Posterior Urethral Valves
Posterior urethral valves (PUV) are the most common cause of bladder outlet obstruction in boys, with an incidence of 1 in 5,000 to 8,000 male births.1 Although the majority of boys with PUV are diagnosed antenatally, approximately one-third will be diagnosed during childhood or adolescence. PUV is the most common obstructive cause of end-stage renal disease (ESRD) in children, and is the etiology for approximately 35% of children who require renal transplantation.2
Embryology and Anatomy
At 5 to 6 weeks’ gestation, the orifice of the mesonephric duct migrates from an anterolateral position in the cloaca to Müller’s tubercle on the posterior wall of the urogenital sinus, occurring simultaneously with division of the cloaca. Remnants of the mesonephric duct normally remain as small distinct, paired lateral folds termed the inferior urethral crest and plicae colliculi. When the insertion of the mesonephric ducts into the cloaca is too anterior, normal migration of the ducts is impeded, and the ducts fuse anteriorly, resulting in abnormal ridges, which become the PUV. A smaller aperture between the leaflets causes more obstruction than those with a larger aperture and a less prominent anterior component.3
Three distinct types of PUV have been described. Type I is an obstructing membrane that radiates distally and anteriorly from the verumontanum toward the membranous urethra, fusing in the midline. Approximately 95% of PUV are type I, in which the valves are thought to be a single membranous structure with the opening positioned posteriorly near the verumontanum. Type III appears as a membranous diaphragm with a central opening at the verumontanum. The obstructing tissue also has been termed a congenital obstructing posterior urethral membrane.4 It is thought that instrumentation with a urethral catheter might disrupt the posterior aspect of the membrane, resulting in the appearance of a type I valve. Type II valves are prominent longitudinal folds of hypertrophied smooth muscle that radiate cranially from the verumontanum to the posterolateral bladder neck, but these are nonobstructive and clinically insignificant.
Antenatal Diagnosis, Management, and Outcomes
About 10% of antenatally diagnosed obstructive uropathy is due to PUV, and approximately two thirds of PUV are diagnosed antenatally. Typical findings on ultrasound (US) include bilateral hydroureteronephrosis, a distended bladder, and a dilated prostatic urethra, called a ‘keyhole’ sign.5 Discrete focal cysts in the renal parenchyma are diagnostic of renal dysplasia. Amniotic fluid volume is variable. Those with normal or slightly reduced amniotic fluid have a better prognosis. In contrast, oligohydramnios suggests significant obstructive uropathy, and pulmonary hypoplasia secondary to renal dysplasia is common. Oligohydramnios prevents normal lung development in utero. Pathologically, this process results in reduced branching of the bronchial tree and reduced numbers and size of alveoli.
The gestational age at which hydronephrosis is recognized influences prognosis. In one study, fetuses with normal appearing renal anatomy before 24 weeks were more likely to have normal renal function than were those with hydronephrosis before 24 weeks.6 One meta-analysis showed the best predictive value for postnatal renal function was the appearance of the fetal renal cortex.7 A more recent study showed that ultrasound parameters alone are not able to predict postnatal renal function.8 Prune-belly syndrome, urethral atresia, and bilateral high-grade vesicoureteral reflux (VUR) can have a similar prenatal sonographic appearance to PUV. Collectively, bladder outlet obstruction found prenatally can result in a significant (57%) incidence of renal failure by 2 years of age.9
In the fetus with suspected PUV and normal amniotic fluid volume, serial fetal sonograms are necessary to monitor the status of the hydronephrosis and amniotic fluid volume. If oligohydramnios develops, bladder drainage may help restore the amniotic fluid and allow normal pulmonary development. Before any intervention, a karyotype should be obtained to confirm the male gender and to detect chromosomal abnormalities, which occur in about 12%.10 Fetal renal function is assessed with serial urinary electrolytes and β2-microglobulin levels. Normally, fetal urine is hypotonic (favorable prognosis), with sodium less than 100 mEq/L, chloride less than 90 mEq/L, osmolality less than 210 mEq/L, and β2-microglobulin levels less than 6 mg/L.5 Elevated fetal urine electrolytes and β2-microglobulin levels are an indication of irreversible renal dysfunction. Sequential bladder aspiration every 48 to 72 hours should be performed because the initial urine sample may be stagnant and fresh urine more accurately reflects the function of the fetal kidneys.11,12
If fetal urine is hypotonic, and oligohydramnios is present, then fetal intervention to restore the amniotic fluid volume should be considered, with the goal of preventing life-threatening pulmonary hypoplasia. This procedure has been performed in the first trimester,13 although the majority of fetuses are diagnosed and treated in the second trimester. If the gestational age of the fetus is ≥32 weeks, early delivery is advisable. If the fetus is <32 weeks’ gestation, however, the urine may be diverted into the amniotic fluid with a percutaneously placed vesicoamniotic shunt (VAS). In a recent meta-analysis, antenatal bladder drainage appears to improve perinatal survival and relieve bladder outlet obstruction, especially in those fetuses with poor prognostic criteria.14 To date, there is no evidence that drainage of the obstructed fetal bladder will improve renal or bladder function.
In theory, VAS does not allow the bladder to cycle. Consequently, when counseling expectant parents, they need to understand that their newborn may have limited renal function or ESRD, even if the drainage procedure is successful. VAS can have complications in up to 45%.5,15 The shunts become obstructed or displaced in 25% of cases, necessitating additional procedures that increase morbidity to the mother and fetus, and there is a 5% procedure-related chance of fetal loss. In addition, omental or bowel herniation through the fetal abdominal wall can occur.
Despite adequate bladder drainage, renal function may be so limited that the amniotic fluid volume remains low. In one study of high-risk fetuses identified in the first trimester with severe bilateral hydroureteronephrosis, bladder distention, and oligohydramnios managed with a VAS, a 60% overall survival rate and a 33% incidence of renal failure were found.16 In a review of 14 fetuses with proven PUV and favorable fetal urinary electrolytes undergoing VAS at a mean gestational age of 22.5 weeks, six deaths occurred before term delivery. Of the surviving eight neonates, three had ESRD, and the other five had an elevated serum creatinine.17 In a more optimistic study of 20 boys with ‘lower urinary tract obstruction’ managed by VAS, the overall 1-year survival was 91%. In this study, the mean birth weight was 2574 g, 40% had acceptable renal function, 20% had mild renal insufficiency, and 30% required dialysis.18 Of this group, seven had PUV and seven had prune-belly syndrome, a nonobstructive condition. This lack of evidence regarding fetal drainage is the impetus for the study on Percutaneous Shunting in Lower Urinary Tract Obstruction (PLUTO) trial, which is an international randomized controlled trial comparing the effect of VAS versus no intervention. This study is evaluating prenatal and perinatal mortality, renal function, and other variables.19
Fetal ultrasound is useful in the diagnosis of lower urinary tract obstruction (LUTO). Sonographic features of LUTO include bilateral hydronephrosis, a thick wall dilated bladder, and dilated upper urethra. Unfortunately, there are a number of etiologies for this sonographic appearance, including urethral atresia, prune-belly syndrome, as well as PUV. The first two causes would not necessarily be an indication for either VAS or in utero endoscopic ablation. Percutaneous endoscopic ablation is now being performed in a few centers in the USA.20–22 One meta-analysis showed a perinatal survival advantage using endoscopic ablation compared to observation, but there was no significant improvement in survival with ablation when compared to VAS.23 Two small studies have evaluated the role of fetal cystoscopy to improve the accuracy of the prenatal ultrasound findings regarding a definite etiology.24,25 In addition, if PUV is identified at cystoscopy, then ablation can be considered.
Clinical Presentation
Neonates with PUV not diagnosed prenatally can present with symptoms of delayed voiding or a reduced urinary stream.15 Also, respiratory distress secondary to pulmonary hypoplasia may be the primary manifestation of PUV. Other postnatal signs and symptoms include an abdominal mass, failure to thrive, lethargy, poor feeding, urinary tract infection (UTI), and urinary ascites. Physical examination in the newborn typically discloses a palpable walnut-sized bladder, secondary to the hypertrophic detrusor muscle. Urinary ascites can result in significant abdominal distention. Older boys can have persistent diurnal incontinence or abdominal distention.
Radiographic Evaluation
Significant bilateral hydroureteronephrosis and a thick-walled, distended bladder are seen on ultrasound. Corticomedullary differentiation is a favorable prognostic sign regarding renal function (Fig. 57-1). Conversely, echogenic kidneys or subcortical cysts and the loss of corticomedullary differentiation are unfavorable signs. Suprapubic or perineal ultrasound may demonstrate a dilated prostatic urethra, which is pathognomonic for PUV.
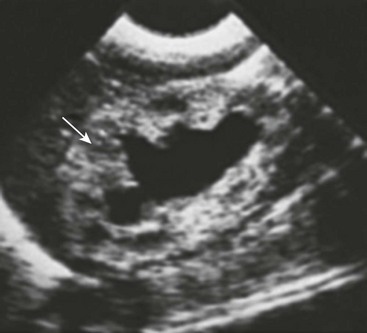
FIGURE 57-1 This renal sonogram demonstrates a hydronephrotic kidney with intact corticomedullary junction (arrow) in an infant with posterior urethral valves.
The voiding cystourethrogram (VCUG) is the only radiographic study that definitively establishes the diagnosis of PUV (Fig. 57-2). The valves appear as a defined lucency in the distal prostatic urethra. The posterior urethra is dilated and elongated. The bladder is trabeculated due to muscular hypertrophy with a clear delineation of the bladder neck. Unilateral VUR is present in 25% and bilateral VUR in 25% of infants with PUV.
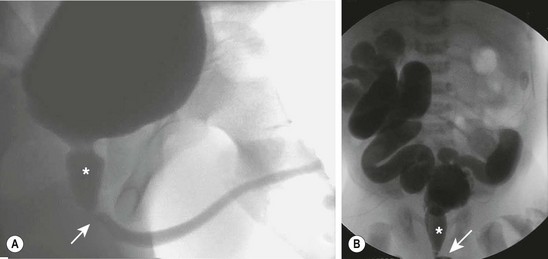
FIGURE 57-2 These two voiding cystourethrograms show varying degrees of obstruction from posterior urethral valves. In both studies the location of the valves is marked with an arrow and the posterior urethra is identified with an asterisk. (A) There is no evidence of vesicoureteral reflux. (B) There is massive, bilateral grade V reflux.
Renal nuclear scintigraphy with a technetium-99m–labeled dimercaptosuccinic acid (99mTc-DMSA) is performed if imaging studies show thin or abnormal parenchyma in either kidney on ultrasound and/or high-grade VUR. The study should be delayed until 6 to 8 weeks of age to allow maturation of renal function. This study is effective in establishing baseline differential renal function. However, if renal function is poor, visualization of the kidneys will be suboptimal. An alternative to renal scintigraphy is dynamic contrast-enhanced magnetic resonance urography (MRU). This study provides high-resolution renal images and assessment of differential renal function, but requires an anesthetic.26
Initial Management
Primary Valve Ablation
Endoscopic valve ablation is performed after the neonate is stabilized. Well-lubricated infant urethral sounds should be passed to gently dilate the meatus and glandular urethra. The neonatal male urethra usually accepts a 9.5 French endoscope. Overly aggressive dilation of the urethra in order to pass a larger endoscope may lead to urethral trauma with subsequent stricture formation, and should be avoided. Vigorous dilation may also result in iatrogenic hypospadias due to splitting of the glans to the subcoronal level.27
An 8 French or 9.5 French cystoscope typically is used with a Bugbee electrode on low cutting current inserted through the operating channel. The valve leaflets should be incised using a low cutting current at the 5 and 7 o’clock positions (Fig. 57-3). Incision at the 12 o’clock position, where the valve leaflets fuse, also may be helpful. An alternative technique employs the Nd : YAG laser.28 In a premature or small neonate, a cystoscope as small as 6.9 French can be used, although visualization of the PUV may be suboptimal. If urethral bleeding develops, coagulation should be performed carefully because injury to the urethra can occur with overzealous cautery. Following valve ablation, a pediatric feeding tube is left for one to two days.
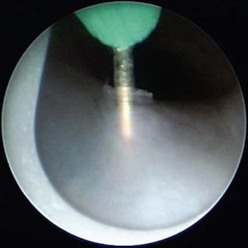
FIGURE 57-3 This cystoscopic view shows valve ablation with an electrode placed through the operating channel of the cystoscope.
A VCUG and renal ultrasound should be obtained two to four weeks after ablation to confirm satisfactory valve disruption and assess the upper urinary tracts. In addition, renal function should be monitored carefully. Valve ablation is successful in more than 90% of patients. The most common complication is incomplete valve ablation in which case repeat cystoscopy and valve incision is necessary. Urethral stricture is uncommon if small endoscopes are used.
Temporary Urinary Diversion
An alternative to primary valve ablation is cutaneous vesicostomy (Fig. 57-4). This approach is appropriate in a small or premature neonate when the pediatric cystoscope is too large for the urethra or if severe hydroureteronephrosis, urinary ascites, or high-grade VUR and poor renal function are present. In these cases, optimal upper urinary tract drainage is necessary to maintain existing renal function. The most popular technique was described by Blocksom and popularized by Duckett.29 A small transverse incision is performed midway between the umbilicus and pubic symphysis, and the dome of the bladder is brought to the skin. The vesicostomy should calibrate to 24–26 French to avoid stenosis. Daily dilation of the stoma with a plastic medicine dropper helps prevent stomal contraction. The vesicostomy allows urine to drain directly into the diaper, obviating the need for a collection device. Complications include stomal stenosis, if the stomal size is less than 24–26 French, and prolapse, if the anterior wall of the bladder is exteriorized rather than the bladder dome.
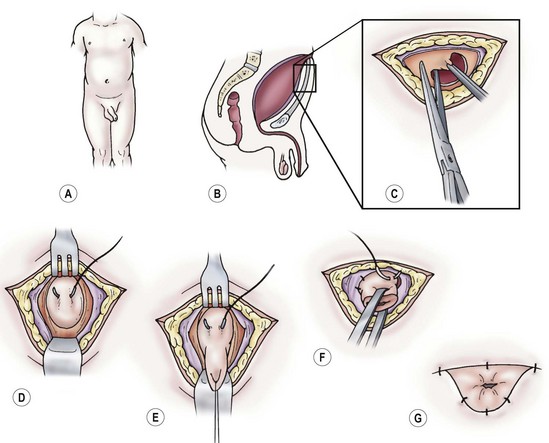
FIGURE 57-4 Technique of cutaneous vesicostomy. (A–C) Transverse incision is made midway between the umbilicus and pubic symphysis. (D,E) Traction sutures are placed through the bladder, and it is mobilized superiorly to the dome of the bladder. (F) The detrusor should be fixed to the rectus fascia. The bladder is opened, and the mucosa is sutured to the skin. (G) Completed vesicostomy should be calibrated to 24 French.
In the past, after insertion of a urinary catheter into the bladder, proximal diversion with cutaneous pyelostomy or cutaneous ureterostomy was advocated for neonates and infants with severe hydronephrosis and a persistently elevated creatinine.30 Theoretically, proximal diversion provides better renal drainage than a vesicostomy, particularly with ureterovesical obstruction, and optimizes the potential for renal function and somatic growth. However, proximal drainage has not been shown to prevent ESRD because at least 85% of these patients have renal dysplasia.31 In addition, by diverting the urine away from the bladder, regular cyclical bladder filling and contraction does not occur which results in a smaller, less compliant bladder.32
Currently, proximal diversion is reserved for the rare case in which valve ablation or vesicostomy fails to improve upper tract drainage. When needed, the preferred method of proximal diversion is the Sober-en-T ureterostomy in which the proximal ureter is divided and exteriorized on the abdominal wall (Fig. 57-5). The proximal end of the distal ureteral segment is then anastomosed to the renal pelvis. The advantage of this form of diversion is that it allows urine to drain into the bladder, thereby maintaining bladder cycling, while providing good upper tract drainage.33 In one retrospective study of 36 boys who underwent bilateral Sober ureterostomies, the mean duration of diversion was 55 months.34 Bladder compliance was normal in 69%, and bladder capacity was normal in 80%.
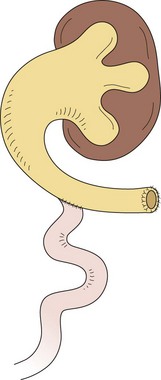
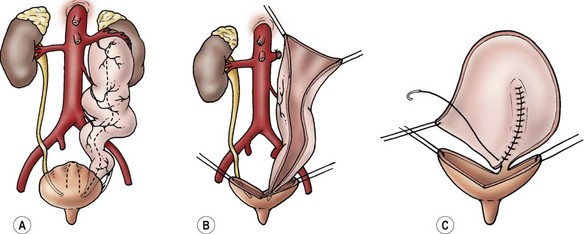
FIGURE 57-5 This diagram describes the Sober-en-T cutaneous ureterostomy, which is the authors’ preferred method for proximal diversion in patients with posterior urethral valves. The proximal ureter is divided and exteriorized on the abdominal wall. The proximal end of the distal ureteral segment is then anastomosed to the renal pelvis.
Rupture of the renal fornix with urinary extravasation and transudation into the peritoneum occurs in 5% to 15% of neonates with PUV.35,36 Some infants develop a perirenal urinoma, whereas others have urinary ascites (Fig. 57-6). The differential renal function of kidneys with urinoma vs. those without is similar. However, with urinary ascites, significant electrolyte abnormalities can result from urinary reabsorption, and respiratory compromise may also occur from the abdominal distention.
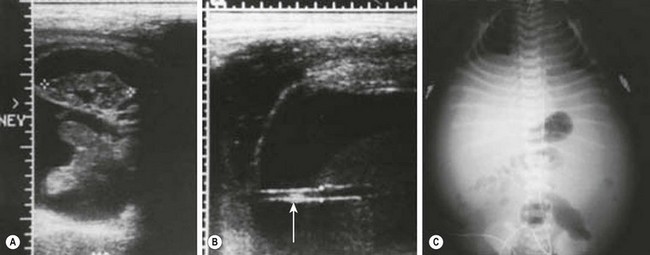
FIGURE 57-6 Posterior urethral valves and ascites. (A) Prenatal ultrasound image demonstrating a perirenal urinoma around the right kidney, which is not hydronephrotic. (B) Prenatal ultrasound image showing ascites and stretched umbilical vessels (arrow). (C) Plain radiograph of the abdomen in a neonate with a distended abdomen from urinary ascites.
Follow-up After Initial Therapy
Early treatment with anticholinergic therapy (oxybutynin) may also be beneficial. In a nonrandomized study of infants with PUV, treatment with oxybutynin for two years resulted in significant reduction in high voiding pressures and significant improvement in bladder capacity.37
Prognosis After Initial Therapy
The prognosis for satisfactory renal function can be predicted from several factors. A serum creatinine concentration less than 0.8 mg/dL one month after initial treatment, or at age 1 year, is associated with favorable renal function.38 Others have shown that the serum creatinine level after four to five days of catheter drainage is also predictive of long-term renal function ultimately.39 Visualization of the corticomedullary junction differentiation on renal ultrasound is also associated with a favorable outcome (see Fig. 57-1).40 This radiologic finding may not be present on the initial ultrasound, but may become apparent during the first few months of life. Achieving diurnal continence by the age of 5 years indicates that minimal or no bladder dysfunction is present and is also a favorable finding.41 Another good prognostic feature is the presence of a pressure pop-off mechanism such as massive VUR into a nonfunctioning kidney (termed the VURD syndrome: valves, unilateral reflux, dysplasia), urinary ascites, or a large bladder diverticulum (Fig. 57-7).42 The concept is that the high intravesical pressure is dissipated, allowing more normal renal development. Although short-term studies have suggested that these mechanisms allow more normal renal development, at age 8 to 10 years only 30% of boys with the VURD syndrome have a normal serum creatinine.43 One important favorable prognostic sign is the normal appearance of the contralateral kidney at diagnosis. Such patients had no UTIs or incontinence with long-term follow-up.44 Finally, absence of reflux on the initial VCUG is also a favorable sign.
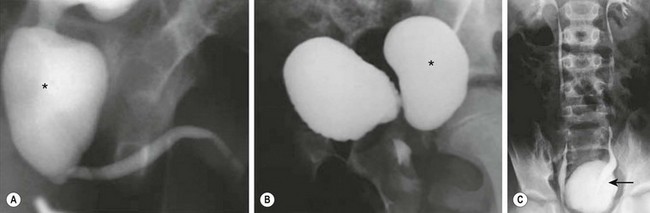
FIGURE 57-7 The VURD syndrome. A 7-year-old boy was found to have posterior urethral valves and a bladder diverticulum. (A) The voiding cystourethrogram demonstrates a large, dilated posterior urethra (asterisk) secondary to the valves. (B) The lateral view of this study shows a trabeculated bladder with a large bladder diverticulum (asterisk). (C) The excretory urogram shows normal upper urinary tracts and deviation (arrow) of the distal left ureter due to the large bladder diverticulum. This boy underwent endoscopic valve ablation, excision of the bladder diverticulum, and left ureteroneocystostomy.
Adverse prognostic factors include bilateral VUR, persistence of the serum creatinine higher than 1.0 mg/dL after initial therapy,45 identification of small subcapsular renal cysts (indicative of renal dysplasia), increased renal echogenicity,46 and loss of corticomedully differentiation.46 In addition, failure to achieve diurnal continence is an indication of bladder instability and detrusor sphincter dyssynergia (DSD), which can result in elevated upper urinary tract pressures and a gradual deterioration in renal function.
Review of studies of long-term renal function is difficult because of variable follow-up among study patients. In one report of 27 boys diagnosed between 1956 and 1970 with a 31- to 44-year follow-up, 18% died at an early age and 11% were lost to follow-up.47 Of the remaining surviving men, 32% were uremic, 21% had moderate renal insufficiency, and 40% had signs of bladder dysfunction. This historical study does not reflect the impact of early diagnosis with prenatal ultrasound and lower tract pharmacotherapy. For example, in a more recent study of 79 cases of PUV prenatally diagnosed between 1987 and 2004, 65 were live births managed with primary valve ablation. In follow-up, only 17% had renal failure and 76% of toilet-trained men were completely continent.48 Early gestational age at diagnosis and the presence of oligohydramnios were negative prognostic factors.
A contemporary retrospective study of 260 boys with PUV from 1992–2008 showed risk factors for progression to ESRD include nadir serum creatinine greater than 1.0 mg/dL, bilateral grade III or higher reflux at diagnosis, recurrent febrile UTIs, and severe bladder dysfunction. About 12% of these boys progressed to ESRD at a mean age of 11 years (range 5–16 years). Nadir serum creatinine and bladder dysfunction were found to be independent risk factors predictive of ultimate progression to ESRD.49 Another recent study confirmed the high prognostic value of an elevated creatinine after primary valve ablation.50
Late Diagnosis
Although late presentation of PUV has been thought to be a more benign entity, studies over the past 15 years have shown that this may not be true. In a study of 47 patients, age 5–35 years, found to have PUV postnatally, common presenting symptoms were daytime incontinence (60%), UTI (40%), and voiding pain (13%).51 Hydronephrosis was found in 40% and VUR in 33%. Serum creatinine was elevated in 35% and 10% had ESRD. If a VCUG had been performed only in those with hydronephrosis, an abnormal ultrasound, or a UTI, 30% of PUVs would not have been diagnosed.
A recent study evaluated the impact of the timing of diagnosis on long-term outcome in 52 patients with PUV who were diagnosed between 1994 and 2008.52 Thirty-nine boys were diagnosed by 1 year of age and 13 were diagnosed after 1 year. There was no statistical difference between groups in the rate of ESRD at a mean of 7.2 years following valve ablation. In the early diagnosis group, 10% required renal transplant, while no patient in the late diagnosis group developed ESRD, suggesting a lower risk of long-term renal insufficiency. Chronic renal failure (CRF) occurred in 52% with early diagnosis who had abnormal renal parenchyma while CRF developed in 33% in the late diagnosis group who had normal appearing kidneys.
A retrospective review was performed on 141 boys with PUV that presented after birth.53 Most (90%) patients were born after 1990 with a mean age of 46 months. The most common symptoms were UTI (28%) and voiding complaints (50%). In long-term follow-up, 12 patients (9%) had chronic renal disease. Five of the 12 had chronic disease at initial presentation without improvement following treatment, and seven of 12 developed chronic kidney disease 5 to 23 years after diagnosis. Disease progression was associated with bilateral hydronephrosis, increased severity of hydronephrosis, and bilateral VUR.
Vesicoureteral Reflux
At the initial presentation of PUV, VUR is present in approximately 50% of boys. Half of these boys will have bilateral VUR and half unilateral. After valve ablation, nearly all patients will show improvement in reflux grade at 1 year.32,54 Another 25% will develop spontaneous VUR. However, in these patients VUR may not resolve for as long as 3 years after initial treatment, and resolution of high-grade VUR is unlikely.55 Antibiotic prophylaxis is continued, and periodic upper tract imaging and cystography should be performed. Renal deterioration without infection may be a sign of bladder dysfunction. Lower tract evaluation with videourodynamics is important.
VUR should be corrected if breakthrough infections occur or if it remains high grade. The efficacy of endoscopic subureteric injection therapy has not been proved, but its use remains an option. Most pediatric urologists are adept at performing ureteral reimplantation, but reimplanting thick, dilated ureters into the abnormal bladder can be challenging. A 15–30% complication rate has been reported, most often persistent reflux or ureteral obstruction.56,57 If bilateral high-grade VUR is found, a transureteroureterostomy can be performed in conjunction with a single, long, tapered reimplant and a psoas hitch. However, if the single reimplanted ureter becomes obstructed, the upper tracts may deteriorate rapidly. If unilateral high-grade reflux into a kidney with reasonable function occurs, transureteroureterostomy into the nonrefluxing ureter is an option.
In boys with unilateral VUR into a dysplastic kidney, a nephrectomy should be performed at some point. The ureter should be removed unless the bladder is small and/or poorly compliant. In this case, an ureterocystoplasty can be considered (Fig. 57-8). Postoperatively, the remaining kidney should be monitored carefully for the development of hydronephrosis because the pressure ‘pop-off’ mechanism has been removed.
Bladder Dysfunction after Initial Therapy
The prognosis for boys with PUV depends on the status of the kidneys and the bladder at the time of diagnosis, and the method of bladder management as the child grows. In as many as 40% with PUV, ESRD or chronic renal insufficiency develops, and the vast majority of these boys have voiding dysfunction.41 Many boys with PUV have a spectrum of urodynamic abnormalities that change over time as they age. For example, in a study of 16 prepubertal boys with PUV who were seen before age 1 year and observed from ages 4 to 14 years, initial bladder overactivity was observed.58 Over time, however, the overactivity improved and the bladder capacity increased. Adolescent boys had a high-capacity bladder with low contractility and incomplete emptying. Other groups have reported similar findings.59,60
The bladder abnormalities seen in boys with PUV are manifested as incontinence and/or persistent hydronephrosis. Boys with significant urodynamic abnormalities most likely will develop severe renal functional impairment.61 The cause of the bladder dysfunction is not completely understood, but experimental evidence suggests that fetal urethral obstruction causes irreversible changes in the smooth muscle cells of the bladder62 and results in deposition of type III collagen in the bladder wall. In a study of fetuses with PUV, a greater than twofold increase in bladder wall thickness was demonstrated when compared to normal controls.63
As many as 50% of boys with PUV have ongoing daytime incontinence into late childhood.41 Significant urodynamic abnormalities may persist following relief of the bladder outlet obstruction. Several potential causes for urinary incontinence are known in boys with PUV,18,56 including the following:
1. Detrusor abnormalities such as (a) an overactive bladder secondary to uninhibited detrusor contractions, (b) overflow incontinence, (c) poor compliance, and (d) myogenic failure
2. High-pressure voiding secondary to incomplete valve ablation
3. DSD in which the sphincter muscle fails to relax during bladder contraction
4. Polyuria secondary to a concentrating defect as a result of long-standing obstructive uropathy that causes renal tubular damage
5. Valve bladder, which is a bladder with poor compliance resulting from fibrosis secondary to long-standing obstruction.64–66 This clinical situation can cause secondary ureteral obstruction with worsening hydronephrosis if the bladder pressure is greater than 35 cmH2O pressure (Table 57-1).
TABLE 57-1
Pathophysiologic Changes in the Valve Bladder Syndrome
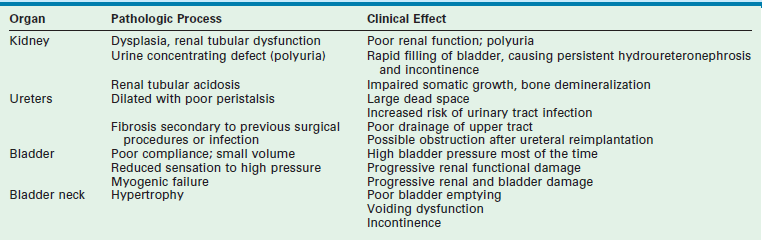
From Close CE. The valve bladder. In: Gillenwater JY, Grayhack JT, Howards SS, et al, editors. Adult and Pediatric Urology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 2311–18.
Consequently, long-term therapy for the boy with PUV includes management of the bladder as well as attention to renal function.67–73 See Chapter 56 for more information about management of bladder dysfunction.
Long-Term Risk of End-Stage Renal Disease
A recent study from Finland reported outcomes in 193/200 males with PUV from 1953 to 2003 with only seven patients lost to follow-up.74 With a median age of 31 years (range 6–69 years), 22.8% had progressed to ESRD. The lifetime risk of ESRD in this cohort was 28.5%. The time to progression to ESRD correlated with the lowest serum creatinine value during the first postoperative year. An increased risk of ESRD was associated with early presentation, pneumothorax, bilateral VUR, and recurrent UTI following valve ablation. No patient progressed to ESRD after their mid 30s. In another series, one-third developed ESRD.41 In a smaller series, 54% developed ESRD.75 With improved neonatal care and management of ESRD in newborns, it is likely that the risk of ESRD will decrease.
Renal Transplantation
In many cases, impaired renal function can be stabilized during childhood. However, during adolescence, there may be insufficient renal reserve, and dialysis or renal transplantation becomes necessary. Retrospective studies of boys with PUV undergoing renal transplantation have suggested that the valve bladder may have a detrimental effect on graft survival. For example, in an older study, a significantly worse 5-year graft survival was noted for patients undergoing transplantation for ESRD due to PUV than was found in patients with nonobstructive abnormalities.76 More recent studies, however, have demonstrated no difference in graft survival or serum creatinine levels between boys with PUV and children with nonobstructive causes of renal failure.77–80 These results may reflect more effective treatment of the valve bladder in the recent past.
Adult Sexual Function and Fertility
Few long-term studies have evaluated the reproductive status of men who were born with PUV. Theoretically, prostate function might be affected because of elevated urethral pressure during embryonic development and ongoing voiding dysfunction. In addition, some boys with PUV have reflux into the seminal vesicles and ejaculatory ducts as well as a history of cryptorchidism, which might contribute to impaired fertility. In a recent report, sexual function was assessed using the International Index of Erectile Function.81 There were 67 men with PUV and 102 age-matched controls with a mean age of 38 years in both groups. Increasing age was the only risk factor for developing erectile dysfunction. Only one of 61 (2%) sexually active men could not ejaculate. In this series, there was no increase in ejaculatory problems in those men who had undergone both PUV ablation and bladder neck incision, which was commonly performed several decades ago. Almost half of the men had children and four of seven with a renal transplant had children. Paternity rates were similar to the general Finnish population. Eight (12%) men had attempted to father children without success.
In another Finnish study, 68 men with PUV and 272 controls with a median age of 38.5 years responded to the Danish Prostatic Symptom Score.82 Mild hesitancy, weak stream, incomplete bladder emptying, and straining were twice as common in patients with PUV than controls. The prevalence of lower urinary tract symptoms (LUTS) was increased about twofold in PUV men when compared to controls. Most of the study group reported little to no symptoms. Infrequently, pyospermia and reduced sperm counts were noted in men with a history of PUV and severe LUTS. Azoospermia was uncommon, but when observed, it was usually related to CRF. Another study of 16 men treated for PUV in infancy reported that sexual function and voiding symptoms were normal, and their semen analysis was adequate for fertility.83
References
1. Malin, G, Tonks, A, Morris, R, et al. Congenital lower urinary tract obstruction: A population-based epidemiological study. BJOG. 2012.
2. Penna, FJ, Elder, JS. CKD and bladder problems in children. Adv Chronic Kidney Dis. 2011; 18:362–369.
3. Krishnan, A, de Souza, A, Konijeti, R, et al. The anatomy and embryology of posterior urethral valves. J Urol. 2006; 175:1214–1220.
4. Dewan, PA, Zappala, PG, Ransley, PG, et al. Endoscopic reappraisal of the morphology of congenital obstruction of the posterior urethra. Br J Urol. 1992; 70:439–444.
5. Elder, JS. Management of antenatal hydronephrosis. In: Puri P, ed. Newborn Surgery. 3rd ed. London: Hodder-Arnold; 2011:856–871.
6. Hutton, KA, Thomas, DF, Arthur, RJ, et al. Prenatally detected posterior urethral valves: Is gestational age at detection a predictor of outcome? J Urol. 1994; 152:698–701.
7. Morris, RK, Malin, GL, Khan, KS, et al. Antenatal ultrasound to predict postnatal renal function in congenital lower urinary tract obstruction: Systematic review of test accuracy. BJOG. 2009; 116:1290–1299.
8. Bernardes, LS, Salomon, R, Aksnes, G, et al. Ultrasound evaluation of prognosis in fetuses with posterior urethral valves. J Ped Surg. 2011; 46:1412–1418.
9. Anumba, DO, Scott, JE, Plant, ND, et al. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenat Diagn. 2005; 25:7–13.
10. Elder, JS, Duckett, JW, Snyder, HM. Intervention for fetal obstructive uropathy: Has it been effective? Lancet. 1987; 2:1007–1010.
11. Johnson, MP, Bukowski, TP, Reitleman, C, et al. In-utero surgical treatment of fetal obstructive uropathy: A new comprehensive approach to identify appropriate candidates for vesicoamniotic shunt therapy. Am J Obstet Gynecol. 1994; 170:1770–1779.
12. Johnson, MP, Corsi, P, Bradfield, W, et al. Sequential urinalysis improves evaluation of fetal renal function in obstructive uropathy. Am J Obstet Gynecol. 1995; 173:59–65.
13. Kim, SK, Won, HS, Shim, JY, et al. Successful vesicoamniotic shunting of posterior urethral valves in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2005; 26:666–668.
14. Morris, RK, Malin, GL, Khan, KS, et al. Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG. 2010; 17:382–390.
15. Cendron, M, Elder, JS. Perinatal urology. In: Gillenwater JY, Grayhack JT, Howards SS, Mitchell M, eds. Adult and Pediatric Urology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002:2041–2127.
16. Freedman, AL, Bukowski, TP, Smith, CA, et al. Fetal therapy for obstructive uropathy: Specific outcomes diagnosis. J Urol. 1996; 156:720–724.
17. Holmes, N, Harrison, MR, Baskin, LS. Fetal surgery for posterior urethral valves: Long-term postnatal outcomes. Pediatrics. 2001; 108:e1–e7.
18. Biard, JM, Johnson, MP, Carr, MC, et al. Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstet Gynecol. 2005; 106:503–508.
19. Morris, RK, Kilby, MD. An overview of the literature on congenital lower urinary tract obstruction and introduction to the PLUTO trial: Percutaneous shunting in lower urinary tract obstruction. Aust NZJ Obstet Gynecol. 2009; 49:6–10.
20. Quintero, RA, Romero, R, Johnson, MP, et al. In-utero percutaneous cystoscopy in the management of fetal lower obstructive uropathy. Lancet. 1995; 346:537–540.
21. Quintero, RA, Shukla, AR, Homsy, YL, et al. Successful in-utero endoscopic ablation of posterior urethral valves: A new dimension in fetal urology. Urology. 2000; 55:774.
22. Clifton, MS, Harrison, MR, Ball, R, et al. Fetoscopic transuterine release of posterior urethral valves: A new technique. Fetal Diagn Ther. 2008; 23:89–94.
23. Morris, RK, Kilby, MD. Long-term renal and neurodevelopmental outcome in infants with LUTO, with and without fetal intervention. Early Hum Dev. 2011; 87:607–610.
24. Welsh, A, Agarwal, S, Kumar, S, et al. Fetal cystoscopy in the management of fetal obstructive uropathy: Experience in a single European centre. Prenat Diagn. 2003; 30:1033–1041.
25. Ruano, R. Fetal surgery for severe lower urinary tract obstruction. Prenat Diagn. 2011; 31:661–674.
26. McMann, LP, Kirsch, AJ, Scherz, HC, et al. Magnetic resonance urography in the evaluation of prenatally diagnosed hydronephrosis and renal dysgenesis. J Urol. 2006; 176:1786–1792.
27. Shapiro, E, Elder, JS. Complications of surgery for posterior urethral valves. In: Taneja SS, Smith RB, Ehrlich RM, eds. Complications of Urologic Surgery. 4th ed. Philadelphia: WB Saunders; 2010:669–683.
28. Bhatnagar, V, Agarwala, S, Lal, R, et al. Fulguration of posterior urethral valves using the Nd:YAG laser. Pediatr Surg Int. 2000; 16:69–71.
29. Duckett, JW, Jr. Cutaneous vesicostomy in childhood: The Blocksom technique. Urol Clin North Am. 1974; 1:485–495.
30. Krueger, RP, Hardy, BE, Churchill, BM. Growth in boys with posterior urethral valves: Primary valve resection vs. upper tract diversion. Urol Clin North Am. 1980; 7:265–272.
31. Tietjen, DN, Gloor, JM, Husmann, DA. Proximal urinary diversion in the management of posterior urethral valves: Is it necessary? J Urol. 1997; 158:1008–1010.
32. Close, CE, Carr, MC, Burns, MW, et al. Lower urinary tract changes after early valve ablation in neonates and infants: Is early diversion warranted? J Urol. 1997; 157:984–988.
33. Liard, A, Seguier-Liqszyc, E, Mitrofanoff, P. Temporary high diversion for posterior urethral valves. J Urol. 2000; 164:145–148.
34. Ghanem, MA, Nijman, RJ. Long-term followup of bilateral high (sober) urinary diversion in patients with posterior urethral valves and its effect on bladder function. J Urol. 2005; 173:1721–1724.
35. Patil, KK, Wilcox, DT, Samuel, M, et al. Management of urinary extravasation in 18 boys with posterior urethral valves. J Urol. 2003; 169:1508–1511.
36. Hekkila, J, Taskinen, S, Rintala, R. Urinomas associated with posterior urethral valves. J Urol. 2008; 180:1476–1478.
37. Casey, JT, Hagerty, JA, Maizels, M, et al. Early administration of oxybutynin improves bladder function and clinical outsomes in newborns with posterior urethral valves. J Urol. 2012.
38. Smith, GH, Canning, DA, Schulman, SL, et al. The long-term outcome of posterior urethral valves treated with primary valve ablation and observation. J Urol. 1996; 155:1730–1734.
39. Denes, ED, Barthold, JS, Gonzalez, R. Early prognostic value of serum creatinine levels in children with posterior urethral valves. J Urol. 1997; 157:1441–1443.
40. Hulbert, WC, Rosenberg, HK, Cartwright, PC, et al. The predictive value of ultrasonography in evaluation of infants with posterior urethral valves. J Urol. 1992; 148:122–124.
41. Parkhouse, HF, Barratt, TM, Dillon, MJ, et al. Long-term outcome of boys with posterior urethral valves. Br J Urol. 1988; 16:59–62.
42. Kaefer, M, Keating, MA, Adams, MC, et al. Posterior urethral valves, pressure pop offs and bladder function. J Urol. 1995; 154:708–711.
43. Cuckow, PM, Dinneen, MD, Risdon, RA, et al. Long-term renal function in the posterior urethral valves, unilateral reflux and renal dysplasia syndrome. J Urol. 1997; 158:1004–1007.
44. Narasimhan, KL, Mahajan, JK, Kaur, B, et al. The vesicoureteral reflux dysplasia syndrome in patients with posterior urethral valves. J Urol. 2005; 174:1433–1435.
45. DeFoor, W, Clark, C, Jackson, E, et al. Risk factors for end stage renal disease in children with posterior urethral valves. J Urol. 2008; 180:1705–1708.
46. Duel, BP, Mogbo, K, Barthold, JS, et al. Prognostic value of initial renal ultrasound in patients with posterior urethral valves. J Urol. 1998; 160:1198–1200.
47. Holmdahl, G, Sillen, U. Boys with posterior urethral valves: Outcome concerning renal function, bladder function and paternity at ages 31 to 44 years. J Urol. 2005; 174:1031–1034.
48. Sarhan, O, Zaccaria, I, Macher, MA, et al. Long-term outcome of prenatally detected posterior urethral valves: Single center study of 65 cases managed by primary valve ablation. J Urol. 2008; 179:307–312.
49. Ansari, MS, Surdas, R, Farai, S, et al. Renal function reserve in children with posterior urethral valve- a novel test to predict long-term outcome. J Urol. 2011; 185:2329–2333.
50. Sarhan, OM, El-Ghoneimi, AA, Helmy, TE, et al. Posterior urethral valves: Multivariate analysis of factors affecting the final renal outcome. J Urol. 2011; 185:2491–2495.
51. Bomalaski, MD, Anema, JG, Coplen, DE, et al. Delayed presentation of posterior urethral valves: A not so benign condition. J Urol. 1999; 162:2130–2132.
52. Kibar, Y, Ashley, RA, Roth, CC, et al. Timing of posterior urethral valve diagnosis and its impact on clinical outcome. J Ped Urol. 2011; 7:538–542.
53. Engel, DL, Pope, JC, IV., Adams, MC, et al. Risk factors associated with chronic kidney disease in patient with posterior urethral valves without prenatal hydronephrosis. J Urol. 2011; 185:2502–2508.
54. Priti, K, Rao, KL, Menon, P, et al. Posterior urethral valves: Incidence and progress of vesicoureteric reflux after primary fulguration. Pediatr Surg Int. 2004; 20:136–139.
55. Hassan, JM, Pope, JC, IV., Brock, JW, III., et al. Vesicoureteral reflux in patients with posterior urethral valves. J Urol. 2003; 170:1677–1680.
56. Warshaw, BL, Hymes, LC, Trulock, TS, et al. Prognostic features in infants with obstructive uropathy due to posterior urethral valves. J Urol. 1985; 133:240–243.
57. El-Sherbiny, MT, Hafez, AT, Ghoneim, MA, et al. Ureteroneocystostomy in children with posterior urethral valves: Indications and outcome. J Urol. 2002; 168:1836–1840.
58. Holmdahl, G, Sillén, U, Hanson, E, et al. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol. 1996; 155:694–698.
59. De Gennaro, M, Capitanucci, ML, Mosiello, G, et al. The changing urodynamic pattern from infancy to adolescence in boys with posterior urethral valves. Br J Urol. 2000; 85:1104–1108.
60. Emir, H, Eroglu, E, Tekant, G, et al. Urodynamic findings of posterior urethral valve patients. Eur J Pediatr Surg. 2002; 12:38–41.
61. Ghanem, MA, Wolffenbuttel, KP, de Vylder, A, et al. Long term bladder dysfunction and renal function in boys with posterior urethral valves based on urodynamic findings. J Urol. 2004; 171:2409–2412.
62. Karim, OM, Cendron, M, Mostwin, JL, et al. Developmental alterations in the fetal lamb bladder subjected to partial urethral obstruction in-utero. J Urol. 1993; 150:1060–1063.
63. Freedman, AL, Qureshi, F, Shapiro, E, et al. Smooth muscle development in the obstructed fetal bladder. Urology. 1997; 49:104–107.
64. Mitchell, ME. Persistent ureteral dilatation following valve resection. Dial Pediatr Urol. 1982; 5:8–10.
65. Close, CE. The valve bladder. In: Gillenwater JY, Grayhack JT, Howards SS, eds. Adult and Pediatric Urology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002:2311–2318.
66. Donohoe, JM, Weinstein, RP, Combs, AJ, et al. When can persistent hydroureteronephrosis in posterior urethral valve disease be considered residual stretching? J Urol. 2004; 172:706–711.
67. Glassberg, KL. The valve bladder syndrome: 20 years later. J Urol. 2001; 166:1406–1414.
68. Capitanucci, M, Marciano, A, Zaccara, A, et al. Long-term bladder function followup in boys with posterior urethral valves: Comparison of noninvasive vs invasive urodynamic studies. J Urol. 2012; 188:953–957.
69. Cain, MP, Wu, SD, Austin, PF, et al. Alpha blocker therapy for children with dysfunctional voiding and urinary retention. J Urol. 2003; 1770:1514–1515.
70. Koff, SA, Mutabagani, KH, Jayanthi, VR. The valve bladder syndrome: Pathophysiology and treatment with nocturnal bladder emptying. J Urol. 2002; 167:291–297.
71. Montane, B, Abitbol, C, Seeherunvong, W, et al. Beneficial effects of continuous overnight catheter drainage in children with polyuric renal failure. BJU Int. 2003; 92:447–451.
72. Johal, NS, Hamid, R, Aslam, Z, et al. Ureterocystoplasty: Long-term functional results. J Urol. 2008; 179:2373–2375.
73. Austin, PF, Lockhart, JL, Bissada, NK, et al. Multi-institutional experience with the gastrointestinal composite reservoir. J Urol. 2001; 165:2018–2021.
74. Hekkila, J, Holmberg, C, Kyllonen, L, et al. Long-term risk of end stage renal disease in patients with posterior urethral valves. J Urol. 2011; 186:2392–2396.
75. Caione, P, Nappo, SG. Posterior urethral valves: Long-term outcome. Pediatr Surg Int. 2011; 27:1027–1035.
76. Reinberg, Y, Gonzalez, R, Fryd, D. The outcome of renal transplantation on children with posterior urethral valves. J Urol. 1988; 140:1491–1493.
77. Indudhara, R, Joseph, DB, Perez, LM, et al. Renal transplantation in children with posterior urethral valves revisited: A 10-year follow-up. J Urol. 1998; 160:1201–1203.
78. DeFoor, W, Tackett, L, Minevich, E, et al. Successful renal transplantation in children with posterior urethral valves. J Urol. 2003; 170:2402–2404.
79. Fine, MS, Smith, KM, Shrivastava, D, et al. Posterior urethral valve treatments and outcomes in children receiving kidney transplants. J Urol. 2011; 185:2507–2511.
80. Kamal, MM, El-Hefnawy, AS, Soliman, S, et al. Impact of posterior urethral valves on pediatric renal transplantation: A single-center comparative study of 297 cases. Pediatr Transplantation. 2011; 15:482–497.
81. Taskinen, S, Heikkilä, J, Santtila, P, et al. Posterior urethral valves and adult sexual function. BJU Int. 2012; 110:E392–E396.
82. Tikkinen, KA, Heikkila, J, Rintala, RJ, et al. Lower urinary tract symptoms in adults treated for posterior urethral valves in childhood: Matched cohort study. J Urol. 2011; 186:660–666.
83. Lopez Pereira, R, Miguel, M, Martinez Urrutia, MJ, et al. Long-term bladder function, fertility and sexual function in patients with posterior urethral valves treated in infancy. J Ped Urol. 2011.