Posterior Lumbar Arthroscopic Discectomy and Rehabilitation
Haideh V. Plock, Ben B. Pradhan, David Pakozdi and Rick B. Delamarter
Lumbar herniated nucleus pulposus (HNP) falls within the spectrum of degenerative spinal conditions and can occur with little or no trauma. Lumbar disc abnormalities increase with age.1,2 The actual incidence of lumbar disc herniations is unknown because many people with herniations are asymptomatic.1,3,4 Approximately 90% of lumbar herniations occur at the L4-L5 and L5-S1 levels.1,5,6 More than 200,000 discectomies are performed in the United States each year, and this number is likely increasing.7 The success of this procedure, as with all surgical procedures, depends vastly on proper patient selection and to a lesser extent on surgical technique. However, it is incumbent on the spinal surgeon to be absolutely meticulous with intraoperative technique once the decision for surgery is made. To this end, the use of a microscope is recommended for lumbar discectomy. Once the learning curve has been mastered, the microscope not only offers advantages over loupes but also forces one to think at a much higher level of clarity about what and where root encroachment pathology is present.8 More importantly, the patient has less morbidity and an earlier hospital discharge compared with standard or limited discectomy.5,9–14
Surgical Indications and Considerations
Pathophysiology
Intervertebral discs cushion and tether the vertebrae, providing both flexibility and stability. The normally gelatinous nucleus pulposus is surrounded by the ligamentous annulus broses. In the young and healthy disc, the nucleus and annulus blend. Degenerative or pathologic changes can cause separations of the two entities, as well as compromise the integrity of the annulus, such that a sufficient load can cause nuclear fragments to migrate and impinge on neural elements.15 This pathophysiology is reflected in a clinical presentation of acute sciatica pain with a prior history of back pain. Lumbar disc herniations may occur with little or no trauma, although patients frequently report a bending or twisting motion as the inciting event, causing the onset of symptoms. Common causes of lumbar herniations include falls, car accidents, repetitive heavy lifting, and sports injuries of all types.
Diagnosis
The radiographic diagnosis of lumbar disc herniation has been made rather simple with magnetic resonance imaging (MRI). The clinical diagnosis is frequently straightforward as well. A patient with a lumbar herniation generally has some element of low back pain with radiation into the buttocks, thigh, leg, and foot. The leg radiation almost always follows a dermatomal distribution. Patients frequently complain of numbness, tingling, or weakness in the affected dermatome. Lying down may relieve the symptoms, whereas sitting, walking, and standing may exacerbate them. Provocative maneuvers that increase abdominal pressure (coughing, sneezing, defecating) may intensify symptoms as well. Complaints of bowel and bladder dysfunction may signal a cauda equina syndrome and may necessitate emergent workup and treatment.
Physical Examination
Visual inspection may reveal lumbar muscle spasm, fasciculations, and postural changes, including listing to the side and a forward flexed position. Gait observation can reveal a listing antalgic walk. Weakness can give a dropped foot type gait (anterior tibialis), buckling of the leg (quadriceps), or Trendelenburg gait (gluteus medius). Range of motion (ROM) testing may be limited secondary to pain. Neurologic testing is extremely important and should include motor, sensory, and reflex testing. Lumbar herniations may cause varying degrees of dermatomal weakness, sensory deficits, and reflex changes. Straight-leg raises (SLRs) are a good indicator of nerve root impingement in lower lumbar herniations, and a positive femoral stretch can indicate an upper lumbar herniation.
Imaging and Other Tests
MRI is clearly the imaging study of choice to diagnose a lumbar disc herniation (Fig. 15-1). Plain radiographs should always be obtained to evaluate overall alignment, bony integrity, and stability. Patients who cannot obtain an MRI can be diagnosed using computed tomography (CT), CT myelogram, or CT discogram. These imaging tests are so sensitive that a discectomy is not indicated if a disc is not found to be herniated by one of these techniques. Other tests can include an electromyogram (EMG) or nerve conduction study.
Management
It is important to understand that most patients with symptomatic herniated lumbar discs will get better over time, regardless of the type of treatment. Weber’s classic study16 reported that sciatica from HNP would improve 60% of the time with nonsurgical methods and 92% of the time with surgery at 1 year. By 4 years out, no statistical difference was found (51% improvement in conservative group versus 66% in the surgical group), and no difference was found at 10-year follow-up. The 5-year outcomes from the Maine Lumbar Spine Study are similar to the 4-year results of the Weber study. At 1-year follow-up, 71% of surgical patients reported relief of leg symptoms compared with 43% of conservatively managed patients.17 They reported long-term follow-up at 5 years, with 70% of patients in the operative group describing improvement versus 56% in the nonoperative group.18 More recently, the Spine Patient Outcomes Research Trial results have contributed to the favorable opinion of surgical outcomes as well. Treatment effects were statistically significant at 2 years and maintained at 4-year follow-up for primary outcomes in favor of surgery.19
In the absence of cauda equina syndrome or progressive or significant neurologic deficits, most practitioners attempt conservative care before suggesting surgical intervention.
Nonoperative Treatment
Indications for Surgery
Surgical indications, as currently recommended by the North American Spine Society (NASS), include a definite diagnosis of ruptured lumbar intervertebral disc and the following23,24:
1 Failure of conservative treatment
2 Unbearable or recurrent episodes of radicular pain (or both)
3 Significant neurologic deficit
Conservative treatment consists of nonoperative management and careful observation. Some may benefit from a short trial of nonoperative treatment even after 8 weeks if no prior care was given. Failed conservative treatment is the most common indication for lumbar discectomy. Those who have not improved sufficiently and are not experiencing continued improvement might then be offered treatment by surgical excision of the disc. Such patients should be advised that this is an elective operation but that delay for longer than 3 to 6 months in the face of persistent and severe symptoms may compromise the best ultimate result.24,25 The other indications (2 to 5) are exceptions to the 4- to 8-week rule. Excruciating pain may not be relieved by nonoperative means and may require earlier surgical decompression. Recurrent sciatica should also receive consideration for surgery: the chance of recurrent sciatica after the second episode is 50% and after the third episode is almost 100%.25 An example of a significant neurologic deficit may be a foot drop or weakness that prevents normal posture, gait, or affects the patient’s profession or a particular skill. Any definite progression of neurologic deficit is an absolute indication for surgery. Cauda equina syndrome is relatively rare. It is reported in 1% to 3% of patients with confirmed disc herniations,26,27 and it is an orthopedic or neurosurgical emergency. Features include rapid progression of neurologic signs and symptoms, bilateral leg pain, caudal sensory deficit, bladder overflow incontinence or retention, and loss of rectal sphincter tone with or without fecal incontinence.
Contraindications for Discectomy
![]() NASS and the American Academy of Orthopaedic Surgeons have identified the following factors as absolute or relative contraindications for discectomy24,28:
NASS and the American Academy of Orthopaedic Surgeons have identified the following factors as absolute or relative contraindications for discectomy24,28:
1 Lack of clear clinical diagnosis, anatomic level of lesion, and radiographic evidence of HNP
2 Lack of trial of nonoperative treatment (with the exceptions mentioned previously)
5 Medical contraindications to surgery (e.g., major comorbidities, unfavorable survival)
6 Disc herniation at a level of instability (may need additional stabilization)
Surgical Procedures
One only has to review the natural history of lumbar disc disease to realize that spinal surgeons play a palliative role in the management of HNP.29–33 Surgical procedures as treatment for lumbar HNP include the following:
1 Lumbar discectomy (microscopic or standard open technique)
a Hemilaminotomy and discectomy
2 Minimally invasive percutaneous techniques
b Percutaneous discectomy (suction, shaver, laser, endoscopic tools)
Use of an Operating Microscope
The attempt to improve visualization and illumination has led many spine surgeons to use loupes and a headlight. We believe the magni cation and illumination built into the microscope offer many surgical advantages, the most important of which is reduced wound size and decreased tissue manipulation (Fig. 15-2). The surgeon can limit the amount of tissue dissection by working through a small exposure directly over the pathology to be removed. Microsurgical techniques can also be used to preserve the ligamentum flavum and epidural fat to minimize postoperative epidural broses and improve clinical results by preserving natural tissue planes.8,34 With this approach, the disc herniation can be easily removed, lateral recess stenosis can be decompressed, and nerve root manipulation is kept to a minimum. The senior author has used this technique since 1986 for most lumbar disc herniations and has found the approach to be safe, with fewer dural tears and nerve root injuries and less postoperative epidural broses than with standard discectomy.5,8,10,35
However, the microscope is not without its disadvantages. Peripheral vision is lost, with the field of vision limited to approximately 4 to 5 cm. Because of this, the surgeon needs to know detailed anatomy of the spine. The line of vision is axed through the microscope. To look over structures (to overcome tissue overhang), the patient or microscope has to be adjusted during the surgery. This can be avoided by proper retraction or dissection of tissue away from the line of vision. Researchers reported increased disc space infection after microsurgery.36,37 This was most likely caused by contamination from unsterile parts of the microscope during surgery, although no one has looked at the potential for an increased infection rate when two surgeons with loupes and headlights bump heads over the wound! Recent reports by those who have experience with the microscope do not show any increased infection rates.5,10,14,38,
Lumbar Microdiscectomy
Microscopic discectomy (microdiscectomy) has become the gold standard for operative treatment of lumbar disc herniations, and the latest minimally invasive percutaneous techniques have not been shown to be more effective.8,39,40 Although no statistical differences can be shown in the ultimate long-term outcomes of microscopic versus standard open discectomies,11,13,14,32,41–43 the microscope provides improved illumination and magni cation, and patients have less morbidity and earlier hospital discharge when compared with standard discectomies.
Operative Setup
General anesthesia is preferable because of patient comfort, as well as airway and sedation control. Another advantage is the option of hypotensive anesthesia. The procedure can also be done under epidural or local anesthesia with sedation, although this is not our preference. The patient’s position is always prone with the abdomen free, thus relieving pressure on the abdominal venous system and, in turn, decreasing venous backflow through the Batson venous plexus into the spinal canal. This has the effect of decreasing bleeding from the epidural veins intraoperatively. Several frames are available for this, but we prefer a Wilson frame on a regular operating table because of the ease of setup.
Identification of Level and Side
A preincision lateral radiograph or fluoroscopy image, with a radiopaque skin marker placed according to preoperative radiographs and anatomic landmarks, will identify the appropriate incision location for the disc space to be exposed. This is best done by placing a spinal needle as straight vertically as possible, approximately 2 cm from midline contralateral to the side of surgery. The side of surgery is usually the more symptomatic side, although occasionally a midline HNP can be approached from either side.
Skin Incision and Interlaminar Space Exposure
A 2- to 3-cm incision is made midline or up to 1 cm lateral to the spinous process on the symptomatic side at a level directly over the disc space based on the localizing lateral radiograph. At L5-S1, this incision tends to be directly over the interlaminar space; but as one moves up the lumbar spine, this incision will be progressively over the cephalad lamina. The dissection is carried down to the lumbodorsal fascia, which is sharply incised. The fascial incision is placed carefully, just lateral to the spinous processes to avoid damage to the supraspinous and interspinous ligament complex. The subperiosteal muscle dissection and elevation are confined to the interlaminar space and approximately half of the cephalad and caudad lamina. The facet capsules are carefully preserved. A Cobb elevator and Bovie cautery are used. A framed retractor is then placed. The surgeon should expose the lateral border of the pars as a landmark for preserving enough of the pars during laminotomy to prevent fracture.
At this time another localizing lateral radiograph should be obtained to confirm the proper level. A forward-angled curette can be placed underneath the cephalad lamina of the interspace. With this intraoperative radiographic verification, wrong-level surgery is impossible. The radiograph will also indicate how much of the cephalad lamina needs to be removed to expose the disc space. The microscope is then brought into position.
Spinal Canal Entry
After exposure of the interlaminar space and placement of the retractor, a high-speed burr is used to remove several millimeters of the cephalad lamina and 2 to 3 mm of the medial aspect of the inferior facet, taking care to leave at least a 6-mm bridge of bone at the level of the pars (Fig. 15-3). Once the cephalad lamina and medial aspect of the inferior facet have been removed, the ligamentum flavum is easily seen because its bony attachments are exposed. The ligamentum attaches at the very cephalad edge of the lower lamina, but approximately halfway up the upper lamina, and it attaches to the medial aspect of the superior facet. Thus the high-speed burr can be used relatively safely on top of the bottom half of the superior lamina, as well as the medial aspect of the inferior facet.
Free Ligamentum Flavum
The ligamentum flavum is then released from the medial edge of the superior facet with a forward-angled curette. It can also be released from the undersurface of the upper and lower lamina (Fig. 15-4). It is safest to start the curette inferolaterally toward the superior aspect of the pedicle (caudal aspect of the foramen).
A ligamentum and epidural fat-sparing approach, performed by creating a flap of the ligamentum as described previously, decreases postoperative epidural broses and can improve results.8,34 However, this can make it more difficult to get a good view of the nerve root. Certainly this is easier with a microscope than without one. The less-experienced surgeon may perform partial removal of these tissues. The ligamentum flap is also not recommended for large midline disc herniations (with or without cauda equina syndrome) and severely stenotic canals because the ligamentum itself occupies more room in the already severely compromised spinal canal and would also interfere with direct visualization for the delicate manipulation of the thecal sac.
Lateral Recess Exposure
After release of the ligamentum flavum, the medial edge of the superior facet is resected with 2- to 4-mm Kerrison rongeurs. This resection goes from the lower pedicle to the tip of the superior facet (Fig. 15-5). This medial facet resection decompresses any lateral recess stenosis at the level of the pedicle and up into the foramen, and it allows easy access to the lateral disc space. If needed, some of the lateral ligamentum flavum, particularly into the foramen, can be removed with the Kerrison rongeurs.
Nerve Root and Ligamentum Retraction
Bipolar cautery can be used at this time to cauterize any epidural bleeding over the lateral disc space, directly cephalad to the pedicle. We recommend finding the pedicle and then using it as a guide to release the epidural nonneural tissues above the disc space. At this point a nerve root retractor can be placed on the disc space, and the ligamentum flavum, epidural fat, and nerve root are retracted toward the midline, generally exposing the herniation (Fig. 15-6). Again, the bipolar cautery can be used to cauterize any epidural veins over the disc herniation. Any free large fragments of disc can now be removed (Fig. 15-7). If needed, a forward-angled curette can be used to scrape the inferior and posterior bony margins of the foramen, using a unidirectional pulling motion. Using the bony pedicle as a starting point ensures that the end of the curette does not include any neural tissue before scraping.
Discectomy
Frequently the annular defect of the disc herniation is all that is necessary to allow cleaning out of any loose nucleus pulposus inside the disc space, although the annulotomy can be enlarged with a No. 11 blade. The herniated nuclear material is then cleaned out with straight or angled pituitary rongeurs and small back-angled curettes. Care should be taken not to damage or curette the endplates. The annulotomy can be performed in various shapes, which are not discussed in detail here.36,44
One unresolved issue is how much disc to remove from the disc cavity. Removal of as much disc as possible implies curettage of the interspace, including possible removal of the cartilaginous endplates. Critics of this approach point out that no matter how long the surgeon works, it is impossible to remove all disc material in this fashion. They also argue that this method increases risk of damage to anterior visceral structures and increases risk of chronic back pain induced by conditions, such as sterile discitis and instability. Although some surgeons believe that extensive intradisc débridement decreases the rate of recurrent HNP, others refute that position.36,45–47 In the end, the only reasonable prospective controlled study is Spengler’s,48 which suggests that limited disc excision is all that is necessary. The advantages of limited disc excision are less trauma to endplates and less dissection, less nerve root manipulation, a lower prevalence of infection, reduced risk of damage to structures anterior to the disc space, and less disc space settling postoperatively (theoretically reducing the incidence of chronic back pain).
Disc Space Irrigation
After the HNP and any remaining loose material is removed, the disc space is irrigated under some pressure with a long angiocatheter; then the pituitary rongeur is again used to remove any loose fragments. The spinal canal is then palpated underneath the nerve root and across the vertebral bodies above and below for any residual fragments. In doing the limited disc excision, one must also be sure to probe under the posterior annulus (both medially and laterally) for loose fragments. This is an important step to ensure that no displaced or sequestered fragments are missed. Residual disc material will feel rough, whereas the native dural surface is quite smooth. In the end the patient must be left with a freely mobile nerve root. The preoperative MRI should be carefully studied for displaced fragments, but it is important to keep in mind that fragments may have moved since the MRI was taken.
Closure
Once the decompression is complete, the entire surgical wound is thoroughly irrigated with antibiotic-containing irrigant. Any final bleeding is controlled with bipolar cautery, thrombin-soaked gel foam, or FloSeal hemostatic gel. After complete hemostasis and removal of all gel foam, the closure is performed in layers. Many attempts have been made to design substances to seal the laminotomy defect and prevent scar formation, including fat grafts, hydrogel, silicone, Dacron, and steroids.49 We simply prefer the ligamentum flap (Fig. 15-8).5,8,25 The dorsal lumbar fascia is closed with No. 1-0 sutures, the subcutaneous layer with 2-0 sutures, and the skin with 3-0 subcuticular sutures. Using this ligamentum flavum–sparing approach, blood loss should be no more than 10 to 20 ml. With good hemostasis, drainage of the surgical wound is not necessary.
Postoperative Course
Many microdiscectomy procedures can be done on an outpatient basis.12,50,51 Most patients are encouraged to walk as tolerated. Sitting is also tolerated but may be more limited. Many return to work within 5 to 10 days, especially those with desk type of work. All patients are required to participate in lumbar physical therapy, primary stabilization, and mobilization beginning at approximately 4 weeks after surgery. Most athletes return to their normal athletic activities within 8 weeks after surgery. However, the postoperative course is variable, and return to normal activities depends on the patient’s overall medical condition, as well as neurologic and overall recovery.16,52,53
Unusual Disc Herniations
Herniated Nucleus Pulposus at High Lumbar Levels (L1-L2, L2-L3, L3-L4)
High lumbar HNPs are uncommon (5%). When they occur they are likely to be foraminal or extraforaminal.25,54 Important skeletal anatomy in the higher lumbar spine for the spinal surgeon to be aware of includes the following: (1) the pars are narrower, and facet integrity is easily lost with excessive laminotomy; (2) the laminae are broader; (3) the interlaminar window is narrower; (4) the inferior border of the lamina overhangs more of the disc space; (5) at L1-L2, the conus cannot be retracted like the cauda equina at lower levels; (6) the nerve roots exit more horizontally and are less mobile; and (7) epidural veins may be more prevalent. At these levels, because of the limited size of the interlaminar space, ligamentum excision rather than sparing is recommended.
Recurrent Disc Rupture
The incidence of recurrent HNP at the same level and side of a previously operated on disc is 2% to 5%.5,55,56 The microscope is especially valuable in this scenario because of the scar between tissue planes, including neural elements. Adequate time must be spent carefully teasing the tissues apart with a blunt instrument (e.g., bipolar, curette, Penfield) before forcefully mobilizing the nerve root. The incidence of complications is understandably higher in revision discectomies.
Cauda Equina Syndrome
The classic teaching in cauda equina syndrome is that (1) it is an orthopedic emergency, and (2) a wide decompression through a bilateral approach is necessary. We agree with the first point but not the second. Few disc herniations are too big to be addressed microsurgically. A wider hemilaminectomy may be needed. The microscope is invaluable when working in the severely stenotic canal. If the disc cannot be easily or totally excised unilaterally, then bilateral hemilaminotomies may be done.26,27
Herniated Nucleus Pulposus in the Adolescent Patient
The risk for recurrence of HNP after surgical excision is higher in adolescents than in adults. Because of the high proteoglycan content in adolescent discs and the prevalence of disc protrusions rather than disc extrusions, some have recommended percutaneous chemonucleolysis rather than surgical intervention in this age group.25,57,58 Studies have been published with controversial results for surgical discectomy in this patient population.59–61 Chemonucleolysis may have merit in the treatment of symptomatic disc protrusions, but discectomy is necessary in the setting of an extruded or sequestered disc causing significant or progressive neurologic deficit or pain. These extruded or sequestered fragments are frequently heavily collagenized.24,62 Long-term follow-up studies of more than 12 years after discectomy in this group have reported good to excellent results in 87% to 92% of patients.63,64
Complications
Complications for the discectomy procedures include dural tears, neural injury, visceral injuries, postoperative infection, recurrence of herniation, inadequate decompression, and iatrogenic instability.
Dural tears occur in 1.0% to 6.7% of cases, although the incidence decreases with experience.5,16,38,65–67 If possible, repair should be done by direct suture (5-0 to 7-0 silk, nylon, or polypropylene) with or without a dural patch.65 The patient should be kept flat for a few days after surgery to lower the hydrostatic pressure in the lumbar thecal sac while the repair seals.
Neural injuries are rare, although the risk is greater with unusual disc herniations as described previously. Visceral injuries occur when an instrument penetrates the anterior annulus. Among these, vascular injuries are the most common.65,68 If these are recognized, then immediate laparotomy for surgical repair is indicated.
Postoperative discitis occurs in 1% of cases or less in experienced hands, although clearly a learning curve exists in developing facility with the microscope. Higher infection rates (up to 7%) have been reported with the use of a microscope during surgery, although in experienced hands this has been shown not to be true.65 An MRI is the best diagnostic imaging tool. An image-guided needle biopsy may be performed to assist in organism-specific antibiotic selection. Reoperation may not be necessary unless the patient develops root compression, cauda equina syndrome, or an epidural abscess.
The literature reports recurrent HNP at a previously operated site occurring anywhere from 2% to 5% after lumbar discectomy.25,69 When reoperating for a recurrent HNP, it is important to get adequate exposure of the dural sac above and below the disc space. Then using a combination of blunt (nerve hook, Penfield, bipolar) and sharp (Kerrison) dissection, the dural sac and nerve root are exposed and mobilized above the HNP.
Iatrogenic mechanical instability is fortunately a rare occurrence after discectomy, even if a decompressive laminectomy was required for a stenotic canal or to excise a large disc.6 Symptomatic mechanical treatment may require surgical stabilization. Suboptimal results after discectomy can be the result of several other problems that, unfortunately, do not have a straightforward medical or surgical treatment. Although very rare, these can include epidural broses, arachnoiditis, and complex regional pain syndrome.65
Discussion
Most modern studies using microscopic techniques for treatment of herniated lumbar discs report 90% to 95% success rates.* A multicenter, prospective trial has proved what cannot be repeated often enough: If the therapist selects patients with dominant radicular pain (compared with back pain), with neurologic changes and painful SLRs, and with a study confirming a disc rupture, then he or she can anticipate a high level of success for discectomy, with or without a microscope.41 The rate of successful outcome drops significantly as more of these inclusion criteria are not met. Persistent back pain occurs in up to 25% of patients who undergo microdiscectomy.66,67 This has led to the opinion that it is important to save the supraspinous and intraspinous ligament complex, remove as little lamina as possible, save the ligamentum flavum as a flap, and do a limited discectomy. These steps theoretically reduce iatrogenic instability, epidural broses, sterile discitis, and loss of disc height. All of these steps are facilitated by the use of a microscope, but no proof exists that these steps reduce the incidence of back pain.
The most frequent cause of poor result from lumbar disc surgery is faulty patient selection because of erroneous or incomplete diagnosis. Technical errors, such as wrong-level surgery, incomplete decompression, and intraoperative complications, explain a small percentage of failures. A 1981 study assigned the following frequency of missed diagnoses as sources of failure: lateral spinal stenosis, 59%: recurrent or persistent herniation, 14%; adhesive arachnoiditis, 11%; central canal stenosis, 11%; and epidural broses, 7%. Finally, the results of repeat surgery are not as good as primary surgery, regardless of the reason or whether a microscope was used, because of scar tissue, higher incidence of complications, or larger dissections. In the past decade, a substantial increase in interest in minimally invasive procedures has occurred in all areas of medicine, particularly for spinal disorders. Several methods to remove HNP have been proposed as alternatives to standard open discectomy. Injected chymopapain can dissolve much of the central nucleus, but is not likely to act on extruded or sequestered fragments, which are often heavily collagenized.24,57,62 Likewise, percutaneous suction discectomies and removal of nucleus (either mechanically or by laser from the center of the disc) may reduce intradisc pressure but are unlikely to influence the effects of extruded or sequestered disc material. Therefore although alternative minimally invasive techniques hold considerable promise, lumbar microdiscectomy is still the gold standard for surgical treatment of lumbar HNP with radiculopathy. However, the skills and technology to remove herniated discs by such alternatives are evolving.24,39,40,71–73
Therapy Guidelines for Rehabilitation
Postoperative spine rehabilitation allows for a safer and faster return to functional activities. The early return to appropriate activities has been encouraged after surgeries of the extremities for many years. The same approach should be applied to the spine. Careful instruction and frequent reevaluation enable a therapist to progress the patient’s functional activities to premorbid levels safely. The therapist should apply a functionally appropriate and suitably aggressive postoperative protocol to the patient recovering from lumbar microdiscectomy.
Lumbar disc herniations can do more than compromise the nerve root. Compensatory movement patterns, altered mechanics of the motion segment, and muscle splinting may result in misleading referred pain patterns (e.g., myofascial trigger points). Furthermore, the literature suggests that abnormal changes in paraspinal muscle activity occur after an HNP.74,75 Triano and Schultz76 found a high correlation between the absence of the flexion-relaxation phenomenon (i.e., the relaxation of the lumbar paraspinal muscles at terminal flexion in standing) and poor results on the Oswestry Pain Disability Scale (Box 15-1).
Microdiscectomy is designed to decompress neural tissues by removing the disc material that is causing the neurologic signs and symptoms not alleviated through aggressive conservative care.77 Surgery cannot correct poor posture and body mechanics, relieve myofascial pain syndromes, or remedy faulty motor patterns of synergistic activity accompanying muscle substitution that occur in many patients with low back pain. Additionally, Hides, Richardson, and Jull78,79 have found that the lumbar multifidi, a primary segmental stabilizer, do not spontaneously recover after low back pain, so it is doubtful that they will spontaneously recover after the trauma of spine surgery. The loss of these crucial active segmental stabilizers may lead to recurrent lumbar pain syndromes. To avoid this and aid the patient’s rehabilitation after spinal surgery, the therapist must tirelessly question and reassess, using a problem-solving approach.
The following guidelines are not intended to be a substitute for sound clinical reasoning. Rather they are intended as a guide for the successful postoperative rehabilitation of patients after lumbar microdiscectomy. The primary goals after a lumbar microdiscectomy are the reduction of pain, prevention of recurrent herniation, restoration of normal muscle activity and biomechanics, maintenance of dural mobility, improvement of function, and early return to appropriate activities. Each patient’s program must be individualized to attain these goals for the following reasons:
1 Patients have slightly different pathoanatomic abnormalities and surgical procedures.
2 Patients have different levels of strength, flexibility, and conditioning after surgery.
Each patient must therefore receive care in accordance with individual needs. To this end the guidelines should be progressed as tolerated, and the therapist should not try to keep the patient “on schedule.”
![]() Increasing lower extremity (LE) symptoms, progressive neurologic deficit, and incapacitating pain are obvious “red flags” that require prompt reevaluation. Although the therapist must not ignore pain, an acceptable level of discomfort is reasonable if the patient is increasing functional activities and progressing in the program as anticipated. Pain should be monitored in three parameters, with the therapist carefully noting the pain pattern (e.g., left lateral thigh to knee), observing the frequency (e.g., constant, intermittent, rare), and having the patient rate the intensity (0 to 10). This allows close tracking of changes in pain with exercise and activity so that the program can be progressed or modified accordingly.
Increasing lower extremity (LE) symptoms, progressive neurologic deficit, and incapacitating pain are obvious “red flags” that require prompt reevaluation. Although the therapist must not ignore pain, an acceptable level of discomfort is reasonable if the patient is increasing functional activities and progressing in the program as anticipated. Pain should be monitored in three parameters, with the therapist carefully noting the pain pattern (e.g., left lateral thigh to knee), observing the frequency (e.g., constant, intermittent, rare), and having the patient rate the intensity (0 to 10). This allows close tracking of changes in pain with exercise and activity so that the program can be progressed or modified accordingly.
Finally, any successful spinal rehabilitation program must not ignore psychosocial factors that negatively affect the program. It has been suggested that the greatest indicator for postoperative results is preoperative psychologic testing, not MRI or clinical signs.80–83 Additionally, patients who have active litigation or workers’ compensation claims have been shown to return to activity later than patients who do not.84 These factors must be considered in evaluating patients, progressing exercise programs, and assessing clinical results.
Phase I (Protective Phase)
TIME: 1 to 3 weeks after surgery
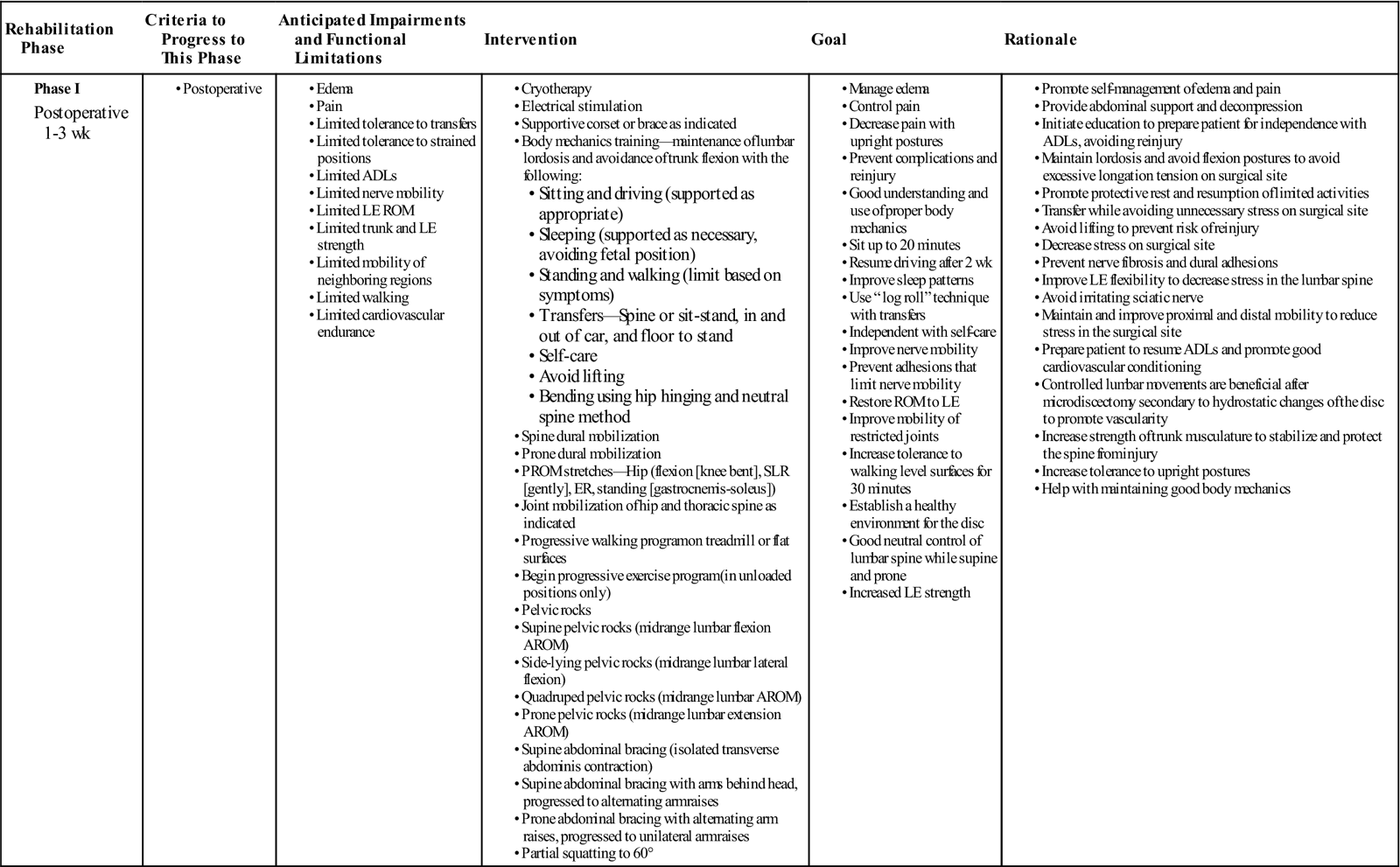
GOALS: Protect the surgical site to promote wound healing, maintain nerve root mobility, reduce pain and inflammation, educate patient to minimize fear and apprehension, establish consistently good body mechanics for safe and independent self-care (Table 15-1)
TABLE 15-1
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase I |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
The first postoperative week typically consists of protective rest, progressive ambulation, and appropriately limited activities. Activity tolerance is the result of progressive activity, not rest. The patient should be encouraged to walk at a comfortable pace for short distances several times a day. Patients are usually allowed to shower 7 days after surgery, depending on wound healing.
![]() Driving is usually not allowed for 1 to 2 weeks, although this may be extended if the right LE is significantly compromised. Typically patients can return to office work within 1 week. Because the patient in phase I has difficulty tolerating sustained positioning, he or she may require support during driving, sitting, and lying postures. Additionally, patients may need to be directly educated in changing positions frequently. Patients may have significant incisional pain, especially with flexion movements.
Driving is usually not allowed for 1 to 2 weeks, although this may be extended if the right LE is significantly compromised. Typically patients can return to office work within 1 week. Because the patient in phase I has difficulty tolerating sustained positioning, he or she may require support during driving, sitting, and lying postures. Additionally, patients may need to be directly educated in changing positions frequently. Patients may have significant incisional pain, especially with flexion movements.
![]() The therapist must avoid all loaded lumbar flexion in patients in phases I and II. The patient can apply cold packs to the surgical site for 15 to 20 minutes several times a day to help control pain, muscle spasm, and swelling.
The therapist must avoid all loaded lumbar flexion in patients in phases I and II. The patient can apply cold packs to the surgical site for 15 to 20 minutes several times a day to help control pain, muscle spasm, and swelling.
The therapist may begin outpatient physical therapy as soon as the patient can comfortably come to the clinic, usually in the second or third week. Treatment begins only after the patient is evaluated to ascertain the following:
• A thorough history of the condition, including previous treatments or surgeries and time out of work
• The status of the wound site
• Anthropometric data, and postural and body mechanics assessment
![]() Limited mechanical testing (Standing motion testing and end-of-range movements are not assessed until after the fifth week postoperatively.)
Limited mechanical testing (Standing motion testing and end-of-range movements are not assessed until after the fifth week postoperatively.)
• Neurologic status (examination includes neural tension testing)
• Baseline core strength testing in nonaggravating position (i.e., supine)
![]() The therapist must take care during the initial evaluation to avoid any testing that may injure an already compromised patient. We typically include Waddell signs85 late in the rehabilitation process to help delineate nonorganic physical signs (Box 15-2).
The therapist must take care during the initial evaluation to avoid any testing that may injure an already compromised patient. We typically include Waddell signs85 late in the rehabilitation process to help delineate nonorganic physical signs (Box 15-2).
![]() The mechanical examination must be very limited in the phase I and phase II patient. It is intended to elicit symptomatic and mechanical responses that suggest mechanical problems and so dictate the treatment course.
The mechanical examination must be very limited in the phase I and phase II patient. It is intended to elicit symptomatic and mechanical responses that suggest mechanical problems and so dictate the treatment course. ![]() Because weight-bearing motion testing and end-of-range movements are typically not performed until after the fifth week postoperatively, the therapist uses responses to positioning and midrange movements in prone, supine, and side-lying positions to determine mechanical problems in the initial weeks.
Because weight-bearing motion testing and end-of-range movements are typically not performed until after the fifth week postoperatively, the therapist uses responses to positioning and midrange movements in prone, supine, and side-lying positions to determine mechanical problems in the initial weeks.
![]() Hip muscle strength testing should be postponed in the early stages of healing to prevent stressing inflamed lumbosacral tissues. Neural tension testing is an integral part of the lumbar evaluation. Therefore the therapist should have the patient perform the SLR, Cram test, femoral nerve tension test (prone knee flexion), and supine dural tension and do the appropriate measuring, recording, and comparison with the opposite limb.
Hip muscle strength testing should be postponed in the early stages of healing to prevent stressing inflamed lumbosacral tissues. Neural tension testing is an integral part of the lumbar evaluation. Therefore the therapist should have the patient perform the SLR, Cram test, femoral nerve tension test (prone knee flexion), and supine dural tension and do the appropriate measuring, recording, and comparison with the opposite limb.
![]() Slump testing should not be performed until after the fourth or fifth week postoperatively. Core strength testing may be performed in a variety of ways; however, Lee86 describes a nice functional approach based on grouping core musculature into slings. A good understanding of soft tissue healing rates, spinal mechanics, and the specific surgical procedure helps avoid needless soft tissue trauma. For further evaluation of the patient’s physical limitations and guidance toward appropriate functional training, the therapist can use the Modified Low Back Pain Oswestry Questionnaire87 (see Box 15-1) or the Roland-Morris Functional Disability Questionnaire.46 These are easily administered and helpful. The therapist should document the patient’s perceived disability status before treatment and at predetermined intervals to monitor functional progress and determine the appropriate direction of functional training exercises.
Slump testing should not be performed until after the fourth or fifth week postoperatively. Core strength testing may be performed in a variety of ways; however, Lee86 describes a nice functional approach based on grouping core musculature into slings. A good understanding of soft tissue healing rates, spinal mechanics, and the specific surgical procedure helps avoid needless soft tissue trauma. For further evaluation of the patient’s physical limitations and guidance toward appropriate functional training, the therapist can use the Modified Low Back Pain Oswestry Questionnaire87 (see Box 15-1) or the Roland-Morris Functional Disability Questionnaire.46 These are easily administered and helpful. The therapist should document the patient’s perceived disability status before treatment and at predetermined intervals to monitor functional progress and determine the appropriate direction of functional training exercises.
After evaluation the therapist thoroughly explains the existing problems and the treatment plan to the patient. Therapist and patient should work together to reach mutual agreement on realistic goals. Patient education is crucial to achieving positive results because the patient ultimately treats himself or herself several hours each day with a home exercise program and self-treatment techniques. Furthermore, avoiding reinjury is perhaps the single most important postoperative factor responsible for a rapid progression in the program and ultimately full recovery. Through patient education, a safe and relatively rapid return to activities can occur. The physical therapist (PT) should educate the patient regarding proper postures, home exercises, self-care techniques, and body mechanics for the safe performance of activities of daily living (ADLs). Proper postures and body mechanics are crucial during the postoperative healing phase. Ideally the therapist should instruct the patient before surgery, but if this does not occur, then the first postoperative task is to teach the patient correct postures and body mechanics.
Proper Postures
The PT teaches the patient to maintain normal lumbar lordosis.
![]() Patients should avoid lumbar flexion in standing or sitting because intradisc pressures are increased and excessive shear forces occur. Intolerance to prolonged postures is typical in phase I, and frequent movement breaks are recommended. The concept of abdominal bracing should be taught early. The therapist also should investigate the ergonomics of the patient’s workstation to avoid potential problems.
Patients should avoid lumbar flexion in standing or sitting because intradisc pressures are increased and excessive shear forces occur. Intolerance to prolonged postures is typical in phase I, and frequent movement breaks are recommended. The concept of abdominal bracing should be taught early. The therapist also should investigate the ergonomics of the patient’s workstation to avoid potential problems.
Sitting and Driving.
The therapist should do the following:
![]() Caution the patient to never slouch while sitting.
Caution the patient to never slouch while sitting.
Sleeping.
The therapist should do the following:
![]() Instruct the patient to avoid sleeping in the fetal position because of the prolonged lumbar flexion.
Instruct the patient to avoid sleeping in the fetal position because of the prolonged lumbar flexion.
![]() Have the patient avoid unsupported prone three-quarter lying positions because of the rotational component.
Have the patient avoid unsupported prone three-quarter lying positions because of the rotational component.
Standing and Walking.
Body Mechanics
To allow the patient to progress rapidly, the therapist should do everything possible to avoid reinjury. Minor setbacks may delay progression of the program, and a major setback may be irreparable. The therapist should pay close attention to the patient’s movements. Patients may say they understand correct mechanics but display incorrect movement patterns. Frequent and critical observation allows the therapist to evaluate the patient’s spinal mechanics and determine whether the patient has integrated the correct postures and mechanics. A checklist of basic functional movements (i.e., rising from lying, rising from sitting, sitting in neutral, reaching overhead, bending to knee level) is helpful to record the performance of these skills and whether the patient requires cues to complete the tasks.
Transfers.
The therapist should do the following:
![]() Remember that all twisting motions are prohibited. Instruct them to move their feet to turn instead.
Remember that all twisting motions are prohibited. Instruct them to move their feet to turn instead.
Dressing.
Hygiene.
Lifting.
Bending.
The therapist should do the following:
• ![]() Advise the patient to avoid all bending at the waist. Lumbar flexion with loading is arguably the most hazardous movement in the first two phases. The interdisk pressures are significantly increased, and tension on the healing posterior annulus compounds the problem. Prolonged or repetitive bending is especially injurious.88
Advise the patient to avoid all bending at the waist. Lumbar flexion with loading is arguably the most hazardous movement in the first two phases. The interdisk pressures are significantly increased, and tension on the healing posterior annulus compounds the problem. Prolonged or repetitive bending is especially injurious.88
Occasionally, patients with low back pain possess poor kinesthetic-proprioceptive coordination. A simple technique to improve the patient’s sense of lumbar movement and position involves the use of tape. First, the therapist places the patient on all fours and has him or her assume a neutral spine position. The therapist places a 12- to 18-cm long piece of tape on the paraspinals parallel to the spine (Fig. 15-9), while avoiding placing the tape directly over the incision site. The therapist then asks the patient to make small movements into flexion and extension, always returning to neutral. The additional feedback from the tape pulling or wrinkling will assist the patient in learning spinal proprioception. Various postures can then be tried, including kneeling, side lying, sitting, and standing, with small motions of the lumbar spine while in each position. The patient then progresses to functional movements (e.g., transfers, walking, bending).
Exercise
Dural mobilization (i.e., mobilization of the nervous system, neurodynamic exercise, nerve root gliding, neural tension exercises) should begin as soon as possible in the first week. The preoperative neural compromise and the postoperative inflammation in and around the epidural space contribute to neural broses and dural adhesions. They are occasionally problematic and are easily preventable. An excellent presentation of neural mobilization principles and techniques can be found in Butler.89
Technique.
The therapist should do the following:
Dural mobilization should be done several times a day. The therapist must caution the patient that this exercise may provoke neural symptoms, and that he or she must allow the pain or tingling to resolve to baseline levels before beginning the next repetition. The patient should not overmobilize the neural tissues. ![]() As with any exercise, self-mobilization of the nervous system at home is inappropriate until a positive response has been established from repeated movements in the clinic. The dural mobilizations are progressed as tolerated to include other components of the affected nerve (e.g., ankle dorsiflexion, hip internal rotation [IR]). Eventually (in phase III) the patient can perform neural mobilizations while sitting (“sitting slump”).
As with any exercise, self-mobilization of the nervous system at home is inappropriate until a positive response has been established from repeated movements in the clinic. The dural mobilizations are progressed as tolerated to include other components of the affected nerve (e.g., ankle dorsiflexion, hip internal rotation [IR]). Eventually (in phase III) the patient can perform neural mobilizations while sitting (“sitting slump”).
The early initiation of a progressive spinal-stabilization program is crucial to the eventual tolerance of more strenuous functional activities and sports skills. Because the lumbar spine is inherently unstable around the neutral zone, the trunk musculature must be sufficiently strong and coordinated to stabilize and protect the spine from injury.90,91 The stabilization program progresses from unloaded spinal positions to partially loaded and eventually fully loaded functional training. The posterior pelvic tilt exercise is the least desirable exercise to obtain active lumbar stability.90,92–94 The transversus abdominis must be isolated from the remaining abdominal musculature because it has consistently been shown to be active before the other abdominal muscles or the primary movers during limb motions, regardless of direction.95,96 In addition, the transversus abdominis can become dysfunctional in patients with low back pain.95,97–100 Therefore the transversus abdominis possesses a superior ability to stabilize the lumbar spine actively and locally.38,101–103 Although the more superficial abdominal muscles (the obliques) are important in lumbar stability, they are trained later in the program for their rotational contribution to limit lateral shear and torsional stresses and create trunk rotation. Early in phase II, the lumbar multifidi are isolated and trained because of their ability to stabilize segmentally.102,104,105 In addition, it has been found that the lumbar multifidi atrophy in patients with low back pain and there is a decrease in muscle thickness change with activation.100,106 Literature has also investigated the importance of the pelvic floor musculature in stabilizing the lumbar spine.80,107,108 Evidence shows that the pelvic floor muscles cocontract with the transverse abdominus; therefore recruiting the pelvic floor muscles should be considered when instructing in bracing. Bracing has been shown to immediately increase posteroanterior spinal stiffness and stability.109 Eventually a cocontraction of transversus abdominis, multifidus, and pelvic floor (abdominal bracing) is performed during all exercises and functional activities.110,111 All the exercises should focus on control and technique and be progressed as tolerated to improve endurance of these primary stabilizers.
The PT should instruct the patient in the neutral spine concept and help the patient find the neutral spine position in various postures. The patient can then be taught to control the transverse abdominus with electromyographic (EMG) biofeedback, pressure biofeedback,* or rehabilitative ultrasound imaging102,112–116 in several positions (e.g., supine, all fours, prone). Feedback available by rehabilitative ultrasound imaging has been shown to improve transverse abdominus and lumbar multifidus muscle recruitment up to 4 months posttraining.117,118 After that, the patient can progress the postures to include sitting and standing and increase the duration of the contractions to 60 seconds.
Based on the information obtained in the history, the responses to various positions, and the limited clinical testing performed during the initial evaluation, the therapist determines which midrange lumbar movements are tolerated and are indicated for exercise. Correct and controlled lumbar movements are beneficial to the patient after microdiscectomy because hydrostatic changes of the disc promote improved vascularity.119,120 To this end, the therapist teaches pelvic rocks in pain-free positions (e.g., all fours, prone). A pelvic rock is a repetitive and continuous pelvic tilt from an anterior to a posterior position. A bias toward lumbar extension is typical in the patient who has undergone microdiscectomy because lumbar extension reduces tangential stress posteriorly. A flexion bias is usually not recommended because the surgical entry is into the posterior disc and flexion positions tend to create stress to this area and tension on the incision. A healthy respect for soft tissue healing periods is essential.
The therapist instructs the patient in most of the following exercises in phase I, but he or she should not prescribe any exercise or position for the home program until repeated trials in the clinic have proven painless. Stabilization, flexibility, coordination, and spinal mobility exercises are included in an attempt to address all parameters. The exercise sequence is important and should be considered by the PT when adding exercises. Good technique and control of movement are essential. No exercise should increase the pain pattern or cause lingering pain. Clearly some muscular soreness may accompany the program, but this should be well tolerated and transient. The typical patient should be able to contract the transversus abdominis for 60 seconds in various positions within approximately 1 week after the initial visit.
The following exercises are taught in phase I:
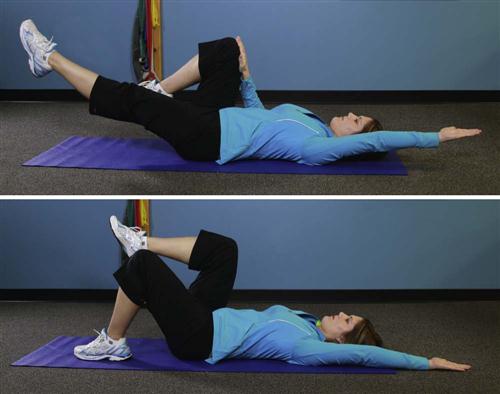
2 Quadruped pelvic rocks (i.e., midrange lumbar active range of motion [AROM])
3 Supine dural stretching (or prone dural stretching for upper lumbar disorders)
5 Supine pelvic rocks (i.e., midrange lumbar flexion AROM)
7 Supine gluteal, hip external rotator, and hamstring stretches to correct myofascial limitations; actively holding neutral spine during these low-load, long-hold exercises is important (Fig. 15-10)
8 Side-lying pelvic rocks (i.e., midrange lumbar lateral flexion AROM)
9 Prone pelvic rocks (i.e., midrange lumbar extension AROM)
11 Gastrocnemius and soleus stretching in standing position (Fig. 15-11)
12 Partial squats to 60° of knee flexion while maintaining neutral spine with abdominal bracing
Spinal Mobilization
Spinal mobilization of the lumbar spine is rarely used in phase I or II. Mobilization of the hips or thoracic spine may be needed and is best addressed on an individual basis. When progress is poor with active movements and deemed secondary to a hypomobile segment, mobilization to restore lumbar movement may be necessary in phase II. In phase III, mobilization can play an important role and is used frequently to reduce pain during specific lumbar motions, especially at end of range. A review of Maitland,121 Mulligan,122 and Paris56 may assist in clinical reasoning.
Cardiovascular Conditioning
Cardiovascular conditioning is an important part of the rehabilitation program and is beneficial both for patients recovering from lumbar microdiscectomy and those with chronic low back pain.123 In addition, endurance training of the LE musculature improves tolerance to prolonged standing and walking. When LE muscles fatigue, poor body mechanics soon follow. The patient typically performs aerobic training by progressive walking (on a treadmill or outdoors without hills), stationary cycling (on recumbent or upright bikes with the patient paying close attention to the maintenance of lordosis and avoidance of hip sway), or swimming (initially only the freestyle stroke with avoidance of “craning” during the breathing phase). Craning is suboccipital extension with rotation (occipitoatlantal to atlantoaxial) or cervical extension with rotation (C2 to C7). Swimming and aqua therapy are usually delayed until the second or third week after surgery to ensure complete wound healing and sufficient lumbar stabilization. ![]() The PT must caution patients never to jump or dive into the water, but rather use the ladder or steps. Aquatic therapy for postoperative lumbar rehabilitation is not covered in this chapter, but Watkins , Williams, and Watkins124 present a good source for the interested clinician.
The PT must caution patients never to jump or dive into the water, but rather use the ladder or steps. Aquatic therapy for postoperative lumbar rehabilitation is not covered in this chapter, but Watkins , Williams, and Watkins124 present a good source for the interested clinician.
The therapist determines the patient’s training heart rate and adheres to this guideline during all conditioning exercises. Patients who have no prior history of aerobic exercise or who are very deconditioned must progress slowly and be carefully monitored. Aerobic conditioning in phase I (with focus on correct postures and mechanics) may include walking, stationary cycling, and water exercises. Patients should avoid stair climbers and cross-country skiing machines until phase II, when adequate trunk stability is usually attained. In addition, rowing, running, and in-line skating should be avoided until phase III, when significant active lumbar stability has been achieved.
The progression of cardiovascular training is highly variable and depends on the patient’s prior level of conditioning and present goals. He or she can usually begin with 5- to 10-minute bouts and progress at 5-minute intervals up to 30 or 60 minutes. The therapist must pay careful attention to patient position because correct postures deteriorate as fatigue increases. Neurologic weakness of the hip flexors or abductors, quadriceps, hamstrings, ankle dorsiflexors, and plantar flexors significantly alters gait and requires modification of the aerobic program to avoid abnormal mechanical stress.
Modalities
In general, the use of passive treatment techniques alone should be avoided; however, occasionally they may be necessary to augment the functional restoration program. The therapist should use pain control modalities only as needed to support the exercise program. Cryotherapy and interferential stimulation applied to the low back for 15 to 20 minutes after an exercise session are helpful. Some therapists may prefer electric myostimulation (EMS), microstimulation, or transcutaneous electrical nerve stimulation for muscle spasm reduction and pain control. However, EMS that is delivered too intensely in the first several weeks after surgery may unwittingly jeopardize the healing paraspinal muscle tissue and should therefore be used judiciously. The patient’s posture during modalities is always important and varies depending on positional tolerance. Supported prone lying or supported supine lying is usually quite comfortable in this phase.
Wound Care
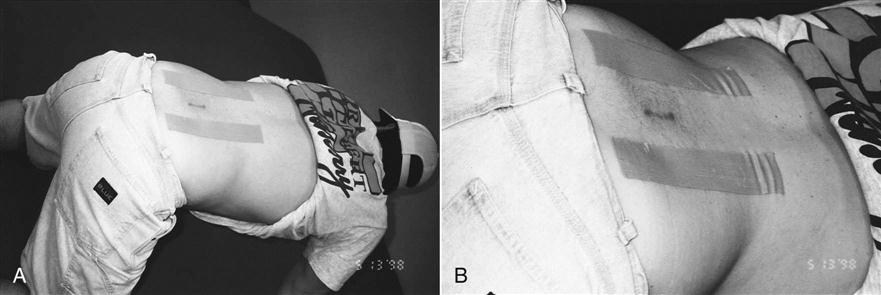
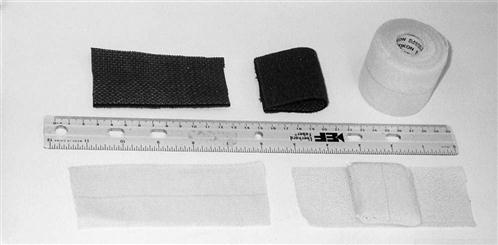
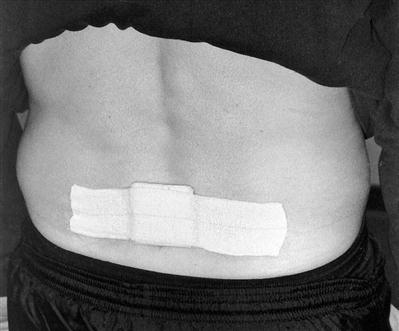
Along with the patient’s history, the inspection of the incision site during the initial evaluation helps determine whether extra measures are needed. Any signs of infection are a red flag that requires prompt medical intervention. Some patients desire “invisible” scars, whereas others are much less concerned. Because patients scar differently, the therapist should monitor their progress and offer solutions to excessive scarring or scar stretching. A compression taping technique can be used to limit hypertrophic scar formation and reduce surgical scar widening. First, the therapist folds a 10- × 6-cm Neoprene pad in half and secures it with a 2-inch wide elastic tape (Elastikon tape)* (Fig. 15-12). The pad is then affixed horizontally over the closed wound (Figs. 15-13 and 15-14) to provide both compression and approximation of the surgical scar. The patient wears the compression patch constantly for 6 to 10 weeks, removing it only to bathe. It should not be applied until the wound site is completely healed (approximately 2 weeks).
Phase II (Functional Recovery Phase)
TIME: 4 to 6 weeks after surgery
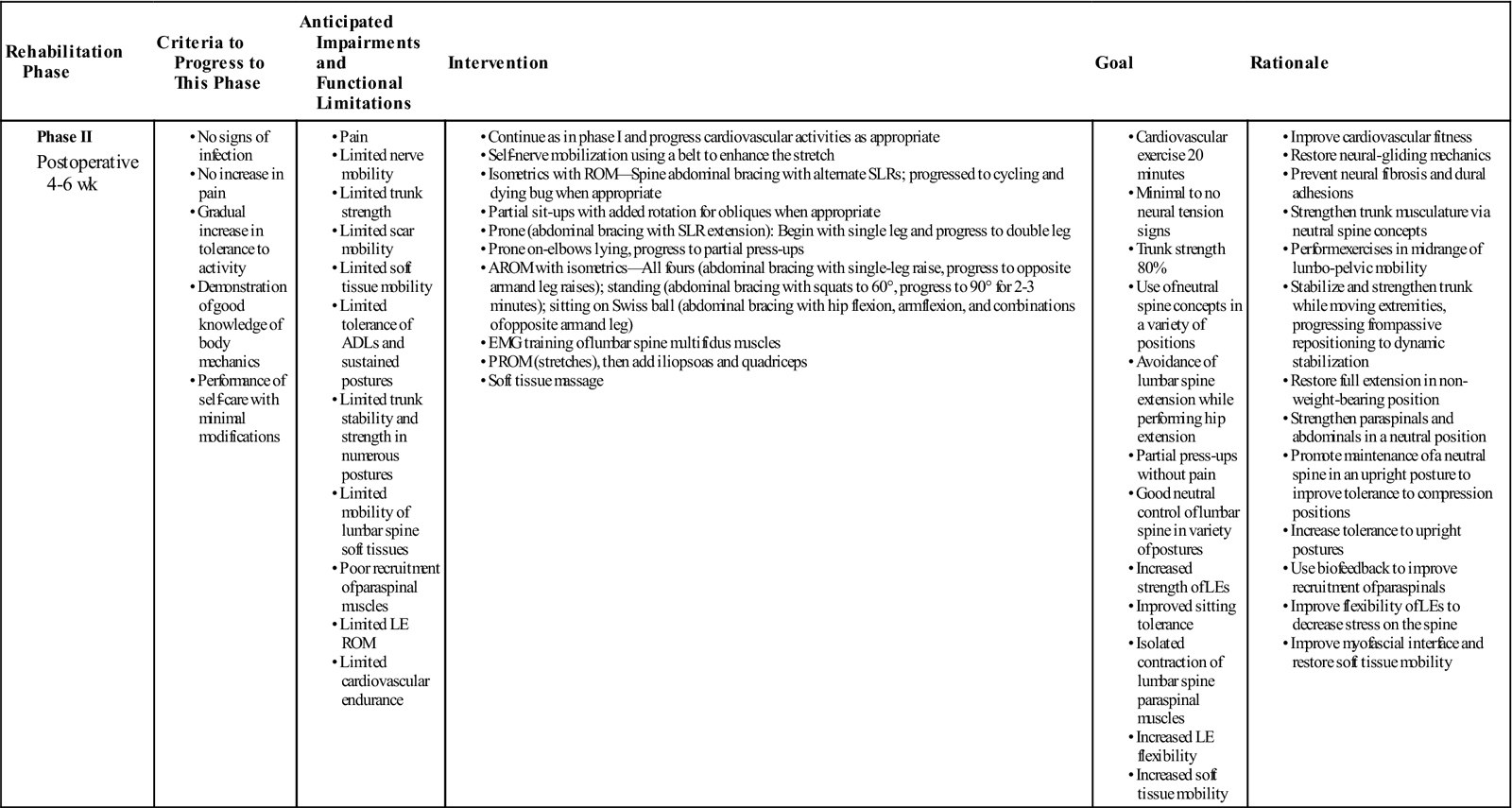
GOALS: Understand neutral spine concept, improve cardiovascular condition, increase trunk strength to 80%, increase soft tissue mobility and LE flexibility and strength, maintain nerve root mobility (Table 15-2)
TABLE 15-2
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
|
Phase II |
• Pain
|
|
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
As surgical site pain diminishes and active spinal stability improves, the PT can increase the patient’s program of functional activities and exercise. The patient in phase II should have complete wound healing, although some tenderness and paraspinal spasm may persist. Neural tension signs should be negative, but neurodynamic testing may reveal limitations. The patient should be gaining tolerance to functional activities and be able to perform all self-care with minor modifications. The patient’s tolerance to aerobic exercise also should be improving. Correct body mechanics and postures should be maintained as functional activity increases. Patients should have confidence in their ability to stabilize the lumbar spine actively in all loaded positions. Pain-free lumbar AROM should be increasing to end-of-range strain only, although terminal flexion may still provoke pain.
![]() The patient should continue to avoid loaded lumbar flexion. Through brief reevaluations in each treatment session, the therapist collects additional lumbar motion data. For example, if prone pelvic rocks are well tolerated, then the patient’s positional tolerance to elbow lying and partial extension in lying can be assessed safely.
The patient should continue to avoid loaded lumbar flexion. Through brief reevaluations in each treatment session, the therapist collects additional lumbar motion data. For example, if prone pelvic rocks are well tolerated, then the patient’s positional tolerance to elbow lying and partial extension in lying can be assessed safely.
![]() The therapist should avoid standing motion testing and sitting testing except for the most conditioned patients who are doing very well.
The therapist should avoid standing motion testing and sitting testing except for the most conditioned patients who are doing very well.
Exercise
A recent Cochrane systematic review of randomized controlled trials concluded that high-intensity exercise programs initiated 4 to 6 weeks postmicrodiscectomy lead to decreases in pain and disability faster than no treatment or low-intensity programs.125 However, although early exercise intervention resulted in faster decreases in pain and disability, clinical outcomes at 1-year follow-up had similar results versus low-intensity or no exercise.126,127 The therapist should instruct the patient in the correct way to contract and control the lumbar multifidus with EMG biofeedback. Special attention to the training of this important segmental stabilizer is essential.128 Retraction of the paraspinal muscles during surgery can denervate the multifidus muscle.129 Fortunately, lumbar microdiscectomy requires a minimal wound opening, so this complication is lessened. The patient should perform abdominal bracing (holding neutral spine with a cocontraction of the transverse abdominis, multifidus, and pelvic floor) in supine, prone, and all-fours positions, progressing to transition movements. Ultimately, the cocontraction is used to stabilize the lumbar spine during all ADLs. The therapist can progress the patient’s midrange lumbar movements and spinal-stabilization program as tolerated, using Swiss ball exercises to improve balance and dynamic lumbar stabilization during sitting. ![]() The patient should continue to avoid axial loading during end-range lumbar flexion or lateral flexion movements. As the patient shows control and tolerance, the exercise level may be increased. Pain during exercise typically requires correction of the technique or exercise modification. In addition, muscle groups that may have been weakened by neurologic compromise (e.g., hip abductors, quadriceps, ankle plantar flexors, dorsiflexors) must be strengthened. The slow twitch fibers are most involved and are easily fatigued. The longer that neural compression and inflammation have been present, the longer the period before regeneration occurs. Careful attention to back-protected positions during strengthening exercises is crucial to avoiding reinjury.
The patient should continue to avoid axial loading during end-range lumbar flexion or lateral flexion movements. As the patient shows control and tolerance, the exercise level may be increased. Pain during exercise typically requires correction of the technique or exercise modification. In addition, muscle groups that may have been weakened by neurologic compromise (e.g., hip abductors, quadriceps, ankle plantar flexors, dorsiflexors) must be strengthened. The slow twitch fibers are most involved and are easily fatigued. The longer that neural compression and inflammation have been present, the longer the period before regeneration occurs. Careful attention to back-protected positions during strengthening exercises is crucial to avoiding reinjury.
Typical Phase II Exercises.
The therapist should do the following:
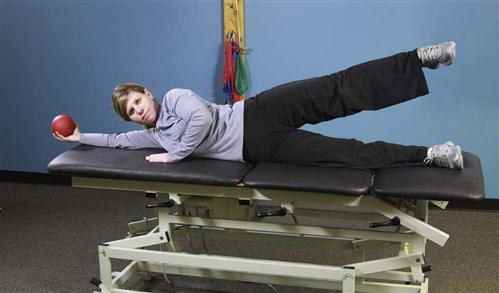
1 Supine abdominal bracing with alternate SLRs, progressed to abdominal bracing with unsupported LE extension (i.e., cycling), progressed to abdominal bracing with unsupported upper extremity (UE) and LE extension (i.e., dying bug) (Fig. 15-15)
3 Supine partial sit-ups, progressed to partial sit-ups with rotation to facilitate oblique strengthening (Fig. 15-16)
4 Double-leg bridging, progressed to single-leg bridging and then to single-leg bridging with opposite knee extended (Fig. 15-17)
5 Prone elbow lying, progressed to partial press-ups
6 Prone abdominal bracing with single-leg raises, progressed to prone double-leg raises
7 Standing repetitive squats to 60°, progressed to 90° for 2 to 3 minutes
8 Abdominal bracing on all fours with single-leg raise, progressed to opposite arm and leg raises (Fig. 15-18)
9 Balance board training on both limbs, progressed in duration
10 Isolated strengthening of neurologically compromised muscles
11 Swiss ball sitting exercise progression (in neutral spine with abdominal brace)
12 Stretching of the quadriceps, gluteals, hip external rotators, iliopsoas, hamstrings, and calves as required to correct myofascial limitations (Figs. 15-19 through 15-22; see also Fig. 15-11)
Soft Tissue Mobilization
Scarring of myofascial elements with collagen cross-fibers or fibrofatty tissue limits muscle broadening during contraction and connective tissue elasticity during movement.130 Muscle spasm and protective guarding of the gluteals and low back musculature may persist. Soft tissue mobilization of the lumbar paraspinals and buttock musculature is frequently needed to improve muscle function and reduce spasm.131 The PT must exercise care when performing soft tissue mobilization to the paraspinals before the third or fourth week after surgery because the tissue healing is incomplete. The mechanical and reflexive effects of soft tissue mobilization are well suited for patients recovering from microdiscectomy, and certain techniques are particularly beneficial before paraspinal strengthening (e.g., side-lying paraspinal pull from midline). Careful questioning and soft tissue examination will uncover gluteal trigger points that can cause buttock or LE pain patterns.76 These myofascial pain syndromes are not uncommon preoperatively and may linger postoperatively. With appropriate treatment they can be relieved so that the functional restoration program may progress. Scar massage to release adherent soft tissue also may be needed.
Spinal Mobilization
Spinal mobilization using nonthrust maneuvers may be beneficial for patients in phase II if they do not have protective muscle spasm, bone disease of the spine, or hypermobile or irritable adjacent motion segments. Muscle energy techniques are usually well tolerated and best suited for phase II.
![]() Thrust maneuvers (grade V or high-velocity manipulations) are not indicated.
Thrust maneuvers (grade V or high-velocity manipulations) are not indicated.
Cardiovascular Conditioning
The PT should continue to progress the cardiovascular program in intensity and duration of aerobic training. The use of cross-country ski machines, stair climbers, and swimming for aerobic exercise is allowed if sufficient trunk stability has been achieved. ![]() Patients should avoid rowing and in-line skating until phase III.
Patients should avoid rowing and in-line skating until phase III.
![]() Running is not recommended until after the twelfth week after surgery because of the degree of spinal stabilization required and the repetitive axial loading sustained by the disc.
Running is not recommended until after the twelfth week after surgery because of the degree of spinal stabilization required and the repetitive axial loading sustained by the disc.
Modalities
The therapist and patient should use modalities only as needed to support the exercise program. Cryotherapy and interferential stimulation to the low back after exercise may be beneficial.
Phase III (Resistive Training Phase)
TIME: 7 to 11 weeks after surgery
GOALS: Ensure patient is independent in self-care and ADLs with minimal alterations, increase tolerance to activities, progress return to previous level of function
The patient in phase III should consistently perform correct body mechanics and postures without prompting and should tolerate almost all functional activities. Prolonged positioning (e.g., unsupported sitting) may still provoke low back pain, but this should be easily relieved with change of position or simple stretching exercises. Soft tissue healing at this stage is largely complete, although some surgical site tenderness may still be present. All self-care and ADLs should be performed confidently and painlessly with minimal modifications. Patients in phase III should have good tolerance to midrange lumbar movements and sufficient spinal stabilization to perform spinal movements in loaded positions.
The resumption of lifting activities must be progressive and occur with careful instruction. Because approximately half of all workers’ compensation claims for low back injury result from lifting objects, this patient group needs proportionally more instruction and functional training.
Because soft tissue healing is nearly complete by phase III, more extensive mechanical testing can be performed to ascertain tolerance to various lumbar movements, as well as the end range sensation.
![]() Standing motion testing (without overpressure) and seated testing can be performed safely on most patients after 6 weeks. The outcome of the movement testing determines to a great extent the treatment and exercise progression. Neural tension signs should be negative unless scarring has occurred. Occasionally some neurologic signs and symptoms persist into the third phase, but with monitoring and calm encouragement the PT can reassure affected patients that these symptoms will subside with continued neural mobilization and time.
Standing motion testing (without overpressure) and seated testing can be performed safely on most patients after 6 weeks. The outcome of the movement testing determines to a great extent the treatment and exercise progression. Neural tension signs should be negative unless scarring has occurred. Occasionally some neurologic signs and symptoms persist into the third phase, but with monitoring and calm encouragement the PT can reassure affected patients that these symptoms will subside with continued neural mobilization and time.
Researchers132 and clinicians note that flexibility, strength (stability), and coordination return at different rates after injury. During spinal rehabilitation, flexibility should precede strength, proximal strength should precede distal strength, and strength should precede coordination. This culminates in the more rapid and fluid functional movements seen in uninjured persons. The PT must be sure to consider the sequence of return of these various elements, the existing limitations uncovered during mechanical testing, and the patient’s realistic goals when planning the progression of the exercise program.
Exercise
Functional training exercises (i.e., sports-specific drills, work-hardening activities) typically begin in phase III. Preset goals determine the kinetic activities that are to be the focus of rehabilitation. The therapist closely supervises the progression of these activities, paying careful attention to the quality of spinal mechanics and lumbar stabilization. Functional training is focused on trunk movements that simulate activities to which the patient will return. Sports-specific training (Figs. 15-23 through 15-26) can begin if the patient has achieved sufficient active lumbar stability and spinal mobility in fully loaded positions, as well as adequate myofascial flexibility and conditioning. The therapist can use proprioceptive training with balance boards and Swiss balls. Initially, athletes who take part in running and jumping activities are most safely trained with unloading devices,* during supervised treadmill running or jump training. These patients are typically well conditioned before surgery and have progressed postoperatively without setbacks.
Golfers need to be trained to hold the neutral spine dynamically during all five phases of the swing. The PT can incorporate specific strength, flexibility, and balance exercises to achieve a safe and mechanically sound golf swing.124
Work-hardening activities for medium to heavy work classifications typically begin at 8 weeks and include lift training from 25 to 50 lb. Workers in these fields need special attention with regard to materials handling and should have a functional capacity evaluation 10 to 12 weeks after surgery to determine appropriate return-to-work status.
The exercise program progresses in intensity and difficulty to include rotational trunk stability, overhead activities, and balance training using a balance board. Training the patient in diagonal patterns in loaded positions better simulates real-life situations. A new stabilization exercise for patients in phase III challenges the obliques and transverse abdominals with minimal stress to passive tissues.76 McGill133 refers to this exercise as isometric side-support on knees or on feet (depending on the degree of difficulty). Therapists should prescribe this exercise for home performance only after proving patient tolerance during clinic sessions.
The spinal mobility program attempts to restore painless and full lumbosacral ROM. The PT should prescribe appropriate exercises and incorporate mobilization to achieve full and pain-free lumbar ROM and continue the stretching exercises needed to attain normal myofascial flexibility.
Soft Tissue Mobilization
Soft tissue mobilization should continue as needed to ensure a pliable surgical scar, proper gluteal and paraspinal muscle function, and soft tissue extensibility.
Spinal Mobilization
Spinal mobilization should be used when necessary to restore motion at hypomobile segments and reduce pain associated with movement. Because the restoration of normal spinal motion is a primary goal, the therapist must identify and correct aberrant arthrokinematics. The expanded mechanical testing in phase III will reveal limitations or provoke symptoms that require attention. Maitland,121 Mulligan,122 and Paris56 can be reviewed to assist in clinical reasoning.
Cardiovascular Conditioning
Cardiovascular conditioning should continue to progress in intensity and duration. The patient’s aerobic fitness program is determined by the ultimate activity goals. A typical sedentary office worker obviously does not train as intensely as a professional athlete. However, the therapist should not underestimate the aerobic demands placed on a manual laborer and should encourage appropriate endurance exercises.
Aerobic conditioning (focusing on correct postures and mechanics) may include treadmill walking, stationary cycling, the use of cross-country ski machines and stair climbers, swimming, and skating (in-line or on ice). Patients who have had previous experience with rowing may resume this exercise. Attention to proper stroke form is important, and modification to maintain lordosis may be necessary.
![]() Patients should not start a running program until after the twelfth week postoperatively because of the high compressive and repetitive axial loads at heel strike. A walk-run program should be initially implemented on a treadmill, with the therapist supervising and analyzing gait. When the patient does resume running, it should be in the morning hours when the disc is maximally hydrated.124
Patients should not start a running program until after the twelfth week postoperatively because of the high compressive and repetitive axial loads at heel strike. A walk-run program should be initially implemented on a treadmill, with the therapist supervising and analyzing gait. When the patient does resume running, it should be in the morning hours when the disc is maximally hydrated.124
Modalities
Cryotherapy may still be beneficial after intensive training sessions. EMS, transcutaneous electrical nerve stimulation, microcurrent, interferential stimulation, and other modalities are seldom necessary.
Discharge Planning
When the anticipated goals and desired outcomes have been attained, the patient is discharged with a home or club exercise program (or with both). The exercise program is to be maintained indefinitely. As always, the postsurgical patient should try to return to premorbid activity levels. Because goals vary dramatically among patients, some may require substantially more training than others, such as overhead lift training, plyometric jump training, or sport-specific skill training. A reasonable level of tolerance to strenuous work activities or recreational sports should be attained before these higher activity level patients are discharged.
The comprehensive lumbar evaluation performed in phase III reveals any limitations in motion, weaknesses, neural restrictions, and painful movements that still need to be addressed. The PT can obtain additional information from computerized testing devices132* that provide objective data on lumbar motion speed, acceleration and deceleration, and degree of ROM. Other testing equipment, such as computerized isokinetic machines, determines objective trunk strength values at various speeds of lumbar ROM. This information can be helpful in guiding the therapist to choose appropriate exercises to remedy any weaknesses or limitations, especially in more physically active patients.
Most patients recovering from lumbar microdiscectomy progress uneventfully if properly educated and carefully rehabilitated. The PT can facilitate the systematic training program to achieve a safe and rapid return of function by applying clinical knowledge and manual skills.
Clinical Case Review
1What are the goals for the first week following microdiscectomy?
(1) Protect the incision site; (2) maintain nerve root mobility; (3) reduce pain and inflammation; (4) educate the patient; (5) establish consistent body mechanics.
2Mikayla has been progressing well over the first couple of weeks following her microdiscectomy. Today she comes in complaining of a general feeling of fatigue. She also reports that she thinks she has had a fever for about a week. What should you do?
You should inspect Mikayla’s wound site for signs of infection. Look for any oozing or discharge from the wound. Note any increased redness or warmth about the incision site. Fever and malaise can be a sign of infection (or just a cold). If you suspect an infection, prompt referral back to her surgeon is indicated.
3Myrna had microdiscectomy 2 weeks ago. She wants to go to her son’s baseball game this weekend. What should her therapist tell her?
Her therapist should tell her the following:
• Caution her to avoid sitting in bleachers or on benches without any back support.
• Suggest she take some type of alternative seating device that has some back support.
• Recommend frequent changes of position (avoid sitting greater than 20 minutes at a time).
4Rick is 41 years old. He has had progressing back pain episodes over the past 2 years. An MRI shows a herniated disc at L4-L5. Rick also has intermittent complaints of left radicular leg pain. He had microdiscectomy surgery 2 weeks ago and has come to outpatient physical therapy for evaluation and treatment. How should a spinal evaluation be altered to assess a patient who has recently had microdiscectomy surgery?
Mechanical testing should be limited (standing motion testing and end-of-range movements are not assessed until 5 weeks after surgery). Hip muscle strength testing should be postponed in the early stages of healing to prevent stressing inflamed lumbosacral tissues. Slump testing is not performed until much later. A good understanding of soft tissue healing rates, spinal mechanics, and the specific surgical procedure helps avoid needless soft tissue trauma.
5Summer continues to have difficulty performing a proper brace. What can you do to assist her?
You can use EMG, pressure biofeedback, or rehabilitative ultrasound imaging to help Summer learn how to properly brace again.
6Edna was a sedentary person before her surgery. You find that she is resistant to doing any exercises and appears afraid of “messing up her surgery.” What should you tell Edna?
Edna should be educated on the changes in muscle function following microdiscectomy. Also, she should know that not properly rehabilitating those muscles can lead to chronic back problems in the future. Assure her that you will be tailoring her exercise program to her individual needs and will be respecting pain.
7Verlyn is a 40-year-old woman. She had microdiscectomy surgery for the L5 disc 6 weeks ago. Back pain is minimal. LE flexibility and strength is gradually improving. Trunk strength also is progressing. She is now seeing a PT for treatment. Previous treatments have included modalities for pain control, LE flexibility exercises, trunk and general strengthening, cardiovascular conditioning, and body mechanics. Verlyn is concerned about the intermittent radicular pain in her right leg. Prolonged sitting, walking, or standing aggravates her right leg. She reports reproduction of calf pain with hamstring stretching. What treatment technique should be used to decrease calf pain frequency and intensity?
Verlyn tested positive for adverse neural tension in the right leg. After several treatments of mobilization to the nervous system, complaints of pain decreased significantly in intensity and frequency.
8Karla has a 5-month-old daughter at home. She arrives at therapy 3 weeks after a microdiscectomy procedure. What should Karla be instructed to do immediately?
Karla should be instructed to do the following:
9Jason works in a warehouse where he must repeatedly carry heavy boxes and walk for most of the day. He had surgery 9 weeks ago and does not understand why his therapist has him riding a bicycle and walking on a treadmill as part of his lumbar microdiscectomy rehabilitation. If he has to do aerobic exercise, he would rather run. What is the therapist’s rationale for these exercises?
The following explains the therapist’s rationale:
10Raquel is having difficulty finding a comfortable position while sleeping after her microdiscetomy. After questioning her you find that she typically sleeps on her side in the fetal position. What should you tell Raquel?
Raquel should avoid sleeping in the fetal position as this places the lumbar spine in prolonged flexion, which you want to avoid after microdiscectomy surgery.
Recommend the following positions instead:
• Supported supine lying with a pillow under the knees
• Supported sidelying with a pillow between the knees and arms
• Supported prone three-quarter lying with the spine straight (see Fig. 16-7, A through C)
11Summer is almost 3 months postmicrodiscectomy. She had been making excellent progress until last week when she returned to her Pilates class. Now she is complaining of low back pain, which occurs when she does a postpelvic tilt as instructed in the class. What should you tell Summer?
Posterior pelvic tilting should be avoided because it puts the lumbar spine in a flexed position. Be aware of your patients’ activities and what they require so that you may adequately prepare them to return to their previous recreational or professional activities. Summer may also need to review the proper way to brace her lumbar spine by engaging her transverse abdominis, multifidi, and pelvic floor muscles.
12Kristy is a 36-year-old mother of two who had a microdiscectomy over 3 months ago. She is currently off work from her job as a waitress where she injured her back. Objectively she appears to have made a great recovery from her surgery; however, she consistently reports high pain levels with any activity. What may be contributing to her report of pain?
Psychosocial factors need to be considered in every patient’s prognosis. Patients who have workers’ compensation claims have been shown to return to activity later than patients who do not.
13Russell is ready to return to running. What is the safest way he can do this following his microdiscectomy 3 months ago?
Initially a walk-run program should be implemented on a treadmill with supervision by the PT analyzing his biomechanics. An unloading device can also be used. Advise Russell to run first thing in the morning—when he progresses to outdoor running—when his discs are maximally hydrated and can offer the most shock absorbing capability.
14Ken had an L4 microdiscectomy 3 weeks ago. Today he has 9/10 pain and return of pain down his posterior leg. Upon examination you note that his knee jerk reflex is absent and he has weakness of his quadriceps. What should you do?
Incapacitating pain and neurologic deficit are “red flags.” Joe should be referred back to his surgeon immediately for reevaluation.
15Lynne had a microdiscectomy over 10 years ago but never had physical therapy. She is now having lower back pain and her physician has sent her to therapy. She has poor segmental motion and inability to stabilize her trunk with extremity motions. Why might this be?
Lumbar segmental stabilizers do not spontaneously recover after injury and this can lead to recurrent lumbar pain syndromes. Also, Lynne may have developed faulty motor programs and muscle substitutions, which could also contribute to her current problem.

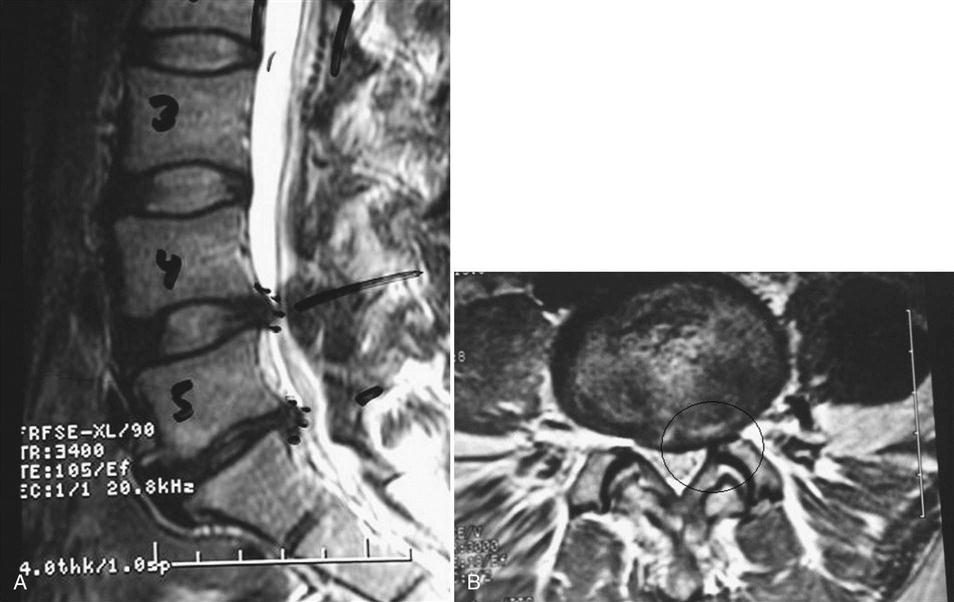
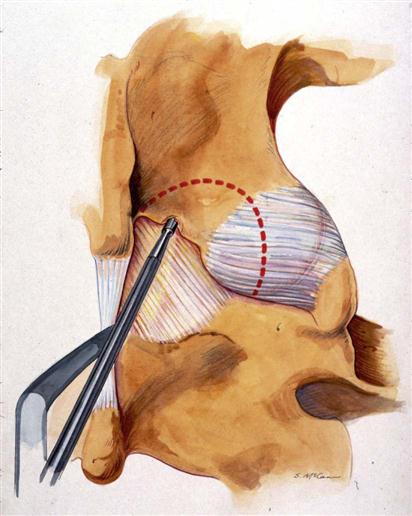
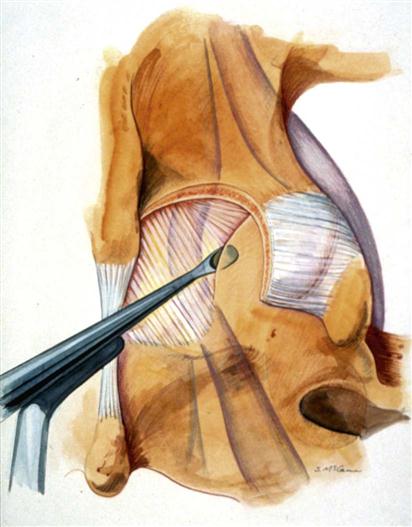
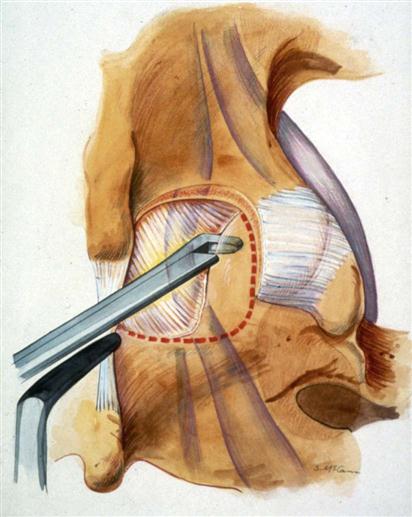
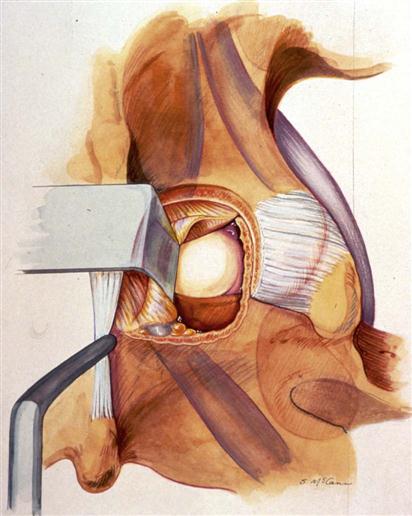
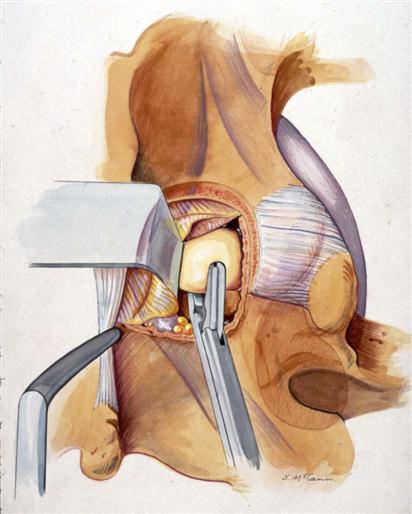
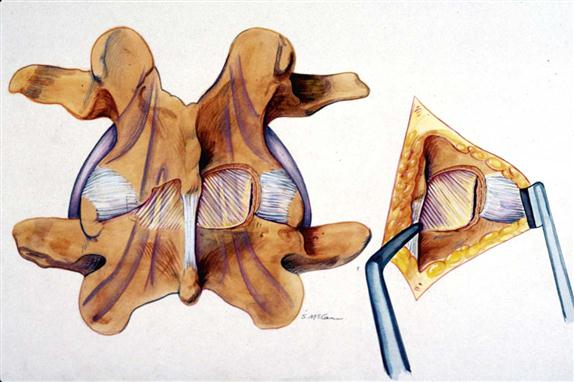
 mile.
mile. mile.
mile. hour.
hour. hour.
hour. hour.
hour.