Chapter 38 Posterior Semicircular Canal Occlusion for Benign Paroxysmal Positional Vertigo
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
Benign paroxysmal positional vertigo (BPPV) is the most common vestibular end organ disorder; in one busy vestibular clinic, BPPV accounted for 17% of all diagnoses.1 Patients complain of brief vertigo spells, often accompanied by nausea, but rarely vomiting. The actual duration of the spells (5 to 15 seconds) is usually much shorter than what the patients describe. Spells are induced by characteristic head movements, such as rolling to the affected side while in bed or extending the neck while upright. Less common precipitating movements include bending forward, arising from a supine position, and rotating the head. When the disease is very active, in addition to the brief positional vertigo episodes, patients may complain of protracted, nonspecific imbalance and dizziness accompanied by mild lassitude.
BPPV is most often an idiopathic disorder. The most common identifiable cause is head or temporal bone trauma.2 Other, less common causes include viral labyrinthitis, vestibular neuronitis, stapedectomy, perilymph fistula, Meniere’s disease, and chronic otitis media.3–8 Various combinations of rotatory, vertical, and oblique nystagmus may be seen in response to the Dix-Hallpike maneuver, depending on the position of the globe within the orbit during nystagmus. The nystagmus profile correlates, however, with the known neuromuscular pathways arising from the crista of the undermost posterior canal.9–12
The classic diagnostic maneuver as described by Dix and Hallpike13 has the patient laying back from a sitting to a head-hanging position. Before lying the patient back, the head is turned 45 degrees toward the side being tested such that when lying back, that side is directed down toward the floor. In some patients, it may be impossible to extend the neck back into a “head-hanging” position because of arthritis, kyphoscoliosis, or vascular disease. For pure diagnostic testing purposes, the lack of hyperextension should not preclude a positive diagnostic test, but it becomes a factor later on when attempting the therapeutic repositioning maneuver (see later). The Dix-Hallpike maneuver serves to rotate the undermost posterior semicircular canal (SCC) in the earth’s vertical plane. This rotation results in an endolymph current and secondary cupular displacement, which produces the characteristic oculomotor response, primarily a rotatory nystagmus.
BPPV must be differentiated from other causes of vertigo and nystagmus. The history is usually typical, and a positive response to the Dix-Hallpike maneuver is virtually diagnostic, assuming that all features are present. Typical posterior canal BPPV, which produces a positive response to the Dix-Hallpike maneuver, must be differentiated from the much less common lateral canal BPPV14 and the extremely rare anterior canal BPPV. Lateral canal BPPV gives rise to more severe and prolonged vertigo spells. It is brought on by head movements that produce gravitational forces on the affected lateral canal and is diagnosed by rolling the patient from one lateral supine position to the other and observing the eyes for horizontal nystagmus. Vertigo usually resolves within days to weeks of onset, but similar to the classic posterior canal variant, it can also be treated with various repositioning maneuvers.15 The remainder of this discussion deals solely with the management of the classic posterior canal BPPV.
Untreated posterior canal BPPV has three clinical courses. Most commonly, patients experience a self-limited single episode that subsides spontaneously over weeks to months. A second group of patients experiences remissions and recurrences ranging from weeks to years. A third, smaller group has the more chronic form of this disorder. In one busy vestibular clinic, about 30% of untreated patients had symptoms lasting longer than 1 year.8
PATHOPHYSIOLOGY
Under normal physiologic conditions, the cupula has the same density as the surrounding endolymph. The SCCs are normally not sensitive to linear acceleration (e.g., gravity). A fixed cupular deposit would render the posterior canal crista sensitive to gravity, however.16 Rotation of the canal in the earth’s vertical plane during the Dix-Hallpike maneuver would produce cupular displacement through the gravitational pull on the deposit, resulting in nystagmus and vertigo. This condition, so-called cupulolithiasis, may represent the extremely rare, more chronic form of this disorder. Cupulolithiasis also seems to be more prevalent in lateral canal BPPV.15
The more common, self-limited variant and the variant with remissions and recurrences likely have a different pathophysiologic mechanism. Free-floating endolymph particles within the posterior canal produce a Dix-Hallpike response identical to that of a fixed cupular deposit.17 Because the posterior canal is the most gravity-dependent part of the vestibular labyrinth, free-floating endolymph particles have a predilection for settling in the posterior canal endolymph.
With the head upright, the most dependent part of the canal is the area just posterior and inferior to the ampulla on the side of the cupula opposite the utricle. As the posterior canal rotates during the Dix-Hallpike maneuver, the particles initially rotate upward because of their inertia. After a short latent period, gravity pulls them down and away from the cupula (utriculofugal) to a more dependent position. Their hydrodynamic drag creates an endolymph current in the same direction, displacing the cupula away from the utricle. Utriculofugal displacement of the posterior canal cupula increases the resting discharge rate of the hair cells and first-order vestibular neurons. As known from previous animal studies, this action produces excitation of the ipsilateral superior oblique and contralateral inferior rectus muscles,9 which causes counterclockwise eye rotation with stimulation of the left posterior crista and clockwise rotation with right-sided stimulation. The compensatory fast component of the induced nystagmus is in the opposite direction, however, corresponding with the clinical findings of typical BPPV.
These free-floating posterior canal particles have been identified in vivo in patients undergoing surgery for BPPV.18,19 This theory of free-floating particles, also referred to as canalithiasis, is an important concept because it relates to the treatment of this condition.
PREOPERATIVE PATIENT COUNSELING AND CONSERVATIVE MANAGEMENT
First, the patient must be reassured that BPPV is an inner ear disorder that is relatively benign and most often self-limited. Medical management for BPPV is mostly ineffective.20 The most efficacious means of vertigo control is avoidance of the specific provocative head movements that induce the attacks. Most patients already use this approach by not lying on the affected side and by not extending the neck to look upward. Patients who stringently avoid these movements may have more prolonged courses because the absence of provocative movements prevents movement within and subsequent dispersement of the particles from the canal.
Most cases of BPPV resolve spontaneously over weeks to months without any treatment. Brandt and Daroff21 recommended a rigorous course of physiotherapy under heavy sedation during several days of hospitalization. They believed that the exercises shook free the otolithic debris from the cupula. Other clinicians could not reproduce their good results. Semont and associates22 reported excellent results using a technique termed the liberatory maneuver. They theorized that this technique liberated deposits from the cupula and reported a 92% success rate following two maneuvers.
The liberatory maneuver is difficult to perform in elderly, frail patients. The particle repositioning maneuver23–25 provides the same benefits as the liberatory maneuver, but is simpler to accomplish. It is based on the free-floating particle (canalithiasis) pathophysiologic theory of BPPV and is adapted from Epley’s canalith repositioning procedure.26 For the purpose of this discussion, it is important to remember that the cupula forms a complete barrier across the ampullated end of the canal that is impermeable to endolymph and free-floating particles. Free-floating posterior canal endolymph particles can enter and exit the canal only through the common crus.
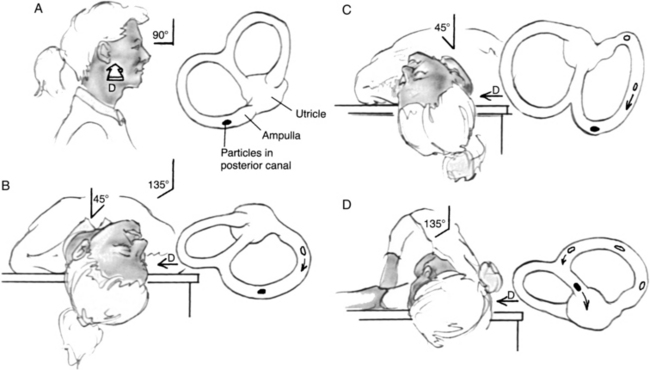
The current particle repositioning technique (Fig. 38-1) begins with the patient seated lengthwise on the examining table. The first part of the Dix-Hallpike maneuver is performed by rotation of the posterior SCC of the undermost (affected) ear in the earth’s vertical axis (see Fig. 38-1B). The examiner should observe the classic nystagmus response, which confirms the diagnosis, and then reassure the patient as the vertigo subsides. The patient maintains this position for 30 to 60 seconds after resolution of the nystagmus, allowing the particles to settle in their new dependent position closer to the common crus. In the second stage, the patient rolls laterally through position C into position D onto the opposite side with the head turned 45 degrees downward. This stage is performed in a smooth, continuous motion, and the neck is kept extended throughout. This method rotates the affected posterior canal 180 degrees in the plane of gravity, allowing the free-floating particles to follow the natural curve of the canal and continue their relative course through the common crus into the utricle, presumably from whence they came.
Conversely, a secondary nystagmus that reverses direction from that of the initial head-hanging position (position B) may occur through two possible mechanisms. In one, the particles reverse their direction of movement because of an improperly performed maneuver, resulting in a utriculopetal endolymph current. This reversal usually occurs when the neck is not hyperextended enough during the roll. Cupulolithiasis is the other possible mechanism underlying reversal nystagmus. The gravitational effect on a fixed cupular deposit results in utriculofugal cupular deflection during the Dix-Hallpike maneuver (see Fig. 38-1B), as would be seen with free-floating particles. The position assumed during the second stage of the particle-repositioning maneuver effectively rotates the posterior canal 180 degrees in the earth’s vertical plane (see Fig. 38-1D). This action serves to flip the whole posterior canal and its cupula upside down, reversing the gravitational pull on the cupula and resulting in utriculopetal cupular displacement and a reversal of the nystagmus response.
After another 30 to 60 seconds, the patient is brought back up to the sitting position, and the eyes are observed for nystagmus. With a successful maneuver, there should be no nystagmus when the patient returns to the upright position because the particles would have been removed from the posterior canal. This result is in contrast with that of the conventional Dix-Hallpike maneuver, in which one notes a reversal of the nystagmus when the patient sits back up. No specific postmaneuver instructions to limit head and neck movements are necessary.15 Patients should be reassessed 1 to 4 weeks after the maneuver, and if the Dix-Hallpike test is still positive, the repositioning maneuver should be repeated. When appropriately administered, repositioning maneuvers should alleviate BPPV in greater than 95% of cases.27
SURGICAL PATIENT SELECTION
Operative intervention is offered for intractable cases in which symptoms are severe enough to affect the patient’s occupation or lifestyle significantly, and when the disorder fails to respond to repeated repositioning maneuvers. Rarely, surgery is offered to patients with severe and frequent recurrences. Until the advent of posterior canal occlusion, transection of the posterior ampullary nerve (singular neurectomy) was the gold standard of operative treatment.28–30 Singular neurectomy is technically difficult, is performed by very few surgeons, and yields variable rates of failure and sensorineural hearing loss.31,32 Ohmichi and colleagues33 showed that the singular nerve would be inaccessible through a tympanotomy approach in 14% of human temporal bones. In a 2007 literature review and temporal bone dissection study comparing posterior canal occlusion with transection of the posterior ampullary nerve, Leveque and associates34 found that posterior canal occlusion presented far fewer technical issues and a far lower risk of hearing loss compared with singular neurectomy.
Posterior SCC occlusion evolved to circumvent the technical challenges and high risk of hearing loss associated with transection of the posterior ampullary nerve. Money and Scott35 initially used this technique in feline vestibular physiology experiments. Plugging individual SCCs blocked their receptivity to angular acceleration without influencing the responses of the other ipsilateral vestibular receptors. Although posterior canal occlusion was at first a theoretical remedy for BPPV, the main concern in applying this technique to humans was its possible detrimental effect on hearing. This problem was not addressed in the original cat studies. Parnes and McClure36 carried out a study in guinea pigs to measure the effect of canal occlusion on hearing using brainstem auditory evoked responses. The hearing responses remained unchanged during follow-up periods lasting 6 months.
The hypothesis that canal occlusion would abolish BPPV was tested when two patients presented with intractable BPPV in ears with coexisting profound sensorineural hearing losses. With no hearing to lose, both patients agreed to undergo what at that time was an experimental procedure. Both patients were relieved of their BPPV and remained symptom-free for at least 4 years, when they were lost to follow-up.37,38 In addition, both patients maintained postoperative lateral SCC function as measured by caloric responses. This important finding indicated that the procedure’s success resulted from the isolated defunctioning of the posterior canal and not from a generalized destructive process of the vestibular labyrinth.
Singular neurectomy eliminates the resting discharge from the posterior SCC crista, creating a static vestibular asymmetry39 between the two posterior canals. This effect results in immediate postoperative vertigo at rest and spontaneous rotatory nystagmus. Posterior canal occlusion does not disturb the resting neuronal discharge from the occluded canal, however; most patients do not have spontaneous postoperative vertigo or nystagmus at rest, unless complicated by other factors. Singular neurectomy and posterior canal occlusion result in a dynamic vestibular asymmetry,39 itself resulting in motion sensitivity. The dynamic asymmetry gradually resolves with central adaptation, which can be hastened with vestibular physiotherapy.
PREOPERATIVE EVALUATION
Preoperative evaluation includes a routine audiogram. The procedure is not recommended in an only or significantly better hearing ear. Preoperative electronystagmography or videonystagmography helps to ensure there is a normal vestibular response from the contralateral ear. A high-resolution computed tomography (CT) scan of the temporal bone defines the anatomy and ensures that the posterior canal is accessible through a transmastoid approach. Preoperative imaging with either CT or magnetic resonance imaging (MRI) rules out central lesions that may mimic BPPV, a very rare occurrence.40
SURGICAL TECHNIQUE
A limited mastoidectomy with a fluted ball drill and suction-irrigation is performed through a standard postauricular incision (Fig. 38-2A). The antrum is opened, providing exposure of the lateral SCC. In most instances, identification of the tegmen and digastric ridge is unnecessary. The bony sigmoid sinus prominence is identified, but in most cases it is unnecessary to bare the sigmoid sinus dura. Bone removal proceeds anteriorly from the sigmoid sinus along the cerebellar plate toward the posterior canal. When the posterior canal otic capsule is identified, the bone is blue-lined (thinned down so much that the transmitted light through the canal’s perilymph creates a dark appearance) with progressively smaller diamond burrs and copious suction irrigation. The target zone for the occlusion is the area at, or just inferior to, a line extending posteriorly from the lateral SCC (Donaldson’s line). Sometimes there are abundant air cells between the posterior canal otic capsule bone and the dura, and sometimes the bony canal directly contacts the dura. The preoperative CT scan helps with navigation through this area.
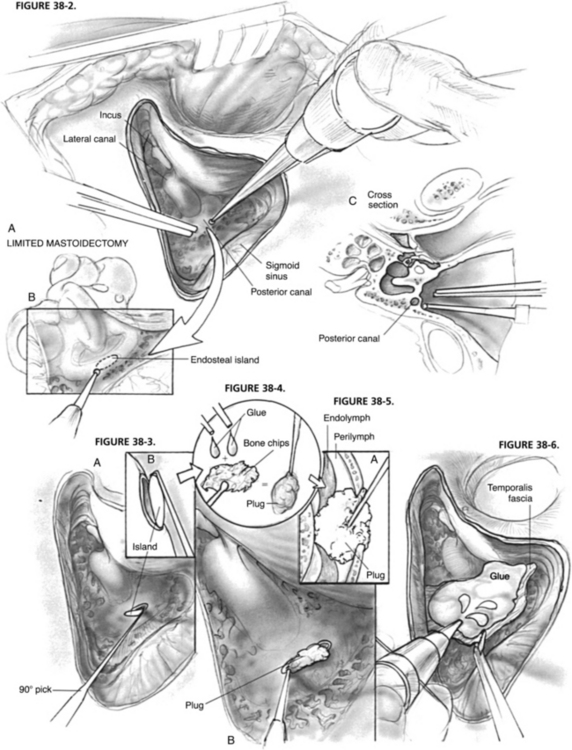
FIGURE 38-3. A, Lifting out endosteal island with a fine 90 degree pick. B, Magnified lateral view.
FIGURE 38-4. Creating plug with two-component fibrinogen glue and mastoid cortex bone chips.
FIGURE 38-6. Covering fenestra and surrounding bone with fascia and glue.
After blue-lining the canal, a 1 mm diamond burr is used to skeletonize a 3 mm segment of the canal 180 degrees around the outer circumference down to endosteum, creating a 1 × 3 mm endosteal island (Fig. 38-2B). Bone removal should proceed evenly along the circumference so that when the endosteum is violated and perilymph is exposed, all drilling can cease. The endosteal island is removed with a fine 90 degree pick to expose the perilymph (Fig. 38-3). Great care must be taken not to suction directly on the perilymph and especially the membranous labyrinth. At this stage, the exact outline and limits of the membranous labyrinth are usually not clearly discernible. Although not essential, perilymph may be gently “wicked” away with a Cottonoid, or gently suctioned with a No. 24 suction with the thumb off the hole to expose the membranous labyrinth, at which time the membranous duct is seen to collapse.
Various materials have been used to occlude the canals, including bone dust alone, bone wax, fascia or other tissue, and bone pâté made from either bone dust and blood or bone dust and fibrin glue. I use dry bone chips gathered from the mastoidectomy mixed with a couple of drops of two-component, fast-acting human fibrinogen glue. Once set (about 30 seconds), it forms an easily workable, but malleable plug with a firm consistency (Fig. 38-4). The plug is gently and firmly inserted through the fenestra with the intention of completely filling the canal lumen and compressing the membranous labyrinth closed (Fig. 38-5). A blunt 45 degree probe works well to pack the plug in the canal tightly. The membranous labyrinth is resistant to shearing and tearing. As has been learned from partial labyrinthectomies in skull base surgery (see next section), controlled resection of the membranous duct is not risky or dangerous to the other inner ear receptors (i.e., hearing), unless the membranous labyrinth is roughly avulsed. The bone chips within the plug lead to intracanal ossification resulting in complete permanent occlusion of the canal.
After completing the plug insertion, the fenestra and surrounding bone are covered with a piece of temporalis fascia, which is kept in place by several more drops of fibrinogen glue (Fig. 38-6). A good tissue seal is necessary to prevent a postoperative perilymph fistula.
In variations of this technique, Anthony41 successfully treated BPPV by applying an HGM argon laser to the blue-lined posterior canal. The laser burns were purported to create fibrous bands within the canal, leading to obstruction of the membranous duct. Kartush and Sargent42 used a CO2 laser–assisted occlusion technique.
RESULTS
In approximately 30% of ears, after the canal has been fenestrated, and the membranous labyrinth has been exposed, particles can be seen freely floating within the endolymph.19 In one previously reported case, the membranous labyrinth was resected with the particles and was subjected to scanning electron microscopy. In this particular case, the particles proved to be degenerating otoconia.19
FURTHER APPLICATIONS OF CANAL OCCLUSION SURGERY
Several modifications of posterior canal occlusion have ensued for various other inner ear and skull base conditions. There are only two reported cases of canal occlusion for intractable BPPV of the other canals: one of a single lateral canal occlusion using an argon laser on a blue-lined canal,43 and another case report of an anterior canal plugging.44 McElveen and colleagues45 described three patients who each underwent a successful hearing-preserving modified translabyrinthine acoustic neuroma resection in which all three SCCs were occluded. Molony and associates46 reported two patients who underwent extended middle fossa approaches and one who underwent an extended suboccipital approach for acoustic tumor removal. Hearing was preserved in all three patients after occluding and resecting the superior SCC and posterior SCC.
Hirsch and colleagues47 first reported on the partial labyrinthectomy, in which the superior and posterior canals of the same labyrinth were occluded and resected for access to various skull base lesions, with consistent hearing preservation. Minor and coworkers48 published their seminal work on the superior SCC dehiscence syndrome in 1998, in which several patients underwent superior SCC occlusion through the middle fossa approach. Several subsequent reports have ensued from their center and other centers showing good hearing preservation rates. Further positive reports on partial labyrinthectomy by Sekhar and associates49 and Kaylie and colleagues50 substantiated the utility and success of labyrinthine partition with multiple canal occlusions, with predictably good hearing preservation rates.
Dehiscence of the posterior canal can also occur (rarely), and it too can be treated successfully with canal occlusion.51 Agrawal and Parnes52 reported their successful results using the transmastoid approach for superior SCC occlusion in three cases of superior SCC dehiscence. In one patient, hearing remained unchanged, whereas in the other two patients hearing improved with the resolution of the conductive component of the hearing loss.
1. Nedzelski J.M., Barber H.O., McIlmoyl L. Diagnoses in a dizziness unit. J Otolaryngol. 1986;15:101-104.
2. Barber H.O., Leigh R.J. Benign (and not so benign) postural vertigo: Diagnosis and treatment. In: Barber H.O., Sharpe A., editors. Vestibular Disorders. Boca Raton, FL: CRC Press; 1988:215-232.
3. Lindsay J.R., Hemenway W.G. Postural vertigo due to unilateral sudden partial loss of vestibular function. Ann Otol Rhinol Laryngol. 1956;65:692-706.
4. Spector M. Positional vertigo after stapedectomy. Ann Otol Rhinol Laryngol. 1961;70:251-254.
5. Stahle J., Terins J. Paroxysmal positional nystagmus. Ann Otol Rhinol Laryngol. 1965;74:69-83.
6. Barber H.O. Positional vertigo and nystagmus. Otolaryngol Clin North Am. 1973;6:169-187.
7. McClure J.A., Rounthwaite J. Vestibular dysfunction associated with benign paroxysmal vertigo. Laryngoscope. 1977;87:1434-1442.
8. Baloh R.W., Honrubia V., Jacobson K. Benign positional vertigo: Clinical and oculographic features in 240 cases. Neurology. 1987;37:371-378.
9. Cohen B., Suzuki J., Bender M.B. Nystagmus induced by electrical stimulation of ampullary nerves. Acta Otolaryngol (Stockh). 1965;60:422-436.
10. Harbert F. Benign paroxysmal positional vertigo. Arch Ophthalmol Head Neck Surg. 1970;84:298-302.
11. Baloh R.W., Sakala S., Honrubia V. The mechanism of benign paroxysmal positional nystagmus. Adv Otorhinolaryngol. 1979;25:161-166.
12. Katsarkas A., Outerbridge J.S. Nystagmus of paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1983;92:146-150.
13. Dix M.R., Hallpike C.S. Pathology, symptomatology and diagnosis of certain disorders of the vestibular system. Proc R Soc Med. 1952;45:341.
14. Fife T.D. Recognition and management of horizontal canal benign positional vertigo. Am J Otol. 1998;19:345-351.
15. Parnes L.S., Agrawal S.K., Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). Can Med Assoc J. 2003;169:681-693.
16. Schuknecht H.F. Pathology of the Ear. Cambridge, MA: Harvard University Press; 1974.
17. Hall S.F., Ruby R.R.F., McClure J.A. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8:151-158.
18. Parnes L.S., McClure J.A. Free-floating endolymph particles: A new operative finding during posterior semicircular canal occlusion. Laryngoscope. 1992;12:988-992.
19. Welling D.P., Parnes L.S., O’Brien B., et al. Particulate matter in the posterior semicircular canal. Laryngoscope. 1997;107:90-94.
20. McClure J.A., Willett J.M. Lorazepam and diazepam in the treatment of benign paroxysmal vertigo. J Otolaryngol. 1980;9:472-477.
21. Brandt T., Daroff R.B. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol Head Neck Surg. 1980;106:484-485.
22. Semont A., Freyss G., Vitte E. Curing the BPPV with a liberatory maneuver. Adv Otorhinolaryngol. 1988;42:290-293.
23. Parnes L.S., Price-Jones G. Particle-repositioning maneuver for benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1993;102:325-331.
24. Parnes L.S., Robichaud J. Further observations during the particle repositioning maneuver for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1997;116:238-243.
25. Fung K., Hall S.F. Particle-repositioning maneuver: Effective treatment for benign paroxysmal positional vertigo. J Otolaryngol. 1996;25:243-248.
26. Epley J.M. The canalith-repositioning procedure: For treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107:399-404.
27. Epley J.M. Positional vertigo related to semicircular canalithiasis. Otolaryngol Head Neck Surg. 1995;112:154-161.
28. Gacek R. Transection of the posterior ampullary nerve for the relief of benign paroxysmal positional nystagmus. Ann Otol Rhinol Laryngol. 1974;83:596-605.
29. Epley J.M. Singular neurectomy: Hypotympanotomy approach. Otolaryngol Head Neck Surg. 1980;88:304-309.
30. Gacek R. Singular neurectomy update. Ann Otol Rhinol Laryngol. 1982;91:469-473.
31. Silverstein H. Singular neurectomy: A treatment for benign positional vertigo. In: Brackmann D.E., editor. Neurological Surgery of the Ear and Skull Base. New York: Raven Press; 1982:331-335.
32. Meyerhoff W.L. Surgical section of the posterior ampullary nerve. Laryngoscope. 1985;95:933-935.
33. Ohmichi T., Rutka J., Hawke M. Histopathologic consequences of surgical approaches to the singular nerve. Laryngoscope. 1989;99:963-970.
34. Leveque M., Labrousse M., Seidermann L., Chays A. Surgical therapy in intractable benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2007;136:693-698.
35. Money K.E., Scott J.W. Functions of separate sensory receptors of nonauditory labyrinth of the cat. Am J Physiol. 1962;202:1211-1220.
36. Parnes L.S., McClure J.A. Effect on brainstem auditory evoked responses of posterior semicircular canal occlusion in guinea pigs. J Otolaryngol. 1985;14:145-150.
37. Parnes L.S., McClure J.A. Posterior semicircular canal occlusion for intractable benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 1990;99:330-334.
38. Parnes L.S., McClure J.A. Posterior semicircular canal occlusion in the normal hearing ear. Otolaryngol Head Neck Surg. 1991;104:52-57.
39. McClure J.A., Lycett P. Vestibular asymmetry. Arch Otolaryngol Head Neck Surg. 1983;109:682-687.
40. Shoman N., Longridge N. Cerebellar vermis lesions and tumours of the fourth ventricle in patients with positional and positioning vertigo and nystagmus. J Laryngol Otol. 2007;121:166-169.
41. Anthony P. Partitioning the labyrinth: Application in benign paroxysmal positional vertigo. Am J Otol. 1991;12:388-393.
42. Kartush J.M., Sargent E.W. Posterior semicircular canal occlusion for benign paroxysmal positional vertigo-CO2 laser-assisted technique: Preliminary results. Laryngoscope. 1995;105:268-274.
43. Nomura Y. Argon laser irradiation of the semicircular canal in two patients with benign paroxysmal positional vertigo. J Laryngol Otol. 2002;116:723-725.
44. Brantberg K., Bergenius J. Treatment of anterior benign paroxysmal positional vertigo by canal plugging: A case report. Acta Otolaryngol. 2002;122:28-30.
45. McElveen J.T.Jr., Wilkins R.H., Erwin A.C., Wolford R.D. Modifying the translabyrinthine approach to preserve hearing during acoustic tumour surgery. J Laryngol Otol. 1991;105:34-37.
46. Molony T.B., Kwartler J.A., House W.F., Hitselberger W.E. Extended middle fossa and retrolabyrinthine approaches in acoustic neuroma surgery: Case reports. Am J Otol. 1992;13:360-363.
47. Hirsch B.E., Cass S.P., Sekhar L.N., Wright D.C. Translabyrinthine approach to skull base tumors with hearing preservation. Am J Otol. 1993;14:533-543.
48. Minor L.B., Solomon D., Zinreich J.S., Zee D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249-258.
49. Sekhar L.N., Schessel D.A., Bucur S.D., et al. Partial labyrinthectomy petrous apicectomy approach to neoplastic and vascular lesions of the petroclival area. Neurosurgery. 1999;44:537-550.
50. Kaylie D.M., Horgan M.A., Delashaw J.B., McMenomey S.O. Hearing preservation with the transcrural approach to the petroclival region. Otol Neurotol. 2004;25:594-598.
51. Mikulec A.A., Poe D.S. Operative management of a posterior semicircular canal dehiscence. Laryngoscope. 2006;116:375-378.
52. Agrawal S.K., Parnes L.S. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29:363-367.