CHAPTER 49 Posterior Lumbar Interbody Fusion
The technique of posterior lumbar interbody fusion (PLIF) has become an important part of the modern spine surgeon’s armamentarium. This was not always so; for nearly half a century after the introduction of this technique, it was performed routinely by only a handful of surgeons. Most surgeons condemned the technique as unnecessary, technically difficult, or even dangerous.1,2 Controversy persists regarding the safety of PLIF compared with other approaches to the intervertebral space and regarding the benefit that PLIF adds to the outcome of surgical treatment for various spinal pathologies.
Historical Perspective
Attempts to identify the first surgeon to perform an interbody fusion from a posterior approach are muddled by several reports appearing in the published literature beginning in 1944,3–5 but no one disputes that credit for initially developing the techniques and key principles of the operation as it is practiced today belongs to Cloward,6 who emphasized the importance of a wide exposure of the spinal canal to minimize nerve root injuries, the use of structural graft to prevent intervertebral collapse, and the complete removal of nuclear material from the disc space and replacement with bone to promote fusion. Cloward was widely criticized for his operation, probably as much because he advocated that it be included as part of the routine surgical treatment for all lumbar disc herniations as for the frequency of poor outcomes and complications when it was attempted by other surgeons. A few surgeons embraced Cloward’s operation, and several large surgical series were published in the literature reporting good outcomes and high fusion rates.7–10 Widespread interest in and acceptance of PLIF as a valuable technique did not occur, however, until the introduction of pedicle screw instrumentation.
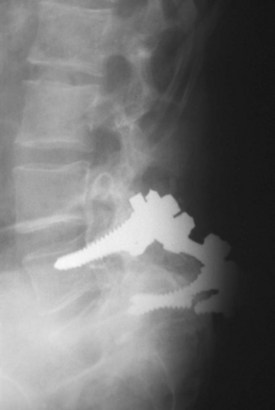
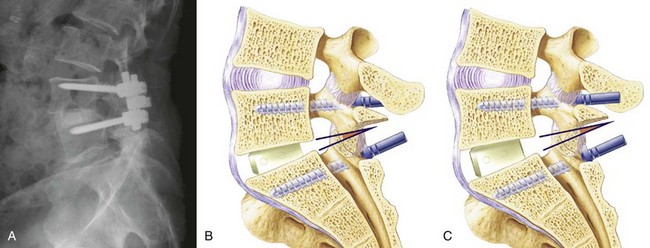
With the early generation of pedicle instrumentation, screw fracture was a common occurrence. Fracture especially occurred if a dramatic change in spinal alignment was achieved, such as in the reduction of a high-grade spondylolisthesis. Even without large changes in alignment, screw fracture occurred commonly in overweight patients or when the disc space was distracted.11 In those cases, the mechanical loads that the instrumentation was subjected to exceeded the fatigue properties of the screws. Surgeons, in particular Steffee and Sitkowski,12 recognized that a structural graft placed in the disc space would divert some of the load, decreasing the forces on the posterior instrumentation, and reduce the frequency of instrumentation failure. Steffee and Sitkowski12 reported their experiences with reduction of high-grade spondylolisthesis. In each of the first three patients treated with pedicle screws, the instrumentation failed, and alignment was lost. In the next 11 patients, an interbody graft was placed. All of these patients developed solid fusions with no loss of alignment. With PLIF, the load-sharing anterior column support could be added to protect the pedicle screws without the need for a separate anterior incision (Fig. 49–1).
Another development that favored the adoption of PLIF was the invention of the interbody fusion cage by Brantigan and the titanium mesh cage by Harms. Their designs eliminated the need to harvest a structural graft from the iliac crest, which was a major source of morbidity previously associated with the PLIF procedure. The cage provided the structure,13 and the osteogenic potential for fusion came from cancellous bone packed into the cage, which could be obtained from the iliac crest more easily than a structural graft, with less injury to the patient. Before the invention of the cages, it was possible to avoid the morbidity associated with graft harvest by using allograft bone, as advocated by Cloward.14,15 Concerns about availability, disease transmission, healing potential, delayed healing, and wide variation in the structural integrity of available grafts all contributed to the limited enthusiasm for the use of allograft bone for interbody fusion.16,17 The availability of cages overcame another common objection to the PLIF operation.
The availability of pedicle screw instrumentation and intervertebral cages contributed to progressively greater acceptance of the operation first pioneered by Cloward. Better alignment and higher fusion rates are routinely achieved using these devices. The training of surgeons and marketing of these devices by their manufacturers have also contributed to wider adoption of PLIF in surgical practice. Not all of these devices have proven to be successful, however. Cylindric threaded fusion cages enjoyed brief popularity as PLIF devices that did not require supplementary fixation, but mediocre results and high complication rates associated with their use resulted in their virtual disappearance as a posterior spinal implant.18 The clinical experience with threaded implants and the study of the relevant biomechanics have helped surgeons understand the PLIF operation better, however, and develop the operation that is currently performed. Although more recent advances, such as the development of the transforaminal and direct lateral approaches to the disc space, have decreased the frequency with which PLIF is performed, PLIF remains an important and powerful tool for spinal surgeons.
Indications for Interbody Fusion
Some surgeons believe that an interbody fusion is indicated whenever a lumbar fusion is done. They argue that the intervertebral space is biologically and mechanically superior for fusion compared with the intertransverse plane because of the larger surface area of the highly vascular bony endplate and because the interbody bone graft is subject to compressive forces. In addition, they criticize the large amount of muscle damage that occurs from exposure of the spine for posterolateral fusion. Despite these theoretical advantages, it is difficult to show clinical superiority of interbody fusions over posterolateral fusions for most lumbar degenerative conditions, and most published studies comparing the techniques reveal similar outcomes regardless of what fusion technique is used.19–21 There are, however, certain circumstances when interbody fusion offers definite advantages. Adding an interbody fusion to a posterolateral fusion increases the rate of achieving successful arthrodesis. This is especially true when a long fusion extends to the sacrum, which is historically associated with a high risk of lumbosacral pseudarthrosis.22
Some authors have argued that interbody fusion should be combined with posterolateral fusion in other patients at high risk for failed fusion, such as smokers.23 In patients with pseudarthrosis after failed posterolateral fusion, an interbody fusion is a good salvage operation, allowing the fusion to occur in a well-vascularized bony bed rather than attempting fusion again in the scarred devascularized posterolateral space.24 Interbody fusion offers a particular advantage in the treatment of scoliosis and kyphosis or flatback deformities. The destabilizing effect of disc space preparation facilitates rotational correction, and distraction of the intervertebral space allows correction of segmental kyphosis and asymmetrical tilt of the vertebrae. In patients with isthmic spondylolisthesis who undergo deformity reduction, it has been well shown that an interbody graft offers a biomechanical advantage, which protects instrumentation by load sharing, helping to maintain the alignment achieved at surgery. Also, the L5 transverse process is often hypoplastic in these patients, so the increased bone surface area of the vertebral endplate offers a vastly larger area to which bone can fuse.
Most series evaluating the role of interbody fusion in isthmic spondylolisthesis show substantial clinical and radiographic benefit.25,26 Finally, the most common indication for interbody fusion, but probably the most controversial, is ablation of the disc space in patients with discogenic pain.27,28 Many advocates for surgical treatment of this entity argue that only interbody fusion can completely remove motion of the painful disc and that complete removal of nuclear material is necessary to eliminate the anatomic source of pain.
Biomechanics of Interbody Fusion
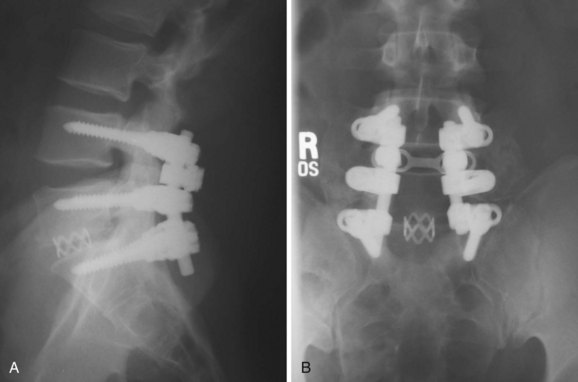
An appreciation of the mechanical properties of the spine after interbody fusion is essential to understanding the benefits and pitfalls of this operation. It seems intuitively obvious that placing a solid block in the disc space would prevent flexion of the vertebral motion segment. This is what occurs—whether the graft is placed from an anterior or a posterior approach. This fact has important implications for protection of posterior instrumentation. Interbody grafts significantly decrease the strain in posterior spinal implants when they are subjected to compression or flexion loads, which are the common modes of failure of these constructs in clinical practice. Various authors have shown that placing intervertebral cages decreases forces and strain in posterior implants by 56% to 80%,29,30 and the clinical benefit of establishing anterior load sharing to prevent implant and construct failure is well established (Fig. 49–2).31
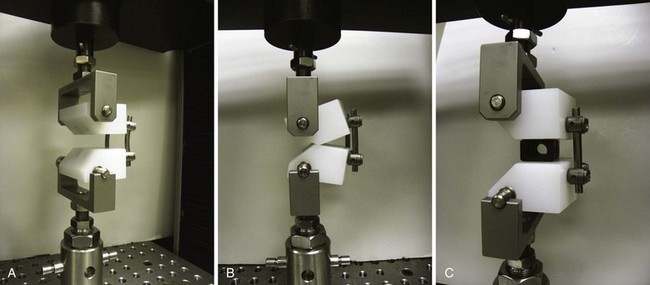
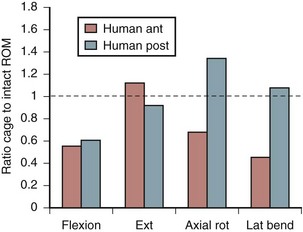
Increasing stability when the spine is subject to flexion loading does not mean that the spine is more stable when subject to forces in every direction. After interbody graft placement, the motion segment is more unstable in certain directions. The pattern of instability is determined to a great extent by the direction from which the graft is placed and represents the destabilizing effect of the surgical approach32 and the preparation of the disc space. Grafts placed from an anterior approach result in increased motion when force is applied in extension; this likely represents the effect of dividing the anterior longitudinal ligament. Grafts placed from a posterior approach result in increased axial rotation of the motion segment owing to the resection of the posterior facet complexes necessary to avoid excessive dural retraction.33–37 The design of the cage—vertical, box, or threaded and whether or not the cage engages the endplates—does not seem to matter with regard to the patterns of instability created by intervertebral placement. Only the structures sacrificed during the approach to the disc are important in determining instability of the final construct (Fig. 49–3).38,39
Most surgeons make the assumption that a PLIF procedure is a stabilizing operation. The biomechanical data show that this is not entirely true. Understanding that placing an interbody graft or cage can lead to destabilization of the motion segment in certain directions is important for understanding why interbody fusion done without supplementary fixation has a high failure rate, especially when done from a posterior approach. Proponents of threaded fusion cages, which were designed to be used without supplementary fixation, argued that distraction of the disc space would stabilize the motion segment through ligamentotaxis. This argument is true. Increasing the size of the cage used to distract the disc space results in greater stabilization of the motion segment on mechanical testing.40 After cyclic loading, some subsidence is inevitable, however, and biomechanical tests done after cyclic loading of threaded cage constructs reveal decreased stability in all directions.41
Cage Characteristics
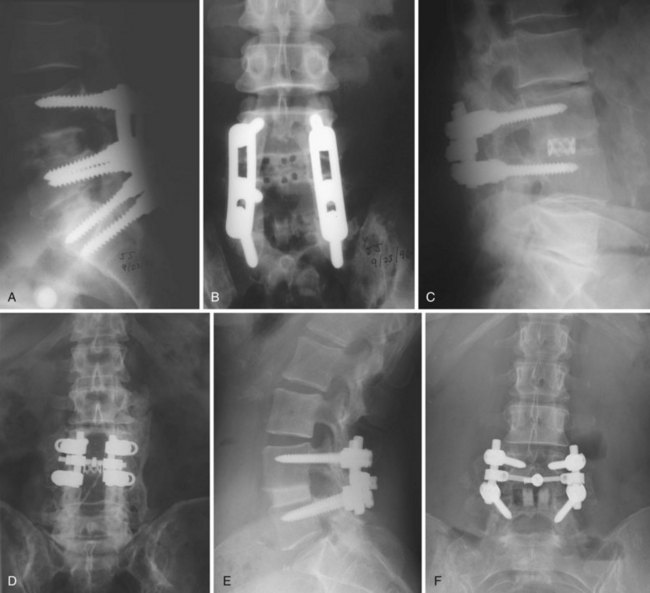
The optimal cage material is unknown. Cloward41a advocated the use of ethylene oxide sterilized allograft bone. Brantigan and colleagues16 tested 18 tricortical iliac specimens obtained from bone banks and determined that 3 of them were not strong enough to sustain anticipated loads without collapse. Current allograft products available for intervertebral use are made primarily of cortical bone and have adequate compressive strength. In designing his cage, Brantigan chose carbon fiber–reinforced polymer because of its strength and because its modulus of elasticity is similar to that of cortical bone.13 In the United States, other materials commonly used in commercially available cages are polyetheretherketone (PEEK), a nonreinforced polymer, and titanium, which has a modulus of elasticity significantly greater than that of cortical bone. The stiffness of the cage is determined not only by the mechanical properties of the material but also by the shape and design characteristics of the cage. The ideal stiffness and its role in limiting subsidence or enhancing fusion are unknown. Finite element data have suggested that stiffer cages are more likely to subside,42 but animal data have not supported this hypothesis,43 and clinical reports have not convincingly shown that metal cages are more likely to subside than cages made of polymer (Fig. 49–4).
Kanayama and colleagues44 studied the effect of cage design on stress shielding of the graft inside the cage. They determined that it was the pore size, not the stiffness of the material, that determined the load experienced by the graft. The total pore size was not important; rather, it was the size of the largest contiguous opening that determined how much force was transferred to the graft. It was implied that minimizing stress shielding is important to promote fusion, but no data were presented to support that presumption. One clinical study consistent with this hypothesis compared narrow and standard carbon fiber–reinforced polymer cages. The pore in the narrow cage was 36% smaller than the pore in the standard cage. There was a small but statistically significant reduction in fusion rate, 91.1% compared with 98.9%, but the conclusions have limitations because of methodologic flaws.45 A larger pore size seems desirable as long as the design of the cage provides adequate structural support for the endplate.
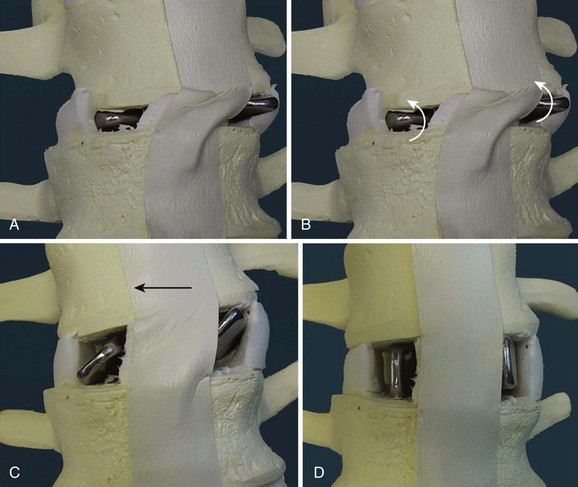
Cage design also influences the alignment achieved. An increasingly large body of data show that maintaining or restoring lumbar lordosis is an important surgical goal and that lordosis correlates with the outcome of surgery.46 Early cage designs were rectangular in shape and tended to force the vertebral endplates into parallel alignment. It is possible to achieve lordosis with rectangular cages, either by resecting bone posteriorly or by compressing the posterior disc space and causing the posterior portion of the cage to subside.47 A disadvantage of this approach is that it results in loss of posterior disc space height, which can lead to foraminal narrowing. A better solution is to use a cage that is tapered to achieve lordosis.48–50 It is commonly said that anterior distraction is necessary to achieve lordosis, but distraction of the disc space with an implant that is not tapered flattens the spine. Placing large cages to achieve lordosis is a mistake. They require more root retraction to place, and this is more likely to result in nerve injury. A better solution is to use a tapered device and not to strive for maximal anterior height restoration (Fig. 49–5).
Subsidence
In addition to achieving fusion, avoiding subsidence to maintain alignment is an important goal of interbody fusion surgery. In mechanical testing, endplate failure has a linear correlation with decreased bone density,51 and severe osteoporosis is considered to be a relative contraindication to interbody fusion because of the risk of endplate collapse. The strength of the endplate is not uniform, and the central portion, where most surgeons place their grafts, is the weakest. Mapping studies have shown that the posterolateral portion of the endplate is most resistant to compression.52,53 Placing the cage or graft in the area where the bone is strongest makes biomechanical sense54 but may not be consistent with the goals of an individual surgical case. If it is necessary to increase lordosis, better results are achieved by anterior placement. Putting the graft in the posterolateral position might be stronger but would block compression of the posterior disc space and limit lordosis. This is especially true with rectangular cage designs or vertical titanium mesh cages.
Cage designs that optimize contact with the strongest portions of the endplate are desirable and include cloverleaf designs and large round cages, both of which have peripheral endplate contact.55 Practical use of large round cages is limited to anterior surgery because of the excessive dural retraction that would be necessary to place such an implant. Lordotic cages placed laterally also achieve near-optimal endplate contact. The surface area of the endplate that needs to be in contact with the graft is unknown but is commonly stated to be 30%. This percentage is based on a study of thoracic vertebrae subjected to a physiologic load of 600 N. Failure of the endplate occurred in 80% of specimens subjected to that load when the grafts were 25% or less of the endplate surface area. When the grafts exceeded 30% of the endplate surface, 88% of the specimens remained intact (Fig. 49–6).56
Preservation of the endplate is important to maintain the structural integrity of the vertebra. Oxland and colleagues57 removed slightly less than 1 mm of cortical endplate with a power bur to expose trabecular bone; this resulted in a 33% reduction in compressive resistance of the vertebra. If the endplate is decorticated where it is not in contact with the cage, the strength of the remaining endplate is preserved.58 Consequently, it is recommended that the endplates be carefully preserved during preparation of the disc space wherever they are in contact with the implant, but that areas of the endplate that are not load bearing can be decorticated to increase contact of graft placed outside the cage with trabecular bone.
Author’s Preferred Technique
The author’s experience with PLIF dates back to 1994, and the method of interbody fusion described here has been used without modification since 1999. Early results were reported in 2000 with an interbody fusion rate of 92%.59 It is the author’s practice always to perform a posterolateral fusion simultaneously with the PLIF to ensure the highest chance of achieving an arthrodesis. The technique for PLIF evolved from that described by Steffee and Biscup60 for insertion of trapezoidal “ramps” made of carbon fiber–reinforced polymer. Their method was to insert the ramps sideways and rotate them into their final position, minimizing the amount of root retraction needed and simplifying placement of a lordotic graft.
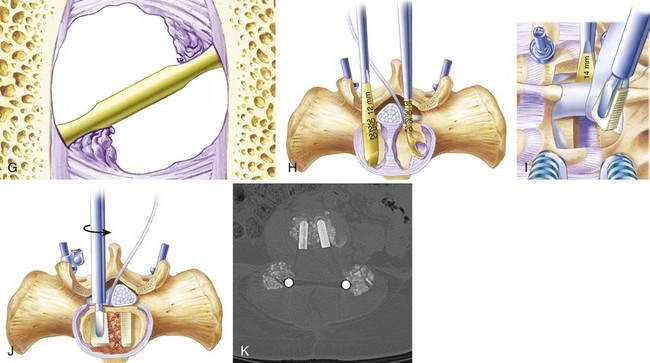
The technique described here for preparation of the intervertebral space is applicable to any intervertebral cage or graft, and the principles outlined help prevent neural injury and other complications that have been associated with this operation. The procedure begins with proper positioning, which is essential to achieve a good outcome. The patient is positioned in hyperextension to help create lumbar lordosis. The abdomen should hang free for unimpeded venous return; this decompresses the epidural venous plexus and helps reduce bleeding. A radiolucent positioning frame is useful so that intraoperative fluoroscopic images can be obtained to confirm correct placement of screws and grafts (Fig. 49–7). Pedicle screws should be placed before beginning the interbody fusion. PLIF destabilizes the motion segment and should not be done unless adequate pedicle fixation can be achieved.
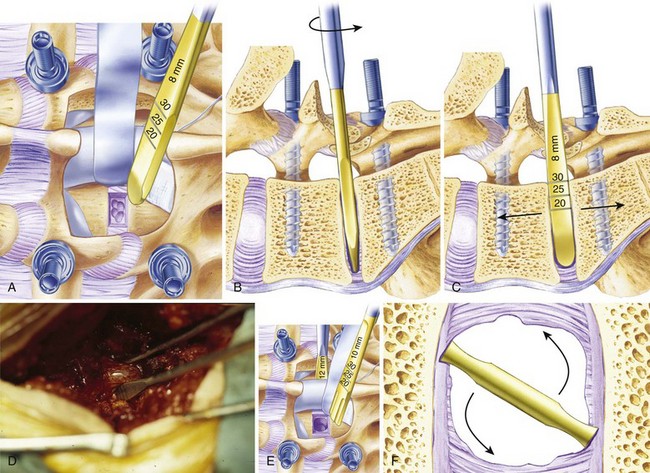
The anulus is opened bilaterally, and the disc space is distracted with intervertebral spreaders. These are flat bars of increasing width with rounded edges. They are inserted into the disc space horizontally and rotated 90 degrees, distracting the disc space. If the disc space is initially very narrow, it is sometimes helpful to use a smaller instrument first, such as a No. 4 Penfield elevator, to identify the path. It is important not to use force so as to avoid inadvertent penetration into the vertebral body. Lateral fluoroscopy can be helpful to confirm that the starting tools are correctly placed in the interspace. The disc is gradually distracted, working from side to side with increasingly large spreaders until resistance is met. The interspace should not be overdistracted, or the endplates may collapse. It is not necessary to maximize distraction to achieve lordosis, and decompression of the nerve roots should be accomplished by aggressive foraminotomy, not aggressive distraction. Placing a maximally tapered graft is the best way to increase lordosis. Trying to place a tall graft has many negative effects. It increases the amount of dural retraction necessary, it increases the volume of bone graft needed to fill the interspace, and it increases the distance over which the fusion must occur (Fig. 49–8).
Cancellous graft is packed from the opposite side into the middle of the interspace; a syringe with the end cut off is useful for this step. Finally, the second trapezoidal allograft is inserted into position. Additional graft is packed around the allograft wedges to fill the disc space maximally with bone. The foramina and the midline under the dura are inspected to ensure no cancellous bone or disc material was displaced into the spinal canal or neural foramen. Lastly, the instrumentation is tightened in gentle compression to secure the grafts and achieve lordosis (Figs. 49-9 through 49-11).
Optimal Graft Material
The “gold standard” for achieving a solid fusion is cancellous autograft bone, regardless of which cage device or intervertebral spacer is chosen. Brantigan61 reported a 97.7% fusion rate when using autologous cancellous graft in the cage he designed. Numerous authors have reported similar fusion rates using bone harvested from the lamina, spinous processes, and facet joints, without using iliac graft.62 Miura and colleagues63 reported a 100% fusion rate at 12 months in 32 patients treated with carbon fiber–reinforced polymer cages. Kim and colleagues64 reported a 95% rate using local bone in titanium vertical mesh cages in a prospective study of 50 patients. Kasis and colleagues65 reported improved clinical outcomes in a prospective comparison when using local bone, attributing at least some of the benefit to avoiding the pain and complications of iliac graft harvest. McAfee and colleagues66 reported a 98% fusion rate in 120 patients using local bone in carbon fiber–reinforced polymer cages placed via a unilateral transforaminal approach.
The aforementioned reports of fusion rates determined radiographically must be interpreted in light of previous studies that suggested that radiographic methods have limited reliability for the determination of fusion success.67,68 Other authors have cautioned that the use of local bone may be associated with pseudarthrosis, and if local bone is used, careful preparation and cleaning is necessary to avoid incorporation of soft tissue with the graft material.69,61 Synthetic ceramic bone graft substitutes combined with iliac crest aspirate have also been reported to be highly effective for achieving interbody fusion,70 but experience is limited.
More recently, there has been a surge of enthusiasm for the use of bone morphogenetic protein to achieve spinal fusion.71 Recombinant human bone morphogenetic protein (rhBMP-2) was shown to be highly effective in achieving spinal fusion when used inside a titanium threaded cage placed anteriorly in the intervertebral space. In a 2-year prospective randomized trial of 279 patients, fusion was achieved in 94.5% of patients compared with 88.7% in the control group in whom iliac autograft was used.72 Although not approved by the U.S. Food and Drug Administration (FDA) for posterior use, many surgeons have extrapolated the outcomes documented in the anterior clinical trial to posterior intervertebral applications. Review of the published data on outcomes of posterior interbody use of this material reveals conflicting results. Although some authors report universal fusion with few complications,73 others have reported a worrisome list of unexpected outcomes.
A clinical trial of rhBMP-2 used in posterior threaded fusion cages was stopped by the FDA because of a high rate of new bone formation (28 of 34 patients) in the spinal canal or neuroforamina, although the authors of the study were enthusiastic about the fusion results and believed that there was no clinical significance to the new bone formation.74 Radiculopathy and neurologic deficit associated with ectopic bone have been reported by other authors,75,76 and radiculitis has been reported with the use of rhBMP-2 even when no ectopic bone has formed. The use of a barrier between rhBMP-2 and the neural elements has been reported to eliminate this complication.77,78 Osteolysis and endplate resorption resulting in subsidence and cage migration have also been reported as a complication of using rhBMP-2. Reports regarding the clinical significance of these radiographic findings vary significantly, and many surgeons continue to advocate for the use of rhBMP-2 in posterior interbody applications.79–82 Optimal dosing and methods of application of this recombinant protein are unclear at this time. Although fusion rates seem to be high with the use of rhBMP-2, questions remain regarding safety, superiority, and cost-effectiveness.
Posterior Lumbar Interbody Fusion Versus Transforaminal Lumbar Interbody Fusion
Although Cloward, Steffee, Branch, and others emphasized the importance of a wide exposure to minimize dural retraction and nerve root injuries during PLIF, other authors described a more medial approach, which was often associated with a high incidence of nerve root injuries. This association was especially true before the advent of posterior instrumentation, when it was often recommended that the facet joints be preserved to avoid destabilizing the motion segment.83 Dural and root injuries were particularly prevalent when threaded fusion cages were used posteriorly because of the emphasis on using large devices to achieve stability through annular distraction.84 Because the cylinders were designed to cut into the endplates, the diameter needed was sometimes 50% greater than the height of the interspace achieved.39 Even when using smaller devices, rates of neurologic complications of 17% were reported.85
In 1997, Harms and colleagues86 reported a technique for performing a posterior interbody fusion through a more lateral approach than previously described.87 These investigators advocated a unilateral approach to the disc space through the neural foramen by removing the facet joint. This approach allowed access to the disc space through the triangular “safe zone” between the exiting nerve root and the lateral dural edge.88 In this way, an interbody fusion could be performed with minimal or no retraction of the dura. The technique has been enthusiastically adopted by many surgeons, and at least one group showed a reduction of nerve root injuries when comparing PLIF and transforaminal lumbar interbody fusion in their hands.89,90
One theoretical disadvantage of the transforaminal lumbar interbody fusion technique is that because only a unilateral approach to the disc space is done, it is impossible to remove the disc material completely. Javernick and colleagues91 showed that, on average, 31% more disc material could be removed when a bilateral approach was done. This finding leads to concern that the fusion rate might not be satisfactory with a unilateral approach. McAfee and colleagues92 showed that for anterior interbody fusions, 100% of patients who underwent complete discectomies achieved a solid arthrodesis, whereas only 86% of patients with incomplete discectomies achieved solid fusion. In an animal model, the inclusion of disc material with autologous bone in cages led to a significant impairment of fusion.93 Despite these concerns, although no prospective randomized comparison has been done, the unilateral approach to the disc space seems to result in adequate fusion rates.
Outcomes and Complications of Posterior Lumbar Interbody Fusion
In large series, fusion rates greater than 95% have routinely been reported with PLIF, regardless of whether carbon fiber–reinforced cages, polymer cages, vertical titanium mesh cages, allograft bone spacers, or stand-alone cylindric threaded cages have been used.19,94–96 In contrast, only 77% of the patients reported by Rivet and colleagues97 achieved solid arthrodesis, despite the use of cancellous iliac graft and supplementary pedicle fixation. Fuji and colleagues98 reported a nonunion rate of 72% in their series of threaded cages placed posteriorly without supplementary fixation. It is unclear whether poor technique or other factors are responsible for the poor fusion results reported in those series.
Probably the most devastating complication that can occur with PLIF procedures is a nerve root injury.99 The reported incidence of this complication varies widely, suggesting that surgical technique is an important factor affecting the frequency with which this complication occurs. Hosono and colleagues, in their review of 240 patients operated on by four different surgeons, correlated complication rates with the experience of the surgeon.85 In that series, 41 patients had some kind of nerve injury, mostly transient. In Brantigan’s series,61 despite previous failed discectomy and the associated epidural scar in 83% of his patients, postoperative radiculitis was reported in only 2 of 100 patients, and one patient experienced a footdrop.
In the larger series of carbon fiber–reinforced polymer cages submitted to the FDA in which 221 patients were operated on by 15 surgeons, 3 cases of reflex sympathetic dystrophy and 3 cases of motor deficit, 1 of which was permanent, were reported.94 Davne and Myers100 reported only a 0.4% rate of traction root injury in their series of 384 PLIF procedures. Krishna and colleagues101 reported postoperative neuralgia in 7.1% of their patients, but they were able to reduce the incidence of this complication by half by modifying their technique and removing the superior facet to widen the exposure. Barnes and colleagues84 reported a 13.6% incidence of permanent nerve root injury when using threaded fusion cages but no nerve root injury when using much smaller allograft wedges. These results reinforce the principle that wide exposure, careful technique, and avoiding oversized grafts can minimize the risk of neurologic injury in PLIF.
Graft displacement and loosening is another complication that was associated with PLIF when the technique was first described. This is a rare complication, however, with the addition of posterior pedicle screw stabilization. Subsidence of the implants can occur with PLIF. Most authors have not carefully documented this phenomenon. Factors predisposing to subsidence include inadequate graft technique and sizing and endplate injury during preparation. Patient factors such as weight and osteoporosis are likely important.102 Implant type may be a factor, but convincing evidence of this has not been presented.103,104 Pedicle screws alone do not prevent subsidence after PLIF.105 Other complications of PLIF are similar to the complications encountered in all lumbar spine surgery. Epidural hematoma, wound infections, and other non–implant-related complications seem to occur with similar frequency in PLIF as in other reconstructive operations on the lumbar spine.
1 White AH. Editorial commentary. In: White AH, Rothman RH, Ray CD, editors. Lumbar Spine Surgery Techniques and Complications. St Louis: CV Mosby; 1987:294-295.
2 Verlooy J, Smedt KD, Selosse P. Failure of a modified posterior lumbar interbody fusion technique to produce adequate pain relief in isthmic spondylolytic grade 1 spondylolisthesis patients. Spine (Phila Pa 1976). 1993;18:1491-1495.
3 Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg Am. 1944;26:125-130.
4 Ovens JM, Williams HG. Intervertebral spine fusion with removal of herniated intervertebral disk. Am J Surg. 1945;70:24-26.
5 Jaslow IA. Intercorporeal bone graft in spinal fusion after disc removal. Surg Gynecol Obstet. 1946;82:215.
6 Cloward RB. The treatment of ruptured intervertebral discs by vertebral body fusion: Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
7 Collis JS. Total disc replacement: A modified posterior lumbar interbody fusion: Report of 750 cases. Clin Orthop Relat Res. 1985;193:64-67.
8 Ma GW. Posterior lumbar interbody fusion with specialized instruments. Clin Orthop Relat Res. 1985;193:57-63.
9 Lin PM, Cautilli RA, Joyce MF. Posterior lumbar interbody fusion. Clin Orthop Relat Res. 1983;180:154-168.
10 Hutter CG. Posterior lumbar interbody fusion, a 25 year study. Clin Orthop Relat Res. 1983;179:86-96.
11 Cohen DS: Etiology of broken pedicle screw instrumentation in the treatment of spondylolisthesis. Proceedings of the North American Spine Society 11th Annual Conference, Vancover, 1996, pp 168-169.
12 Steffee AD, Sitkowski DJ. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988;227:99-102.
13 Brantigan JW, Steffee AD, Geiger JM. A carbon fiber implant to aid interbody lumbar fusion: Mechanical testing. Spine (Phila Pa 1976). 1991;16(6 Suppl):S277-S282.
14 Cloward RB. Gas-sterilized cadaver bone grafts for spinal fusion operations: A simplified bone bank. Spine (Phila Pa 1976). 1980;5:4-10.
15 Cloward RB. Posterior lumbar fusion updated. Clin Orthop Relat Res. 1985;193:16-19.
16 Brantigan JW, Cunningham BW, Warden K, et al. Compression strength of donor bone for posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1993;18:1213-1221.
17 Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976). 1994;19:1271-1279.
18 Barnes B, Rodts GE, Haid R, et al. Allograft implants for posterior lumbar interbody fusion: Results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191-1198.
19 Kim KT, Lee SH, Lee YH, et al. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006;31:1351-1357.
20 Fritzell P, Hägg O, Wessberg P, et al. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2002;27:1131-1141.
21 Jacobs WC, Vreeling A, DeKleuver M. Fusion for low-grade adult isthmic spondylolisthesis: A systematic review of the literature. Eur Spine J. 2006;15:391-402.
22 Byrd JA3rd, Scoles PV, Winter RB, et al. Adult idiopathic scoliosis treated by anterior and posterior spinal fusion. J Bone Joint Surg Am. 1987;69:843-850.
23 DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130-139.
24 Cohen DB, Chotivichit A, Fujita T, et al. Pseudarthrosis repair: Autogenous iliac crest versus femoral ring allograft. Clin Orthop Relat Res. 2000;371:46-55.
25 Molinari RW, Bridwell KH, Lenke LG, et al. Anterior column support in surgery for high-grade, isthmic spondylolisthesis. Clin Orthop Relat Res. 2002;394:109-120.
26 Suk SI, Lee CK, Kim WJ, et al. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 1997;22:210-219.
27 Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976). 2000;25:853-857.
28 Nachemson A, Zdeblick TA, O’Brien JP. Lumbar disc disease with discogenic pain: What surgical treatment is most effective? Spine (Phila Pa 1976). 1996;21:1835-1838.
29 Lowery GL, Harms J. Principles of load sharing. In: Bridwell KH, Dewald RL, editors. The Textbook of Spine Surgery. 2nd ed. Philadelphia: Lippincott-Raven; 1997:155-165.
30 Lee JY, Milne EL, Shufflebarger HL, et al: Anterior column support in long segment kyphosis constructs. In Proceedings of the Scoliosis Research Society 32nd Annual Meeting, St. Louis, 1997, Paper #50.
31 McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744.
32 Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: A literature review. Eur Spine J. 2000;9:S95-S101.
33 Tencer AF, Hampton D, Eddy S. Biomechanical properties of threaded inserts for lumbar interbody spinal fusion. Spine (Phila Pa 1976). 1995;20:2408-2414.
34 Voor MJ, Mehta S, Wang M, et al. Biomechanical evaluation of posterior and anterior lumbar interbody fusion techniques. J Spinal Disord. 1998;11:328-334.
35 Dimar JR, Beck DJ, Glassman SD, et al. Posterior lumbar interbody cages do not augment segmental biomechanical stability. Am J Orthop. 2001;8:636-639.
36 Brodke DS, Dick JC, Kunz DN, et al. Posterior lumbar interbody fusion: A biomechanical comparison, including a new threaded cage. Spine (Phila Pa 1976). 1997;22:26-31.
37 Pitzen T, Geisler FH, Matthis D, et al. Motion of threaded cages in posterior lumbar interbody fusion. Eur Spine J. 2000;9:571-576.
38 Lund T, Oxland TR, Jost B, et al. Interbody cage stabilisation in the lumbar spine: Biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351-359.
39 Tsantrizos A, Baramki HG, Zeidman S, et al. Segmental stability and compressive strength of posterior lumbar interbody fusion implants. Spine (Phila Pa 1976). 2000;25:1899-1907.
40 Goh JC, Wong HK, Thambyah A, et al. Influence of PLIF cage size on lumbar spine stability. Spine (Phila Pa 1976). 2000;25:35-39.
41 Kettler A, Wilke HJ, Dietl R, et al. Stabilizing effect of posterior lumbar interbody fusion cages before and after cyclic loading. J Neurosurg. 2000;92(1 Suppl):87-92.
41a Cloward RB. Gas sterilized cadaver bone grafts for spinal fusion operations. A simplified bone bank. Spine. 1980;5:4.
42 Vadapalli S, Sairyo K, Goel VK, et al. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion: A finite element study. Spine (Phila Pa 1976). 2006;31:E992-E998.
43 van Dijk M, Smit TH, Sugihara S, et al. The effect of cage stiffness on the rate of lumbar interbody fusion: an in vivo model using poly(l-lactic Acid) and titanium cages. Spine (Phila Pa 1976). 2002;27:682-688.
44 Kanayama M, Cunningham BW, Haggerty CJ, et al. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. Neurosurgery. 2000;93(2 Suppl):259-265.
45 Fogel GR, Toohey JS, Neidre A, et al. Outcomes of posterior lumbar interbody fusion with the 9-mm width lumbar I/F cage and the variable screw placement system. J Surg Orthop Adv. 2009;18:77-82.
46 Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30:2024-2029.
47 Groth AT, Kuklo TR, Klemme WR, et al. Comparison of sagittal contour and posterior disc height following interbody fusion: Threaded cylindrical cages versus structural allograft versus vertical cages. J Spinal Disord Tech. 2005;18:332-336.
48 Sears W. Posterior lumbar interbody fusion for lytic spondylolisthesis: Restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J. 2005;5:161-169.
49 Gödde S, Fritsch E, Dienst M, et al. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2003;28:1693-1699.
50 Brantigan JW, Neidre A. Achievement of normal sagittal plane alignment using a wedged carbon fiber reinforced polymer fusion cage in treatment of spondylolisthesis. Spine J. 2003;3:186-196.
51 Jost B, Cripton PA, Lund T, et al. Compressive strength of interbody cages in the lumbar spine: The effect of cage shape, posterior instrumentation and bone density. Eur Spine J. 1998;7:132-141.
52 Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976). 2001;26:889-896.
53 Lowe TG, Hashim S, Wilson LA, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976). 2004;29:2389-2394.
54 Labrom RD, Tan JS, Reilly CW, et al. The effect of interbody cage positioning on lumbosacral vertebral endplate failure in compression. Spine (Phila Pa 1976). 2005;30:E556-E561.
55 Tan JS, Bailey CS, Dvorak MF, et al. Interbody device shape and size are important to strengthen the vertebra-implant interface. Spine (Phila Pa 1976). 2005;30:638-644.
56 Closkey RF, Parsons JR, Lee CK, et al. Mechanics of interbody spinal fusion: Analysis of critical bone graft area. Spine (Phila Pa 1976). 1993;18:1011-1015.
57 Oxland TR, Grant JP, Dvorak MF, et al. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine (Phila Pa 1976). 2003;28:771-777.
58 Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976). 2000;25:1077-1084.
59 Lebwohl NH, Green BA, Buck BE: Technique indications complications and outcomes of allograft PLIF. In Proceedings of the Scoliosis Research Society 35th Annual Meeting, Cairns, Australia, 2000, Exhibit #66.
60 Steffee A: Personal communication, October 1994.
61 Brantigan JW. A prospective study of 100 consecutive cases. In: Brantigan JW, Lauryssen C, editors. Intervertebral Fusion Using Carbon Fiber Reinforced Polymer Implants. St Louis: Quality Medical Publishing; 2006:231-248.
62 Arai Y, Takahashi M, Kurosawa H, et al. Comparative study of iliac bone graft and carbon cage with local bone graft in posterior lumbar interbody fusion. J Orthop Surg. 2002;10:1-7.
63 Miura Y, Imagama S, Yoda M, et al. Is local bone viable as a source of bone graft in posterior lumbar interbody fusion? Spine (Phila Pa 1976). 2003;28:2386-2389.
64 Kim KT, Lee SH, Lee YH, et al. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006;31:1351-1357.
65 Kasis AG, Marshman LA, Krishna M, et al. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine (Phila Pa 1976). 2009;34:572-577.
66 McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976). 2005;30(6 Suppl):S60-S65.
67 Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976). 1993;18:1186-1189.
68 Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine (Phila Pa 1976). 1991;16(6 Suppl):S261-S265.
69 Togawa D, Bauer TW, Lieberman IH, et al. Lumbar intervertebral body fusion cages: Histological evaluation of clinically failed cages retrieved from humans. J Bone Joint Surg Am. 2004;86:70-79.
70 Peterson M, Weinman C, Lewis M. The use of ultraporous a-tricalcium phosphate supplementing local autograft in lumbar interbody fusion surgery. Spine J. 2005;5(Suppl):S171.
71 Rihn JA, Gates C, Glassman SD, et al. The use of bone morphogenetic protein in lumbar spine surgery. J Bone Joint Surg Am. 2008;90:2014-2025.
72 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
73 Geibel PT, Boyd DL, Slabisak V. The use of recombinant human bone morphogenic protein in posterior interbody fusions of the lumbar spine: a clinical series. J Spinal Disord Tech. 2009;22:315-320.
74 Haid, RW Jr, Branch CLJr, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527-538.
75 Wong DA, Kumar A, Jatana S, et al. Neurologic impairment from ectopic bone in the lumbar canal: A potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8:1011-1018.
76 Chen NF, Smith ZA, Stiner E, et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40-46.
77 Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9:623-629.
78 Villavicencio AT, Burneikiene S, Nelson EL, et al. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3:436-443.
79 Smoljanovic T, Bojanic I, Delimar D. Adverse effects of posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2009;18:920-923.
80 Vaidya R, Sethi A, Bartol S, et al. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557-562.
81 Rihn JA, Makda J, Hong J, et al. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: A clinical and radiographic analysis. Eur Spine J. 2009;18:1629-1636.
82 Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17:1735-1744.
83 Lin PM. Posterior lumbar interbody fusion technique: Complications and pitfalls. Clin Orthop Relat Res. 1985;193:90-102.
84 Barnes B, Rodts, GE Jr, Haid RWJr, et al. Allograft implants for posterior lumbar interbody fusion: Results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191-1198.
85 Hosono N, Namekata M, Makino T, et al. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: Analysis of risk factors. J Neurosurg Spine. 2008;9:403-407.
86 Harms J, Jeszenszky D, Stoltze D, et al. True spondylolisthesis reduction and monosegmental fusion in spondylolisthesis. In: Bridwell KH, Dewald RL, editors. The Textbook of Spine Surgery. 2nd ed. Philadelphia: Lippincott-Raven; 1997:1337-1347.
87 Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol. 1998;10:90-102.
88 Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991;58:159-164.
89 Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976). 2001;26:567-571.
90 Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: Transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2:118-126.
91 Javernick MA, Kuklo TR, Polly DWJr. Transforaminal lumbar interbody fusion: Unilateral versus bilateral disk removal—an in vivo study. Am J Orthop. 2003;32:344-348.
92 McAfee PC, Lee GA, Fedder IL, et al. Anterior BAK instrumentation and fusion: Complete versus partial discectomy. Clin Orthop Relat Res. 2002;394:55-63.
93 Li H, Zou X, Laursen M, et al. The influence of intervertebral disc tissue on anterior spinal interbody fusion: An experimental study on pigs. Eur Spine J. 2002;11:476-481.
94 Brantigan JW, Steffee AD, Lewis ML, et al. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: Two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976). 2000;25:1437-1446.
95 Arnold PM, Robbins S, Paullus W, et al. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer: A prospective, multicenter trial with 2-year follow-up. Am J Orthop. 2009;38:E115-E122.
96 Kuslich SD, Danielson G, Dowdel JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976). 2000;25:2656-2662.
97 Rivet DJ, Jeck D, Brennan J, et al. Clinical outcomes and complications associated with pedicle screw fixation-augmented lumbar interbody fusion. J Neurosurg Spine. 2004;1:261-266.
98 Fuji T, Oda T, Kato Y, et al. Posterior lumbar interbody fusion using titanium cylindrical threaded cages: Is optimal interbody fusion possible without other instrumentation? J Orthop Sci. 2003;8:142-147.
99 Wetzel FT, LaRocca H. The failed posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1991;16:839-845.
100 Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine (Phila Pa 1976). 1992;17(6 Suppl):S184-S189.
101 Krishna M, Pollock RD, Bhatia C. Incidence, etiology, classification, and management of neuralgia after posterior lumbar interbody fusion surgery in 226 patients. Spine J. 2008;8:374-379.
102 Okuda S, Oda T, Miyauchi A, et al. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88:2714-2720.
103 Abbushi A, Cabraja M, Thomale UW, et al. The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J. 2009;18:1621-1628.
104 Tokuhashi Y, Ajiro Y, Umezawa N. Subsidence of metal interbody cage after posterior lumbar interbody fusion with pedicle screw fixation. Orthopedics. 2009;32:259.
105 Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976). 2004;19:1271-1279.