;77-B:626-629.
13 Ramberg N, Sahlstrand T. Early and Long-term follow-up after automated percutaneous lumbar discectomy. J Spinal Discord. 2001;14:511-517.
14 Choy DS, Asher PW, Ran HS, et al. Percutaneous laser decompression: a new therapeutic modality. Spine. 1992;17:949-956.
15 Valls J, Ottolenghi CE, Schajowicz F. Aspiration biopsy in diagnosis of lesions of vertebral bodies. JAMA. 1948;136:376-382.
16 Craig FS. Vertebral-body biopsy. J Bone Joint Surg (Am). 1956;38A:93-102.
17 Kambin P, Gellman H. Percutaneous lateral discectomy of the lumbar spine. Clin Orthop. 1983;174:127-132.
18 Hijikata S, Yamagishi N, Nakayama T, et al. Percutaneous discectomy: a new treatment method for lumbar disc herniation. J Toden Hosp. 1975;5:5-13.
19 Yeung AT, Yeung CA. In vivo endoscopic visualization of patho-anatomy in Painful degenerative conditions of the lumbar spine. Surgical Tech Int. 2006;XV:243-256.
20 Onik GM, Helms C, Hoaglund F, et al. Successful percutaneous lumbar discectomy using a new aspiration probe: a case report. Am J Radiol. 1985;6:290-293.
21 Yeung AT, Yeung CA. Posterolateral selective endoscopic discectomy: the YESS Technique. In: Kim D, Fessler R, Regan J, editors. Endoscopic Spine Surgery and Instrumentation: Percutaneous Procedures. New York: Thieme; 2004:201-211.
22 Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome and complications in 307 consecutive cases. Spine. 2002;27:722-731.
23 Tsou PM, Yeung AT. Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: outcome and technique. Spine J. 2002;2:41-48.
24 Kambin P, Bradger MD. Percutaneous posterolateral discectomy: anatomy and mechanism. Clin Orthop. 1987;223:145-154.
25 Kambin P. Arthroscopic Microdiscectomy: Minimal Intervention in Spinal Surgery. Baltimore: Urban and Schwarzenberg; 1991.
26 Kambin p, McCullen G, Parke W, et al. Minimally invasive arthroscopic spinal surgery. Instr Course Lect. 1997;46:1443-1461.
27 Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine. 1995;20:1965-1971.
28 Kambin P, Schaffer JL. Percutaneous posterolateral discectomy and decompression with a 6.9mm cannula. J Bone Joint Surg (Am). 1991;73-A:822-831.
29 Kambin P. Arthroscopic microdiscectomy. Arthroscopy. 1992;8:287-295.
30 Kambin P, O’Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop. 1998;347:150-167.
31 Kambin P, Savitz MH. Arthroscopic microdiscectomy: an alternative to open disc surgery. Mount Sinai J Med. 2000;67:283-287.
32 Kambin P, Zhou L. Arthroscopic discectomy of the lumbar spine. Clin Orthop. 1997;337:49-57.
33 Kambin P, Zhou L. History and current status of percutaneous arthroscopic disc surgery. Spine. 1996;21:57S-61S.
34 Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mount Sinai J Med. 2000;67:327-332.
35 Mathews HH. Transforaminal endoscopic microdiscectomy. Neurosurg Clin North Am. 1996;7:59-63.
36 Tsou PM, Yeung CA, Yeung AT. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J. 2004;4:564-573.
37 Ito M, Abumi K, Kotani Y, et al. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine. 2007;32:200-206.
38 Kambin P, Casey K, O’Brien E, et al. Transforaminal arthroscopic decompression of the lateral recess stenosis. J Neurosurg. 1996;84:462-467.
39 Lew SM, Mehalic TF, Fagone KL. Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniation. J Neurosurg. 2001;94:216-220.
40 Choi G, Lee SH, Bhanot A, et al. Percutaneous endoscopic discectomy for extraforaminal lumbar disc herniations. Spine. 2007;32:93-99.
41 Jang JS, An SH, Lee SH. Transforaminal percutaneous endoscopic discectomy in the Treatment of foraminal and extraforaminal lumbar disc herniations. J Spinal Discord Tech. 2006;19:338-343.
42 Sasani M, Oktenoglu T, Canbulat N, et al. Percutaneous endoscopic discectomy for far lateral lumbar disc herniations: prospective study and outcome of 66 patients. Minim Invas Neurosurg. 2007;50:91-97.
43 Ahn Y, Lee SH, Park WM, et al. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29:326-332.
44 Hoogland T, van den Brekel-Dijkstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: a prospective, cohort evaluation of 262 consecutive cases. Spine. 2008;33:973-978.
45 Casey KF, Chang MK, O’Brien ED, et al. Arthroscopic microdiscectomy: comparison of preoperative and postoperative imaging studies. Arthroscopy. 1997;13:438-445.