Chapter 13 Portal Hypertensive Bleeding
Introduction
Portal hypertension is a clinical syndrome defined as an increase in portal venous pressure greater than 5 mm Hg.1,2 It can arise from any condition interfering with the blood flow within the portal system. Among several complications of portal hypertension, including ascites, hepatic encephalopathy, and renal insufficiency, variceal hemorrhage is the most dramatic with a high mortality. Under physiologic conditions, collaterals exist between the portal venous system and the systemic venous circulation; in portal hypertension, these collaterals enlarge in an attempt to decompress the portal circulation and form varices. This chapter focuses on the gastrointestinal (GI) bleeding that results from portal hypertension; other complications of portal hypertension are reviewed separately.
Etiopathogenesis
The portal vein supplies about 1050 mL of blood into the liver sinusoids every minute. An additional 300 mL of blood is supplied by the hepatic artery with the total averaging around 1350 mL/min, which amounts to about 27% of the cardiac output. The liver receives fresh oxygenated blood from the hepatic artery and nutrient-rich blood from the stomach, spleen, and small and large intestine via the portal vein. Measuring approximately 8 cm in length, the portal vein is formed by the union of the superior mesenteric vein and splenic vein behind the neck of the pancreas. Immediately before reaching the liver, the portal vein divides into right and left branches. The right branch receives the cystic vein and enters the right lobe of the liver. The left branch receives the umbilical and paraumbilical veins, which can dilate to form caput medusae in cirrhosis. Blood from the hepatic artery and blood from the portal vein mix for the first time in the hepatic sinusoids. After mixing, the blood is drained from the lobule by the central vein, a branch of the hepatic vein. With this understanding, the portal system can be divided anatomically into presinusoidal, sinusoidal, and postsinusoidal. Portal hypertension can arise from any condition interfering with blood flow at any level within the portal venous system (Table 13.1).
| Prehepatic | Hepatic | Posthepatic |
|---|---|---|
| Portal vein thrombosis | Presinusoidal | Inferior vena cava obstruction |
| NCPF | ||
| IPH | ||
| Sarcoidosis | ||
| Schistosomiasis | ||
| Nodular regenerative hyperplasia | ||
| Myeloproliferative disorders | ||
| Splenic vein thrombosis | Sinusoidal | Cardiac failure |
| Cirrhosis | ||
| Hepatoportal arteriovenous fistula | Postsinusoidal | Constrictive pericarditis |
| Budd-Chiari syndrome | ||
| Venoocclusive disease | ||
| Splenomegaly | ||
IPH, Idiopathic portal hypertension; NCPF, noncirrhotic portal fibrosis.
Cirrhosis is the most common cause of portal hypertension in the Western world,3,4 accounting for about 90% of cases; schistosomiasis is the leading cause in many developing countries. Other causes of portal hypertension include portal vein thrombosis, polycystic liver disease, nodular regenerative hyperplasia of the liver, congenital hepatic fibrosis, myeloproliferative disorders, hepatic sarcoidosis, and hereditary hemorrhagic telangiectasia. The primary factors influencing increased pressure in the portal hepatic system are resistance and flow. In cirrhosis, both resistance and flow are altered. Increased portal venous resistance is central to the development of portal hypertension in cirrhosis. This increased resistance can be attributed to two specific factors. The first is mechanical obstruction to flow secondary to fibrotic disruption of the liver architecture and regenerative nodules. The second factor is a dynamic component produced by active contraction of vascular smooth muscles and activated stellate cells, believed to be due to a relative deficiency of nitric oxide (vasodilator),5,6 and high levels of endothelin-1 (vasoconstrictor) leading to increased sinusoidal resistance.7–9 Also, cirrhosis results in a hyperdynamic circulatory state that is characterized by peripheral and splanchnic vasodilation, reduced mean arterial pressure, and increased cardiac output. Nitric oxide–mediated splanchnic vasodilation8–16 produces an increase in inflow of systemic blood into the portal circulation, which causes an increase in portal pressure.10,11
The correlation between collateral blood flow and the transmural pressure gradient in the varix is expressed as: P1 − P2 = Q × R, where P1 and P2 are the pressures within and outside the varix, Q is the blood flow per unit of time, and R is the resistance to flow through the varix. Poiseuille’s formula states that the resistance to flow may be expressed as follows: R = 8 nl/p r4, where n is blood viscosity, l is length, and r is radius of the vessel. The transmural pressure and radius of the varix along with the mural thickness of the varix (w) determines the wall tension of the varix according to the law of Laplace: Wall tension = Q × (8 nl/p r4) × r/w. The propensity for a varix to bleed is directly linked to its wall tension. Theoretically, large long varices with thin walls and high rates of flow are most prone to bleed. Decreasing collateral flow (by decreasing portal pressure) should decrease the risk of variceal rupture.12,13
Clinical Presentation
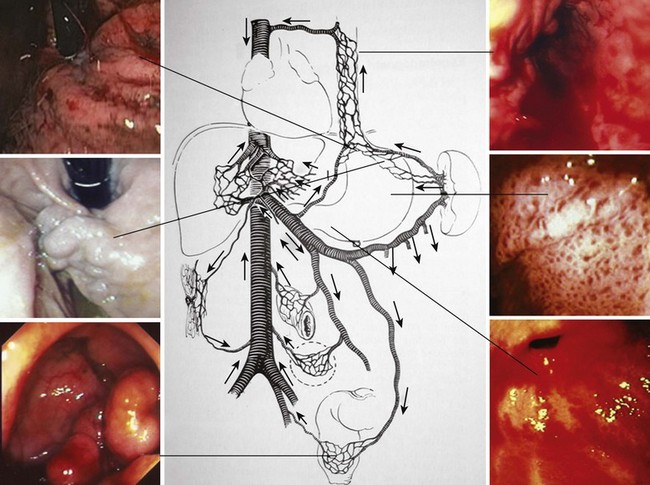
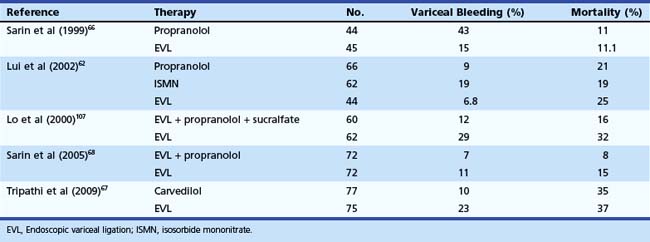
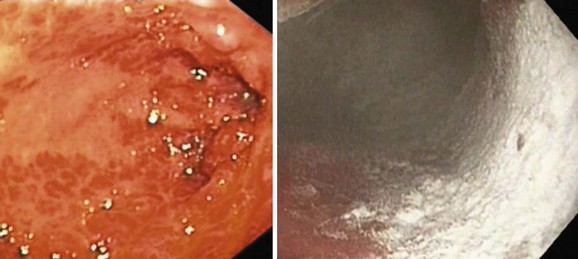
Portal hypertension should be suspected in all patients with GI bleeding in conjunction with signs of liver disease, such as ascites, jaundice, spider angiomata, gynecomastia, palmar erythema, testicular atrophy, and splenomegaly. Variceal bleeding classically manifests as effortless and recurrent hematemesis with or without dark red stools (melena). In the stomach, portal hypertension may manifest as portal hypertensive gastropathy (PHG)14,15 or gastric antral vascular ectasia (GAVE).16,17 Bleeding from either of these two conditions is usually more indolent and chronic (Fig. 13.1).
Variceal bleeding is usually associated with systemic effects including hypotension, shock, new onset of spontaneous bacterial peritonitis, or worsening encephalopathy. Spontaneous bacterial peritonitis after variceal bleeding is believed to be secondary to translocation of bacteria across the compromised mucosae.18,19 Patients with spontaneous bacterial peritonitis who were not given antibiotics had a high failure to control bleeding and higher rates of rebleeding.20 Also, in patients with ascites, infections may induce acute impairment in systemic circulatory function and hepatorenal syndrome. It is proposed that endotoxins released during bacterial infection result in an increase in portal pressure through the introduction of endothelin (a potent substance for contraction of the stellate cells), vasoconstrictive cyclooxygenase products, and contraction of hepatic stellate cells. Endotoxin-induced nitric oxide and endothelin-induced prostacyclin could inhibit platelet aggregation and reduce hemostasis at the level of the varix. Hepatic encephalopathy, which is a serious and potentially reversible disorder and known to be associated with advanced liver failure, can also be exacerbated by portal hypertension–associated GI bleeding. GI bleeding and consequent cerebral adaptation to gut-derived neurotoxins is one possible explanation. Increased levels of ammonia and mercaptans by the intestinal bacteria lead to impaired neural function secondary to cellular swelling and depletion of glutamate.
Diagnosis
Endoscopy
EGD is the most effective method for screening and surveillance of portal hypertension in patients with cirrhosis.21 The distal esophagus is the most common location for varices; however, varices can also develop in the stomach and small and large intestine.2,22 Esophageal varices occur in about 50% of all patients with cirrhosis. Gastric varices are found in approximately one of five patients with portal hypertension. About 5% to 10% of patients with gastric varices may not have esophageal varices.23 Although endoscopic evaluation of small or large bowel is not recommended in patients who are suspected to have portal hypertension (unless symptoms warrant), varices could be encountered in the small or large intestine (see Fig. 13.1).
Although EGD is recommended for all patients with a new diagnosis of cirrhosis to screen for varices, the procedure is costly, may be unpleasant, and has potential risk for complications. EGD is often performed under sedation,24–26 which includes its own cost and complications that may be more severe in patients with cirrhosis. Unsedated EGD performed with small-diameter endoscopes is being evaluated as an alternative to conventional endoscopy to avoid the direct and indirect costs of conscious sedation.27,28
Hepatic Vein Pressure Gradient
The hepatic vein pressure gradient (HVPG) is more invasive but is an accurate tool to measure portal hypertension. Portal venous pressure can be measured either directly by portal venography or indirectly by HVPG. HVPG is the difference between the wedged hepatic venous pressure (a measure of portal venous pressure) and the free hepatic vein pressure.29,30 In acute variceal bleeding, the measurement of HVPG provides prognostic information and identifies difficult-to-treat patients. In patients with acute variceal bleeding, HVPG of 20 mm Hg or greater measured soon after admission was associated with a significantly longer intensive care unit (ICU) stay, longer hospital stay, greater transfusion requirements, and worse short-term and long-term survival (1-year mortality 64% vs. 20%; P > .002).31 HVPG greater than 10 mm Hg is a good predictor of the development of varices. A threshold value of 12 mm Hg has been identified for variceal rupture.32 In cirrhotic patients with portal hypertension, the reduction of HVPG to less than 12 mm Hg or by 20% or more of the baseline value significantly reduces the risk of bleeding. Most importantly, a reduction of HVPG 20% or greater of baseline reduces the risk of death.33,34
Other Methods
Several methods have been proposed as alternatives to conventional EGD or HVPG for noninvasive diagnosis of esophageal varices,35 including platelet count-to-spleen diameter ratio, Fibrotest, and Fibroscan. Multidetector computed tomography (CT) esophagography and esophageal capsule endoscopy are considered minimally invasive. Transient elastography (Fibroscan) is a novel noninvasive method for assessing liver stiffness and is useful for diagnosis of advanced fibrosis and portal hypertension. Transient elastography is painless, rapid, and easy to perform at the bedside or in the outpatient setting. Results are expressed in kilopascals (kPa), and values range from 2.5 to 75 kPa.36 A study assessed the correlation between liver stiffness with Fibroscan and HVPG in diagnosing significant portal hypertension in 150 patients who underwent a liver biopsy and hemodynamic measurements.37 In patients with significant portal hypertension (HVPG >10 mm Hg), area under the receiver operating characteristic curves for Fibroscan was 0.945. The cutoff value of 21 kPa accurately predicted significant portal hypertension in 92% of patients for whom measurements were successful. Larger studies are needed to define precisely the role of Fibroscan in the diagnosis of portal hypertension.
Esophageal capsule endoscopy is a practical, safe, well-tolerated, and accurate method for the diagnosis of esophageal varices.38 However, interpretation of esophageal capsule endoscopy can be compromised by the presence of secretions or bubbles, and it does not detect gastric varices. An unpredictable transit time and poor interobserver agreement could account for a significant rate of failure (6% in some studies). The use of multidetector CT esophagography to grade esophageal varices has been evaluated more recently.39,40 The esophagus is insufflated with air via a catheter passed through the mouth. In the study by Kim and associates39 that included 90 patients with cirrhosis, 30 patients had large esophageal varices. CT scan performance for the diagnosis of large varices was 0.931 to 0.958 (estimated by the area under the receiver operating characteristic). Perri and colleagues40 prospectively evaluated 102 patients who underwent both CT and endoscopic screening for gastroesophageal varices. CT was found to have approximately 90% sensitivity for the identification of large esophageal varices but only about 50% specificity. The sensitivity of CT in detecting gastric varices was 87%.
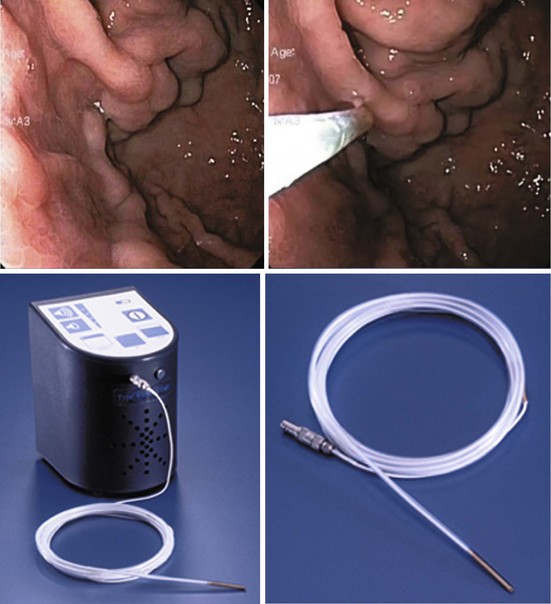
Gastric varices are harder to detect by endoscopy, especially if they are small and isolated. Small varices in the fundus are often mistaken for a mucosal fold. Their identity as varices is based on their shape (grapelike clusters) and their bluish tinge. At endoscopic ultrasound (EUS), they can be seen as circular or linear anechoic channels within the gastric wall. The addition of color Doppler or through the scope Doppler probe can differentiate gastric varices from mucosal folds.41
Grading and Classification of Varices
Varices are easily diagnosed by upper GI endoscopy and are categorized by their location into esophageal and gastric varices. Varices are most often seen in the distal esophagus and may extend beyond the Z line into the gastric cardia. The distal esophagus must be insufflated with air while assessing the variceal size. The most recent guidelines by the American Association for the Study of Liver Diseases (AASLD) recommend classifying esophageal varices as either small (<5 mm) or large (>5 mm) (Fig. 13.2).42
Esophageal varices can also be graded using several classifications for documentation and research purposes.43–45 According to Conn,43 the number of varices is graded as follows: 1+, a single varix; 2+, two to three varices; 3+, four to six varices; and 4+, more than six varices. The size of the varices is graded as follows: 1+, small varices detectable only on performing Valsalva maneuver; 2+, small varices (approximately 1 to 3 mm in diameter) visible without Valsalva maneuver; 3+, varices of moderate size (3 to 6 mm in diameter); and 4+, large varices (>6 mm). Extent of esophageal involvement is graded as follows: 1+, terminal 3 cm; 2+, terminal 6 cm; 3+, terminal 9 cm; and 4+, involving more than the terminal 9 cm.
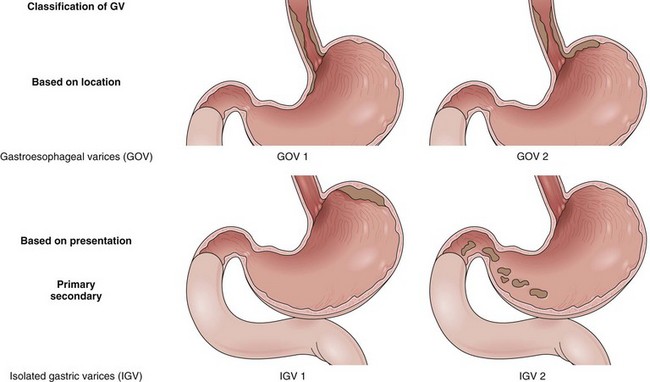
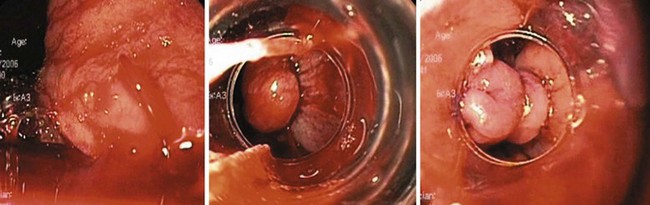
Certain types of varices are more likely to appear depending on the cause of portal hypertension. Isolated gastric varices located on the fundus (IGV1) classically occur in association with splenic vein thrombosis. Duodenal and biliary varices can occur more often with extrahepatic causes of portal hypertension.46 A simple classification of gastric varices depending on their anatomic location in the stomach can also help in understanding their natural history and approach to management.47 Gastroesophageal varices are located in the esophagus and extend in continuity to the lesser curve (GOV1) or greater curve (GOV2) of the stomach. Isolated gastric varices are located usually on the fundus (IGV1) or greater curve or other sites in the stomach or first part of duodenum (IGV2) (Fig. 13.3). In more recent publications as well as in our own experience, the use of a through the scope Doppler probe not only facilitates the diagnosis, but it also confirms successful therapy of gastric varices (Fig. 13.4).41
Predictors of Variceal Bleed
The North Italian Endoscopic Club uses the extent of liver disease, large variceal size, and the presence of red color signs on endoscopy as an index for the likelihood of first variceal bleeding.48 Of the above-mentioned indicators, variceal size seems to be the most important predictor of first variceal bleeding. The probability of variceal bleeding is significantly greater in patients with larger esophageal varices (P = .0001), with more severe red wale marks (P = .0001), or with more severe liver dysfunction according to the Child-Pugh classification (P = .007).49 However, because only one-third of patients who present with variceal hemorrhage have the previously mentioned risk factors, a better definition of predictive factors is needed.
Sarin and Kumar47 examined 12 clinical, endoscopic, and hemodynamic variables in 126 patients with portal hypertension (72 bleeders and 54 nonbleeders). The variceal size and intravariceal pressures were the most important predictors of hemorrhage. In another study, the risk of bleeding was 0% when the variceal pressure was 13 mm Hg or less, 9% when the variceal pressure was 13 to 14 mm Hg, 17% when the variceal pressure was 14 to 15 mm Hg, 50% when the variceal pressure was 15 to 16 mm Hg, and 72% when the variceal pressure was greater than 16 mm Hg.50
HVPG is a measure of portal pressure and intravariceal pressure in patients with cirrhosis. Garcia-Tsao and colleagues32 found that HVPG was significantly greater in 49 patients who had bled from esophageal varices than in 44 patients with cirrhosis who did not bleed (20.4 ± 5.1 mm Hg vs. 16.0 ± 5.2 mm Hg). They also found that none of the patients who had bled had HVPG less than 12 mm Hg. In one study, the portocaval pressure gradient decreased after transjugular intrahepatic portosystemic shunt (TIPS) procedure (from 19.7 ± 4.6 mm Hg to 8.6 ± 2.7 mm Hg), and a subsequent increase in pressure greater than 12 mm Hg (18.4 ± 7.46 mm Hg) was associated with rebleeding.51 Moitinho and coworkers31 reported that patients admitted because of variceal bleeding who had HVPG greater than 20 mm Hg measured within 48 hours had a fivefold increased risk of (1) failure to control bleeding with the emergency treatment or (2) experiencing early rebleeding. Dynamic measurements of HVPG are more valuable to assess the influence of therapeutic interventions or alcohol abstinence. Active bleeding at endoscopy was statistically shown to be an independent risk factor in predicting failure to control variceal bleeding.52
Natural History of Varices
Gastroesophageal varices are the most significant portosystemic collaterals because their rupture results in variceal hemorrhage, the most common lethal complication of cirrhosis.53 The prevalence and rate of growth of varices in cirrhotics are often related to the severity of liver disease. All patients should be screened for varices at the time of diagnosis of cirrhosis. Patients who have no varices on screening endoscopy should be rescreened every 2 to 3 years if their liver function is stable or earlier if there are signs of hepatic decompensation. Patients who have small varices on screening endoscopy should be rescreened every 1 to 2 years because the development of large varices is greater in patients with small varices on initial endoscopy compared with patients with no varices.42
About one-third of patients with varices experience a variceal hemorrhage. The 2-year bleeding risk in patients with cirrhosis and moderate to large varices is 25% to 30%. Lifelong risk of variceal bleeding is almost 50%.54 In portal vein obstruction, the bleeding rate is even higher. After varices start bleeding, hemorrhage spontaneously stops in only 50% of cases. Patient’s with Child C cirrhosis and actively spurting varices are particularly likely to continue to bleed without active intervention. After cessation of active bleeding, the risk of rebleeding is higher for approximately 6 weeks. The risk of early rebleeding is greatest within the first 48 hours, and about half of all early rebleeding episodes occur during this time. Risk factors for early rebleeding include large varices, age older than 60 years, severity of initial bleed, renal failure, ascites, active bleeding on endoscopy, and red wale marks (defined as longitudinal dilated venules resembling whip marks on the variceal surface). Aggressive volume replacement may exacerbate portal hypertension and precipitate early rebleeding.
Of patients who bleed from esophageal varices, the variceal hemorrhage is fatal in roughly 20% of patients. The incidence of new varices is approximately 5% to 15% per year, depending on the Child-Turcotte-Pugh class1 and the etiology of the liver disease.2,22 The risk of gastric variceal bleeding is lower than the risk of esophageal variceal bleeding, but gastric variceal bleeding, especially fundal varices, tends to be more severe, to require more transfusions, and to have a higher rate of mortality.23 Gastric variceal bleeding has been reported to account for 3% to 30% of all acute variceal bleeding episodes. The risk of bleeding from gastric varices depends on the location. Although GOV1 constitute more than 70% of gastric varices, only 11% of GOV1 ever bleed. In contrast, although IGV1 constitute less than 8% of all gastric varices, 80% of IGV1 bleed.19 IGV1 are often fed by spontaneous large collaterals that partly decompress the portal vein, and IGV1 are associated with lower portal pressures than esophageal varices. IGV2 are rare (4.7% of all gastric varices); commonly seen in antrum (53%), duodenum (32%), or at both sites (11%); and rarely in body and fundus (4%). Overall, gastric varices bleed less often but more severely than esophageal varices.55
Management
Endoscopic and Pharmacologic Therapy
Primary Prophylaxis to Prevent Esophageal Variceal Bleeding
Primary prophylaxis of variceal bleeding or prevention of the first episode of variceal hemorrhage is the most logical approach to decrease morbidity and mortality after a variceal bleed. Because only about 10% to 20% of patients bleed, it is essential to select carefully patients who are at a high risk of variceal hemorrhage. In patients without varices, nonselective β blockers were proposed with the aim of preventing the development of varices.56 In a large multicenter study, 213 cirrhotic patients with portal hypertension (HVPG >5 mm Hg) but without varices were randomly assigned to receive timolol or placebo in double-blind conditions for a median of 55 months.57 The rate of the development of esophageal varices did not differ between the two groups, and the timolol group reported more adverse events. These results do not support the universal use of β blockers in cirrhotics without varices. In patients with no varices on endoscopy, there is no indication for treatment to prevent the formation of varices.
Patients who have varices may benefit from the interventions directed toward prevention of first bleeding. Endoscopic sclerotherapy, which is not commonly used at the present time, was compared with no treatment in 20 randomized trials that included 1756 patients, most of whom had medium-sized or large-sized varices. In the sclerotherapy group, there was a significant reduction in bleeding in 5 trials, increase in bleeding in 2 trials, and no difference in 13 trials.58 Because of significant heterogeneity in the results with regard to bleeding and mortality among the different trials, prophylactic sclerotherapy is not recommended for primary prophylaxis.59,60 In patients who have small varices that have not bled, β blockers can be used to prevent the progression of varices and bleeding. A meta-analysis evaluating nonselective β blockers in the prevention of first variceal bleed investigated the results of three trials that included patients with small varices.61 The group receiving β blockers showed a reduction in the incidence of first variceal hemorrhage (2% over 2 years) compared with the nontreated group (7% over 2 years), but the reduction was not statistically significant.
Prophylaxis is important in patients who are considered at high risk for bleeding because of advanced liver disease and the presence of red wale marks on varices.21 Regarding patients with medium to large varices, a meta-analysis of 11 trials comprising 1189 patients evaluated nonselective β blockers (i.e., propranolol, nadolol) versus placebo in the prevention of first variceal bleeding and showed that the risk of bleeding was significantly reduced by β blockers (30% in control vs. 14% in β blocker group). This meta-analysis indicates that one bleeding episode is avoided for every 10 patients treated. More importantly, mortality was lower in the β blocker group compared with the control group, and this difference has been shown to be statistically significant.62 β blockers might not be tolerated by all patients, and there might be some contraindications, such as in patients with asthma, insulin-dependent diabetes, and peripheral vascular disease. The most common side effects in cirrhotics are lightheadedness, fatigue, and shortness of breath.
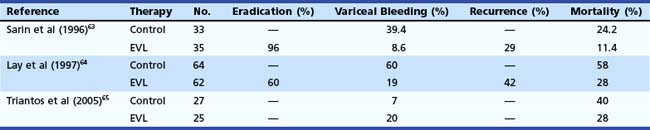
In the last 2 decades, EVL has essentially replaced sclerotherapy as a mode of variceal eradication, and it has been evaluated for preventing the first bleed from esophageal varices. In a series of 68 patients, Sarin and coworkers63 showed the superiority of EVL compared with no therapy to prevent first variceal bleed (Table 13.2). Their results were subsequently confirmed by Lay and colleagues,64 who found significant reduction in the incidence (19% vs. 60%) and mortality (28% vs. 58%) of first bleed when EVL was compared with no therapy. In contrast to these reports, a more recent study showed no benefit of EVL; however, the patients included in this study were either intolerant to or had contraindication for the use of β blockers.65
Table 13.2 Endoscopic Variceal Ligation (EVL) versus No Treatment for Prevention of First Bleeding from Esophageal Varices
After the initial encouraging reports, it was logical to assess whether EVL is comparable to the current therapy for primary prophylaxis—β blocker therapy. Sarin and coworkers66 randomly assigned 89 patients with high-risk varices to receive EVL or propranolol. The actuarial probability of bleeding at 18 months was significantly lower in the EVL group (15.8%) compared with the propranolol group (43%). There was no survival benefit with EVL, however. Lui and colleagues62 assigned 172 patients to EVL (n = 44), propranolol (n = 66), or isosorbide mononitrate (ISMN) (n = 62) therapy (Table 13.3). On intention-to-treat analysis, variceal bleeding was observed in 7% of patients randomly assigned to EVL, 14% of patients randomly assigned to propranolol, and 23% of patients randomly assigned to ISMN; the difference between EVL and nitrates was significant. A significant number of patients reported side effects with drug treatments (45% propranolol and 42% ISMN vs. 2% EVL), resulting in withdrawal from treatment in 30% of patients receiving propranolol and 21% of patients receiving ISMN. There was no significant difference in mortality rates in the three groups.
A more recent randomized controlled trial found carvedilol to be more effective in preventing first variceal bleed than EVL in patients with high-risk esophageal varices.67 Sarin and coworkers68 noted that although addition of propranolol to EVL does not decrease the probability of first bleed or death, the recurrence of varices is lower if propranolol is added to EVL. Several meta-analyses have been performed comparing EVL with β blockers.69–71 The meta-analysis performed by Imperiale and Chalasani69 included five trials with 601 patients. Khuroo and associates70 included eight trials comprising 596 patients. More recently, Gluud and coworkers71 performed a meta-analysis of 15 randomized controlled trials comprising 1174 patients. Imperiale and Chalasani69 and Khuroo and associates70 concluded that in patients with large varices that are high risk for bleeding, EVL decreases bleeding episodes; however, there was no reduction effect on mortality. Gluud and coworkers71 noted that the positive effects associated with the EVL group were more commonly seen in the trials that had shorter follow-up.
There are several distinct advantages of EVL over β blockers, as follows:
Teran and colleagues72 examined the cost-effectiveness of various treatments in primary prophylaxis of variceal bleeding. They found that β blockers were more cost-effective than sclerotherapy or shunt surgery in patients with cirrhosis with a cost savings between $440 and $1460. The other treatments were not cost-effective. Spiegel and coworkers found empiric β blocker therapy for primary prophylaxis of variceal hemorrhage to be a cost-effective measure because the use of screening endoscopy to guide therapy adds significant cost with only marginal increase in effectiveness.
The Baveno IV consensus recommended that nonselective β blockers should be considered as first-choice treatment to prevent first variceal bleeding in patients with high-risk varices, and EVL should be offered to patients with contraindications or intolerance to β blockers.21 HVPG can also be monitored while receiving treatment with a β blocker. If HVPG is reduced by 25% or more or the gradient is less than 12 mm Hg after 4 to 6 weeks of therapy, the drug should be continued. A more noninvasive method of measuring efficacy of β blockers is by noting a decrease in the patient’s pulse rate. AASLD and American College of Gastroenterology also consider β blockers as first choice in patients with medium to large varices that have not bled and are not high risk. In patients with high-risk varices, the combination of EVL and β blockers is considered first-choice therapy.
Management of Active Esophageal Variceal Bleeding
Active bleeding has been defined by the Baveno II Consensus Workshop as oozing or spurting at the time of endoscopy. Clinically significant bleeding is defined as bleeding with a transfusion requirement of 2 or more units of blood within 24 hours of time zero, together with a systolic blood pressure less than 100 mm Hg or a postural change of greater than 20 mm Hg or pulse rate greater than 100 beats/min at time zero.73
Pharmacotherapy
The advantage of pharmacotherapy is that it is generally applicable and can be administered as soon as the diagnosis of variceal hemorrhage is suspected. Vasoactive therapy should be initiated before diagnostic EGD. Several vasoactive agents have been evaluated extensively. Vasopressin and its analogue terlipressin and somatostatin and its analogues octreotide, lanreotide, and vapreotide have been studied. β blockers should not be used in the acute setting because they decrease blood pressure and blunt a physiologic increase in heart rate associated with bleeding. The use of vasoactive drugs alone in acute variceal bleeding has not proved to be more effective than endoscopic treatment. Adding octreotide to EVL in patients with acute variceal bleeding was beneficial in one study but not in another.74,75 A meta-analysis of 15 trials comparing emergency sclerotherapy and pharmacologic treatment (vasopressin with or without nitroglycerin, terlipressin, somatostatin, or octreotide) showed similar efficacy but with fewer side effects in the pharmacologic therapy group.76
The use of somatostatin and analogues such as octreotide and vapreotide also produces splanchnic vasoconstriction, which is thought to be indirect via the inhibition of the release of vasodilatory peptides, primarily glucagon. More recent studies imply that octreotide has local vasoconstrictive effects. Somatostatin and its analogues have the advantage of being safe and can be used continuously for 5 days or longer. Terlipressin is a synthetic analogue of vasopressin and has the benefit of longer biologic activity with significantly fewer side effects. Terlipressin should be the first choice because it is the only drug that has been shown to improve survival; however, it is unavailable in the United States. Terlipressin is also the only pharmacologic treatment that has been shown to improve prognosis of variceal bleeding in placebo-controlled randomized controlled trials and meta-analysis. The most common side effect is abdominal pain; more serious side effects such as peripheral or myocardial ischemia may occur in less than 3% of patients.77 Of the somatostatin analogues, only octreotide is available in the United States; it may be used as an adjunct to endoscopic therapy. The combination of pharmacologic and endoscopic therapy is considered to be the most effective treatment for acute variceal bleeding at the present time.
Acid Suppression
Many uncontrolled studies suggest a beneficial effect of acid suppression as an adjunct to sclerotherapy. In three controlled studies, the effect of sucralfate on healing of sclerotherapy-induced ulcers or preventing ulcer bleeding was found to be controversial. In one controlled study, ranitidine had a significant effect on ulcer healing, whereas omeprazole had no effect in another study.78
Antibiotics
Patients with cirrhosis are prone to developing spontaneous bacterial peritonitis among other infections. Consequently, antibody prophylaxis is an integral part of therapy for patients with variceal bleeding and should be started at admission.21 Patients with Child class B and C cirrhosis are at the highest risk for developing bacterial infections. The use of prophylactic antibiotics is associated with a decrease in the rate of infections and improved survival. The improved survival is considered to be related to a decrease in the incidence of early rebleeding in patients who receive prophylactic antibiotics. Oral norfloxacin (400 mg twice daily) or intravenous ciprofloxacin (in patients in whom oral administration is not feasible) is the recommended antibiotic.79,80 Antibiotics must be started on admission even if there is no apparent ascites.
Endoscopic Variceal Ligation Therapy
The current recommendation is to perform EVL after initial resuscitation when the patient is stable and the bleeding has ceased or slowed. Endoscopic sclerotherapy has largely been replaced by EVL. Sclerotherapy can be used when poor visualization prevents effective band ligation for bleeding varices (Fig. 13.5). Rarely, in cases with failed band ligation, our group and others have attempted mechanical closure of the bleeding site with hemoclips (Fig. 13.6).81
Endoscopic Sclerotherapy
Endoscopic sclerotherapy consists of injection of a sclerosing agent into the lumen of a varix (intravariceal) or immediately adjacent to the vessel to tamponade flow (paravariceal).82 The injected sclerosant achieves its immediate hemostatic effect by coagulation necrosis, and variceal thrombosis and subsequently the inflammation of the surrounding tissue and scarring lead to variceal obliteration. The most commonly used sclerosants, with nearly comparable efficacy, are ethanolamine oleate, polidocanol, absolute alcohol, sodium tetradecyl sulfate, and sodium morrhuate.22,82–84 Absolute alcohol is a potent, aqueous, readily available low-cost agent and is comparable to 5% ethanolamine oleate and 3% sodium tetradecyl sulfate.82,84 Endoscopic sclerotherapy is performed using freehand technique. An injector with a retractile needle is passed through the operating channel of the endoscope, and the sclerosant is injected into the varix. During an acute bleed, injections are directed at the bleeding site. Endoscopic sclerotherapy can effectively arrest active esophageal variceal bleeding in about 95% of cases. Sclerotherapy is associated with significant complications, which have led to infrequent use of this modality in present-day practice. Its use is mainly limited to actively bleeding cases in which banding is not feasible or has failed.
Endoscopic Variceal Ligation versus Sclerotherapy
EVL and sclerotherapy have been compared and shown to be effective in the control of acute variceal bleeding. Two randomized trials compared EVL versus sclerotherapy in acute variceal bleeding.85,86 In the first trial, 71 cirrhotics with active variceal bleeding were randomly assigned to receive EVL (37 patients) or sclerotherapy (34 patients) immediately after endoscopic examinations. Treatment failure within 1 month was 8% in the EVL group versus 30% in the sclerotherapy group (P = .02). Blood transfusion requirements were significantly lower in the EVL group than in the sclerotherapy group (3.2 ± 1.2 U vs. 4.5 ± 1.8 U, P < .01). There were significant complications in 5% in the EVL group and 30% in the sclerotherapy group (P = .007). In the second trial, patients admitted with acute GI bleeding and with suspected cirrhosis received somatostatin infusion for 5 days. Patients underwent endoscopy within 6 hours, and patients with esophageal variceal bleeding were randomly assigned to receive either sclerotherapy (n = 89) or EVL (n = 90). Therapeutic failure occurred in 21 patients treated with sclerotherapy (24%) and in 9 patients treated with EVL (10%) (relative risk 2.4, 95% confidence interval 1.1 to 4.9). Failure to control bleeding occurred in 15% versus 4% (P = .02). Side effects occurred in 28% of patients receiving sclerotherapy versus 14% receiving EVL (relative risk 1.9, 95% confidence interval 1.1 to 3.5); the side effects were serious in 13% versus 4% (P = .04). The 6-week survival probability without therapeutic failure was better with EVL (P = .01).
The efficacy of EVL for the control of acute bleeding has been debated technically because it may be difficult to visualize and band a bleeding varix. This technical difficulty could be overcome by placing four bands, one in each quadrant, just above the gastroesophageal junction and a second set 3 to 4 cm proximal. Stiegmann and coworkers87 randomly assigned patients who bled from esophageal varices to sclerotherapy or EVL. The control of active bleeding was comparable—77% in the sclerotherapy group and 86% in the EVL group. Mortality was higher in the sclerotherapy group (45%) compared with the EVL group (28%). Avgerinos and colleagues88 randomly assigned 71 patients with cirrhosis and active variceal bleeding to EVL or sclerotherapy. The bleeding was controlled in 97% of patients in the EVL group and 76% of patients in the sclerotherapy group.
Adjuvant and Alternative Treatments to Endoscopic Modalities in the Treatment of Acute Variceal Bleeding
Balloon tamponade using a triple-lumen Sengstaken-Blakemore tube or a four-lumen Minnesota tube stops acute variceal bleeding effectively. The Zimmon tube allows endoscopy through the inflated rim of the balloon that abuts against the gastroesophageal junction and compresses the bleeding varices. Balloon tamponade is not routinely used before therapeutic endoscopy because it has a high rate of complications.54,89–91
Secondary Prophylaxis to Prevent Recurrent Esophageal Variceal Bleeding
All patients who survive an episode of acute variceal bleeding should undergo secondary prophylaxis. The rebleeding rate in untreated patients is around 60% within 1 to 2 years of the index hemorrhage. The risk of rebleeding is greatest within the first 6 weeks, with more than half of rebleeding episodes occurring within 3 to 4 days. Several risk factors have been recognized and include severe initial bleeding as defined by a hemoglobin less than 8 mg/dL, gastric variceal bleeding, active bleeding at endoscopy, and elevated HVPG.92,93 It is vital that preventive therapy should be initiated before discharge from the hospital in patients who have recovered from an episode of hemorrhage for at least 24 hours.
Pharmacologic Therapy
Pharmacologic therapy using nonselective β blockers with ISMN has been proposed as a modality to prevent recurrent variceal bleeding. The combination therapy has synergistic effects on reducing portal pressures. Gournay and colleagues94 tested the effectiveness of ISMN as an adjunct to propranolol for the prevention of variceal rebleeding. The study included 95 patients randomly assigned to combination treatment (46 patients) or propranolol alone (49 patients). The group receiving combination therapy had less rebleeding (33% vs. 41%); however, it was not statistically significant.
Endoscopic Variceal Ligation
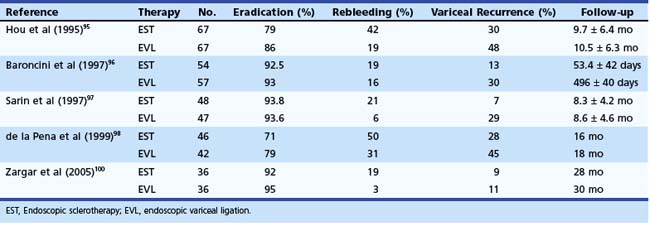
EVL has essentially replaced sclerotherapy owing to faster reduction and obliteration of varices and by requiring fewer procedures with lower rates of complications (Table 13.4).95–99 EVL was compared with sclerotherapy for the prevention of recurrent bleeding in 18 studies (n = 1509 patients). Meta-analyses have shown that the number of sessions, the time for obliteration, and the rebleeding rates are significantly lower with EVL compared with sclerotherapy. Sarin and associates97 observed a faster variceal eradication with EVL requiring fewer sessions but with a higher recurrence rate compared with sclerotherapy group over a follow-up period of 8.5 ± 4.4 months. The higher recurrence with EVL is probably because ligation does not occlude the perforators as is done by injecting a sclerosant. The incidence of PHG was greater after sclerotherapy than after EVL (20.5% vs. 2.3%). The infrequent occurrence of PHG after EVL may be due to the fact that band ligation does not occlude the esophageal perforators, which allow blood to be drained away to paraesophageal collaterals, resulting in less congestion of gastric microcirculation but a higher rate of recurrence of esophageal varices. EVL has also been found effective in extrahepatic portal venous obstruction.100
Endoscopic Variceal Ligation and Sclerotherapy
EVL and sclerotherapy together during the same session (synchronous combination) for obliteration of esophageal varices has been proposed as a more effective management option compared with their individual use. Two meta-analyses, one comprising seven trials and a more recent one comprising eight trials, showed no differences in rebleeding, death, or number of sessions to variceal obliteration between groups and a higher incidence of esophageal strictures in the combination therapy group.101,102 Consequently, EVL should not be combined with sclerotherapy.
Endoscopic Variceal Ligation and Pharmacotherapy
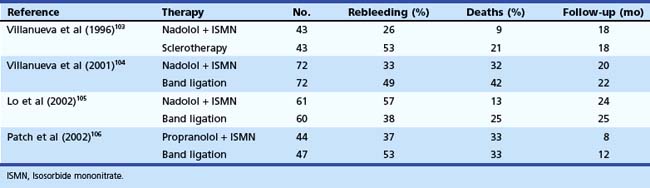
Combined EVL and pharmacotherapy using β blockers have also been proposed for secondary prophylaxis of variceal hemorrhage. The results of four trials are presented in Table 13.5.103–106 EVL was also compared with the combination of EVL, β blockers, and sucralfate; 62 patients received EVL alone, and 60 received combination therapy.107 After a median follow-up of 21 months, rebleeding and variceal recurrence occurred in 30% and 50% of patients in the EVL group and in 11.6% and 26% of patients in the combination group (P < .05). In addition, a large meta-analysis from Spain, which included 1860 patients in 23 trials, showed that combination therapy reduced overall and variceal rebleeding in cirrhotics more than either therapy alone,108 supporting that this is a better approach for secondary prophylaxis.
Argon Plasma Coagulation and Microwave Coagulation
Argon plasma coagulation and microwave coagulation are other approaches to improve the rate of long-term eradication of esophageal varices after EVL by causing fibrosis of the esophageal wall. The techniques of argon plasma coagulation, low-power diode laser treatment, and microwave are still experimental.109–113
Gastric Varices
Bleeding from gastric varices is less frequent than bleeding from esophageal varices, but it is usually more severe and is associated with a higher mortality rate. Gastric varices develop in approximately 20% of patients with portal hypertension.55 The management of bleeding gastric varices depends on the natural history of different types of gastric varices. Although GOV1 disappear with the obliteration of esophageal varices in nearly 60% of patients, GOV2 and IGV1 require specific therapy.114 GOV1 can also be managed with EVL in either an antegrade or retrograde fashion (Fig. 13.7). The risk of bleeding from IGV1 was shown to correlate with variceal size (>10 mm), Child class, and the presence of red color signs on varices.22 IGV2 are uncommon varices, rarely bleed, and can be managed similar to IGV1.
Endoscopic Sclerotherapy
Endoscopic sclerotherapy has not been highly successful in the treatment of gastric varices. The reason has been the high incidence of complications associated with sclerotherapy, which include gastric ulceration and perforation and recurrent bleeding rates of 37% to 53%.115 Endoscopic sclerotherapy does not achieve thrombosis of the entire varix, and the necrosis caused by it may induce massive bleeding from the nonthrombosed high-flow gastric varix, leading to high mortality.60
Endoscopic Variceal Ligation
EVL and detachable snares have also been used by some groups to control acute gastric variceal bleeding and reduce the risk of rebleeding. Limited efficacy data are available, however.116,117
Tissue Adhesive Agents
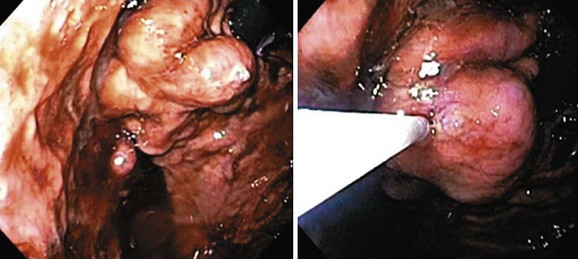
Tissue adhesive agents have been reported to be effective in treating bleeding gastric varices. Compared with endoscopic sclerotherapy or EVL, endoscopic variceal obturation with tissue adhesive such as N-butyl-cyanoacrylate, isobutyl-2-cyanoacrylate, or thrombin is more effective for acute gastric variceal bleeding, especially fundal varices, with better control of initial hemorrhage and lower rates of rebleeding (Fig. 13.8). Native cyanoacrylate (N-butyl-2-cyanoacrylate [Histoacryl]) is a liquid with a consistency similar to water and lends itself to intravariceal injection. On contact with a physiologic medium such as blood, it rapidly polymerizes, forming a hard substance that plugs the bleeding varix and serves as a useful treatment option. However, the rapid polymerization can block the needle or damage the endoscope. Histoacryl must be diluted with lipiodol (0.5 : 0.8 mL) to delay the polymerization reaction to complete the injection and remove the needle. The individual dosage of Histoacryl per injection should be limited to 2 mL for fundal varices. Obliteration is tested by palpating the varix with the needle retracted. If soft, the varix is injected with additional aliquots of Histoacryl; after 3 to 4 days of injection, necrosis occurs on the variceal walls, and the cast is slowly extruded. The extrusion process may last several weeks and sometimes even months.
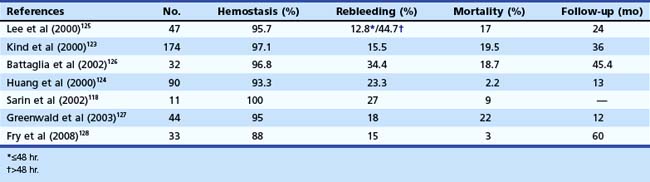
With Histoacryl, control of active bleeding from gastric varices has been reported to range from 93% to 100% of patients, and rate of rebleeding is generally less than 30%.118–125 Huang and coworkers124 reported 94% hemostasis in 90 patients with active or recent variceal bleeding from fundal varices. Tumorous gastric varices had a higher rebleeding rate than the tortuous and nodular type (34.4% vs. 17.2%). Kind and associates123 reported their 12-year experience with the use of Histoacryl in 174 patients with bleeding gastric varices. The hemostasis rate was 97.1% with an early rebleeding rate of 15.5% and a hospital mortality rate of 19.5%. Complications including chest pain and treatment-related, ulcer-induced bleeding occurred in 2.9% of patients. However, these investigators reported high rebleeding rates in patients with prehepatic block with IGV1-type varices. Table 13.6 summarizes other studies that have used Histoacryl.118,123–128
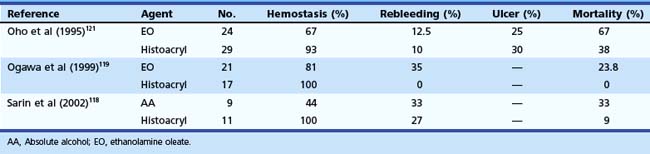
The use of glue has also been compared with other modalities.121 A large prospective, randomized trial comparing gastric variceal obturation with Histoacryl versus EVL in patients with acute gastric variceal hemorrhage showed that control of active bleeding was similar in both groups but that rebleeding over a follow-up period of 1.6 to 1.8 years occurred significantly less frequently in the GVO group (23% vs. 47%), with an average of only 1.5 sessions (range 1 to 3).129 Sarin and coworkers118 found Histoacryl to be more effective than absolute alcohol for controlling acute bleeding (89% vs. 62%) and obliteration of gastric varices (100% vs. 44%) (Table 13.7). Histoacryl has also been shown to be superior to ethanolamine.119 Combination of a sclerosing agent (ethanolamine oleate) and Histoacryl has not been found to be of added advantage.120,130 Glue injection has also been found superior to EVL of gastric varices.122 Repeat glue injection into the gastric varix to obliterate it completely may be better than injection on demand (or response to recurrent bleed).125
Table 13.7 Endoscopic Sclerotherapy versus Histoacryl Injection for Active Gastric Variceal Bleeding
Histoacryl injection has some adverse effects. Although uncommon, embolization of the adhesive into the lung, spleen, portal vein, renal vein, inferior vena cava, or brain poses a serious problem. The incidence varies from 2% to 5%; most often, the embolization is small. Because of the fear of embolization, some operators prefer to inject undiluted Histoacryl. However, the disadvantage of using undiluted adhesive is that the device channel of the endoscope may get blocked, or the injector needle may get glued into the varix.130,131 Modifications of the injection technique, such as applying a silicone coating of the injection needle and sheath and new cyanoacrylate compounds such as acrylic glue (2-octyl-cyanoacrylate), can reduce the risk of such complications. Cases of visceral fistula have also been described after glue injection.132 EUS with or without Doppler probe could be used to guide the injection of glue into gastric varices and to assess adequate obliteration. EUS could also help in assessing the paraesophageal collaterals and obliteration of perforators in esophageal variceal sclerotherapy or ligation.133
Other agents, including thrombin alone or in combination with fibrinogen (“fibrin glue”), are useful to stop excessive surface bleeding.134 Poly-N-acetyl glucosamine (P-GLc NAc) isolated from marine microalgae is a polysaccharide polymer that was shown to stop variceal hemorrhage quickly in dogs. An open trial of endoscopic gastric intravariceal injection treatment with Beriplast (fibrin sealant) enrolled 15 patients presenting with gastric variceal bleeding and followed them up to 1 month after endoscopic treatment. There was failure to control bleeding in one patient. Four patients had rebleeding after the index bleed. All patients were followed for 30 days. There were 14 patients who were discharged from the hospital after the first episode of gastric variceal bleeding. None of the patients had injection-induced complications. Beriplast injection seems to be a safe, simple, and effective endoscopic treatment in acute gastric variceal bleeding. It is undergoing further evaluation.135
Ectopic Nongastroesophageal Varices
Anorectal Varices
Anorectal varices result from collaterals between the superior hemorrhoidal vein (portal) and the middle and inferior hemorrhoidal veins (systemic). Hemorrhoids must be differentiated from anal varices; the former appear as purple, well-vascularized mucosa in the lower 4 cm of the anal canal. Varices in the anal canal appear as either discrete veins or saccular blue or slate gray swellings. Rectal varices start above the dentate line and are easy to diagnose. Anorectal varices are more common in patients with extrahepatic portal venous obstruction and noncirrhotic portal fibrosis (89%) compared with cirrhosis (56%) and are more common in patients who are bleeders than in nonbleeders.136
In contrast to esophageal varices, anorectal varices rarely bleed, but if they bleed, they often bleed massively. The incidence of bleeding from rectal varices was 1.4%, 6.7%, and 18% in three studies, with more bleeding in patients with noncirrhotic portal hypertension.136–138 For bleeding anorectal varices, endoscopic injection sclerotherapy, EVL, embolization, cryosurgery, and underrunning of the varices with sutures all have been tried but often with limited success. EVL seems to be a safe and effective therapy, but this requires further study to become the standard of care. Portosystemic shunt or TIPS is the other option if EVL fails to decompress and prevent rectal variceal bleeding.46,82
Duodenal, Jejunal, Ileal, and Colonic Varices
Duodenal, jejunal, ileal, and colonic varices are rare and are usually found incidentally at the time of endoscopy, more often in patients with extrahepatic portal vein obstruction or in cirrhotics with portal vein thrombosis. Usually the afferent vessel of the duodenal varices is the superior or inferior pancreaticoduodenal vein originating in the portal vein trunk or superior mesenteric vein. The efferent vein drains into the inferior vena cava. In a review of 169 cases of bleeding ectopic varices, 17% occurred in the duodenum, 17% in the jejunum or ileum, 14% in the colon, 8% in the rectum, and 9% in the peritoneum.139
Duodenal varices more often are secondary; that is, they appear after obliteration of esophageal varices. When a patient with portal hypertension has melena without hematemesis, the possibility of a bleeding duodenal varix should be considered. Sometimes these patients present with active upper GI bleed. Successful control of bleeding after injection of cyanoacrylate, sclerosant, or thrombin and sclerosant combination has been reported.46,139 Data regarding EVL of duodenal varices and other ectopic varices are limited. Even in patients with reasonable liver function, surgical mortality rates for treating actively bleeding duodenal varices range from 30% to 40%. Radiologic procedures such as selective embolization and TIPS may be effective but have not been extensively studied. Jejunal and ileal varices involving the small intestine are rare. They are commonly found at the anastomotic sites and in adhesions.
Patients who present with bleeding jejunal or ileal varices typically have a history of portal hypertension, prior abdominal surgery, and hematochezia without hematemesis. Small bowel varices can occur in the absence of cirrhosis when there is mesenteric or splenic vein thrombosis and even with concomitant nonbleeding esophageal varices. The common locations are ileum (47%) and jejunum (39%). Push enteroscopy and venous phase of angiography may reveal the bleeding source. Small bowel varices can also be identified by wireless capsule endoscopy and retrograde ileoscopy. Successful sclerotherapy and glue injection to treat bleeding jejunal varices have been reported.140
Stomal Varices
Biliary Varices
Biliary varices have been reported in the gallbladder and the bile ducts, more often in patients with portal vein thrombosis. Usually varices of the biliary tree are found incidentally during ultrasound, Doppler, CT scan, or endoscopic retrograde cholangiopancreatography (ERCP) imaging. Bile duct varices may produce narrowing, irregularity, and nodular extrinsic defects termed portal biliopathy on ERCP.141 Spontaneous bleeding from biliary varices is rare. Portal decompressive measures should relieve venous dilation and consequent obstructive jaundice, if present.
Portal Hypertensive Gastropathy
PHG, known earlier as congestive gastropathy, relates to gastric mucosal changes seen endoscopically in patients with portal hypertension. Mild PHG manifests as a mosaiclike pattern without discrete red spots and is unlikely to cause significant blood loss. In severe PHG, mucosal red spots become confluent and give rise to deeply erythematous areas that are susceptible to bleeding. PHG can also be seen in patients without portal hypertension and in healthy subjects.142 PHG is a dynamic condition that can progress from mild to severe and vice versa or even disappear completely. Acute bleeding from PHG is relatively rare, and bleeding from PHG is less severe than bleeding from gastroesophageal varices. After the first bleed from PHG, rebleeding seems to be very common, with reported figures of 62% and 75%. Effective treatment of PHG requires a reduction in the increased portal pressure, which can be accomplished pharmacologically by β blockers with or without nitrates, radiologically by TIPS, by surgical shunts, or by liver transplantation. Argon plasma coagulation through endoscopy was found to be effective in two of nine patients when performed every 2 to 3 weeks in one small series, but this is not an established therapy.143
Gastric Antral Vascular Ectasia
Two endoscopic manifestations of GAVE are currently recognized: (1) watermelon stomach and (2) diffuse antral vascular ectasia. The endoscopic description of watermelon stomach is diagnostic and includes the presence of longitudinal rugal folds traversing the antrum and converging on the pylorus; overlying these folds are myriad angioectasias linearly arrayed, the aggregate resembling the stripes of a watermelon. Some workers have described a more diffuse endoscopic form of GAVE (Figs. 13.9 and 13.10) that sometimes is difficult to differentiate from PHG.144 Table 13.8 outlines the differences and the management options for PHG and GAVE. Patients with GAVE present with significant occult or overt blood loss. Severe anemia is one of the most common presentations, and about 25 mL of blood is estimated to be lost per day by these lesions.145
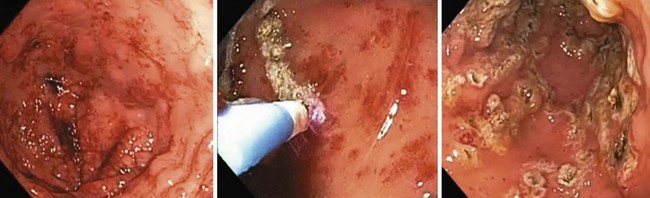
Fig. 13.9 Paintbrush technique to treat gastric antral vascular ectasia using argon plasma coagulator.
Table 13.8 Portal Hypertensive Gastropathy (PHG) versus Gastric Antral Vascular Ectasia (GAVE)
| PHG | GAVE | |
|---|---|---|
| Distribution in stomach | Proximal | Distal |
| Mosaic pattern | Present | Absent |
| Red signs | Present | Present |
| Biopsy | ||
| Thrombi | — | +++ |
| Spindle cell proliferation | + | ++ |
| Fibrohyalinosis | + | +++ |
| Treatment | β blockers | APC |
| TIPS | Transplant |
APC, Argon plasma coagulation; TIPS, transjugular intrahepatic portosystemic shunt.
Courtesy of Patrick Kamath.
The etiology of GAVE is uncertain, but it has been proposed that gastric peristalsis causes prolapse of the loose antral mucosa into the duodenum with consequent elongation and ectasia of the mucosal vessels.146,147 GAVE is more prevalent among patients with chronic liver disease but not infrequently occurs in patients without hepatic pathology. When associated with cirrhosis, the evolution to portal hypertension does not seem to be a prerequisite. Reduction in portal pressure does not improve the endoscopic appearance or bleeding propensity of GAVE. However, liver transplantation leads to rapid regression of the lesions. These features indicate that portal hypertension per se is not the sole pathogenetic mechanisms for GAVE. At the present time, treatment of GAVE is unsatisfactory because reduction of portal pressure by β blockers is of limited help. Drugs such as tranexamic acid, glucocorticoids, and estrogen-progesterone combination may help in reducing transfusion requirements, but their true efficacy remains equivocal.
Our group had reported the use of endoscopic neodymium:yttrium-aluminium-garnet (Nd:YAG) laser coagulation to be effective in 12 of 13 patients after a median period of 6 months, eliminating the need for transfusion.145 Similar experience was reported by others without complications.148 However, success in the diffuse form of GAVE is limited. Cryotherapy is used as an experimental technique, but experience is limited.149,150 Although surgical antrectomy can cure GAVE, liver transplantation is the best option.
Portal Hypertensive Enteropathy and Colonopathy
Portal hypertension–related mucosal abnormalities affecting the small intestine are less frequent with low bleeding propensity.151 Diarrhea and protein-losing enteropathy, which improved after TIPS placement, could be other manifestations of portal hypertensive enteropathy. Kozarek and coworkers152 reported that 14 (70%) of 20 cirrhotics had multiple vascular-appearing lesions (10 with cherry red spots, 6 with spider telangiectasias), 4 of whom also had endoscopic features suggesting a mild, chronic colitis, most commonly involving the right colon. Other investigators have observed colonopathy in 52% of patients with portal hypertension, more often in bleeders (52%) than in nonbleeders (12.5%).137 Portal hypertensive colonopathy is rarely a cause of significant portal hypertension–related bleeding. Colonopathy is usually diagnosed endoscopically and not histologically. Endoscopic lesions of portal hypertensive colonopathy include colonic angioectasias, mucosal abnormalities such as erythema, friability, and edema.153,154
Transjugular Intrahepatic Portosystemic Shunt in the Management of Bleeding Related to Portal Hypertension
TIPS creates a communication between the hepatic vein and an intrahepatic branch of the portal vein using an expandable metallic stent and decompresses high portal pressures. The shunt is placed by an interventional radiologist via a transjugular approach and dilated as needed to reduce the portacaval pressure gradient to less than 12 mm Hg. At the present time, coated stents are used more often to reduce the frequency of shunt stenosis. Immediate complications of TIPS placement include intraperitoneal hemorrhage, sepsis, and cardiopulmonary failure from excessive blood flow into the right heart. Early complications include shunt thrombosis or migration, hepatic encephalopathy, progressive hepatic failure, and pulmonary artery hypertension. Late complications include shunt stenosis, progressive hepatic encephalopathy, portal vein thrombosis, and heart failure. The best indicator of failure of TIPS is recurrence of GI bleeding. TIPS should be avoided in patients with a Model for End-Stage Liver Disease (MELD) score greater than 24 because these patients have a reduced survival.155,156 In patients with a MELD score of 14 or less, survival is excellent. Severe hepatic encephalopathy is a relative contraindication for TIPS.
The best method to evaluate the patency of TIPS is by hepatic venogram and measurement of the portacaval pressure gradient. TIPS has been compared with sclerotherapy in two meta-analyses, involving 811 patients and 750 patients.157,158 The median follow-up period ranged from 10 to 32 months in various trials. Both meta-analyses concluded that TIPS is more effective in preventing variceal rebleeding than endoscopic sclerotherapy (19% vs. 47%). However, because of the increased risk of posttreatment encephalopathy and the lack of improvement in survival, TIPS is not recommended as first-line treatment for preventing variceal rebleeding. A high rate of stent dysfunction requiring revision is another limitation of TIPS.
TIPS is better than the combination of ISMN and propranolol and better than endoscopic therapy in the prevention of variceal rebleeding, although at the expense of an increased risk of encephalopathy.63,64 Also, there is no increased survival benefit. A more recent trial showed that although pharmacologic (propranolol plus nitrates) therapy was less effective than TIPS in preventing rebleeding, it was associated with less encephalopathy, identical survival, and more frequent improvement in Child-Pugh class with lower costs than TIPS.159 These findings are supported further by a meta-analysis of 11 published randomized controlled trials.158 TIPS is considered a salvage therapy for patients who bleed despite adequate medical and endoscopic treatment. TIPS is very effective in the treatment of bleeding gastric varices. It has a success rate of greater than 90% for initial hemostasis and a very low rebleeding rate.160,161 A more recent trial has shown that TIPS is more effective than cyanoacrylate glue injection in preventing rebleeding; however, the rate of complications and survival was not different.162
Surgical Management of Portal Hypertensive Bleeding
When endoscopic, pharmacologic, and radiologic options are unsuccessful in stopping acute or recurrent variceal bleeding, portal system decompression created by a shunt between the portal and systemic venous circulation is considered. Portosystemic shunts can be classified as selective, nonselective, or partial depending on how much hepatic portal flow is preserved. Selective shunt—chiefly, the distal splenorenal shunt (DSRS)—involves an anastomosis between the distal splenic vein and the renal vein and interruption of all collaterals connecting the superior mesenteric and the gastrosplenic components of the portal system. Although DSRS can decrease portal pressure, it may worsen ascites from continuous mesenteric venous hypertension. It is relatively contraindicated in patients with a splenic vein diameter less than 7 mm because of a high incidence of shunt thrombosis. DSRS has been compared with nonselective shunts in several controlled trials, none of which have shown a survival advantage for either procedure.163 Encephalopathy seems to be less with DSRS compared with nonselective shunts, and DSRS can be recommended even for alcoholic patients. Survival after DSRS is higher among patients with nonalcoholic cirrhosis, however, possibly because of better preservation of hepatic portal perfusion.
Partial shunt using polytetrafluoroethylene (PTFE) is a small-diameter shunt that attempts to decompress varices while preserving hepatic portal flow. When partial PTFE shunt is combined with coronary vein ligation and division of collaterals, it offers a fixed resistance and is more likely to maintain hepatopetal portal flow.164 Nonselective shunts divert all portal blood flow and in doing so decompress the entire portal venous system. Examples of nonselective surgical shunts include end-to-side shunt, side-to-side shunt, and conventional splenorenal shunt. The end-to-side shunt, also known as Eck fistula, was compared with medical therapy in four randomized clinical trials, none of which showed a survival advantage. Patients in the shunted group had excellent control of bleeding but at the expense of increased encephalopathy. Bleeding was the most common cause of death in the medically treated group, whereas accelerated hepatic failure was the leading cause of death in the surgical group.89
Surgical options also have been proposed as secondary prophylaxis. Orozco and coworkers165 compared elective treatment of variceal hemorrhage with β blockers (propranolol), endoscopic sclerotherapy, and portal blood flow–preserving surgical procedures (selective shunts and the Sugiura-Futagawa operation). The investigators found that rebleeding rate was significantly lower in the surgical group compared with the other two groups. Survival was better for low-risk patients (Child A) in the three groups, but when the three options were compared, no significant difference was found. A randomized controlled trial compared distal splenorenal shunt with TIPS and found similar efficacy in the control of refractory variceal bleeding in Child-Pugh class A and B patients. Reintervention was significantly greater for TIPS compared with the shunt.166
1 Bosch J. The sixth Carlos E. Rubio Memorial Lecture. Prevention and treatment of variceal hemorrhage. P R Health Sci J. 2000;19:57-67.
2 Sass DA, Chopra KB. Portal hypertension and variceal hemorrhage. Med Clin North Am. 2009;93:837-853.
3 Garcia-Tsao G. Portal hypertension. Curr Opin Gastroenterol. 2006;22:254-262.
4 Cardenas A, Gines P. Portal hypertension. Curr Opin Gastroenterol. 2009;25:195-201.
5 Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351.
6 Sarela AI, Mihaimeed FM, Batten JJ, et al. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44:749-753.
7 Angus PW. Role of endothelin in systemic and portal resistance in cirrhosis. Gut. 2006;55:1230-1232.
8 Yokomori H, Oda M, Ogi M, et al. Enhanced expression of endothelin receptor subtypes in cirrhotic rat liver. Liver. 2001;21:114-122.
9 Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233-240.
10 Iwakiri Y. The molecules: Mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41(Suppl 3):S288-S294.
11 Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934.
12 Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120-1126.
13 Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology. 2006;43:S121-S131.
14 Thuluvath PJ, Yoo HY. Portal hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973-2978.
15 Perini RF, Camara PR, Ferraz JG. Pathogenesis of portal hypertensive gastropathy: Translating basic research into clinical practice. Nat Clin Pract Gastroenterol Hepatol. 2009;6:150-158.
16 Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): An update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77:131-137.
17 Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866-872.
18 Wong F, Bernardi M, Balk R, et al. Sepsis in cirrhosis: Report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725.
19 Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433.
20 Hou MC, Lin HC, Liu TT, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: A randomized trial. Hepatology. 2004;39:746-753.
21 de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. J Hepatol. 2005;43:167-176.
22 Luketic VA, Sanyal AJ. Esophageal varices. I. Clinical presentation, medical therapy, and endoscopic therapy. Gastroenterol Clin North Am. 2000;29:337-385.
23 Sarin SK, Lahoti D. Management of gastric varices. Baillieres Clin Gastroenterol. 1992;6:527-548.
24 Keeffe EB, O’Connor KW. 1989 ASGE survey of endoscopic sedation and monitoring practices. Gastrointest Endosc. 1990;36:S13-S18.
25 Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: Results of a nationwide survey. Gut. 1991;32:12-15.
26 Froehlich F, Gonvers JJ, Fried M. Conscious sedation, clinically relevant complications and monitoring of endoscopy: Results of a nationwide survey in Switzerland. Endoscopy. 1994;26:231-234.
27 Saeian K, Staff D, Knox J, et al. Unsedated transnasal endoscopy: A new technique for accurately detecting and grading esophageal varices in cirrhotic patients. Am J Gastroenterol. 2002;97:2246-2249.
28 Dumortier J, Napoleon B, Hedelius F, et al. Unsedated transnasal EGD in daily practice: Results with 1100 consecutive patients. Gastrointest Endosc. 2003;57:198-204.
29 Bosch J, Garcia-Pagan JC, Berzigotti A, et al. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362.
30 Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev. Gastroenterol Hepatol. 2009;6:573-582.
31 Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-631.
32 Garcia-Tsao G, Groszmann RJ, Fisher RL, et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424.
33 D’Amico G, Garcia-Pagan JC, Luca A, et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: A systematic review. Gastroenterology. 2006;131:1611-1624.
34 Triantos CK, Nikolopoulou V, Burroughs AK. The therapeutic and prognostic benefit of portal pressure reduction in cirrhosis. Aliment Pharmacol Ther. 2008;28:943-952.
35 de Franchis R. Non-invasive (and minimally invasive) diagnosis of oesophageal varices. J Hepatol. 2008;49:520-527.
36 Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8-14.
37 Bureau C, Metivier S, Peron JM, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268.
38 Lapalus MG, Ben Soussan E, Gaudric M, et al. Esophageal capsule endoscopy vs. EGD for the evaluation of portal hypertension: A French prospective multicenter comparative study. Am J Gastroenterol. 2009;104:1112-1118.
39 Kim SH, Kim YJ, Lee JM, et al. Esophageal varices in patients with cirrhosis: Multidetector CT esophagography—comparison with endoscopy. Radiology. 2007;242:759-768.
40 Perri RE, Chiorean MV, Fidler JL, et al. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for esophageal varices. Hepatology. 2008;47:1587-1594.
41 Wong RC, Farooq FT, Chak A. Endoscopic Doppler US probe for the diagnosis of gastric varices (with videos). Gastrointest Endosc. 2007;65:491-496.
42 Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
43 Conn HO. Ammonia tolerance in the diagnosis of esophageal varices: A comparison of endoscopic, radiologic, and biochemical techniques. J Lab Clin Med. 1967;70:442-451.
44 Westaby D, Macdougall BR, Melia W, et al. A prospective randomized study of two sclerotherapy techniques for esophageal varices. Hepatology. 1983;3:681-684.
45 Paquet KJ. Prophylactic endoscopic sclerosing treatment of the esophageal wall in varices—a prospective controlled randomized trial. Endoscopy. 1982;14:4-5.
46 Kotfila R, Trudeau W. Extraesophageal varices. Dig Dis. 1998;16:232-241.
47 Sarin SK, Kumar A. Gastric varices: Profile, classification, and management. Am J Gastroenterol. 1989;84:1244-1249.
48 North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices: A prospective multicenter study. N Engl J Med. 1988;319:983-989.
49 Merkel C, Zoli M, Siringo S, et al. Prognostic indicators of risk for first variceal bleeding in cirrhosis: A multicenter study in 711 patients to validate and improve the North Italian Endoscopic Club (NIEC) index. Am J Gastroenterol. 2000;95:2915-2920.
50 Nevens F, Bustami R, Scheys I, et al. Variceal pressure is a factor predicting the risk of a first variceal bleeding: A prospective cohort study in cirrhotic patients. Hepatology. 1998;27:15-19.
51 Casado M, Bosch J, Garcia-Pagan JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: Correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-1303.
52 Ben-Ari Z, Cardin F, McCormick AP, et al. A predictive model for failure to control bleeding during acute variceal haemorrhage. J Hepatol. 1999;31:443-450.
53 Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102.
54 McKiernan PJ. Treatment of variceal bleeding. Gastrointest Endosc Clin N Am. 2001;11:789-812.
55 Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349.
56 Sarin SK, Groszmann RJ, Mosca PG, et al. Propranolol ameliorates the development of portal-systemic shunting in a chronic murine schistosomiasis model of portal hypertension. J Clin Invest. 1991;87:1032-1036.
57 Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488.
58 Pagliaro L, D’Amico G, Sorensen TI, et al. Prevention of first bleeding in cirrhosis: A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59-70.
59 Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med. 1991;324:1779-1784.
60 Dagher L, Burroughs A. Variceal bleeding and portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 2001;13:81-88.
61 D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: An evidence-based approach. Semin Liver Dis. 1999;19:475-505.
62 Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: A randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology. 2002;123:735-744.
63 Sarin SK, Guptan RK, Jain AK, et al. A randomized controlled trial of endoscopic variceal band ligation for primary prophylaxis of variceal bleeding. Eur J Gastroenterol Hepatol. 1996;8:337-342.
64 Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology. 1997;25:1346-1350.
65 Triantos C, Vlachogiannakos J, Armonis A, et al. Primary prophylaxis of variceal bleeding in cirrhotics unable to take beta-blockers: A randomized trial of ligation. Aliment Pharmacol Ther. 2005;21:1435-1443.
66 Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
67 Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833.
68 Sarin SK, Wadhawan M, Agarwal SR, et al. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol. 2005;100:797-804.
69 Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology. 2001;33:802-807.
70 Khuroo MS, Khuroo NS, Farahat KL, et al. Meta-analysis: Endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther. 2005;21:347-361.
71 Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: Systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-2848.
72 Teran JC, Imperiale TF, Mullen KD, et al. Primary prophylaxis of variceal bleeding in cirrhosis: A cost-effectiveness analysis. Gastroenterology. 1997;112:473-482.
73 de Franchis R. Definition and diagnosis in portal hypertension—continued problems with the Baveno consensus? Aliment Pharmacol Ther. 2004;20(Suppl 3):2-6.
74 Vlavianos P, Westaby D. Management of acute variceal haemorrhage. Eur J Gastroenterol Hepatol. 2001;13:335-342.
75 Jenkins SA, Shields R, Davies M, et al. A multicentre randomised trial comparing octreotide and injection sclerotherapy in the management and outcome of acute variceal haemorrhage. Gut. 1997;41:526-533.
76 D’Amico G, Pietrosi G, Tarantino I, et al. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: A Cochrane meta-analysis. Gastroenterology. 2003;124:1277-1291.
77 Escorsell A, Ruiz del Arbol L, Planas R, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: The TEST study. Hepatology. 2000;32:471-476.
78 McCormack G, McCormick PA. A practical guide to the management of oesophageal varices. Drugs. 1999;57:327-335.
79 Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-748.
80 Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology. 1999;29:1655-1661.
81 Yol S, Belviranli M, Toprak S, et al. Endoscopic clipping versus band ligation in the management of bleeding esophageal varices. Surg Endosc. 2003;17:38-42.
82 Russo MW, Brown RSJr. Endoscopic treatment of patients with portal hypertension. Gastrointest Endosc Clin N Am. 2001;11:1-14.
83 Sarin SK, Kumar A. Sclerosants for variceal sclerotherapy: A critical appraisal. Am J Gastroenterol. 1990;85:641-649.
84 Kochhar R, Goenka MK, Mehta S, et al. A comparative evaluation of sclerosants for esophageal varices: A prospective randomized controlled study. Gastrointest Endosc. 1990;36:127-130.
85 Lo GH, Lai KH, Cheng JS, et al. Emergency banding ligation versus sclerotherapy for the control of active bleeding from esophageal varices. Hepatology. 1997;25:1101-1104.
86 Villanueva C, Piqueras M, Aracil C, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560-567.
87 Stiegmann GV, Goff JS, Michaletz-Onody PA, et al. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527-1532.
88 Avgerinos A, Armonis A, Manolakopoulos S, et al. Endoscopic sclerotherapy versus variceal ligation in the long-term management of patients with cirrhosis after variceal bleeding: A prospective randomized study. J Hepatol. 1997;26:1034-1041.
89 D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: A meta-analytic review. Hepatology. 1995;22:332-354.
90 Vargas HE, Gerber D, Abu-Elmagd K. Management of portal hypertension-related bleeding. Surg Clin North Am. 1999;79:1-22.
91 Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut. 2000;46(Suppl 3–4):III1-III15.
92 de Franchis R, Primignani M. Why do varices bleed? Gastroenterol Clin North Am. 1992;21:85-101.
93 McCormick PA, Jenkins SA, McIntyre N, et al. Why portal hypertensive varices bleed and bleed: A hypothesis. Gut. 1995;36:100-103.
94 Gournay J, Masliah C, Martin T, et al. Isosorbide mononitrate and propranolol compared with propranolol alone for the prevention of variceal rebleeding. Hepatology. 2000;31:1239-1245.
95 Hou MC, Lin HC, Kuo BI, et al. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: A prospective randomized trial. Hepatology. 1995;21:1517-1522.
96 Baroncini D, Milandri GL, Borioni D, et al. A prospective randomized trial of sclerotherapy versus ligation in the elective treatment of bleeding esophageal varices. Endoscopy. 1997;29:235-240.
97 Sarin SK, Govil A, Jain AK, et al. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: Influence on gastropathy, gastric varices and variceal recurrence. J Hepatol. 1997;26:826-832.
98 de la Pena J, Rivero M, Sanchez E, et al. Variceal ligation compared with endoscopic sclerotherapy for variceal hemorrhage: Prospective randomized trial. Gastrointest Endosc. 1999;49:417-423.
99 Wong T, Pereira SP, McNair A, et al. A prospective, randomized comparison of the ease and safety of variceal ligation using a multiband vs. a conventional ligation device. Endoscopy. 2000;32:931-934.
100 Zargar SA, Javid G, Khan BA, et al. Endoscopic ligation vs. sclerotherapy in adults with extrahepatic portal venous obstruction: A prospective randomized study. Gastrointest Endosc. 2005;61:58-66.
101 Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding: A meta-analysis. Ann Intern Med. 1995;123:280-287.
102 Karsan HA, Morton SC, Shekelle PG, et al. Combination endoscopic band ligation and sclerotherapy compared with endoscopic band ligation alone for the secondary prophylaxis of esophageal variceal hemorrhage: A meta-analysis. Dig Dis Sci. 2005;50:399-406.
103 Villanueva C, Balanzo J, Novella MT, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. N Engl J Med. 1996;334:1624-1629.
104 Villanueva C, Minana J, Ortiz J, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med. 2001;345:647-655.
105 Lo GH, Chen WC, Chen MH, et al. Banding ligation versus nadolol and isosorbide mononitrate for the prevention of esophageal variceal rebleeding. Gastroenterology. 2002;123:728-734.
106 Patch D, Sabin CA, Goulis J, et al. A randomized, controlled trial of medical therapy versus endoscopic ligation for the prevention of variceal rebleeding in patients with cirrhosis. Gastroenterology. 2002;123:1013-1019.
107 Lo GH, Lai KH, Cheng JS, et al. Endoscopic variceal ligation plus nadolol and sucralfate compared with ligation alone for the prevention of variceal rebleeding: A prospective, randomized trial. Hepatology. 2000;32:461-465.
108 Gonzalez R, Zamora J, Gomez-Camarero J, et al. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109-122.
109 Nakamura S, Mitsunaga A, Murata Y, et al. Endoscopic induction of mucosal fibrosis by argon plasma coagulation (APC) for esophageal varices: A prospective randomized trial of ligation plus APC vs. ligation alone. Endoscopy. 2001;33:210-215.
110 Hino S, Kakutani H, Ikeda K, et al. Low power diode laser treatment using indocyanine green for eradication of esophageal varices. Endoscopy. 2001;33:873-875.
111 Seewald S, Mendoza G, Seitz U, et al. Variceal bleeding and portal hypertension: Has there been any progress in the last 12 months? Endoscopy. 2003;35:136-144.
112 Cipolletta L, Bianco MA, Rotondano G, et al. Argon plasma coagulation prevents variceal recurrence after band ligation of esophageal varices: Preliminary results of a prospective randomized trial. Gastrointest Endosc. 2002;56:467-471.
113 Monici LT, Meirelles-Santos JO, Soares EC, et al. Microwave coagulation versus sclerotherapy after band ligation to prevent recurrence of high risk of bleeding esophageal varices in Child-Pugh’s A and B patients. J Gastroenterol. 2010;45:204-210.
114 Sarin SK, Jain AK, Lamba GS, et al. Isolated gastric varices: Prevalence, clinical relevance and natural history. Dig Surg. 2003;20:42-47.
115 Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: An eleven-year experience. Gastrointest Endosc. 1997;46:8-14.
116 Shiha G, El-Sayed SS. Gastric variceal ligation: A new technique. Gastrointest Endosc. 1999;49:437-441.
117 Yoshida T, Harada T, Shigemitsu T, et al. Endoscopic management of gastric varices using a detachable snare and simultaneous endoscopic sclerotherapy and O-ring ligation. J Gastroenterol Hepatol. 1999;14:730-735.
118 Sarin SK, Jain AK, Jain M, et al. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol. 2002;97:1010-1015.
119 Ogawa K, Ishikawa S, Naritaka Y, et al. Clinical evaluation of endoscopic injection sclerotherapy using N-butyl-2-cyanoacrylate for gastric variceal bleeding. J Gastroenterol Hepatol. 1999;14:245-250.
120 Miyazaki S, Yoshida T, Harada T, et al. Injection sclerotherapy for gastric varices using N-butyl-2-cyanoacrylate and ethanolamine oleate. Hepatogastroenterology. 1998;45:1155-1158.
121 Oho K, Iwao T, Sumino M, et al. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: a nonrandomized study. Endoscopy. 1995;27:349-354.
122 Lo GH, Lai KH, Cheng JS, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060-1064.
123 Kind R, Guglielmi A, Rodella L, et al. Bucrylate treatment of bleeding gastric varices: 12 years’ experience. Endoscopy. 2000;32:512-519.
124 Huang YH, Yeh HZ, Chen GH, et al. Endoscopic treatment of bleeding gastric varices by N-butyl-2-cyanoacrylate (Histoacryl) injection: Long-term efficacy and safety. Gastrointest Endosc. 2000;52:160-167.
125 Lee YT, Chan FK, Ng EK, et al. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174.
126 Battaglia G, Morbin T, Patarnello E, et al. Visceral fistula as a complication of endoscopic treatment of esophageal and gastric varices using isobutyl-2-cyanoacrylate: Report of two cases. Gastrointest Endosc. 2000;52:267-270.
127 Greenwald BD, Caldwell SH, Hespenheide EE, et al. N-2-butyl-cyanoacrylate for bleeding gastric varices: A United States pilot study and cost analysis. Am J Gastroenterol. 2003;98:1982-1988.
128 Fry LC, Neumann H, Olano C, et al. Efficacy, complications and clinical outcomes of endoscopic sclerotherapy with N-butyl-2-cyanoacrylate for bleeding gastric varices. Dig Dis. 2008;26:300-303.
129 Tan PC, Hou MC, Lin HC, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-697.
130 Binmoeller KF. Glue for gastric varices: Some sticky issues. Gastrointest Endosc. 2000;52:298-301.
131 Bhasin DK, Sharma BC, Sriram PV, et al. Endoscopic management of bleeding ectopic varices with histoacryl. HPB Surg. 1999;11:171-173.
132 Gostout CJ. What we need is a reliable plug. Am J Gastroenterol. 2002;97:1281-1283.
133 Lo GH, Lai KH, Cheng JS, et al. Prevalence of paraesophageal varices and gastric varices in patients achieving variceal obliteration by banding ligation and by injection sclerotherapy. Gastrointest Endosc. 1999;49:428-436.
134 Yang WL, Tripathi D, Therapondos G, et al. Endoscopic use of human thrombin in bleeding gastric varices. Am J Gastroenterol. 2002;97:1381-1385.
135 Kulling D, Vournakis JN, Woo S, et al. Endoscopic injection of bleeding esophageal varices with a poly-N-acetyl glucosamine gel formulation in the canine portal hypertension model. Gastrointest Endosc. 1999;49:764-771.
136 Chawla Y, Dilawari JB. Anorectal varices—their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut. 1991;32:309-311.
137 Ganguly S, Sarin SK, Bhatia V, et al. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology. 1995;21:1226-1231.
138 Goenka MK, Kochhar R, Nagi B, et al. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol. 1991;86:1185-1189.
139 Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology. 1998;28:1154-1158.
140 Getzlaff S, Benz CA, Schilling D, et al. Enteroscopic cyanoacrylate sclerotherapy of jejunal and gallbladder varices in a patient with portal hypertension. Endoscopy. 2001;33:462-464.
141 Chandra R, Kapoor D, Tharakan A, et al. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086-1092.
142 Sarin SK, Misra SP, Singal A, et al. Evaluation of the incidence and significance of the “mosaic pattern” in patients with cirrhosis, noncirrhotic portal fibrosis, and extrahepatic obstruction. Am J Gastroenterol. 1988;83:1235-1239.
143 Viggiano TR, Gostout CJ. Portal hypertensive intestinal vasculopathy: A review of the clinical, endoscopic, and histopathologic features. Am J Gastroenterol. 1992;87:944-954.
144 Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118-123.
145 Gostout CJ, Ahlquist DA, Radford CM, et al. Endoscopic laser therapy for watermelon stomach. Gastroenterology. 1989;96:1462-1465.
146 Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: The watermelon stomach. Gastroenterology. 1984;87:1165-1170.
147 Toyota M, Hinoda Y, Nakagawa N, et al. Gastric antral vascular ectasia causing severe anemia. J Gastroenterol. 1996;31:710-713.
148 Bourke MJ, Hope RL, Boyd P, et al. Endoscopic laser therapy for watermelon stomach. J Gastroenterol Hepatol. 1996;11:832-834.
149 Ripoll C, Garcia-Tsao G. Treatment of gastropathy and gastric antral vascular ectasia in patients with portal hypertension. Curr Treat Options Gastroenterol. 2007;10:483-494.
150 Clarke JO, Thuluvath PJ. Endoscopic frontiers in the field of hepatology. Minerva Gastroenterol Dietol. 2007;53:101-109.
151 Nagral AS, Joshi AS, Bhatia SJ, et al. Congestive jejunopathy in portal hypertension. Gut. 1993;34:694-697.
152 Kozarek RA, Botoman VA, Bredfeldt JE, et al. Portal colopathy: Prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192-1197.
153 Tam TN, Ng WW, Lee SD. Colonic mucosal changes in patients with liver cirrhosis. Gastrointest Endosc. 1995;42:408-412.
154 Chen LS, Lin HC, Lee FY, et al. Portal hypertensive colopathy in patients with cirrhosis. Scand J Gastroenterol. 1996;31:490-494.
155 Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871.
156 Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470.
157 Papatheodoridis GV, Goulis J, Leandro G, et al. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: A meta-analysis. Hepatology. 1999;30:612-622.
158 Luca A, D’Amico G, La Galla R, et al. TIPS for prevention of recurrent bleeding in patients with cirrhosis: Meta-analysis of randomized clinical trials. Radiology. 1999;212:411-421.
159 Escorsell A, Banares R, Garcia-Pagan JC, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: A randomized controlled trial. Hepatology. 2002;35:385-392.
160 Chau TN, Patch D, Chan YW, et al. “Salvage” transjugular intrahepatic portosystemic shunts: Gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114:981-987.
161 Barange K, Peron JM, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139-1143.
162 Lo GH, Liang HL, Chen WC, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685.
163 Jin GL, Rikkers LF. Selective variceal decompression: Current status. HPB Surg. 1991;5:1-15.
164 Collins JC, Rypins EB, Sarfeh IJ. Narrow-diameter portacaval shunts for management of variceal bleeding. World J Surg. 1994;18:211-215.
165 Orozco H, Mercado MA, Chan C, et al. A comparative study of the elective treatment of variceal hemorrhage with beta-blockers, transendoscopic sclerotherapy, and surgery: A prospective, controlled, and randomized trial during 10 years. Ann Surg. 2000;232:216-219.
166 Henderson JM, Boyer TD, Kutner MH, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: A randomized trial. Gastroenterology. 2006;130:1643-1651.