91 Pneumonia and Respiratory Infections
Etiology and Pathogenesis
Microbiology
The common causes of CAP in healthy children in the developed world vary by age group, although an extensive number of pathogens can cause CAP (Table 91-1). Respiratory viruses such as respiratory syncytial virus (RSV); influenza A and B; parainfluenza 1, 2, and 3; adenovirus; and human metapneumovirus (hMPV) can be identified in up to half of patients admitted to the hospital for CAP. These viral pathogens may be identified alone or as part of a co-infection with bacteria. First described in 2001, hMPV, similar to RSV infection in young children, causes a spectrum of respiratory disease ranging from mild bronchiolitis to severe pneumonia.
Table 91-1 Common Bacterial and Viral Causes of Community-Acquired Pneumonia by Age in Healthy Children in the Developed World
| ≤3 Months Old | 3 Months to 5 Years Old | ≥5 Years Old |
|---|---|---|
| Bacteria | Bacteria | Bacteria |
| Group B streptococcus | Streptococcus pneumoniae | Streptococcus pneumoniae |
| Enteric gram-negative bacilli | Mycoplasma pneumoniae | Mycoplasma pneumoniae |
| Streptococcus pneumoniae | Chlamydophila pneumoniae* | Chlamydophila pneumoniae* |
| Bordetella pertussis | Staphylococcus aureus | Staphylococcus aureus |
| Chlamydia trachomatis Staphylococcus aureus |
Haemophilus influenzae (nontypable) | |
| Lower Respiratory Viruses | Lower Respiratory Viruses | Lower Respiratory Viruses |
| Respiratory syncytial virus | Respiratory syncytial virus | Influenza A and B |
| Influenza A and B | Influenza A and B | |
| Parainfluenza viruses 1, 2, 3 | Parainfluenza viruses 1, 2, 3 | |
| Human metapneumovirus | Human metapneumovirus | |
| Rhinovirus | Rhinovirus | |
| Adenovirus | Adenovirus | |
| Bocavirus | Bocavirus | |
| Coronaviruses | Coronaviruses |
* Formerly Chlamydia pneumoniae.
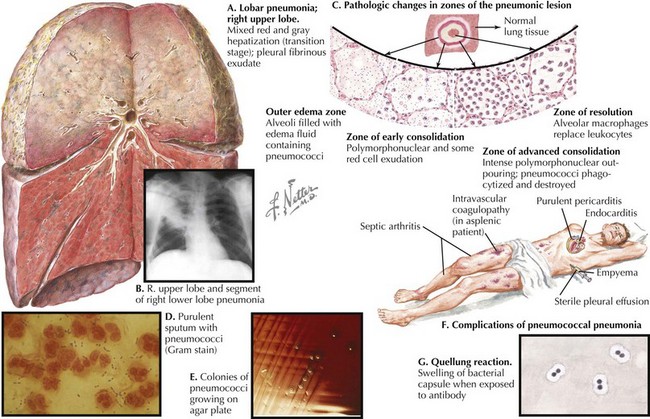
Streptococcus pneumoniae is the most common bacterial cause of childhood CAP (Figure 91-1). Randomized trials of the heptavalent pneumococcal conjugate vaccine (PCV7) demonstrated that the incidence of radiographically confirmed pneumonia was reduced by 20% in vaccine recipients compared with placebo recipients, suggesting that S. pneumoniae causes at least 20% of CAP cases. Postlicensure epidemiologic studies have shown a 39% decrease in all-cause pneumonia hospitalizations in children younger than 2 years of age but nonsignificant decreases in older children. Thus, the significant role of pneumococcus as a cause of childhood CAP drives the choice of empiric antibiotic therapy for younger children.
Pathogenesis
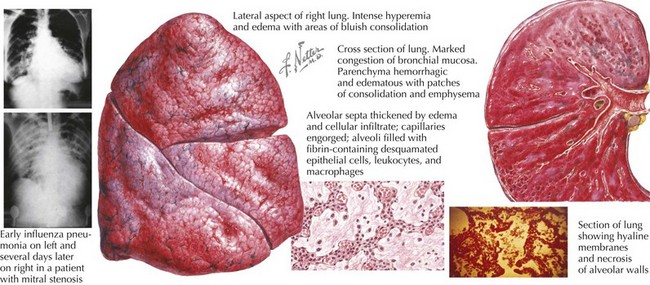
Viral illness alone, such as influenza, can cause severe and necrotizing pneumonia (Figure 91-2). Preceding viral illness may play a part in the pathogenesis of bacterial pneumonia. One study demonstrated that rates of invasive pneumococcal disease each winter season rose in close association with respiratory viral illness diagnoses (RSV, influenza, and hMPV), suggesting that the respiratory damage caused by viral respiratory illness may allow for subsequent bacterial pneumonia. Such data do not prove the direct causation of invasive bacterial pneumonia as a consequence of viral respiratory infection, however. Likewise, a randomized trial of children receiving a pneumococcal vaccine found fewer admissions for both pneumococcal pneumonia and hMPV pneumonia among vaccine recipients compared with placebo recipients, suggesting that hospitalizations for hMPV may involve co-infection with pneumococcus.
Clinical Presentation and Differential Diagnosis
The differential diagnosis of lower respiratory infection includes pulmonary anatomic abnormalities, foreign bodies and chemical irritants, autoimmune diseases, and malignancies, among others (Box 91-1).
Evaluation and Management
Diagnostic Methods
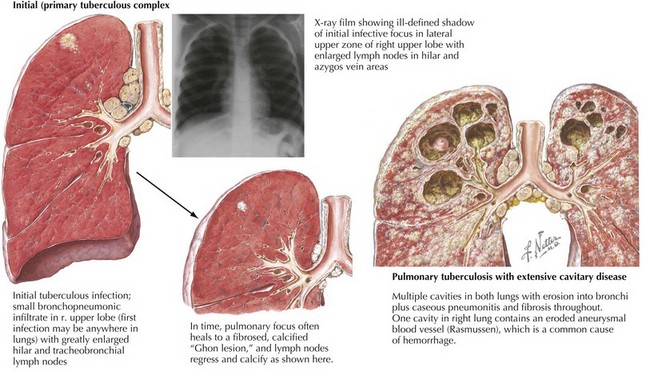
Tuberculin skin testing should be considered for all patients with appropriate tuberculosis risk factors (i.e., travel to an endemic area, contact with a person with active tuberculosis, or contact with people who work or reside in prison or health care settings) and chest radiography findings (Figure 91-3). Newer diagnostic methods that measure serum interferon-γreleased by T-cells stimulated by M. tuberculosis antigens are becoming available but are not validated in children, and they cannot distinguish latent from active disease. Of note, children younger than 2 years of age are more likely to have nonspecific chest radiography findings and disseminated or miliary tuberculosis at presentation.
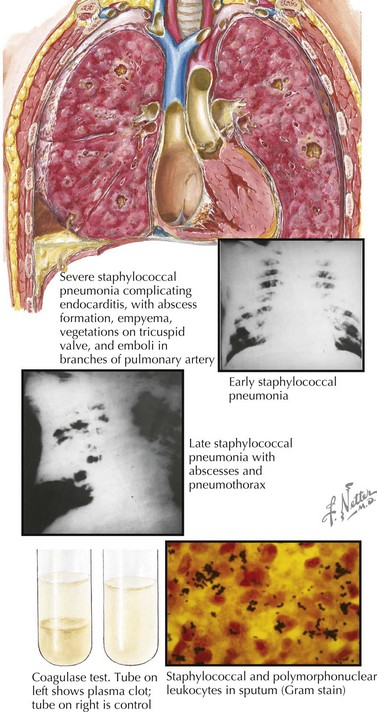
Chest radiography should be considered in highly febrile patients without another identifiable source, especially those with tachypnea or a peripheral blood leukocytosis. Specific chest radiography findings in patients with lower respiratory infection can suggest the etiology. Lobar pneumonia is most common with bacterial pneumonia, although atypical bacterial pathogens occasionally cause lobar infiltrates. Patchy bronchopneumonia with or without effusion or empyema can be seen with S. aureus infection (Figure 91-4). Interstitial infiltrates on chest radiography are most frequently seen with viral and atypical bacterial pathogens such as M. pneumoniae or L. pneumophila. Simple parapneumonic effusions can be seen with almost any pathogen, and severe bacterial pneumonias can also be complicated by complex pleural effusions or empyemas. Hilar lymphadenopathy and nodular disease suggest unusual etiologies such as M. tuberculosis; P. jiroveci; and endemic mycoses such as Histoplasma, Coccidioides, or Blastomyces spp. Pneumatoceles are air-filled cavities caused by alveolar rupture that can be visualized on chest radiography and can be seen in S. aureus pneumonia (and occasionally in pneumonia caused by enteric gram-negative bacilli). More detailed imaging with chest computed tomography (CT) should be sought if an underlying pulmonary malformation (e.g., a congenital cystic adenomatoid malformation) is being considered. Bilateral decubitus chest radiographs are also useful in diagnosing aspiration of radiolucent foreign bodies: the foreign body will act as a ball-valve mechanism, trapping air in the affected lung and allowing it to remain paradoxically inflated when it is in the dependent (and therefore typically deflated) position.
Treatment
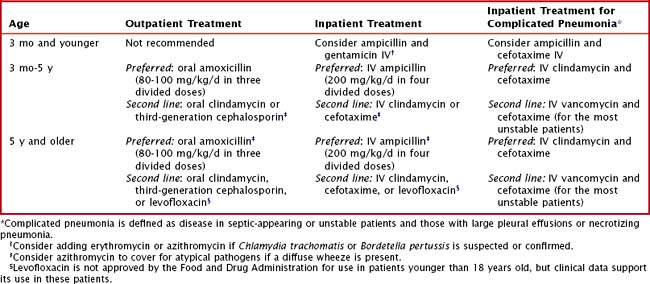
Distinguishing between viral and bacterial pneumonia clinically can be extremely difficult. It is therefore reasonable to provide empiric antimicrobial therapy to outpatients with a clinical diagnosis of pneumonia. Amoxicillin remains the drug of choice for outpatient pediatric pneumonia (Table 91-2); there are no data to support the choice of empiric standard-dose amoxicillin versus high-dose amoxicillin. If chosen, high-dose amoxicillin or ampicillin should have continued efficacy against resistant S. pneumoniae, whose resistance is mediated by alterations in penicillin-binding proteins and can be overcome at higher drug concentrations.
Ampofo K, Bender J, Sheng X, et al. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122:229-237.
Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810-815.
Cardoso MR, Nascimento-Carvalho CM, Ferrero F, et al. Penicillin-resistant pneumococcus and risk of treatment failure in pneumonia. Arch Dis Child. 2008;93:221-225.
Grant GB, Campbell H, Dowell SF, et al. Recommendations for treatment of childhood non-severe pneumonia. Lancet Infect Dis. 2009;9:185-196.
Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179-1186.
Haider BA, Saeed MA, Bhutta ZA: Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2:CD005976, 2008.
Kabra SK, Lodha R, Pandey RM: Antibiotics for community acquired pneumonia in children. Cochrane Database Syst Rev 3:CD004874, 2006.
Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455-1463.
Madhi SA, Ludewick H, Kuwanda L, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193:1236-1243.
McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701-707.