Chapter 20 Polycystic ovarian syndrome
CLASSIFICATION AND AETIOLOGY
Polycystic ovarian syndrome (PCOS) is a term to describe a constellation of clinical and biochemical features. For many of these the aetiology remains poorly understood. Several factors also preclude difficulties in diagnosis of PCOS, including a heterogeneous range of symptoms that can change over time and the lack of a precise and uniform consensus on diagnosis. In 2003 a consensus workshop indicated PCOS to be present if two out of three criteria are met: oligoovulation and/or anovulation, excess androgen activity (as determined by elevated free androgen index) and polycystic ovaries (by gynaecological ultrasound); and other endocrine disorders such as hyperprolactinaemia are excluded.1 Elevated fasting insulin or high insulin levels in glucose tolerance tests may also be used to suggest diagnosis. Other blood tests may be suggestive but not diagnostic, for example if the LH:FSH ratio is greater than 1:1, or if there are low levels of sex hormone binding globulin. The presence of ovarian cysts does not automatically imply a diagnosis of PCOS. The prevalence of PCOS is thought to be between 5 and 10% of women and is one of the main causes of infertility in Western women.2,3
The symptoms of PCOS usually appear upon menarche, and are associated with early puberty brought about by early secretion of androgens.4 This may also be associated with low birth weight. However, the condition can develop a considerable time after menarche in the presence of other environmental factors such as weight gain and subsequent insulin resistance.
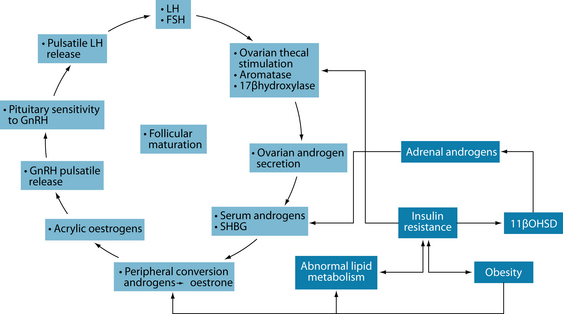
Increased ovarian androgen biosynthesis in the polycystic ovary syndrome results from abnormalities at all levels of the hypothalamic–pituitary–ovarian (HPO) axis. Androgen excess in women with PCOS may be of either ovarian or adrenal origin. It is also postulated that insulin may induce overactivity of 11b-hydroxysteroid dehydrogenase, resulting in excessive adrenal androgen production.5 Androgens may be converted to oestrone in fatty tissue, causing blood oestrone and ultimately stimulating LH production, which triggers ovarian androgen production. Increased frequency of luteinising hormone (LH) pulses in PCOS may result from an increased frequency of hypothalamic gonadotrophin-releasing hormone (GnRH) pulses, resulting in higher production of LH compared with follicle-stimulating hormone (FSH).The increase in pituitary secretion of LH can lead to an increase in androgen production by ovarian theca cells. Increased efficiency in the conversion of androgenic precursors in theca cells leads to enhanced production of androstenedione, which can then be converted by 17b-hydroxysteroid dehydrogenase (17bOHSD) to form testosterone or aromatised by the aromatase enzyme to form oestrone, which can be further converted to oestradiol by 17bOHSD (see Figure 20.1).5
Insulin acts synergistically with LH to enhance androgen production. Insulin also inhibits hepatic synthesis of sex hormone-binding globulin (which ordinarily binds to testosterone) and therefore increases the proportion of testosterone that is biologically available. Testosterone inhibits and oestrogen stimulates hepatic synthesis of sex hormone-binding globulin.5
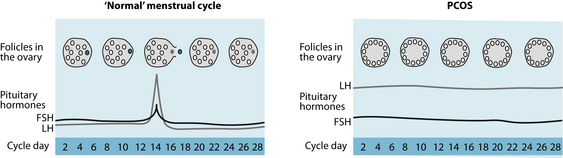
Polycystic ovaries in PCOS develop when the ovaries are stimulated to produce excessive amounts of androgens—particularly testosterone—through the release of excessive luteinising hormone (LH) by the anterior pituitary gland or through high levels of insulin in the blood.6 This causes the follicle to begin maturation but the lack of LH surge results in anovulation, meaning the ovum does not release, and ultimately a cyst is formed (see Figure 20.2).
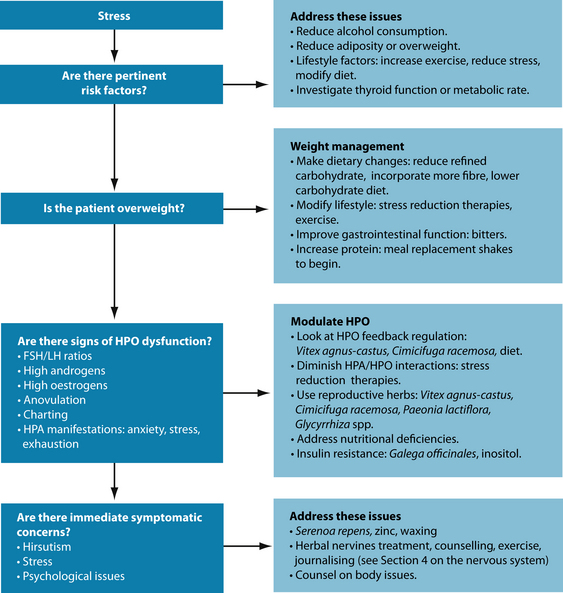
RISK FACTORS
A majority of, though not all, patients in Western settings with PCOS have insulin resistance and are often overweight.7 Elevated insulin levels contribute to or cause the abnormalities seen in the hypothalamic–pituitary–ovarian (HPO) axis that lead to PCOS. Hyperinsulinaemia may increase GnRH pulse frequency, LH dominance over FSH, increased ovarian androgen production, decreased follicular maturation, and decreased SHBG binding—all of which can lead to the development of PCOS.5 Insulin resistance is a common finding among patients of normal weight as well as overweight patients.
It is easy to view the typical PCOS patient with insulin hypersensitivity as the conventional overweight type. However, insulin resistance is also common in lean women with PCOS.8 It is also easy to view PCOS as a primarily androgen-dependent disorder; however, oestrogen dominance is also commonly present in women with PCOS. Although weight loss is generally associated with good clinical effect and being overweight is common in women with PCOS, it should never be assumed that women with PCOS will always be overweight. This trend should be noted to prevent the misdiagnosis of the condition in women who are not overweight.
Thyroid problems may make PCOS symptoms worse9 and women with PCOS also have a high prevalence of autoimmune thyroid conditions.10 Thyroid function should therefore also be checked in patients with PCOS (see Chapter 17 on thyroid abnormalities).
Liver support may also be required in women with PCOS. Approximately 30% of women with PCOS have raised liver enzymes, and diabetes and insulin resistance also increase the risk of non-alcoholic liver disease.11,12 Hyperinsulinaemia may inhibit the production of SHBG in the liver.
CONVENTIONAL TREATMENT
Due to the functional nature of diagnosis, conventional treatment in PCOS is generally focused on a number of goals. These may include reducing hyperinsulinaemia; restoring normal menstruation, reproductive function and fertility; and reducing associated symptoms such as hirsutism.13
If pregnancy is desired, assisted reproductive techniques, including radical drug therapy with clomiphene or a similar agent, or in vitro fertilisation techniques, are also commonly prescribed (see Chapter 31 on fertility, preconception care and pregnancy).13
NATUROPATHIC DIAGNOSIS TECHNIQUES
Charting
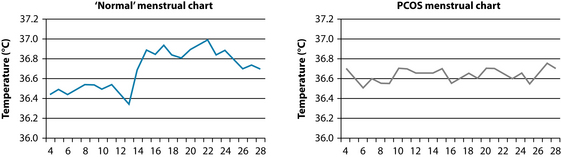
Due to the often significant timeframes encountered when treating patients with PCOS, tools such as charting may be employed to observe hormonal status changes over time. Menstrual cycle charting based on basal body temperature (BBT) has been traditionally used in naturopathic practice to ascertain changes in levels of hormone levels in reproductive females (see Figure 20.3). It is thought to reflect the menstrual cycle in two ways: (1) within 1–2 days before LH surge there should be a low point in BBT; and (2) following ovulation women should generally see a sustained increase in BBT of between 0.2 and 0.5°C.14 This rise is most likely due to the thermogenic effect of a metabolite of progesterone.
It should be noted that focusing on individual components of charting is very often inadequate.14 However, when integrated they can form a useful clinical tool that provides a broad overview of reproductive function and actively involves patients in their treatment. Despite the fact that more specific and accurate investigations currently exist, charting may still have a role to play in clinical practice as it is inexpensive, non-invasive and generally reliable. It also actively involves the patient in the therapeutic and diagnostic process. However, interpretation can be subjective and difficult and in more complex cases other investigative techniques may be more appropriate.
Consistently low temperature (under 36.5°C) may indicate a hypothyroid condition, which may play a role in poor reproductive function (see Chapter 17 on thyroid abnormalities).
Progesterone
Adequate progesterone levels will result in a sustained, noticeable and prompt rise in BBT. A small or unsustained rise with dips in temperature will be indicative of poor progesterone levels. However, temperature levels alone do not confirm appropriate levels of progesterone. The luteal phase needs to be at least 11 days to indicate adequate progesterone levels. Progesterone levels need to peak approximately 7 days after ovulation to ensure adequate uterine lining for implantation.
KEY TREATMENT PROTOCOLS
Follicle stimulating hormone and luteinising hormone regulation
Some herbal medicines may affect levels of FSH and LH and may therefore proffer some benefit in the treatment of PCOS. Despite its status as a phytoestrogen, Cimicifuga racemosa does not seem to affect the release of prolactin and FSH, though it does reduce LH, in the limited research currently conducted.15 This central effect is thought to be due to its role on dopaminergic regulation of reproductive hormones rather than its effects on oestrogen receptors.16,17 The British Herbal Pharmacopeia lists C. racemosa’s main actions as being useful in ovarian dysfunction and ovarian insufficiency.18
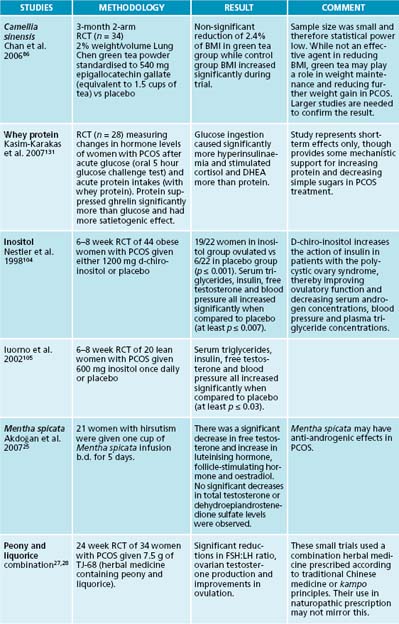
Vitex agnus-castus has had conflicting results, with some studies showing no change in FSH or LH, and another suggesting increased LH release.19–21 V. agnus-castus is thought to have antiandrogenic properties. Humulus lupulus also reduces LH with continued use and may therefore be useful to reduce androgens in PCOS.22 A review of soy studies suggests that soy consumption has no effects on FSH or TSH.23
Unpublished (though publicly available) data suggest that supplementation with the herb Tribulus terrestris for 3 months may normalise ovulation and result in pregnancy in women with endocrine infertility.24 Mentha spicata (via tea therapy—2 cups per day for 5 days) use has been associated with increases in LH, FSH and oestrogen levels in women with PCOS.25
Glycyrrhiza glabra is a commonly used herb in the treatment of PCOS. Though trials for its stand-alone treatment in PCOS are lacking, there exists a theoretical basis behind its use. G. glabra has been demonstrated to reduce testosterone levels in healthy women.26 Various forms of Glycyrrhiza has been used in a combination product (as the key ingredient with Paeonia lactiflora) in the treatment of PCOS in some small trials that showed reduction in FSH:LH ratio, ovarian testosterone production and improvements in ovulation.27,28 However, the situation in the combination product has been confused further by the fact that while this research has focused on G. uralensis clinical application is still dominated by G. glabra. The clinical effects of these minor differences are unknown. G.racemoza may also exert further potent phytoestrogenic activity independent of these effects.29 Glycyrrhiza glabra is also demonstrated to reduce body fat mass in normal weight subjects.30 A Paeonia lactiflora and Glycyrrhiza glabra combination has been found to have numerous effects on PCOS, including regulating FSH:LH ratios (possibly through stimulation of pituitary dopamine receptors),27 lowering testosterone levels and improving oestradiol to testosterone ratios.28,31
Exogenous melatonin has been demonstrated to enhance LH secretion, LH pulse amplitude and LH sensitivity to GnRH.32–34 This was thought to be the result of melatonin supplementation mimicking the effects of PCOS—possibly due to the effects of melatonin on increasing cortisol levels. This effect is known to increase in hypo-oestrogenic states.35 However, while most studies have been in postmenopausal or older women, melatonin supplementation in younger women has been associated with return to menstrual regularity, as well as the reduction of LH levels in younger women with high baseline levels, in clinical settings.36,37 This has led to calls for a possible role of melatonin in the treatment of PCOS and, despite its popularity in some streams of naturopathic medicine, it remains unclear at this stage what role it may play; further research is required.38 Other factors known to affect melatonin levels should also be considered (see Chapter 14 on insomnia).
Other factors may also affect LH levels. Various human studies have suggested that inadequate nutrition or short periods of fasting reduces LH pulsing, though not always LH levels.39–41 This is thought to be due to relative increases in cortisol caused by these states.42 Animal studies seem to suggest that increased endotoxin load can reduce plasma LH levels.43
HPO and hypothalamic–pituitary–adrenal (HPA) axis interaction
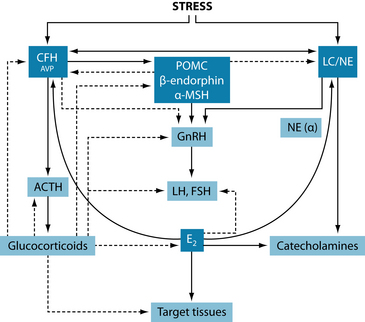
It is well known that women with PCOS exhibit abnormalities in cortisol metabolism as well as higher levels than controls.44 It is also known that a return to states of normal cortisol from high cortisol level in women with amenorrhoea or menstrual disturbances often precedes the resumption of normal ovarian activity.45 These findings further support the role for stress reduction in regulating LH and FSH function in the treatment of reproductive disorders such as PCOS.
The reproductive axis is inhibited at all levels by various components of the HPA axis. Corticotrophin-releasing hormone (CRH) can either directly or indirectly (through β-endorphin) suppress gonadotrophin-releasing hormone. Glucocorticoids may also exert inhibitory effects by rendering target tissues resistant to reproductive hormones, inhibiting GnRH and LH secretion and inhibiting ovarian oestrogen and progesterone biosynthesis.5 The effects of the HPA axis interaction with the female reproductive system can result in idiopathic or hypothalamic amenorrhoea (for example, that associated with stress, depression, anxiety or eating disorders) in its own right, or result in the hypogonadism associated with Cushing’s syndrome,5 though it may also result in further indirect complication of other disorders of hormonal dysregulation like PCOS.
However, these interactions can also be bidirectional. Corticotrophin-releasing hormone, for example, is regulated to some extent in reproductive tissue by oestrogen. Corticotrophin-releasing hormone is responsible for a number of functions in reproductive tissue (see Table 20.1), and disorders or events (such as chronic stress adaptation) that affect these levels may also have clinically relevant effects on reproductive function. Due to this bidirectional activity, a multifaceted approach to disorders, focusing on regulation of both reproductive and adrenal hormones, may be more successful in conditions such as PCOS rather than targeting one system alone, particularly considering PCOS may often be a disorder of oestrogen excess (via adipose tissue) as well as androgen excess. For this reason, generalised hormone-balancing protocols may also be beneficial in PCOS. Further information on balancing reproductive hormones can be found in Chapter 19 on endometriosis.
Table 20.1 Reproductive corticotrophin-releasing hormone, potential physiological roles and potential pathogenic effects46
| REPRODUCTIVE CRH | POTENTIAL PHYSIOLOGICal ROLES | POTENTIAL PATHOGENIC EFFECTS |
|---|---|---|
| Ovarian CRH |
Weight management
Although not all people presenting with PCOS are overweight, in those that are, weight loss is an essential part of PCOS treatment. Not only can realistic weight loss result in dramatic improvement in the condition, but being overweight can also make treatment less effective.47,48 Weight loss also proffers more effectiveness than current medication
for insulin resistance and related disorders.49 Specific individual pharmacotherapy of any kind—including that of dietary and herbal supplements—is generally clinically ineffective in weight loss in the insulin-resistant individual50 and therefore therapy should focus on an integrated approach to weight management.
As little as 2–5% reduction in weight can be enough to improve metabolic and reproductive indices in women with PCOS.51 This modest improvement can restore ovulatory function and improve insulin sensitivity by over 70%.52 A 5–10% reduction in weight can reduce central fat stores by 30% and weight loss also increases SHBG concentration, reduces testosterone concentration and androgenic stimulation of the skin (resulting in reduction in hirsutism), improves menstrual function and conception rates and reduces miscarriage rates in women with PCOS.53–59
High protein diets are typically associated with excellent weight loss results in insulin-resistant and PCOS women.60,61 Although higher-protein or lower-carbohydrate diets are often successful in weight loss in women with PCOS, this weight loss may not automatically equate to improvements in insulin parameters or ovarian function.62 However, Mediterranean-style diets have been associated with both weight loss and improved insulin parameters in both generic insulin resistant and PCOS patients.63,64
An Israeli study comparing three common diets—low-fat, low-carbohydrate and Mediterranean—found that all diets were effective.65 However, the low-carbohydrate and Mediterranean diets appeared to be more effective than the low-fat diet over 2 years. This suggests that dietary interventions may best be individualised to patient needs rather than protocol-driven.
Long-term modest weight loss is far more important in PCOS than acute weight loss. In fact drastic weight loss in women with PCOS may have negative effects on reproductive function.66 Patient compliance may be improved in low-carbohydrate or higher-protein diets compared to low-fat diets. This is evidenced by a systematic review of dietary interventions that suggested that participant attrition was higher in low-fat diet
clinical trials.67 Patients who have consistent eating habits were more likely to maintain their desired weight than those who follow stricter, but variable, protocols or gave themselves more flexibility during holidays.68
Diets higher in mono-unsaturated and polyunsaturated fats did not result in higher weight regain after crash dieting than low-fat diets and also resulted in significantly better lipid and insulin profiles.69 Studies of low-carbohydrate diets in patients with blood sugar dysregulation have also routinely demonstrated improvements in blood sugar and insulin profiles.70–74 There appears to be no negative long-term consequences in insulin sensitivity in these diets.75 However, composition of diets may be important, whereas saturated fats can induce insulin resistance, and mono-unsaturated fats can improve insulin sensitivity.75
Although short-term trials do not seem to indicate macronutrient composition is as important as caloric restriction in short-term weight loss in women with PCOS,62,76 the improved longer-term outcomes and compliance suggest a role for Mediterranean-style or high-protein dietary changes. However, rather than advocating drastic low-carbohydrate measures in diet, a more prudent approach is to increase protein, which can improve satiety and reduce carbohydrate intake by default.
However, it is not just a reduction in carbohydrate that is required. Changes in the types of carbohydrate consumed—shifting towards complex rather than simple carbohydrates—can also improve outcomes in weight management,77 as can increasing fibre and separation of carbohydrate intake from protein intake.78,79 Eating breakfast was also associated with successful weight maintenance.68,80
Care needs to be particularly taken with many foods advertised as ‘low fat’ or similar as these may often be high in sugar or other carbohydrates to compensate for lost taste advantage. Patients need to be adequately counselled on how to identify appropriate dietary additions. Care also needs to be taken when advising particular supplements for weight loss for patients with PCOS. Very few supplements have demonstrated success in weight loss despite many manufacturers making unsubstantiated claims. 5-hydroxy-tryptophan supplementation, for example, has normalised eating patterns in obese patients by reducing their intake of fat, energy and carbohydrate even when diets during the studies were unrestricted.81–84 There is also some evidence to suggest that green tea (from Camellia sinensis) can raise metabolic rates, increase thermogenesis, speed up fat oxidation and improve insulin sensitivity and glucose tolerance.85–88 Its effects on insulin sensitivity and weight loss may be related to the combination of catechins and caffeine,89 though there is speculation that its association with weight management is thought to be related to its caffeine content alone.90 Milk can reduce the glucose tolerance actions of tea by up to 90%.91 However, these should not be relied upon as primary treatments and rather should be adjuncts to a diet and lifestyle modification prescription.
Dietary counselling and exercise may result in a trend towards normalisation of hormone levels (as observed by LH:FSH ratio) in PCOS patients, even in the absence of weight loss.92 In overweight, infertile women with amenorrhoea or anovulation, 12 weeks of exercise and diet therapy were also associated with development of menstrual regularity and normalisation of E1:E2 ratio (see Chapter 19 on endometriosis).93
The role of leptin
Leptin is a hormone secreted by adipocytes and regulates body weight via its effects on metabolism and satiety. However, levels of this hormone appear to be related to the overweight status of the patient. Leptin levels are higher in overweight women with and without PCOS.94,95 Leptin also acts directly on ovarian function through specific receptors.96–99 Generally, leptin inhibits the hypothalamic–pituitary–adrenal axis and stimulates the reproductive system, which may result in ovarian over-production of androgens.
Impaired postprandial cholecystokinin (CCK) secretion, possibly associated with increased levels of testosterone, may also play a role in the greater frequency of binge eating and being overweight in women with PCOS.100 The hormone ghrelin—implicated in appetite regulation—was found to be lower in PCOS women than in controls, indicating alterations in satiety.101 In overweight women with PCOS a dysfunctional leptin resistance may be observed and this may be one reason weight loss is a successful intervention in PCOS.102,103
Insulin resistance
The treatment of insulin resistance is covered in more depth in Chapter 16 on diabetes type 2, though some advances have been made specifically in relation to PCOS. Inositol has the ability to increase ovulation and reduce hyperandrogenism in women with PCOS, including those without weight issues, as well as improving insulin parameters.104–107 Bitters are traditionally used by naturopaths to both improve digestive function and regulate blood sugar levels.108 Vinegar (for example, apple cider vinegar) may be particularly helpful to reduce postprandial blood glucose levels and improve insulin sensitivity.109–112
Galega officinales is traditionally used to treat insulin resistance and contains chromium salts as well as guanidines.113,114 Early research established hypoglycaemic activity in these guanidine compounds.18,115 The drug metformin, used successfully in conventional treatment of PCOS, is a synthetic guanidine derivative. Other herbs that may show promise in the treatment of insulin resistance more generally include Momordica indica, Gymnema sylvestre and Aloe vera.116 Interventions useful in insulin resistance are covered in more depth in Chapter 16 on diabetes type 2.
Chromium was found to improve insulin resistance parameters, though not ovulation, in women with PCOS.117,118 Insulin resistance may be associated with higher aromatase activity.119 This is explored further in Chapter 19 on endometriosis.
Hirsutism
Hirsutism is common in women with PCOS and often weight loss alone has been associated with reducing the effects of hirsutism.53,57,58 An infusion of Mentha spicata was found to lower free testosterone, while not lowering total testosterone or DHEA, in a small study of women with PCOS and hirsutism.25,120 Serenoa repens has also been found to reduce the severity of androgenic dermatological conditions (alopecia) in both men and women and may therefore be useful in PCOS.121,122 Zinc may also be of some value in reducing androgenic activity in skin.123 Hormonal modulation more generally will also assist with skin conditions associated with excess androgens, and is discussed in more detail in Chapter 24 on acne.
INTEGRATIVE MEDICAL CONSIDERATIONS
Acupuncture
Women with PCOS treated with acupuncture have shown improvements in hormone levels, ovulation and basal body temperature.124 This is thought to be particularly related to the effects of acupuncture on the sympathetic nervous system in PCOS.
Asian herbal medicine
There has been much discussion surrounding the successful treatment of PCOS with the herbs P. lactiflora and G. glabra. This is based on successful treatment of PCOS in a series of small uncontrolled studies in a combination herbal product of which these two herbs were only two components of many.27,28 A Japanese combination (unkei-to) has also demonstrated some effect in women with PCOS,125,126 as have a variety of other combination herbal products.127,128
These remedies have a strong tradition of use in PCOS which is being reinforced through modern research; however, they need to be properly prescribed in accordance with traditional Chinese medicine or kampo principles. It should also be remembered that, due to the difficulties in developing a uniform diagnosis of PCOS, these formulations may not be applicable to all populations with or manifestations of PCOS. It is known, for example, that the metabolic characteristics and responses to treatment of Asian women with PCOS may be significantly different to those seen in Western populations.127 Their use in contemporary naturopathic practice needs to take these principles into consideration.
Homoeopathy
A case series has found that individualised homoeopathic treatment may be successful at restoring ovulation in women with amenorrhoea.129
Psychotherapeutic options
Some women with PCOS fail to fall within social norms regarding outer appearance and may feel stigmatised and feel a loss of feminine identity—this may affect mood, sexual function and quality of life more generally.130 Appropriate counselling and psychotherapeutic options may help lessen the impact on quality of life in PCOS patients.
Expected outcomes and follow-up protocols
Reduction of hirsutism is also a long-term treatment. Hair follicles will often take at least 3–6 months (their life cycle) before effects are observed in women with PCOS. It may be prudent to counsel them to make cosmetic adjustments until then if this is a big concern, in addition to counselling them on the long timeframe of treatment.
Kalantaridou S., et al. Stress and the female reproductive system. J Reprod Immunol. 2004;62(1–2):61-68.
Giallauria F., et al. Androgens in polycystic ovary syndrome: the role of exercise and diet. Semin Reprod Med. 2009;27(4):306-315.
Huang S., Chen A. Traditional Chinese medicine and infertility. Curr Opin Obstet Gynecol. 2008;20(3):211-215.
Trickey R. Women, hormones and the menstrual cycle: herbal and medical solutions from adolescence to menopause. Sydney: Allen & Unwin; 2003.
Wilkes S., Murdoch A. Obesity and female fertility: a primary care perspective. J Fam Plann Reprod Health Care. 2009;35(3):181-185.
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
1. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
2. Asuncion M., et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434-2438.
3. Knochenhauer E., et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078-3082.
4. Ibanez L., et al. Polycystic ovary syndrome after precocious pubarche: ontogeny of the low-birthweight effect. Clin Endocrinol (Oxf). 2001;55:667-672.
5. Speroff L., Fritz M. Clinical gynecologic endocrinology and infertility, 7th edn. Philadelphia: Lippincott Williams & Wilkins; 2005.
6. Kovacs, G., Norman, R., editors. Polycystic ovarian syndrome. Cambridge: Cambridge University Press, 2008.
7. Wilkes S., Murdoch A. Obesity and female fertility: a primary care perspective. J Fam Plann Reprod Health Care. 2009;35(3):181-185.
8. Altuntas Y., et al. Reactive hypoglycemia in lean young women with PCOS and correlations with insulin sensitivity and with beta cell function. Eur J Obstet Gynecol Reprod Biol. 2005;119(2):198-205.
9. Ghosh S., et al. Subclinical hypothyroidism: a determinant of polycystic ovary syndrome. Horm Res. 1993;39:61-66.
10. Jannsen O., et al. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150:363-369.
11. Schwimmer J., et al. Abnormal aminotransferase activity in women with polycystic ovary syndrome. Fertil Steril. 2005;83:494-497.
12. El-Serag H., et al. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
13. Edmonds K., editor. Obstetrics and gynaecology. London: Blackwell, 2007.
14. Martinez A., et al. The reliability, acceptability and applications of basal body-temperature (BBT) records in the diagnosis and treatment of infertility. Eur J Obstet Gynecol Reprod Biol. 1992;47:121-127.
15. Duker E., et al. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and ovariectomized rats. Planta Med. 1991;57:420-424.
16. Borrelli F., et al. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003;73:1215-1229.
17. Jarry H., et al. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44:S31-S38.
18. Scientific Committee of the British Herbal Medical Association. British herbal pharmacopoeia, 1st edn. Bournemouth: British Herbal Medicine Association; 1983.
19. Laurintzen C., et al. Treatment of premenstrual tension with Vitex agnus-castus: controlled, double blind study versus pyroxidin. Phytomedicine. 1997;4:183-189.
20. Milewicz A., et al. [Vitex agnus-castus extract in the treatment of luteal phase defects due to latent hyperprolactinemia. Results of a randomized placebo-controlled double-blind study] [in German]. Arzneimittelforschung. 1993;43(7):752-756.
21. Jarry H., et al. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus: direct evidence for a dopaminergic principle by the dopamine receptor assay. Exp Clin Endocrinol. 1994;102(6):448-454.
22. Okamato R., Kumai A. Antigonadotrophic activity of hop extract. Acta Endocrinol. 1992;127(4):371-377.
23. Balk E., et al. Effects of soy on health outcomes. Evid Rep Technol Assess (Summ). 2005;126:1-8.
24. Tabakova P., et al. Clinical study of Tribestan® in females with endocrine sterility. Sofia: Bulgarian Pharmacology Group; 2000.
25. Akdoğan M., et al. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res. 2007;21(5):444-447.
26. Armanini D., et al. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69(11–12):763-766.
27. Takahashi K., Kitao M. Effects of TJ-68 (shakuyaku-kanzo-to) on polycystic ovarian disease. Int J Fertil Menopausal Stud. 1994;39(2):69-76.
28. Takahashi K., et al. Effects of traditional medicine (Shakuyaku-kanzo-to) on testosterone secretion in patients with polycystic ovary syndrome detected by ultrasound. Nippon Sanka Fujinka Gakkai Zasshi. 1988;40(6):789-796.
29. Setchell K., Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S-767S.
30. Armanini D., et al. Effect of licorice on the reduction of body fat mass in healthy subjects. J Endocrinol Invest. 2003;26(7):646-650.
31. Yaginuma T., et al. Effect of traditional herbal medicine on serum testosterone levels and its induction of regular ovulation in hyperandrogenic and oligomenorrheic women. Nippon Sanka Fujinka Gakkai Zasshi. 1998;34(7):939-944.
32. Cagnacci A., et al. Exogenous melatonin enhances luteinizing hormone levels of women in the follicular but not in the luteal menstrual phase. Fertil Steril. 1995;63:996-999.
33. Cagnacci A., et al. Melatonin enhances the luteinizing hormone and follicle-stimulating hormone responses to gonadotropin-releasing hormone in the follicular, but not in the luteal, menstrual phase. J Clin Endocrinol Metab. 1995;80:1095-1099.
34. Cagnacci A., et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf). 2001;54:339-346.
35. Cagnacci A., et al. Melatonin enhances cortisol levels in aged women: reversible by estrogens. J Pineal Res. 1997;22(2):81-85.
36. Bellipanni G., et al. Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp Gerontol. 2001;36(2):297-310.
37. Bellipanni G., et al. Effects of melatonin in perimenopausal and menopausal women: our personal experience. Ann N Y Acad Sci. 2005;1057:393-402.
38. Cagnacci A., Volpe A. A role for melatonin in PCOS? Fertil Steril. 2002;77(5):1089.
39. Loucks A., Heath E. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78:910-915.
40. Olson B., et al. Short-term fasting affects luteinizing hormone secretory dynamics but not reproductive function in normal-weight sedentary women. J Clin Endocrinol Metab. 1995;80:1187-1193.
41. Alvero R., et al. Effects of fasting on neuroendocrine function and follicle development in lean women. J Clin Endocrinol Metab. 1998;83:76-80.
42. Bergendahl M., et al. Short-term fasting suppresses leptin and (conversely) activates disorderly growth hormone secretion in midluteal phase women—a clinical research center study. J Clin Endocrinol Metab. 1999;84(3):883-894.
43. Daniel J.A., et al. Regulation of the growth hormone and luteinizing hormone response to endotoxin in sheep. Domest Anim Endocrinol. 2002;23(1–2):361-370.
44. Yildiz B., Azziz R. Adrenocortical dysfunction in polycystic ovary syndrome. In Kovacs G., Norman R., editors: Polycystic ovary syndrome, 2nd edn., Cambridge: Cambridge University Press, 2007.
45. Kondoh Y., et al. A longitudinal study of disturbances of the hypothalamic-pituitary-adrenal axis in women with progestin-negative functional hypothalamic amenorrhea. Fertil Steril. 2001;76(4):748-752.
46. Kalantaridou S., et al. Stress and the female reproductive system. J Reprod Immunol. 2004;62(1–2):61-68.
47. Dale O., et al. The impact of insulin resistance on the outcome of ovulation induction with low-dose follicle stimulating hormone in women with polycystic ovarian syndrome. Hum Reprod. 1998;13:567-570.
48. Homburg R. Adverse effect of luteinizing hormone on fertility: fact or fantasy. Bailleres Clin Obstet Gynecol. 1998;12:555-563.
49. Knowler W., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
50. Norris S., et al. Efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2004;164(13):1395-1404.
51. Moran L., et al. Effects of lifestyle modification in polycystic ovarian syndrome. Reprod Biomed Online. 2006;12:569-578.
52. Huber-Buchholz M., et al. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470-1474.
53. Kiddy D., et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36:105-111.
54. Pasquali R., et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767-2774.
55. Crosignani P., et al. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928-1932.
56. Moran L., et al. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812-819.
57. Piacquadio D., et al. Obesity and female androgenic alopecia: cause and effect? J Am Acad Dermatol. 1994;30:1028-1030.
58. Ruutiainen K., et al. Influence of body mass index and age on the grade of hair growth and hormonal parameters of hirsute women. Int J Gynecol Obstet. 1988;24:361-368.
59. Clark A., et al. Weight loss results in significant improvement in pregnancy ovulation and outcome rates in anovulatory obese women. Hum Reprod. 1995;10:2705-2712.
60. Mathers J., Daly M. Dietary carbohydrates and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 1998;1:553-557.
61. Skov A., et al. Randomized trial on protein vs. carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528-536.
62. Stamets K., et al. A randomized trial of the effects of two types of short term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81:630-637.
63. Carmina E., et al. Difference in body weight between American and Italian women with polycystic ovary syndrome: influence of diet. Hum Reprod. 2003;18:2289-2293.
64. Esposito K., et al. Effect of a mediterranean-style diet on endothelial dysfuntion and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
65. Shai I., et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229-241.
66. Tsagareli V., et al. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril. 2006;86:227-229.
67. Hession M.R., et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10(1):36-50.
68. Wing R., Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(Supp 1):222S-225S.
69. Due A., et al. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88:1232-1241.
70. Brinkworth G.D., et al. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2004;28(5):661-670.
71. Sharman M.J., et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132(7):1879-1885.
72. Wolever T.M., Mehling C. Long-term effect of varying the source or amount of dietary carbohydrate on postprandial plasma glucose, insulin, triacylglycerol, and free fatty acid concentrations in subjects with impaired glucose tolerance. Am J Clin Nutr. 2003;77(3):612-621.
73. Volek J.S., et al. Fasting lipoprotein and postprandial triacylglycerol responses to a low-carbohydrate diet supplemented with n-3 fatty acids. J Am Coll Nutr. 2000;19(3):383-391.
74. Gannon M.C., Nuttall F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53:2375-2382.
75. Lara-Castro C., Garvey W.T. Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab. 2004;89(9):4197-4205.
76. Moran L., et al. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77-87.
77. Hung T., et al. Fat versus carbohydrate in insulin resistance, obesity, diabetes and cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2003;6:165-176.
78. Landin K., et al. Guar gum improves insulin sensitivity, blood lipids, blood pressure, and fibrinolysis in healthy men. Am J Clin Nutr. 1992;56:1061-1065.
79. Vuksan V., et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23(1):9-14.
80. Wyatt H., et al. Long-term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res. 2002;10:78-82.
81. Ceci F., et al. The effects of oral 5-hydroxytryptophan administration on feeding behavior in obese adult female subjects. J Neural Transm. 1989;76(2):109-117.
82. Cangiano C., et al. Effects of oral 5-hydroxy-tryptophan on energy intake and macronutrient selection in non-insulin dependent diabetic patients. Int J Obes Relat Metab Disord. 1998;22(7):648-654.
83. Cangiano C., et al. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Am J Clin Nutr. 1992;56(5):863-867.
84. Cangiano C., et al. Effects of 5-hydroxytryptophan on eating behavior and adherence to dietary prescriptions in obese adult subjects. Adv Exp Med Biol. 1991;294:591-593.
85. Venables M., et al. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87(3):778-784.
86. Chan C., et al. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Investig. 2006;13(1):63-68.
87. Dulloo A., et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70(6):1040-1045.
88. Chantre P., Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9(1):3-8.
89. Dulloo A., et al. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252-258.
90. Kovacs E., et al. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr. 2004;91:431-437.
91. Anderson R., Polansky M. Tea enhances insulin activity. J Agric Food Chem. 2002;50:7182-7186.
92. Bruner B., et al. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol Nutr Metab. 2006;31(4):384-391.
93. Miller P., et al. Effect of short-term diet and exercise on hormone levels and menses in obese, infertile women. J Reprod Med. 2008;53(5):315-319.
94. Telli M., et al. Serum leptin levels in patients with polycystic ovary syndrome. Fertil Steril. 2002;77(5):932-935.
95. Takeuchi T., Tsutsumi O. Basal leptin concentrations in women with normal and dysfunctional ovarian conditions. Int J Gynecol Obstet. 2000;69(2):127-133.
96. Mitchell M., et al. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130(5):583-597.
97. Finn P., et al. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139(11):4652-4662.
98. Cioffi J., et al. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod. 1997;3(6):467-472.
99. Brannian J., Hansen K. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin Reprod Med. 2002;20(2):103-112.
100. Hirschberg A., et al. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol. 2004;19(2):79-87.
101. Moran L., et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab. 2004;89:3337-3344.
102. Spritzer P., et al. Leptin concentrations in hirsute women with polycystic ovary syndrome or idiopathic hirsutism: influence on LH and relationship with hormonal, metabolic, and anthropometric measurements. Hum Reprod. 2001;16(7):1340-1346.
103. Moschos S., et al. Leptin and reproduction: a review. Fertil Steril. 2002;77(3):433-444.
104. Nestler J., et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340(17):1314-1320.
105. Iuorno M., et al. Effects of D-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8(6):417-423.
106. Nestler J., et al. Role of inositolphosphoglycan mediators of insulin action in the polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2000;13(Suppl 5):1295-1298.
107. Gerli S., et al. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double-blind placebo controlled trial. Eur Rev Med Pharmacol Sci. 2003;7:151-159.
108. Mills S., Bone K. Principles and practice of phytotherapy. Edinburgh: Churchill Livingstone; 2000.
109. Brighenti F., et al. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur J Clin Nutr. 1995;49(5):242-247.
110. Ostman E., et al. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59(9):983-988.
111. Johnston C., et al. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004;27(1):281-282.
112. Liljeberg H., Björck I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr. 1998;52(5):368-371.
113. Neef H., et al. Hypoglycaemic activity of selected European plants. Phytother Res. 1995;6(2):45-48.
114. Neef H., et al. Inhibitory effects of Galega officinalis on glucose transport across monolayers of human intestinal epithelial cells (Caco-2). Pharm Pharmacol Lett. 1996;6(2):86-89.
115. Muller H., Reinwein H. Pharmacology of galegin. Arch Expll Path Pharm. 1927;125:212-228.
116. Yeh G., et al. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277-1294.
117. Lucidi R., et al. Effect of chromium supplementation on insulin resistance and ovarian and menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril. 2005;84(6):1755-1777.
118. Lydic M., et al. Chromium picolinate improves insulin sensitivity in obese subjects with polycystic ovary syndrome. Fertil Steril. 2006;86(1):243-246.
119. la Marca A., et al. Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril. 2002;78(6):1234-1239.
120. Grant P. A randomised clinical trial of the effects of spearmint herbal tea on hirsutism in females with polycystic ovarian syndrome. Endoce Abst. 2008;15:P282.
121. Prager N., et al. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J Altern Complement Med. 2002;8(2):143-152.
122. Morganti P., et al. Effect of gelatine-cystine and Serenoa repens extract on free radical levels and hair growth. J Appl Cosmetol. 1998;16:57-64.
123. Stamatiadis D., et al. Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. Br J Dermatol. 1988;119:627-632.
124. Stener-Victorin E., et al. Acupuncture in polycystic ovary syndrome: current experimental and clinical evidence. J Neuroendocrinol. 2008;20(3):290-298.
125. Ushiroyama T., et al. Effects of switching to wen-jing-tang (unkei-to) from preceding herbal preparations selected by eight-principle pattern identification on endocrinological status and ovulatory induction in women with polycystic ovary syndrome. Am J Chin Med. 2006;34(2):177-187.
126. Ushiroyama T., et al. Effects of unkei-to, an herbal medicine, on endocrine function and ovulation in women with high basal levels of luteinizing hormone secretion. J Reprod Med. 2001;46(5):451-456.
127. Yu Ng E., Ho P. Polycystic ovary syndrome in Asian women. Semin Reprod Med. 2008;26(1):14-21.
128. Huang S., Chen A. Traditional Chinese medicine and infertility. Curr Opin Obstet Gynecol. 2008;20(3):211-215.
129. Cardigno P. Homoeopathy for the treatment of menstrual irregularities: a case series. Homeopathy. 2009;98(2):97-106.
130. Janssen O., et al. Mood and sexual function in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):45-52.
131. Kasim-Karakas S., et al. Relation of nutrients and hormones in polycystic ovary syndrome. Am J Clin Nutr. 2007;85(3):688-694.