Chapter 37 Polypharmacy and pain management
OVERVIEW
Herbal medicines and other complementary therapies are currently experiencing a renewed interest and popularity in developed nations worldwide.1–3 This increased trend in usage suggests that adverse drug–herb interactions may be of significant public health consequence,4 and highlights the importance of sustained pharmacovigilance and evidence based appraisal with the concurrent use of herbs and drugs in a clinical setting.5
Statistics
The Adverse Drug Reactions Advisory Committee (ADRAC) of the Australian Therapeutic Goods Administration received their first report of an adverse drug reaction from a complementary medicine in 1979. Since this time, some 988 reports of suspected adverse drug reactions to herbal medicine have been collected in Australia. As of September 2008, there have been 205,787 reported adverse drug reactions to all forms of medicine.6 While reported statistics on documented herbal adverse drug reactions can be viewed as being comparatively low in contrast to orthodox medications, this may be a reflection of both the relative safety of complementary medicines and the under-reporting of potential drug–herb interactions to regulatory agencies.7,8 Complementary practitioners must remain assiduous in identifying and reporting suspected adverse drug reactions to the regulatory authorities.
Drug–herb interaction mechanisms
Given the multifarious array of substances currently used as medicines it is not surprising that this correlates with many complex factors that can modify drug responses. Current pharmacological opinion suggests that three heterogeneous interaction mechanisms exist: pharmacokinetic, pharmacodynamic and physicochemical models7,9 (see Figure 37.1).
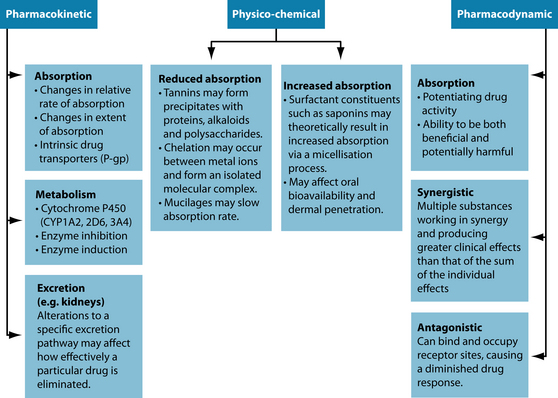
Figure 37.1 Interaction mechanisms
Source: Adapted from Brown L, cohen M. Herbs and natural supplements. 2nd edn. Marrickville Elsevier, 2007.
‘Pharmacokinetics’ is defined as the quantitative study of the absorption, distribution, metabolism and excretion of a medicine by the body (that is, the effects the body has on the medicine).10 Pharmacokinetic interactions occur when there is a modification to any of the four aforementioned processes, resulting in a change in the concentration
of drug at target tissues or receptor sites.7 Herbal medicines have the ability to modify the pharmacokinetic profile of a drug by either increasing or reducing its availability within the body and therefore modifying biochemical and physiological responses. Representative examples of pharmacokinetic interactions include the inhibition/induction of certain isoenzymes of the hepatic cytochrome P450 system (particularly 1A2, 2D6 and 3A4), or the inhibition/induction of the drug transporter P-glycoprotein (P-gp). As interactions of this model are largely unpredictable11 they are of far greater clinical concern in patients taking pharmaceutical medicines, especially those with a narrow therapeutic index (see the box above). Other factors such as age-related changes to pharmacokinetics due to physiological deterioration, individual variability, patient constitution, reduced homeostatic mechanisms, gender, body composition, pregnancy and organ function can also affect drug response,9,12–15 and should be carefully considered in the assessment of potential drug–herb interactions.16
‘Pharmacodynamics’ is concerned with the biochemical and physiological effect the drug has upon the body and includes the relationship between drug concentration and the magnitude of drug effect. Pharmacodynamic interactions transpire when one drug alters the sensitivity or responsiveness of tissues to another drug via receptor binding affinity and intrinsic activity, resulting in additive (agonistic), synergistic or antagonistic drug effects.7 These drug effects have both positive and negative clinical repercussions, with synergistic and additive interactions frequently being used to improve patient outcomes.17 Different to pharmacokinetic interactions, pharmacodynamic interactions may theoretically predictable and can be identified by analysing the therapeutic profiles and mechanisms of action of both the drugs and the herbs used.
The physicochemical model represents the interaction of substances that have conflicting physical or chemical properties. Physicochemical interactions can have a positive or negative effect upon the absorption of one or both substances and their subsequent assimilation within the body. Examples of this type of interaction extend to herbal substances high in tannins, polyphenols, mucilages and saponins,17 and also metal ions via the process of chelation. Decreases or increases in absorption based on these substances are of particular importance when narrow therapeutic index drugs and alkaloid-based herbs or medications are being used.
Challenges
The evidence available to guide naturopaths in the decision-making process for the assessment of drug–herb interactions is multifaceted and incorporates a variety of resources including human clinical trials, in vivo studies and adverse drug reaction database entries (see Table 37.1). Clinical adverse events that have been ascribed to drug–herb interactions are further complicated by such realities as botanical substitution (i.e. either intentional or accidental) of a different plant species and contamination of the herb with pharmaceutically derived medications or other non-herbal substances,4 which should be taken into consideration before jumping to conclusions. This helps separate what one study4 aptly termed true ‘interaction from overreaction’.
Table 37.1 Comparison of the evidence from studies of herb–drug interactions
| STUDY DESIGN | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Controlled clinical trial in patients |
Source: Coxeter PD, McLachlan AJ, Duke CC, Roufogalis BD. Herb–drug interactions: an evidence based approach. Current Medicinal Chemistry 2004;11:1513–1525.
With conclusive evidence to substantiate drug–herb interactions often lacking, it is usually the clinician that is left to speculate on a potential interaction between a herb and a drug.4 Current academics in the field of herbal pharmacology suggest that much greater research and funding to assess the clinical significance of drug–herb interactions is required, and that until such a database exists it is wise to closely monitor patients who are elderly, taking multiple medications,4 taking narrow therapeutic index drugs or suffering from serious diseases, conditions or disorders.
APPROACHING PATIENTS ON MEDICATIONS
Dealing with patients taking medications is one of the most challenging areas of complementary medicine practice, especially where polypharmacy is concerned.9 A methodical and systematic approach (the INQUIRE method) to prescribing complementary medicines to patients taking pharmaceutical medications is proposed by this author as being best practice (see the box below). The method was designed specifically for students of herbal and naturopathic medicine to reduce the potential for oversight and to implement foundational skills that can be honed while under supervision. The INQUIRE method is discussed in more detail below.
Investigate: All medications must be suitably researched and investigated to provide a rational foundation to identify potential interactions using appropriate pharmacological resources (e.g. MIMS). This allows the naturopath to be cognisant of
particularly problematic medications and therefore assists the selection of appropriate CAM therapies. Should the naturopath still be uncertain or need clarification, they should contact their local pharmacist for an expert opinion. Pertinent questions include:
QUICK GUIDE TO MEDICAL PRESCRIBING TERMS
q.d.: every day (quaque die, once daily)
b.i.d.: twice daily (bis in die)
t.d.s.: to be taken three times a day—used for oral medications (ter die sumendus)
t.i.d.: thrice daily—used for external medications (ter in die)
q.i.d.: four times daily (quater in die)
PAIN MANAGEMENT
Pain is a disagreeable subjective physiological and psychosocial experience that often serves a biological purpose (warning of injury).18–20 It incorporates both the perception of a painful stimulus and the response to the aforementioned perception.21 Physiologically, it can be classified into either somatogenic (organic) or psychogenic (non-organic) origin.22 Somatogenic pain occurs as a result of a direct physiological mechanism or insult (such as osteoarthritis) and can be further divided into nociceptive pain (for example, ongoing activation of visceral nociceptors) or neuropathic pain (neurological dysfunction such as nerve compression).22 Pain can further be explained by duration of effect, being either acute (lasting less than 4 weeks) or chronic (pain lasting longer than 12 weeks).
Further compounding the deleterious physiological effects of pain are social, emotional and psychological23 ramifications, which are especially prevalent in chronic pain, causing debilitating sequelae such as affective disorders and comorbid depression.22 Factors such as age, gender, race and socioeconomic status are also important to consider in pain perception.24 Pain sufferers are often impaired in their ability to initiate or maintain fundamental daily activities such as eating, shopping and cleaning,25 further isolating the patient and increasing morbidity scales (decreasing quality of life).
The perception of pain is physiologically mediated mainly by myelinated A-delta (Aδ) fibre and unmyelinated C-fibre terminal nerve endings, in combination with the neurotransmitter glutamate.18,24 In response to mechanical stimuli and subsequent chemical release at the site of injury, these nerve endings increase firing rates and cause pain by either direct stimulation or sensitising nerve endings.18 Both A-delta and C-fibres transmit electrical impulses from the peripheral nervous supply through the dorsal root and into the dorsal horn of the spinal cord.24 Myelination causes the A-delta fibres to transmit impulses extremely quickly, compared to the slower travelling unmyelinated C-fibres. Acute and sharp pain travels via the A-delta fibres in contrast to the more chronic, dull pain generally associated with C-fibre transmission.18 Other fibres, such as silent nociceptors and A-beta fibres, occur in joints and the periosteum, and can also augment the pain response.26
Our physiological understanding of the source of pain, especially in osteoarthritis, is not well understood,24 yet seems to encompass a wide range of contributing local and systemic factors. Examples of such mechanisms include the loss of hyaline articular cartilage and subsequent joint space narrowing, inflammatory mediators (such as bradykinin, histamine, prostaglandins, lactic acid, substance P, vasoactive intestinal peptide and calcitonin gene related peptide), nerve innervation, pain sensitisation and the cortical experience27 of pain.
With such a mixture of factors potentially implicated in the pain response, both orthodox and complementary management plans use multiple treatment options. This provides a unique opportunity for collaboration and integration between both systems of medicine to provide the best patient outcomes. Figure 22.3 in Chapter 22 elaborates on the current medical management of osteoarthritis to include other complementary medicine modalities and outlines the potential for this integrated relationship. Benefits of such an approach include the potential to reduce current workloads on GPs (for mild to moderate patient presentations) and lessen the side effects experienced by many patients limited to drug therapy alone.
Acupuncture
With a history of use spanning millennia, acupuncture has proven a useful alternative treatment option for pain management in a variety of conditions. Clinical evidence suggests acupuncture, in combination with primary care when required, is associated with marked clinical improvement in patients with chronic osteoarthritis-associated pain of the knee or hip.28 Improvement in WOMAC scores, increase in joint mobility and function,29,30 and quality of life improvements have all been identified through clinical studies.31 One review,32 however, suggests that acupuncture requires more evidence of effectiveness in osteoarthritis and what evidence exists is dubious.
Exercise
Reduced mobility has been correlated with an increase in osteoarthritis progression and pain. Findings33 identify high levels of evidence to support land-based therapeutic exercise as being beneficial in reducing knee pain and increasing physical function in osteoarthritis participants. The treatment effect was considered small, but was still comparable to previous reports specific to NSAID based trials.33 Further research comparing both high and low intensity aerobic exercise in osteoarthritis symptoms found similar efficacy in improving pain, aerobic capacity, gait and patient functional status between the two groups.34
Aquatic exercise does not share similar long-term symptomatic benefits in contrast to land-based exercise regimens; however, it does appear to have some beneficial short-term effects for patients with osteoarthritis of the knee or hip.35 Based on these findings, clinicians should consider encouraging patients to undergo aquatic exercise as the first stage of a longer exercise program for patients with arthritic conditions.35
Herbal medicines
Herbal medicines have a long established traditional usage in complaints such as osteoarthritis. Plasters of chilli (Capsicum spp.) have been applied topically for centuries, with modern research now identifying the proposed mechanism of action. Capsaicin, an alkaloidal constituent in the chilli plant, is able to reversibly deplete C-fibre afferent neurons of the neuropeptide substance P,36 which leads to subsequent nerve desensitisation37 in amounts of just 0.025% in topical creams. A clinical study including glyceryltrinitrate in combination with capsaicin in a topical preparation found a potentially synergistic additive effect in reducing pain,38 with participants experiencing less discomfort than capsaicin alone.
Many herb-based medicines for internal ingestion share similar positive findings. Preparations of Phytodolor (an alcohol-based formulation containing Populus tremula, Fraxinus excelsior and Solidago virgaurea),39,40 Harpagophytum procumbens,41 Zingiber officinale,41 Rosa canina41 and avocado/soy bean unsaponifiables40–42 have all shown benefit in the symptomatic management of osteoarthritis. Furthermore, apart from a long traditional history of use in managing pain and fever, Salix alba preparations have shown improvement in pain scores in osteoarthritis when standardised to salicin content.43
Traditional evidence should not be discounted. Herbs such as Eschscholzia californica, Piscidia erythrina, Corydalis ambigua, Uncaria tomentosa44 and Urtica dioica folia have a long history of use for pain and joint disorders and are worthy of inclusion in treatment plans; however, from a scientific perspective they require more clinical studies to demonstrate efficacy.
Pharmaceutical medicines
Clinical evidence obtained from Cochrane systematic reviews suggests improvements in pain associated with osteoarthritis of the knee and patient quality of life for drugs such as diacerein,45 tramadol,46 rofecoxib,47 acetaminophen,48 viscosupplementation (hyaluronan and hylan derivatives)49 and corticosteroids.50
A statistically significant difference was noted between diacerein and placebo in relation to pain measured by the visual analogue scale, with further evidence suggestive of a slowing of disease progression (in hip osteoarthritis only).45 Likewise, tramadol was shown to reduce pain intensity (a reduced WOMAC score), improve joint function and address symptomatic relief; however, the benefits were described as minimal by the research team.45
Twenty-six randomised controlled trials (RCTs) were included in the rofecoxib (Vioxx) review. Evidence indicated that rofecoxib was more effective than placebo but was associated with higher amounts of adverse events.47 Rofecoxib and valdecoxib were withdrawn from the worldwide market in 2004 due to evidence that they increased the risk of heart attack and stroke.47 As such they are no longer clinically relevant.
Fifteen RCTs met inclusion criteria for the systematic review of acetaminophen with a total of 5986 participants. Trials compared acetaminophen (paracetamol) to either placebo or NSAIDs such as diclofenac, naproxen sodium, ibuprofen and celecoxib.48 Acetaminophen was found to be superior to placebo, but less effective than NSAIDs in moderate to severe disease presentations.48
As has been demonstrated, a key detrimental factor associated with medication usage is adverse events. Side effects such as diarrhoea (42% of participants), heartburn, soft stools, stomach pain and frequent bowel movements were reported in the diacerein45 study, with nausea, vomiting, dizziness, constipation, tiredness and headache being reported with tramadol usage.46 Adverse events of tramadol often caused participants to stop taking the medication, therefore limiting medication efficacy.46 For this reason, it would be considered more beneficial to patient welfare to try complementary medicine therapy first for mild to moderate presentations of the disease before having to resort to drug therapy.
Nutraceuticals
Glucosamine is the best-studied substance out of the previously listed interventions. A randomised, placebo-controlled clinical trial with 1500 mg of glucosamine sulfate on osteoarthritis progression found that responders within the treatment group experienced significant improvement in pain via the WOMAC scale, as well as reductions in joint stiffness and limitations of physical function.51 Evidence to support this has been provided by various other research teams52–54 and a Cochrane review.55 The Cochrane meta-analysis comprised 25 RCTs with a total of 4963 patients. Results favoured glucosamine with a 22% (change from baseline) improvement in pain (SMD –0.47; 95% CI –0.72 to –0.23) and a 11% (change from baseline) improvement in function using the Lequesne index.56 Glucosamine was also considered as safe as placebo in terms of the number of adverse events experienced by participants. Some research is also supportive of glucosamine having the ability to modify joint space narrowing57 via a supposed chondroprotective activity.
Chondroitin sulfate has been shown to exhibit an anti-inflammatory activity58 and exhibit positive benefits in osteoarthritis management alone or in combination with glucosamine.59 One study suggested that this anti-inflammatory effect potentially occurs via mechanisms such as diminishing the expression of phospholipase A2 and COX-2 and also reducing concentrations of prostaglandin E2, which may reduce pain.58 Clinical trials on chondroitin sulfate have demonstrated that, at doses between 800 mg and 1 g per day, reduction in pain and improved joint function60–62 have been experienced, along with prevention of knee joint space narrowing.60,62 While chondroitin sulfate has been classified as a slow-acting chondroprotective agent for osteoarthritis, some authors feel that more conclusive evidence is required.63
The lipids derived from fish oils, rich in both eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibit anti-inflammatory activity, providing support for their use in osteoarthritis pain management. Research suggests that fish oils inhibit the formation of the cyclooxygenase products thromboxane A2 (TXA2) and prostaglandin E2 (PGE2) derived from arachidonic acid.64 Arthritic patients should be encouraged to supplement with fish oil, along with weight loss and exercise to retain muscle mass, to see if they experience any symptom amelioration.65
Methylsulfonylmethane has implied analgesic and anti-inflammatory activities,66 and is a popular arthritic supplement in the United States of America. Pilot clinical trial results suggest that methylsulfonylmethane caused statistically significant reductions in WOMAC pain and physical function impairment without notable adverse events.67 A systematic review supports the idea that methylsulfonylmethane is superior to placebo for osteoarthritis, yet also highlights the need for more rigorous clinical studies to be conducted.68
Surgical interventions
In patients with moderate to severe pain due to unicompartmental osteoarthritis of the knee, a Cochrane review based on 13 studies concluded that valgus high tibial osteotomy improved knee function and reduced pain.69 In stark contrast, a controlled trial investigating arthroscopic lavage and debridement of the knee in osteoarthritis patients versus sham surgery revealed no difference in outcome measures being experienced between the two groups.70 A systematic review of arthroscopic debridement found gold-level evidence that arthroscopic debridement provides no benefit in either mechanical or inflammatory osteoarthritis.71
Over 35,000 total knee replacement surgeries are performed in the United States of America every year.72 This surgery has numerous short- and long-term benefits, with relief in pain and improvement in joint function being experienced in over 90% of patients.73 The prosthetic replacements last for over 10 years in 90% of patients and the surgery has an incredibly low mortality rate (< 1%).73 A study pointed out that total knee replacement surgeries should not always be viewed as a last resort, but rather should be investigated when the patient’s quality of life is compromised sufficiently to make the risks of surgery worth the gain in function and reduction in pain.73
Non-surgical interventions
Numerous non-surgical interventions are currently used in the management of osteoarthritis, each with mixed outcomes. Results from a systematic review investigating the effectiveness of therapeutic ultrasound in osteoarthritis patients (n = 294) versus placebo or active therapy showed that there was no difference in range of motion, pain or gait velocity after 4 weeks of treatment.74
Viscosupplementation injections of either hyaluronan or hylan derivatives have been extensively studied in clinical trials for knee osteoarthritis. Over 76 studies on viscosupplementation met systematic review inclusion criteria, comparing against placebo (saline), intraarticular corticosteroids, NSAID usage and physical therapy.75 Results indicated that viscosupplementation has benefit in reducing pain and improving joint function, with longer therapeutic benefit than corticosteroids and fewer systemic adverse events.75
Twenty-eight trials (n = 1973) comparing intraarticular corticosteroid use against placebo, intraarticular hyaluronan/hylan derivatives, joint lavage and against other intraarticular corticosteroids were included in a systematic review.50 Outcome measures indicated that while the short-term benefits of corticosteroids such as pain reduction are well established, viscosupplementation appears more durable50 in the long-term management of osteoarthritis.
Physiotherapy
Physiotherapy is a well-recognised allied health modality that has a strong history of success in managing mild to moderate symptomatic osteoarthritis pain. Objective improvements in knee strength (47%), reduced pain (76%) and range of motion (53%) have been observed in studies using manual physiotherapy for osteoarthritis.76 Other adjunctive therapies such as balneotherapy,77 pulsed electromagnetic fields78 and transcutaneous electrical nerve stimulation (TENS)79 may also provide short-term relief and improvement in function,80 although more studies of better methodological quality are needed. Further studies investigating orthotics and braces have also shown minor benefit.81
Key treatment protocols
The above case study represents a clinical example of a patient trying to manage multiple health conditions and pain, in addition to a cocktail of pharmaceuticals. The presenting patient, currently on long-term prednisolone and salbutamol therapy, suggests she has severe and persistent asthma.82 With a general paucity of evidence-based research in herbal medicines and severe asthma, and after consultation with the patient’s GP, the following was decided:
Herbal formula
| Harpagophytum procumbens 1:2 | 50 mL |
| Zingiber officinale 1:2 | 15 mL |
| Curcuma longa 1:1 | 35 mL |
| 5 mL t.i.d./a.c. | 100 mL |
| Valeriana officinalis and Humulus lupulus combination 1 tablet (before bed) | |
| Capsaicin 0.025% topical analgesic cream (apply 3–4 times per day to affected area) | |
As the patient is being withdrawn from prednisolone, diazepam and paracetamol with codeine within the first month of treatment, statin withdrawal will be withheld until the patient is stabilised on her new management regimen. The use of Zingiber officinale and Curcuma longa in this initial treatment plan carries secondary advantages as they are known hypocholesterolaemics,94,95 which may require a reduction in statin dosage. To complement this hypocholesterolaemic activity, and to further offset the known coenzyme Q10 depletion caused by HMG-Co-reductase inhibitors,97–99 a supplement of coenzyme Q10 will also be added to this patient’s management plan. After 2 months of treatment, blood tests can be re-ordered to assess the impact of the CoQ10, and a decision made as to whether herbs such as Cynara scolymus and Allium sativum should be incorporated as the statin usage is lowered and eventually withdrawn.
Expected outcome and follow-up protocols
After 1 month since her initial consultation with a medical practitioner, the patient is expected to do well on her new asthma therapy. Blood test results and spirometry are aimed to be within acceptable limits. With good kidney function, the diuretic herb Urtica dioica folia could be included in her regimen as an infusion to increase the clearance of metabolic waste (in line with traditional naturopathic treatment principles for osteoarthritis). Good compliance is desired in all areas of diet, medication, supplementation and exercise. Both paracetamol with codeine and diazepam are aimed to be reduced or stopped (with medical consultation). Signposts of improvement are a reduction of knee pain to the point of it being manageable, and her sleep to be of better quality, with less grogginess in the mornings. A general increase in her appetite and energy levels is ideal, with increased ambulation (within the limitations of discomfort). Two months into treatment if the patient is progressing well, a reduction of rescue medication (naproxen) may be achieved. If her sleep patterns are stabilised, her valerian and hops prescriptions may be required only 3–4 nights per week. Blood tests to investigate total cholesterol, triglycerides and HDL:LDL ratio can be ordered to assess whether the herbal formulation and CoQ10 have had any effect on lipid profile. The new focus of treatment can now be concentrated around replacement of atorvastatin for her cholesterol management, building immune function and integrating supportive herbs such as Albizzia lebbeck into her asthma management.
KEY POINTS
Blumenthal M., editor. Herbal medicine: expanded Commission E monographs. Massachusetts: Integrative Medicine Communications, 2000.
Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide, 2nd edn. Marrickville: Elsevier; 2007. 91–105
Coxeter P.D., et al. Herb–drug interactions: an evidence based approach. Curr Med Chem. 2004;11:1513-1525.
ESCOP Monographs. European Scientific Cooperative on Phytotherapy, 2nd edn. Stuttgart and New York: Exeter and G. Thieme Verlag; 2003.
Mills S, Bone K. The essential guide to herbal safety. St Louis: Churchill Livingstone:50–87.
Stargrove et al., Stargrove M, et al. Herb, nutrient, and drug interactions—clinical implications and therapeutic strategies. St Louis: CV Mosby.
WHO monographs on selected medicinal plants, Vol 1. Geneva: World Health Organization, 1999.
1. MacLennan A.H., et al. The escalating cost and prevalence of alternative medicine. Prev Med. 2002;35:166-173.
2. Thomas K., et al. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med. 2001;9:2-11.
3. Eisenberg D., et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575.
4. Coxeter P.D., et al. Herb–drug interactions: an evidence based approach. Curr Med Chem. 2004;11:1513-1525.
5. Patel J., Gohil K.J. Herb–drug interactions: a review and study based on assessment of clinical case reports in literature. Indian J Pharmacol. 2007;39(3):129-139.
6. Personal communication, 2008. Adverse Drug Reaction Advisory Committee: Canberra.
7. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide, 2nd edn. Marrickville: Elsevier; 2007.
8. Farnsworth N. Relative safety of herbal medicines. HerbalGram. 1993;29:36.
9. Bryant B., et al. Pharmacology for health professionals. In Marrickville NSW Australia. Elsevier; 2003.
10. Mills S., Bone K. Principles and practice of phytotherapy. In London UK. Churchill Livingstone; 2000.
11. Mills S., Bone K. The essential guide to herbal safety. St Louis: Churchill Livingstone; 2005. 684
12. Schmucker D. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging. 2001;18(11):837-851.
13. Bressler R., Bahl J.J. Principles of drug therapy for the elderly patient. Mayo Clin Proc. 2003;78(12):1564-1577.
14. Klotz U. Effect of age on pharmacokinetics and pharmacodynamics in man. Int J Clin Pharmacol Ther. 1998;36(11):581-585.
15. Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol. 2003;38(8):843-853.
16. Mallet L., et al. A prescribing in elderly people 2: the challenge of managing drug interactions in elderly people. Lancet. 2007;370(9582):185-191.
17. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide. Marrickville: Elsevier Australia; 2005.
18. Kumar P., Clark M., editors. Clinical medicine, 6th edn, Edinburgh: Elsevier Saunders, 2005.
19. Fauci A.S., et al, editors. Harrison’s principles of internal medicine, 17th edn, New York: McGraw Hill Medical, 2008.
20. Tortora G.J., Derrickson B., editors. Principles of anatomy and physiology. New York: John Wiley & Sons, Inc, 2009.
21. Thomas C.L. Pain. In: Thomas C.L., Taber C.W., editors. Taber’s cyclopedic medical dictionary. Philadelphia: F. A. Davis Company, 1993.
22. Beers M.H., Berkow R., editors. The Merck manual of diagnosis and therapy, 17th edn, Whitehouse Station New York: Merck Research Laboratories, 1999.
23. Keefe F.J., et al. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. J Consult Clin Psychol. 2002;70(3):640-655.
24. Hunter D.J., et al. The symptoms of osteoarthritis and the genesis of pain. Med Clin North Am. 2008;93(1):83-100.
25. Katz P.P. The impact of rheumatoid arthritis on life activities. Arthritis Care Res. 1995;8:272-278.
26. Schaible H., et al. Pathophysiology and treatment of pain in joint disease. Adv Drug Deliv Rev. 2006;58(2):323-342.
27. Treede R.-D., et al. The cortical representation of pain. Pain. 1998;79:105-111.
28. Witt C.M., et al. Acupuncture in patients with osteoarthritis of the knee or hip: a randomized, controlled trial with an additional nonrandomized arm. Revista Internacional de Acupuntura. 2007;1(1):40-41.
29. Witt C, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 366(9480):136–143.
30. Berman B.M., et al. The evidence for acupuncture as a treatment for rheumatologic conditions. Rheum Dis Clin North Am. 2000;26(1):103-115.
31. Ezzo J., et al. Acupuncture for osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2001;44(4):819-825.
32. Ernst E. Acupuncture: what does the most reliable evidence tell us? J Pain Symptom Manage. 2009;37(4):709-714.
33. Fransen M., McConnel S..Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008(4):1-133.
34. Brosseau L., et al. Intensity of exercise for the treatment of osteoarthritis. Cochrane Database Syst Rev. 2003;2:1-16.
35. Bartels E., et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2007;4:1-47.
36. Rains C., Bryson H.M. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7(4):317-328.
37. Hayman M., Kam P.C. Capsaicin: a review of its pharmacology and clinical applications. Curr Anaesth Crit Care. 2008;19(5–6):338-343.
38. McCleane G. The analgesic efficacy of topical capsaicin is enhanced by glyceryl trinitrate in painful osteoarthritis: a randomised, double blind, placebo controlled study. Eur J Pain. 2000;4:355-360.
39. Ernst E., Chrubasik S. Phyto-anti-inflammatories. A systematic review of randomized, placebo-controlled, double-blind trials. Rheum Dis Clin North Am. 2000;26:13-27.
40. Hochberg M. Non-conventional treatment of osteoarthritis by herbal medicine. Osteoarthritis Cartilage. 2008;16(Suppl 1):S2-S4.
41. Chrubasik J., et al. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother Res. 2007;21:675-683.
42. Little C., et al. Herbal therapy for treating osteoarthritis. Cochrane Database Syst Rev. 1, 2000. CD002947
43. Schmid B., et al. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: randomized, placebo-controlled, double blind clinical trial [translated from German]. Z Rheumatol. 2000;59:314-320.
44. Hardin S. Cat’s claw: an Amazonian vine decreases inflammation in osteoarthritis. Complement Ther Clin Pract. 2007;13:25-28.
45. Fidelix T., et al. Diacerein for osteoarthritis. Cochrane Database Syst Rev. 1, 2006. CD005117
46. Cepeda M., et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 3, 2006. CD005522
47. Garner S., et al. Rofecoxib for osteoarthritis. Cochrane Database Syst Rev. 1, 2005. CD005115
48. Towheed T., et al.Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006(1):1-76.
49. Bellamy N., et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2, 2005. CD005321
50. Bellamy N., et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2, 2006. CD005321
51. Reginster J.Y., et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo-controlled clinical trial. Lancet. 2001;357:251-256.
52. Pavelka K., et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomised, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113-2123.
53. Bruyere O., et al. Correlation between radiographic severity of knee osteoarthritis and future disease progression. Results from a 3-year prospective, placebo-controlled study evaluating the effect of glucosamine sulfate. Osteoarthritis Cartilage. 2003;11:1-5.
54. Anon. Glucosamine sulfate. Altern Med Rev. 1999;4(3):193-195.
55. Towheed T., et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2, 2005. CD002946
56. Towheed T., et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;1:1-76.
57. Pavelka K., et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomised, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113-2123.
58. Iovu M., et al. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 2008;16(Suppl 3):S14-S18.
59. Clegg D., et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808.
60. Uebelhart D., et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthritis Cartilage. 2004;12(4):269-276.
61. Mazieres B., et al. Chondroitin sulfate in osteoarthritis of the knee: a prospective, double blind, placebo controlled multicenter clinical study. J Rheumatol. 2001;28(1):173-181.
62. Uebelhart D., et al. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarthritis Cartilage. 1998;6(Suppl A):39-46.
63. Distler J., Anguelouch A. Evidence-based practice: review of clinical evidence on the efficacy of glucosamine and chondroitin in the treatment of osteoarthritis. J Am Acad Nurse Pract. 2006;18:487-493.
64. Zurier R.R., RG. Botanical and marine oils for treatment of arthritis. In: Watson R., editor. Complementary and Alternative Therapies and the Aging Population. USA: Elsevier, 2009. Chapter 1:1–14.
65. Rayman M., Pattison D.J. Dietary manipulation in musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2008;22(3):535-561.
66. Debi R., et al. The role of MSM in knee osteoarthritis: a double blind, randomized prospective study. Osteoarthritis Cartilage. 2007;15(Suppl 3):C231.
67. Kim L., et al. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage. 2006;14(3):286-294.
68. Brien S., et al. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2008;16(11):1277-1288.
69. Brouwer R.W., et al. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev. 3, 2007. CD004019
70. Moseley J., et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
71. Laupattarakasem W., et al.Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008(1)::1-48.
72. Healthcare Cost and Utilisation Project. Agency for healthcare research and quality. Rockville Maryland USA. 2002.
73. Katz J. Total joint replacement in osteoarthritis. Best Pract Res Clin Rheumatol. 2006;20(1):145-153.
74. Welch V., et al.Therapeutic ultrasound for osteoarthritis of the knee. Cochrane Database Syst Rev. 2001(3):1-17.
75. Bellamy N., et al. Viscosupplementation for the treatment of ostearthritis of the knee. Cochrane Database Syst Rev. 2, 2005. CD005321
76. Marks R., Cantin D. Symptomatic osteoarthritis of the knee: the efficacy of physiotherapy. Physiotherapy. 1997;83(6):306-312.
77. Verhagen A., et al. Balneotherapy for osteoarthritis. Cochrane Database Syst Rev. 4, 2003. CD000518
78. Hulme J., et al. Electromagnetic fields for the treatment of osteoarthritis. Cochrane Database Syst Rev. 1, 2002. CD003523
79. Osiri M., et al. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database Syst Rev. 4, 2000. CD002823
80. Fransen M. When is physiotherapy appropriate? Best Pract Res Clin Rheumatol. 2004;18(4):477-489.
81. Brouwer R., et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 1, 2005. CD004020
82. Beers M.H., Berkow R., editors. The Merck manual of diagnosis and therapy, 17th edn, Whitehouse Station New Jersey: Merck Research Laboratories, 1999.
83. McPherson J., editor. Manual of use and interpretation of pathology tests. Sydney: The Royal College of Pathologists of Australasia, 1997.
84. Kumar P., Clark M., editors. Clinical medicine, 6th edn, Edinburgh: Elsevier, 2005.
85. Adams N.P., et al. Beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 4, 2004. CD002738
86. Otulana B.A., et al. High dose nebulized steroid in the treatment of chronic steroid-dependent asthma. Respir Med. 1992;86(2):105-108.
87. Adams N., et al. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database Syst Rev. 1, 2001. CD000195
88. Adelroth E., et al. High dose inhaled budesonide in the treatment of severe steroid-dependent asthmatics. A two-year study. Allergy. 1985;40(1):58-64.
89. Abdullah A.K., Khan S. Evidence-based selection of inhaled corticosteroid for treatment of chronic asthma. J Asthma. 2007;44(1):1-12.
90. Cates C.J., Cates M.J. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 3, 2008. CD006363
91. Boulet L.P., et al. Effects of long-term use of high-dose inhaled steroids on bone density and calcium metabolism. J Allergy Clin Immunol. 1994;94(5):796-803.
92. Blumenthal M., editor. Herbal medicine: expanded Commission E monographs. Newton: Integrative Medicine Communications, 2000.
93. WHO monographs on selected medicinal plants, Vol 1. Geneva: World Health Organization; 1999.
94. Barnes J., et al. Herbal medicines, 3rd edn. London: Pharmaceutical Press; 2007.
95. ESCOP Monographs, 2nd edn. Exeter: European Scientific Cooperative on Phytotherapy; 2003.
96. Mills S., Bone K. Principles and practice of phytotherapy. London: Churchill Livingstone; 2000. 602
97. Bargossi A.M., et al. Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG-CoA reductase inhibitors. Mol Aspects Med. 1994;15:s187-s193.
98. Folkers K., et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. 1990;87(22):8931-8934.
99. Mortensen S.A., et al. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18:s137-s144.