Chapter 8 Positron Emission Tomography Imaging
Use of Positron Emission Tomography in Respiratory Medicine
The most common application of FDG-PET is in investigation of respiratory malignancies. The indications for PET in this setting are listed in Box 8-1.
Box 8-1
Current and Innovative Indications for Positron Emission Tomography in Respiratory Oncology
Principles of Positron Emission Tomography Imaging
Positron Emission Tomography Cameras
A PET camera produces three-dimensional images that represent the distribution of radioactivity in the body. Any molecule that can be labeled with a positron-emitting radioisotope can be used to generate PET images (more than 400 PET tracers are listed in the NIH Molecular Imaging and Contrast Agent Database [MICAD], available at www.ncbi.nlm.nih.gov/books/NBK5330/).
Interpretation of Positron Emission Tomography Images
False-Positive Results
The major causes of false-positive results (Box 8-2) in chest pathology are infectious, inflammatory, and granulomatous disorders. Iatrogenic procedures, such as thoracocentesis, placement of a chest tube, percutaneous needle biopsy, mediastinoscopy, thoracoscopy, and talc pleurodesis, also may give false-positive results.
False-Negative Results
False-negative results are less common and may be due to lesion-dependent or technical factors (see Box 8-2). A critical mass of metabolically active malignant cells is required for PET detection. Interpretation thus is a critical process with tumors exhibiting decreased FDG uptake such as small, very well-differentiated adenocarcinoma, bronchioloalveolar carcinoma, or carcinoid tumors. FDG-avid lesions smaller than 5 mm may be false-negative as a consequence of the limitations in spatial resolution and partial volume effect. In the lower lung fields, the detection limit may even go down to 10 mm, owing to additional respiratory motion. CT-based AC can cause artifacts in the event of misregistration between the CT and the PET data, which can lead to occultation of liver metastasis on the AC images.
Positron Emission Tomography in Diagnosis
The value of FDG-PET in differentiating benign from malignant lung lesions (Figure 8-1) has been studied in many prospective studies and documented in different metaanalyses. In these series, in which a standardized uptake value (SUV) cutoff of 2.5 often was used to suggest malignancy, a sensitivity of about 90% to 95% (range, 83% to 100%), a specificity of about 80% (range, 52% to 100%), and an accuracy of about 90% (range, 86% to 100%) were reported. Differences in the results can be explained mainly by the prevalence of malignancy in the study population, which is the result of the varying epidemiology of solitary pulmonary nodules (SPNs) in different areas of the world (e.g., regions with more tuberculosis or histoplasmosis), and by the inclusion criteria of the different series (e.g., a lower sensitivity can be expected in series with smaller nodules). The causes for false-negative and false-positive findings in SPNs are listed in Box 8-2.
FDG-PET should be used in relation to other clinical (age, smoking history) and radiologic (spiculation) factors determining the likelihood of malignancy. In clinical decision algorithms, FDG-PET will mostly add information for SPNs with an intermediate probability of malignancy. It is important to be aware of possibilities and limitations (see Box 8-2). Strong data point to use of FDG-PET for characterization of solid pulmonary nodules larger than 2 cm in diameter. Sensitivity is around 95%, negative predictive value (NPV) is very high, malignancy can be excluded correctly in the vast majority of cases, and unnecessary invasive procedures can be avoided. In order to minimize the chance of missing malignancy in smaller or faint SPNs, any lesion with FDG uptake resulting in higher-than-background activity should be considered suspect, and the overall use of the “magic” SUV threshold of 2.5 should be abandoned.
Finally, specificity and positive predictive value (PPV) are suboptimal, and clinicians should be aware that a false-positive scan is possible in the conditions listed in Box 8-2, which should be evaluated by appropriate tests. In case of doubt, lesions with increased FDG uptake should be considered to be malignant until proven otherwise and should be managed accordingly.
Positron Emission Tomography in Staging
Tumor-Node-Metastasis Status
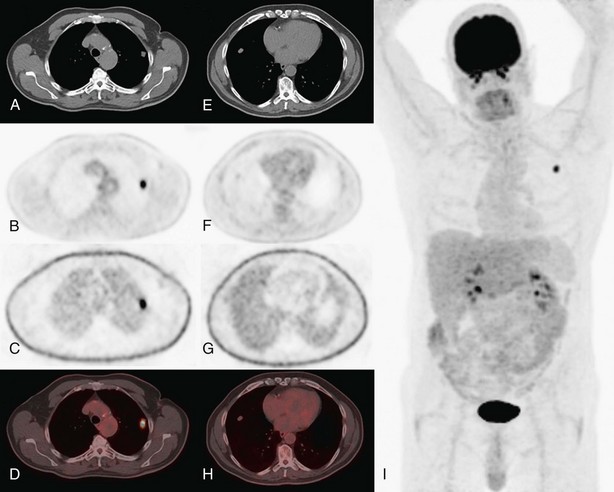
For the T factor, the detailed images of modern multislice CT allow evaluation of the relationship of the tumor to the fissures (which may determine the type of resection), to mediastinal structures, or to the pleura and chest wall. The integrated FDG-PET–CT images (Figure 8-2) may allow more precise evaluation of chest wall and mediastinal infiltration or correct differentiation between tumor and peritumoral inflammation or atelectasis.
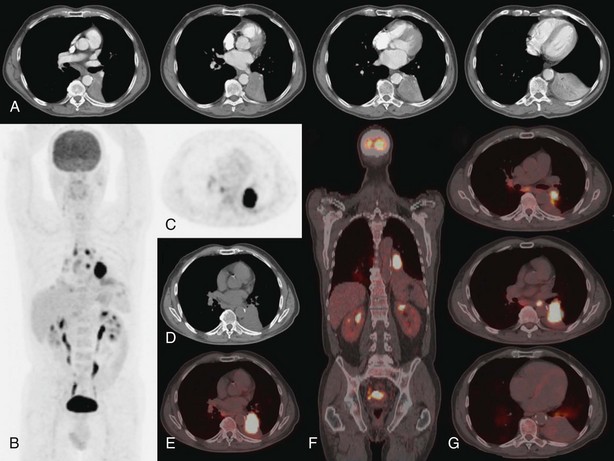
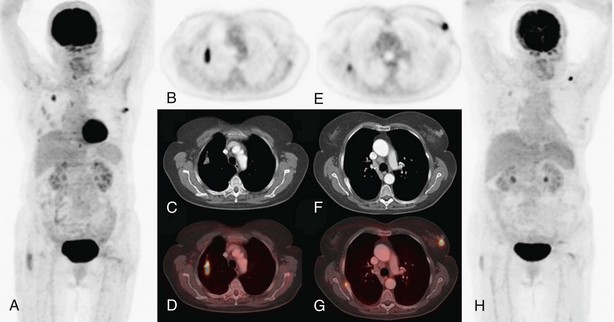
On the other hand, the combination of FDG-PET and CT illustrates the location of suspect lymph nodes, thereby helping to direct tissue sampling procedures such as endobronchial ultrasound–guided transbronchial needle aspiration or cervical mediastinoscopy. Because false-positive results are possible with lymph node imaging (under the conditions listed in Box 8-2), histopathologic proof of lymph node involvement should be sought in most patients with imaging-positive mediastinal nodes on FDG-PET, except those with obvious bulky nodes on imaging (Figure 8-3).
For bone metastases, FDG-PET is more accurate than technetium 99m–tagged medronate disodium (formerly methylene diphosphonate) (99mTc-MDP) bone scan: Sensitivity is at least as good (90% to 95%), and specificity is far better (95%, versus 60% for bone scan). Limitations are the restricted area of imaging for PET (only from the head to just below the pelvis) and possibility of a false-negative result with osteoblastic lesions (rare in lung cancer). For adrenal gland metastases, FDG-PET has a high sensitivity for detection of such disease, so an equivocal-appearing lesion on CT without FDG uptake usually will not be metastatic. FDG-PET also can be of help for investigation of hepatic lesions that remain indeterminate by conventional studies. PET also may reveal metastases in sites that escape attention on conventional staging (e.g., soft tissue lesions, retroperitoneal lymph nodes, barely palpable supraclavicular nodes, painless bone lesions). Exclusion of malignancy requires caution in case of smaller lesions (less than 1 cm in diameter) (see Box 8-2). In this respect, the small contralateral lung nodule(s)—a common finding in the era of spiral multislice CT—often still requires invasive sampling, such as by thoracoscopy.
Influence on Treatment Choices
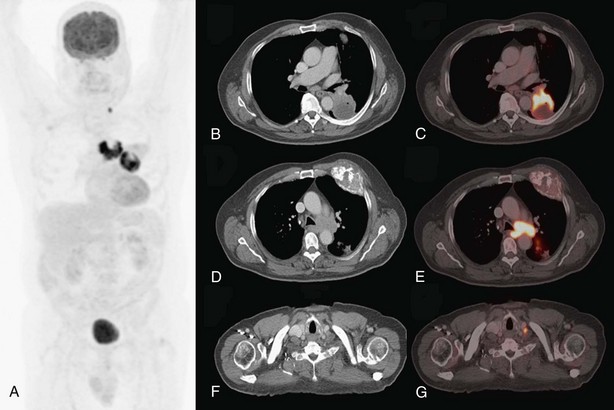
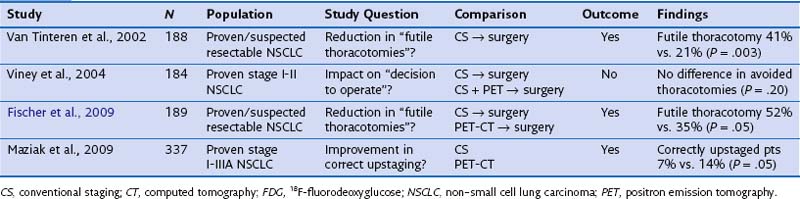
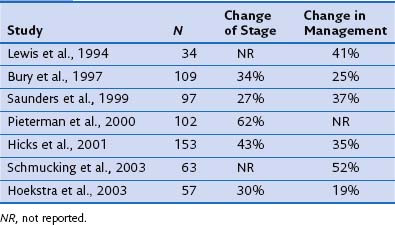
FDG-PET has a significantly complementary role to that of CT for two reasons: First, PET may detect unexpected lymph node or distant organ metastatic spread (Figure 8-4). After a negative result on conventional staging, unknown metastases are found on PET-CT in 5% to 20% of the patients, in increasing numbers from clinical stage I to III tumors. Second, FDG-PET is able to determine the nature of some equivocal lesions on conventional imaging. Consequently, PET induces a change of stage in 27% to 62% of patients with non–small cell lung carcinoma (NSCLC), in general more upstaging than downstaging, related mainly to the detection of unexpected distant lesions (Table 8-1). Such findings lead to a change in management in 25% up to 52% of the patients. Documented changes have involved both treatment intent (curative versus palliative) and choice of treatment modalities (chemotherapy versus radiotherapy, radical radiotherapy versus surgery).
Table 8-1 Impact of Positron Emission Tomography on Stage Designation and Patient Management in Non–Small Cell Lung Carcinoma
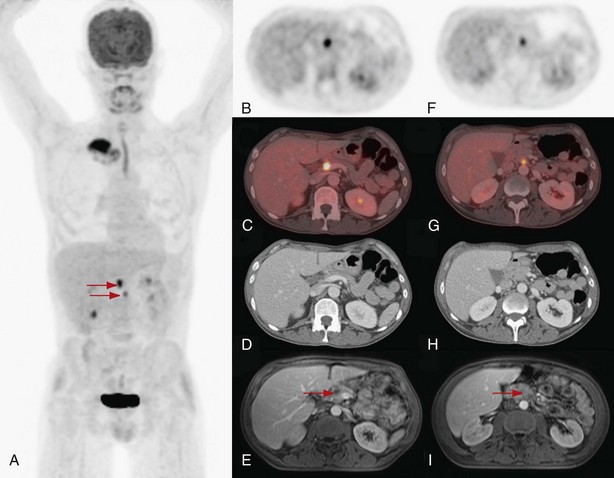
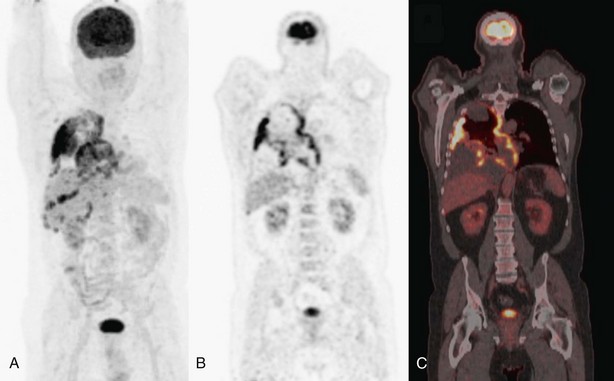
No problem arises in interpretation when whole-body FDG-PET shows multisite metastases, but presence and character of an isolated suspect lesion that determines radical treatment intent should always be verified by other tests or tissue sampling, because of the ever-present risk of false-positive lesions (see Box 8-2) or a second primary tumor. In one large retrospective series, solitary extrathoracic lesions were documented in approximately 20% of the patients. About half of these were metastatic; the other half were not related to lung cancer, as either inflammatory or other benign lesions, or were second primary tumors (Figure 8-5).
The effect of adding FDG-PET or FDG-PET–CT was investigated in several randomized controlled trials (Table 8-2). Two earlier trials that looked at the addition of PET alone reported seemingly contradictory results. Clear differences in trial design probably accounted for this finding. In the Dutch trial, the end point “futile thoracotomy” was clearly defined (benign disease, explorative thoracotomy, pathologic stage IIIA-N2/IIIB, or postoperative relapse or death within 12 months), whereas in the Australian study, there were no benign lesions, surgery was considered to be of use in some patients with stage IIIA-N2 disease, and no strict follow-up terms were predefined. The Australian trial focused on patients with clinical stage I and II disease only, where less additional benefit of PET was demonstrated in previous nonrandomized accuracy studies. The later trials used FDG-PET–CT imaging in addition to standard workup. The study by Fischer and colleagues largely reproduced the Dutch experience, with a significant reduction in futile thoracotomies. However, the number of nonfutile thoracotomies and the overall survival rate were similar in both treatment groups. Finally, the study reported by Maziak and associates mainly looked at correct upstaging and met this primary end point. Even in this study, which was the largest, no significant survival difference at 3 years was noted. This apparent lack of improvement was attributed by the investigators to the fact that larger patient numbers may be needed, and to the fact that some patients did not have their planned surgery.
Other Indications for Positron Emission Tomography
Mesothelioma
Integrated FDG-PET–CT imaging is playing an increasing role in the assessment of suspected or known malignant pleural mesothelioma (MPM) (Figure 8-6). PET-CT is useful in the correct differentiation of malignant (mainly MPM) from benign pleural diseases in asbestos-related CT findings, with an overall accuracy greater than 90% and a high NPV of more than 90%. PET-CT is significantly more accurate in baseline TNM staging in patients who are appropriate candidates for multimodality therapy based on spiral CT findings alone. Although PET-CT does not provide additional information about the primary tumor beyond that supplied by CT alone, it identifies a higher number of metastatic mediastinal lymph nodes and/or unknown distant metastatic disease in up to two thirds of patients, with a significant clinical impact on treatment planning. Early evidence also suggests that PET-CT may have a role in evaluating response to therapy in MPM. This is an interesting possibility, because the assessment of response in patients with MPM according to standard response criteria on CT is far from simple. More work to define response criteria for MPM on FDG-PET is needed. Furthermore, a prospective study in patients with nonsarcomatoid malignant pleural mesothelioma observed that baseline total glycolytic volume on PET was more predictive of survival than CT-assessed TNM stage on multivariate analysis. These observations of prognostic capability still require prospective validation.
Therapy Assessment
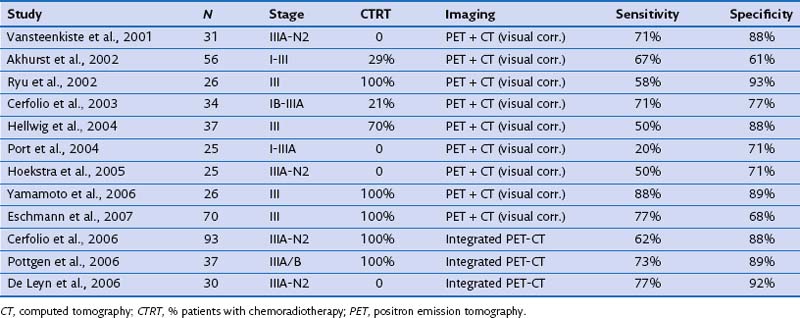
Restaging of locally advanced lung cancer after neoadjuvant or induction therapy has been extensively studied (Table 8-3). In the restaging of mediastinal lymph nodes, FDG-PET added to CT was more accurate than CT alone but with more moderate results than when used for baseline nodal staging (sensitivity of 50% to 80% and specificity of 60% to 90%). Later studies using integrated PET-CT found better sensitivity (up to 77%) with an increase in specificity (up to 92%). Mediastinal restaging with PET-CT thus reaches an accuracy level of some clinical value but is especially useful in directing additional tissue sampling techniques such as endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), esophageal ultrasound–guided fine needle aspiration (EUS-FNA), or mediastinoscopy, which usually need to be added to certify the nodal status.
Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:662–671.
De Cabanyes-Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIA lung cancer: prediction of pathologic stage. J Thorac Oncol. 2010;5:389–398.
Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumour response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–1134.
Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32–39.
Hicks J. Role of 18F-FDG in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50(Suppl 1):31S–42S.
Mavi A, Lakhani P, Zhuang H, et al. Fluorodeoxyglucose-PET in characterizing solitary pulmonary nodules, assessing pleural diseases, and the initial staging, restaging, therapy planning, and monitoring response of lung cancer. Radiol Clin North Am. 2005;43:1–21.
Thomson D, Hulse P, Lorigan P, Faivre-Finn C. The role of positron emission tomography in management of small cell lung cancer. Lung Cancer. 2011;73:121–126.
Van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev. 2006;32:245–260.
Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–540.
Vansteenkiste J, Dooms C. Positron emission tomography in nonsmall cell lung cancer. Curr Opin Oncol. 2007;19:78–83.