Chapter 28 Pneumonia in the Non–HIV-Infected Immunocompromised Patient
Overview
The respiratory tract is continually exposed to a range of microorganisms that normal immune responses generally prevent from causing infection. Hence, it is not surprising that patients with significantly impaired immunity frequently develop lung infections. Immunodeficient patients also frequently have several other risk factors that contribute to an increased risk for development of pneumonia, including low-level microaspiration of oropharyngeal contents, mucosal damage caused by cytotoxic therapy, underlying lung damage, and poor nutrition. As a consequence, lung infections are a common and frequently serious complication in patients with significant impairment of the immune system. Pulmonary infiltrates occur in 25% of patients with neutropenia developing after chemotherapy, and up to 5% of patients undergoing hematopoietic stem cell transplantation (HSCT) will die as a result of pneumonia. The range of potential pathogens causing lung infections in immunocompromised patients is much broader than for the usual pathogens causing community-acquired pneumonia and includes organisms such as Aspergillus fumigatus and human cytomegalovirus (CMV); the diagnosis of infections due to these microbes can be difficult, and the required therapies frequently are toxic. The large number of potential pathogens and the background disease together make management of lung infection in immunocompromised patients considerably more complex, and the use of invasive diagnostic tests such as bronchoscopy frequently is necessary. This chapter focuses on the common causative pathogens and the clinical approach to pulmonary infections in patients who have been severely immunocompromised by chemotherapy, organ transplantation, or hematologic disease (Box 28-1) but not human immunodeficiency virus (HIV) infection (discussed in Chapter 29). Pneumonia in patients with milder degrees of immunosuppression due to myeloma, low-dose cytotoxic therapy, or disease-modifying agents administered for rheumatologic conditions generally should be managed as community- or hospital-acquired pneumonia, but with recognition that the disease could be due to various opportunistic pathogens, as discussed in this chapter.
Box 28-1
Diseases and Conditions Associated With Severe Immunocompromise*
Hematopoietic stem cell transplantation (HSCT)
Other causes of neutropenia (fewer than 1500 cells/mL)
Inherited disorders of immune function (e.g., chronic granulomatous disease, recent chemotherapy)
High-dose corticosteroid treatment (greater than 30 mg prednisolone for 21 days or longer)
Treatment with immunosuppressive therapy (e.g., tacrolimus, cyclosporine)
Treatment with cytotoxic therapy (e.g., cyclophosphamide, mycophenolate)
General Principles of the Clinical Approach
Potential pathogens causing lung infection in the immunocompromised patient are listed in Table 28-1. Empirical therapy that is active against all these possible pathogens is not feasible, especially given the potential toxicity of some treatments. Hence, the challenge in managing these patients is to (1) reduce the scope of the differential diagnosis to include the most likely problems and thus allow relatively targeted empirical therapy and (2) identify when and which type of invasive diagnostic test(s) should be used to provide the most useful data. The likely potential causative pathogens can be defined by ascertaining the following:
• The clinical and radiologic presentation
• The speed of onset of the infection
• The type, duration, and severity of the patient’s immune defect
• Positive results on microbiologic studies
• Associated factors of importance, including any recent local infective epidemics and the patient’s prophylaxis regimen, ethnic background, and travel history
Table 28-1 Range of Pathogens Commonly Associated With Pneumonia in the Non–HIV-Infected Immunocompromised Patient
| Pathogen | Cases (%) |
|---|---|
| Gram-Negative Pyogenic Bacteria | |
| Escherichia coli | 6 |
| Proteus, Enterobacter, Serratia, and Citrobacter spp. | 2 |
| Haemophilus influenzae | <1 |
| Klebsiella pneumoniae | <1 |
| Pseudomonas aeruginosa | 8 |
| Acinetobacter spp. | 2 |
| Stenotrophomonas maltophilia | <1 |
| Gram-Positive Pyogenic Bacteria | |
| Streptococcus pneumoniae | 2 |
| Viridans streptococci | <1 |
| Staphylococcus aureus | 12 |
| Enterococcus spp. | 4 |
| Other Bacteria | |
| Anaerobes | <1 |
| Legionella pneumophila | 2 |
| Chlamydia spp. | 2 |
| Mycoplasma pneumoniae | <1 |
| Mycobacterium tuberculosis | 4 |
| Nontuberculous mycobacteria | <1 |
| Nocardia spp. | 2 |
| Fungi | |
| Pneumocystis jirovecii | 3 |
| Candida spp. | 9 |
| Aspergillus spp. | 24 |
| Rarer molds (e.g., Mucor, Penicillium, Fusarium) | 2 |
| Endemic fungi (e.g., Histoplasma, Coccidioides) | <1 |
| Protozoa | |
| Toxoplasma gondii | <1 |
| Helminths | |
| Strongyloides stercoralis | <1 |
| Viruses | |
| Cytomegalovirus | 6 |
| Herpes simplex virus, varicella-zoster virus | 2 |
| Respiratory viruses (respiratory syncytial virus, adenovirus, influenza virus, parainfluenza virus, human metapneumovirus) | 8 |
Modified from Rañó A, Agustí C, Jimenez P, et al: Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures, Thorax 56:379–387, 2001.
The exact type of immune defect is determined by a combination of the disease and the treatment received and, fortunately, can generally be predicted (Table 28-2). The three main categories of immune defect are absolute or functional neutropenia, defects in cell-mediated immunity, and deficiencies in antibody responses (a clinically less severely affected category). Each is associated with a particular range of pathogens (see Table 28-2). Patients with neutropenia are mainly at risk for infection with extracellular pathogens such as pyogenic bacteria and filamentous fungi. Defects in cell-mediated immunity tend to predispose affected patients to development of infections with intracellular pathogens such as viruses and mycobacteria, as well as some unusual extracellular infections such as Pneumocystis pneumonia (PCP). Deficiencies in antibody responses result in a high incidence of infections due to encapsulated bacteria such as Streptococcus pneumoniae and to herpesviruses. Individual patients may have a combination of these immune defects; for example, lymphoma can cause impairment of cell-mediated immunity that can be combined with neutropenia if the patient receives chemotherapy.
Table 28-2 Type of Immune Defect According to Disease/Treatment and Range of Pathogens Commonly Associated With Infections in Patients with Such Immune Defects
| Immune Defect | Cause | Associated Pathogens |
|---|---|---|
| Neutropenia/functional neutrophil defects |
HSCT, hematopoietic stem cell transplantation.
* Allografts up to 1 month, autografts usually less than 14 days.
† Patients usually have a normal neutrophil count but have defects in their function and/or a poor response to infection.
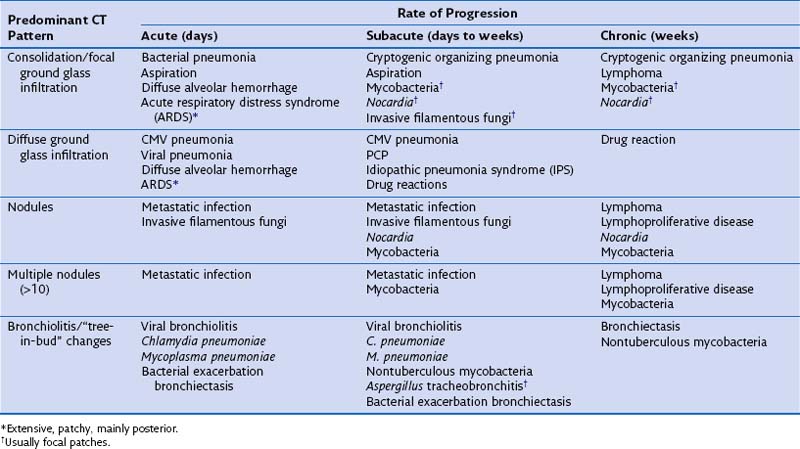
By combining the clinical pattern of presentation with knowledge of the patient’s immune defect, a differential diagnosis of likely pathogens can be suggested. For example, infections developing rapidly over 1 to 3 days with a marked rise in serum inflammatory markers such as C-reactive protein (CRP), pronounced fever, and focal radiologic changes are very likely to be due to infection with pyogenic bacteria. In a patient with a defect in cell-mediated immunity, however, widespread ground glass infiltrations in both lungs developing over several days could represent CMV pneumonitis or PCP. In general, the more severe and prolonged the immune defect, the greater the range of possible causative pathogens and the less typical the clinical presentation for a particular pathogen, prompting the need for early invasive investigation if the initial therapy is failing to effect improvement. Furthermore the character of the disease can be dictated by the severity of the immune defect. For example, infections due to filamentous fungi such as Aspergillus progress faster with increasing severity of neutropenia, but may regress and become more focal when the neutrophil count recovers. Table 28-3 shows the common conditions that should be considered for different presentations of lung complications in immunocompromised patients. Many patients will be found to have dual pathologic processes, either involving two separate pathogens or with simultaneous noninfective (fluid overload being the most common) and infective problems, which will be responsible for separate elements of the clinical picture.
Table 28-3 Differential Diagnosis for Computed Tomography (CT) Pattern and Rate of Development of Clinical Problem
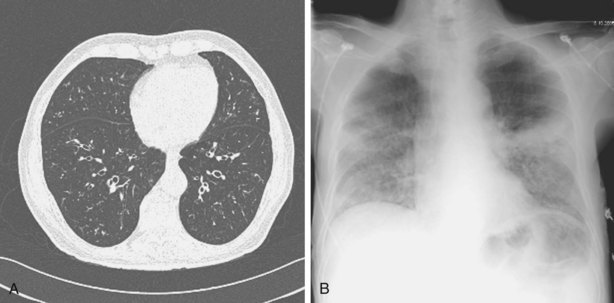
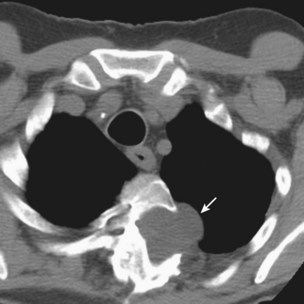
Other important factors that need to be taken into account include the patient’s present antibiotic prophylaxis regimen, travel history, and ethnic background. A patient compliant with co-trimoxazole prophylaxis will rarely develop PCP, and fluconazole prophylaxis predisposes to non-albicans Candida infection. Patients with certain ethnic backgrounds or a pertinent travel history are more at risk of TB, endemic mycoses such as histoplasmosis, or parasitic diseases such as disseminated strongyloidiasis. Finally, the patient’s background lung structure and function should be considered, because existing structural abnormalities may make certain pathogens more likely. For example, bronchiectasis could predispose affected persons to pneumonia caused by P. aeruginosa (Figure 28-1), and preexisting lung cavities to Aspergillus infection.
Clinical Assessment and Diagnostic Protocols
All patients with suspected new lung infection require blood and sputum cultures, as well as routine blood tests, and in many cases nasopharyngeal aspirate studies for identification of viruses, assessment of CMV status, and serum testing for fungal antigens (galactomannan or β-D-glucan). An important question for the respiratory physician is when to utilize more invasive investigations, either bronchoscopy with bronchoalveolar lavage (BAL) and possibly transbronchial biopsy, percutaneous radiologically guided biopsy, or, on occasion, surgical biopsy, usually video-assisted thoracoscopic surgery (VATS). Which test should be used and when will depend to a large extent on the type of radiologic presentation—consolidation, diffuse ground glass infiltrates, tree-in-bud changes, and nodules—in accordance with the algorithms for each provided in Figures 28-2, 28-4, 28-6, and 28-7, respectively. These suggested protocols balance the likelihood and necessity of a positive yield against the clinical probability that a particular disease will be identified with the potential complications of the investigations. These protocols are for general guidance; atypical presentations and dual pathology are not uncommon, and an individual patient often will require a modified approach. Immunocompromised patients are also at high risk for development of a range of noninfective causes of lung disease, including pulmonary edema, idiopathic pneumonia syndrome (IPS), and diffuse alveolar hemorrhage (see Chapter 59). These conditions should always be considered in the differential diagnosis for new lung disease. The protocols are discussed in detail next.
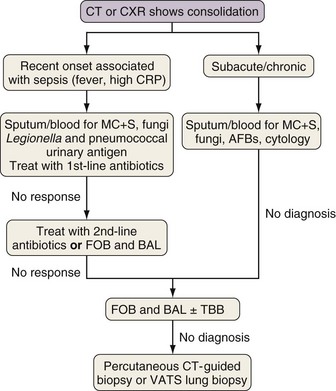
Investigation of Consolidation
Rapidly developing focal consolidation usually is due to bacterial pneumonia and initially can be treated empirically with broad-spectrum antibiotics (Figure 28-2). The most likely reason for a lack of improvement in the patient’s condition within 48 to 96 hours is infection with bacteria resistant to first-line antibiotics. At this point, treatment with second-line antibiotics effective against likely resistant organisms should be started. Consideration also should be given to starting antibiotics that are effective against MRSA and anaerobic pathogens if this has not been done already. In patients at high risk for invasive fungal infection, especially those with CT evidence of associated nodular disease or patchy or infarct-shaped consolidation, failure of first-line therapy warrants testing for fungal antigens (galactomannan or β-D-glucan) and either fiberoptic bronchoscopy (FOB) with BAL or percutaneous CT-guided biopsy (mainly for evaluation of dense consolidation adjacent to the pleura). These investigations also will be necessary in patients whose pneumonia fails to respond to second-line antibiotic therapy or those with subacute or chronic consolidation in whom sputum microbiologic or cytologic testing is nondiagnostic. With progressive disease and lack of a definitive diagnosis after the foregoing tests, surgical biopsy should be seriously considered.
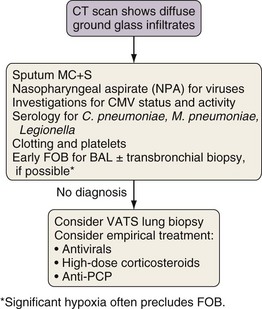
Investigation of Diffuse Ground Glass Infiltration
The differential diagnosis for ground glass infiltrates and centrilobular nodules (Figures 28-3 and 28-4) is wide in scope and includes CMV infection, viral pneumonias, PCP, extensive bacterial infection, and noninfective causes such as acute respiratory distress syndrome (ARDS), drug toxicity, and IPS associated with HSCT (see Chapter 77). As a consequence, unless nasopharyngeal aspirate studies identify a respiratory viral infection, early FOB with BAL and, if possible, transbronchial biopsy should be attempted. Often, however, these patients are markedly hypoxic, increasing the risk associated with the procedure. Negative results on FOB do not exclude infective causes, and a decision then needs to be taken about empirical treatment versus VATS lung biopsy. CT-guided biopsy for investigation of diffuse lung disease carries a high risk of complications coupled with low diagnostic yield and therefore is not appropriate in this setting.
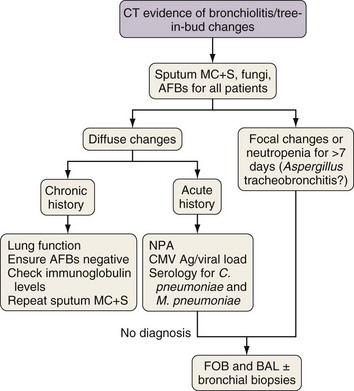
Investigation of Tree-in-Bud Changes
Tree-in-bud changes (Figures 28-5 and 28-6) suggest small airway pathology, which in immunocompromised patients is likely to be caused by respiratory virus infection, Chlamydia pneumoniae, Mycoplasma pneumoniae, or, especially if the changes are focal and associated with nodular changes, Aspergillus tracheobronchitis. Subacute or chronic changes also could reflect bacterial infection in bronchiectasis or nontuberculous mycobacteria infection. If results of nasopharyngeal aspirate studies are negative, then early FOB for BAL and bronchial biopsy of macroscopically inflamed bronchial mucosa should be performed. Aspergillus tracheobronchitis usually is obvious at FOB and is readily confirmed by culture and cytologic examination of bronchial washings and by bronchial biopsy. Infections due to respiratory viruses, C. pneumoniae, and M. pneumoniae often are difficult to diagnose but usually are either self-limiting illnesses or readily controlled with macrolide therapy.
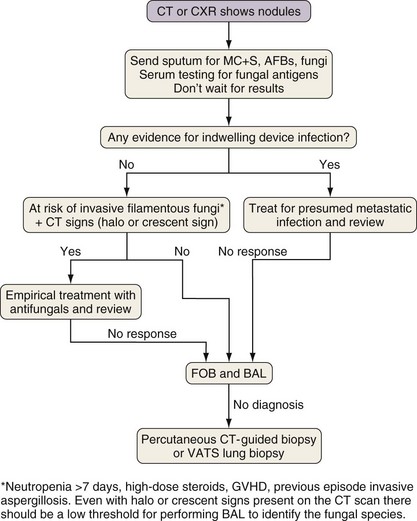
Investigation of Pulmonary Nodules
Considerations in the differential diagnosis for pulmonary nodules include infection with Aspergillus (or other invasive filamentous fungi), Nocardia, or mycobacteria (Figure 28-7). Aspergillus and Nocardia tend to cause a small number of nodules, whereas mycobacteria also can cause large numbers of small nodules. In addition, blood-borne spread of bacteria or Candida from infected indwelling devices can cause a variable number (often numerous) of pulmonary nodules. Serum testing for fungal antigens (galactomannan or β-D-glucan) should be performed. Nodules caused by pyogenic bacterial and viral pneumonia tend to be associated with other radiographic changes findings such as ground glass infiltrates or consolidation. The other major causes of nodules are underlying malignant disease and lymphoproliferative disorders secondary to prolonged immunosuppression. In the absence of CT signs suggestive of invasive filamentous fungal infection (the halo and crescent signs), nodules in patients with line infections or a positive blood culture may be treated empirically as for metastatic infection. For a majority of other patients with lung nodules, FOB with BAL is necessary, although for patients at high risk for infection with invasive filamentous fungi, if CT changes suggestive of Aspergillus infection are present, then empirical antifungal therapy is a reasonable alternative strategy, especially if the result of fungal antigen testing is positive. If FOB findings are unhelpful, the patient should progress to percutaneous CT-guided or VATS lung biopsy, because infections due to fungi, Nocardia, or mycobacteria are accompanied by specific histopathologic changes, and biopsy frequently is diagnostic.
Specific Clinical Entities
Bacterial Pneumonia
General Considerations
Pneumonia due to bacterial pathogens is the most common lung infection in the immunocompromised patient, causing about 40% to 50% of infective episodes. The main risk factor is neutropenia, but cell-mediated immune defects and functional defects in phagocyte responses due to cytotoxic or immunosuppressive therapy also will markedly increase the risk for development of pneumonia. Antibody deficiencies associated with lymphoproliferative disorders, myeloma, and HSCT predispose affected patients to development of pneumonia with encapsulated organisms such as S. pneumoniae and Haemophilus influenzae. Many cases of bacterial pneumonia are nosocomial infections in patients who have been hospitalized for long periods and who previously have been treated with antibiotics so that the normal oropharyngeal flora has been replaced mainly by gram-negative bacteria. As a consequence, the range of potential organisms causing bacterial pneumonia is very different from that seen in community-acquired pneumonia, with a high frequency of resistant organisms such as P. aeruginosa and MRSA (see Table 28-1).
Clinical Features and Diagnosis
Bacterial pneumonia often manifests with a clinical picture similar to that seen in immunocompetent patients, with new-onset fever, cough, chest and radiologic signs of lobar consolidation, and a rapid rise in the level of inflammatory markers such as CRP. However, a more insidious or diffuse presentation that is more difficult to differentiate from viral or fungal infection is not uncommon. CT scans showing dense focal consolidation with a lobar distribution are helpful in differentiating bacterial from fungal or viral pneumonia (Figure 28-8), and blood cultures will be positive in about 20% of cases. Antigen testing of the urine may identify Legionella pneumophila or S. pneumoniae infections. Patients with an atypical clinical presentation or those who do not respond to first- or second-line antibiotics should have FOB and BAL, which has a reasonably high diagnostic yield for bacterial pneumonia. Protected specimen brush procedures probably add little information over and above that obtained with directed BAL. Histologic examination is unlikely to reveal specific features, and lung biopsy is mainly used to exclude other causes of lung infiltrates.
Cytomegalovirus Infection
Clinical Features and Diagnosis
CMV pneumonitis commonly manifests with insidious onset of fever, malaise, cough, and dyspnea with hypoxia. The chest radiograph may be normal in appearance or show nonspecific diffuse bilateral infiltrates. CT scan is more sensitive at identifying pulmonary infiltrates and classically shows bibasal symmetric ground glass opacities, septal line thickening, and multiple small centrilobular nodules (Figure 28-9). More asymmetric changes, consolidation, and effusions are not uncommon, however. The main considerations in the differential diagnosis are IPS, drug-induced pneumonitis, and, in patients not receiving effective prophylaxis, PCP. Serologic determination of either IgG or IgM has no place in the diagnosis of CMV disease, because these antibodies merely reflect previous exposure. However, CMV reactivation is nowadays readily detected by identifying significant viremia through either measuring the level of pp65 antigenemia or CMV DNA using PCR assay in the blood. CMV viremia usually is detectable 2 to 5 days before any clinical manifestations. Unfortunately, CMV viremia is not always present in patients with CMV pneumonitis; conversely, evidence of reactivation does not necessarily mean that new lung infiltrates are due to CMV pneumonitis. The chance that CMV infection is responsible for new lung infiltrates is proportional in part to the level of CMV in the blood, especially if the viral load increased rapidly. Definitive confirmation that CMV is causing pneumonia requires identification of CMV in the respiratory tract by FOB for BAL and preferably a transbronchial biopsy, or possibly a VATS lung biopsy. The presence of “owl’s eye” intranuclear inclusions on cytologic examination is pathognomonic for CMV infection and although this modality is rapid, it is a relatively insensitive test. CMV can be cultured from respiratory samples using fibroblast cell culture to look for the distinctive cytopathogenic effect, but this takes at least a week and therefore is of little clinical benefit. The main diagnostic techniques for CMV pneumonitis are the relatively sensitive rapid tests for CMV antigens using BAL samples directly, or indirectly by probing cell cultures inoculated with the samples after 24 to 48 hours incubation (the shell vial or early antigen detection assays). Quantitative PCR assay on BAL fluid has been used to improve the predictive value for the diagnosis of CMV pneumonitis but requires further investigation. Despite this range of diagnostic techniques, a well-validated standard for identifying CMV pneumonitis is lacking, except for VATS biopsy.
Treatment
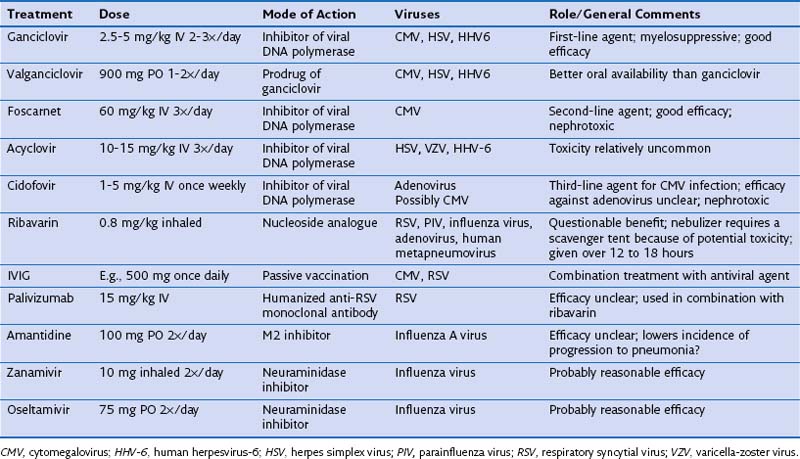
The currently available antiviral agents for treatment of CMV infection and disease are acyclovir, valacyclovir, ganciclovir, valganciclovir, foscarnet, and cidofovir (Table 28-4). A regimen based on the purine analogue ganciclovir is the preferred treatment; this agent, once phosphorylated within infected cells, competitively inhibits viral DNA polymerase. Ganciclovir has significant marrow-depressant effects and may be too toxic for use in hematopoietic stem cell transplant recipients or in patients with existing pancytopenia. Oral absorption is poor, but oral therapy with the valine ester valganciclovir has excellent bioavailability. The second-line agent foscarnet also inhibits the activity of viral DNA polymerase (by binding to the pyrophosphate-binding site). Foscarnet frequently causes significant renal toxicity. Alternatively, cidofovir has a broad activity against DNA viruses including CMV, acting as a competitive inhibitor of viral DNA polymerases. Cidofovir causes both myelosuppression and renal toxicity, and patients should be prehydrated and given probeniced before initiation of treatment. Patients with CMV disease also are frequently given hyperimmune intravenous immunoglobulin (IVIG) as passive vaccination therapy. Very few studies have compared these drugs for efficacy in CMV pneumonia. Treatment lasts from 14 to 21 days and should be guided by blood tests measuring the level of CMV viremia. Patients at risk for such infection often are given antiviral prophylaxis such as with valganciclovir. The mortality rate for established CMV pneumonia is up to 50% in hematopoietic stem cell transplant recipients but is less in other types of immunocompromised patients. Detection of CMV viremia frequently leads to preemptive therapy before symptomatic CMV infection develops, increasing the numbers of patients requiring treatment but improving overall outcome.
Infections With Respiratory Viruses
Clinical Features and Diagnosis
Respiratory virus lung infections often cause a bronchiolitis, manifesting with cough, fever, wheeze, and inspiratory squeaks. They frequently are preceded by a few days of coryzal symptoms. The chest radiograph may be normal in appearance, but a CT scan will show evidence of small airway involvement, with widespread tree-in-bud changes (Figure 28-10). The main considerations in the differential diagnosis in this clinical scenario are Chlamydia or Mycoplasma infection, extensive bacterial bronchitis (usually associated with bronchiectasis), and possibly Aspergillus tracheobronchitis. With more severe disease associated with significant pneumonitis, CT evidence will include small, poorly defined centrilobular nodules and usually bilateral patchy areas of peribronchial ground glass opacity and consolidation. The differential diagnosis in such cases is much broader in scope, including bacterial pneumonia, CMV pneumonitis, PCP, and noninfective causes of pneumonitis. In many patients, the diagnosis of respiratory viral infection can be rapidly confirmed by noninvasive testing using nasopharyngeal aspirate samples for either immunofluorescence for viral antigens or PCR assay for viral nucleic acids. A negative result on nasopharyngeal aspirate studies should lead to FOB, because these tests are more sensitive with BAL fluid than with nasopharyngeal aspirate samples. Respiratory viral infections in immunocompromised patients are often very prolonged but not too severe, with symptoms and positive results on nasopharyngeal aspirate studies persisting for several weeks.
Treatment and Special Considerations with Specific Viruses
With the exception of influenza, treatment options for respiratory viruses are limited and depend on the underlying virus (see Table 28-4). In the absence of pneumonia, the mortality associated with respiratory virus infection in immunocompromised patients is relatively low, and patients can be given supportive therapy only. By contrast, pneumonia due to respiratory viruses after HSCT is associated with significant mortality (up to 40%). Secondary infections are common, and patients frequently need concurrent treatment with antibiotics.
Invasive Aspergillosis
Clinical Features
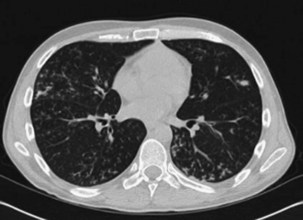
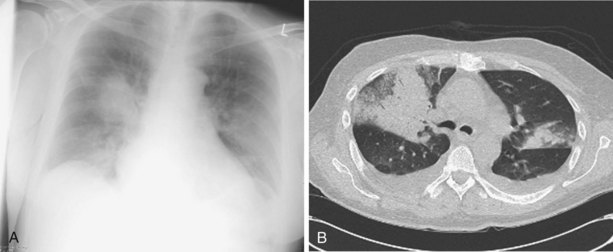
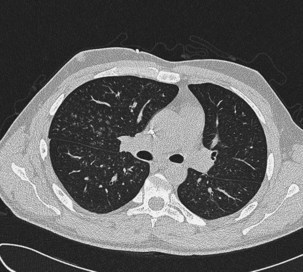
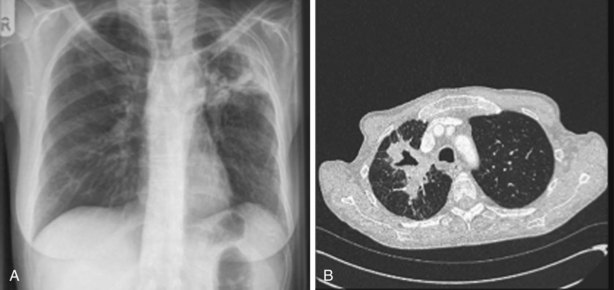
The speed of development of IPA is proportional to the level of immunosuppression, but the disease usually evolves relatively slowly over days and weeks. Fever may be the only symptom of IPA, although cough, pleuritic chest pain, and hemoptysis are common. Chest radiographs show expanding patches of irregular consolidation (often infarct shaped) or nodules that can cavitate. CT scans are very helpful, because they will define the nodular nature of infiltrates, may show specific signs associated with IPA, and can identify the lung as the source of infection in patients with pyrexia and a normal chest radiograph. Specific CT appearances for IPA include the halo sign, an area of lower attenuation shadowing around a nodule or patch of consolidation reported in 50% of cases of IPA and usually occurring in the first week of infection (Figure 28-11); the air crescent sign, a partial cavity formed by infarcted necrotic lung, which is a very specific but later-onset sign occurring around the third week of infection; and an intrapulmonary cavity containing a fungal ball, especially associated with recovery of the patient’s neutrophil count (see Figure 28-11). The halo sign may be detected in other infections, with neoplasms (adenocarcinoma, bronchoalveolar carcinoma, Kaposi sarcoma, and metastases), and in vasculitis. Aspergillus has a predilection for growing into blood vessels, and patients with IPA may suffer fatal massive hemorrhage. Other manifestations of invasive Aspergillus infections affecting the lung include Aspergillus tracheobronchitis, chronic necrotizing pulmonary aspergillosis (CNPA), and chronic cavitary pulmonary aspergillosis (CCPA).
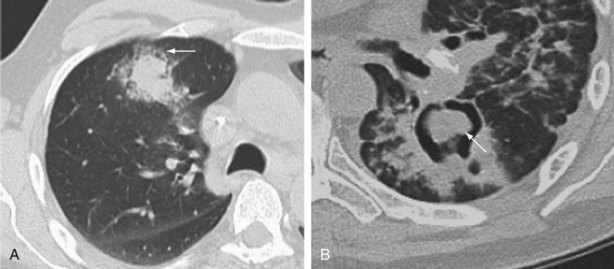
With Aspergillus tracheobronchitis, infection is restricted to the tracheobronchial tree and manifests with a severe unremitting cough and pyrexia. CT scans may show focal areas of bronchial wall thickening and tree-in-bud small airway disease (Figure 28-12). FOB usually is diagnostic, with a distinctive macroscopic appearance of patchy, highly inflamed mucosa with necrotic white slough. Aspergillus can be found in cultures and/or cytology of bronchial washings, and there may be evidence of fungal invasion of the respiratory mucosa in bronchial biopsy specimens.
The more indolent forms of invasive aspergillosis such as CCPA or CNPA are increasing in incidence and are associated with milder degrees of immunosuppression, including steroid and cytotoxic therapies, chronic lung disease or cystic fibrosis. Affected patients present with a long history of cough and marked systemic symptoms of malaise, fatigue, and weight loss. On chest radiographs, CNPA manifests as an indolent patch of consolidation with or without cavitation that progresses over weeks or months (Figure 28-13), whereas CCPA manifests with an expanding upper lobe dry cavity with a thickened and irregular wall. There may be associated pleural thickening. Aspergillus infection also can cause a progressive upper lobe fibrosis.
Microbiologic Diagnosis
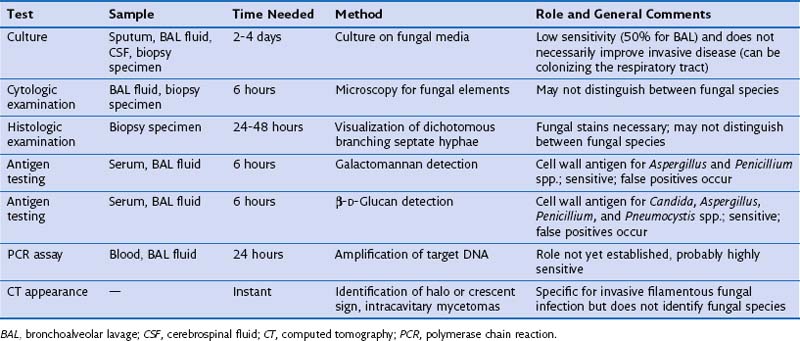
Investigations that can be helpful in making the diagnosis of IPA are described in Table 28-5. In high-risk patients with a compatible clinical syndrome and CT findings highly suggestive of IPA (halo and crescent signs), the diagnosis can be made clinically and may not require confirmation by invasive investigations. However, some Aspergillus species (e.g., A. terreus) are resistant to amphotericin B, and a clinical presentation similar to that in IPA can be caused by rarer filamentous fungi with drug sensitivities that differ from those demonstrated for Aspergillus. Hence, microbiologic confirmation is reassuring, because it ensures that appropriate treatment is given. A microbiologic diagnosis of IPA can be made by culture, cytologic, or histologic appearances in BAL or lung biopsy specimens, or by the detection of fungal cell wall antigen (galactomannan or β-D-glucan) or DNA (by PCR assay) in blood or BAL fluid. Isolation of Aspergillus from BAL in a high-risk immunodeficient patient is highly predictive of IPA but is relatively insensitive, identifying only 50% of cases of IPA. Culture from sputum is even less sensitive, and blood cultures are only rarely positive. Percutaneous CT-guided or VATS biopsy is highly sensitive, because histopathologic examination of the sample can readily identify fungal hyphae infiltrating through lung tissue if fungal stains are used. Hence, strong consideration should be given to biopsy of lung nodules not responding to conventional antibacterial antibiotics, especially if BAL was nondiagnostic. To decrease mortality by allowing rapid identification of cases of IPA before the fungal load is high, several noninvasive tests have been developed. These include detection of galactomannan or β-D-glucan cell wall antigen in the blood (or BAL fluid if FOB has been performed), or PCR assay for Aspergillus DNA from the blood or BAL fluid. These tests are highly sensitive and when used as surveillance in high-risk patients could lead to preemptive antifungal therapy before clinically apparent disease has developed, leading to an improved mortality. However, detection of fungal DNA in a single sample of peripheral blood is a poor indicator of early invasive fungal infection, and galactomannan antigen is not specific for Aspergillus spp., frequently giving false-positive results (especially in patients treated with piperacillin-tazobactam or in young children). Despite these caveats, a negative galactomannan assay result makes active IPA infection unlikely. Serologic tests for antibodies to Aspergillus usually yield negative results in immunocompromised patients with IPA.
Treatment
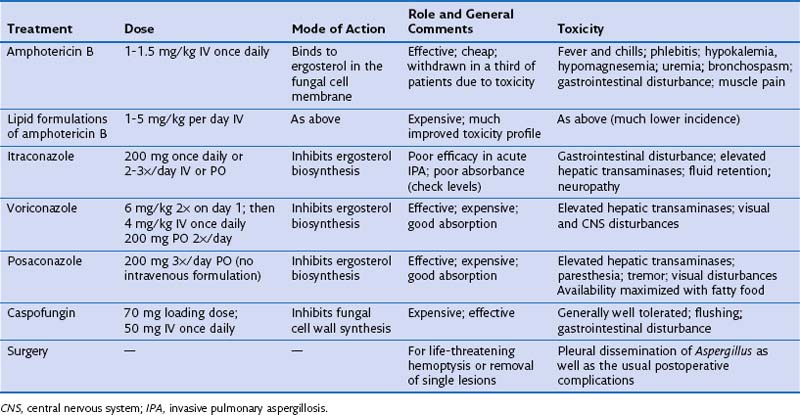
The treatment options for invasive aspergillosis have been considerably improved by the introduction of new azoles, voriconazole and more recently posaconazole, and a new class of antifungal agents, the echinocandins, the first example of which is caspofungin. Doses, modes of action, and common toxicities for the different treatment options are given in Table 28-6. Voriconazole may be more effective than amphotericin B for treating IPA, but which drug is used depends on the patient’s tolerance for that agent and any preexisting medical conditions (e.g., amphotericin B should be avoided in patients with renal problems, and azoles in patients with liver disease). Itraconazole is less efficacious and should be reserved for oral treatment in patients recovering from IPA after induction treatment with amphotericin B, caspofungin, or voriconazole or for the long-term treatment required for chronic forms of invasive aspergillosis. Drug levels probably should be monitored for patients receiving itraconazole, voriconazole, and posaconazole, at least for patients on prolonged therapy. The role of combination therapy remains unclear, but this strategy is potentially attractive in view of different mechanisms of action for the three effective classes of drugs. Urgent surgical resection should be considered in patients with major hemoptysis, because fatal bleeding is not uncommon. Elective resection can be used as primary therapy for single lesions due to IPA, especially those containing an intracavitary mycetoma that might lead to reactivation of IPA during subsequent immunosuppression. Treatment of IPA for several weeks often is necessary, followed by prophylactic therapy in cases with persisting radiologic changes. Treatment of CNPA and CCPA will need to be continued for months if not years (and even lifelong). Owing to a combination of uncontrolled infection and the underlying disease, the mortality rate for IPA is around 50%, and with chronic forms of aspergillosis, the infection rate remains as high as 33%. Azoles can be used for prophylaxis in at-risk groups, including hematopoietic stem cell transplant recipients. Fluconazole is not effective at preventing mold infections such as IPA, and despite its efficacy against Aspergillus, voriconazole seems to offer no clinical benefit over prophylaxis with fluconazole. However, prophylaxis with the newer extended-spectrum triazole posaconazole reduced the incidence of invasive aspergillosis in patients with prolonged neutropenia, from 7% to 1%. Acquired resistance of A. fumigatus to posaconazole has emerged during the course of therapy, which may potentially limit its use for prophylaxis.
Other Fungal Infections
Pneumocystis jirovecii Infection
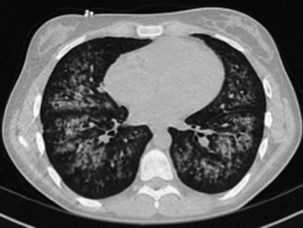
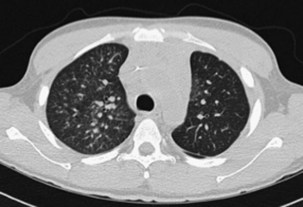
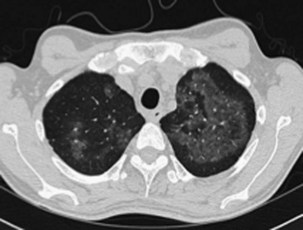
In addition to its close association with HIV infection, PCP is an important type of pneumonia in non–HIV-infected immunocompromised patients with defects in cell-mediated immunity or who are receiving systemic corticosteroid therapy equivalent to greater than 20 mg daily of prednisolone. The clinical presentation generally is insidious in onset, with cough and progressive dyspnea developing over weeks, but can be more fulminant. Impaired gas transfer and hypoxia on exertion with progression to respiratory failure are common. The chest radiograph usually shows bilateral infiltrates but may be normal in appearance. Generally the CT scan will show ground glass infiltrates, which classically mainly affect the upper lobes and spare the lung peripheries (Figure 28-14). On rare occasions, however, even the CT scan may show only minimal abnormalities. The diagnosis is made by recognition of the clinical picture in an at-risk patient and should be confirmed by cytologic identification of the characteristic P. jirovecii cysts in BAL fluid. Diagnosis can be improved using immunohistochemistry assay for Pneumocystis antigens, and perhaps by PCR analysis, although the latter will have a significant false-positive rate as a consequence of colonization in the absence of disease.
Controversies and Pitfalls
• Exactly when bronchoscopy should be used for diagnosing respiratory infections in immunocompromised patients remains controversial, with no clear consensus on early versus late bronchoscopy. The approach described in this chapter based on the type of lung shadowing aims to target bronchoscopy to the patients for whom it may be most helpful.
• CMV pneumonitis is an especially difficult diagnosis to confirm; the specific diagnostic tests often are inconclusive, and the clinician is left with presumptive evidence of involvement of CMV consisting of the combination of a pneumonitis with blood test results indicating reactivation of the virus.
• A major pitfall to avoid is failure to consider noninfective causes of respiratory problems in the non-HIV-infected immunocompromised patient; pulmonary edema, alveolar hemorrhage, ARDS, drug-related or idiopathic pneumonitis, and rapidly progressive airway obstruction due to lung graft-versus-host disease (often precipitated by respiratory viral infections) often need to be included in the differential diagnosis.
• Although most infective causes of lung problems in the non–HIV-infected immunocompromised patient generally have fairly characteristic presentations and affect selected risk groups, considerable overlap between clinical presentations for illnesses due to specific infectious agents is typical. This is especially true for the more severely immunosuppressed patient, in whom the usual clinical presentations become less characteristic.
• Finally, the clinician needs to remember that non–HIV-infected immunocompromised patients often have two simultaneous pathologic conditions (e.g., a bacterial pneumonia complicated by ARDS, or a respiratory viral infection with lung graft-versus-host disease).
Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467.
Denning DW, Riniotis K, Dobrashian R, et al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003;1(Suppl 3):S265–S280.
Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011;66(Suppl 1):i25–i35.
Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–622.
Kanne JP, Godwin JD, Franquet T, et al. Viral pneumonia after hematopoietic stem cell transplantation: high-resolution CT findings. J Thorac Imaging. 2007;22:292–299.
Maertens J, Meersseman W, Van Bleyenbergh P. New therapies for fungal pneumonia. Curr Opin Infect Dis. 2009;22:183–190.
Maschmeyer G, Beinert T, Buchheidt D, et al. Diagnosis and antimicrobial therapy of pulmonary infiltrates in febrile neutropenic patients. Ann Hematol. 2003;82(Suppl 2):S118–S126.
Rañó A, Agustí C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001;56:379–387.
Rubin RH. The pathogenesis and clinical management of cytomegalovirus infection in the organ transplant recipient: the end of the ‘silo hypothesis. Curr Opin Infect Dis. 2007;20:399–407.
Tomblyn M, Chiller T, Hermann E, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238.