Chapter 8 Pleural Tumors
Introduction
MPM is an uncommon neoplasm arising from mesothelial cells of the pleura. The annual incidence is 3000 cases in the United States. The worldwide figure is expected to increase in the coming decade owing to the patterns of occupational exposure to asbestos and latency period of up to 50 years.1 There is currently no universally accepted standard therapy for MPM and the prognosis is poor, with a median survival of 9 to 17 months after diagnosis.2 However, important advances in the treatment of patients with MPM have occurred over the past few years, including a unified staging system, novel targeted agents, improved radiation therapy techniques for local control, and decreased morbidity and mortality in patients who undergo curative surgical resection.1,3 Furthermore, multimodality regimens combining chemotherapy, radiotherapy, and surgery are being used more frequently because of the failure of single-modality therapy. In cases of limited disease, there has been an increasing tendency to perform surgical resection as part of the treatment algorithm. Extrapleural pneumonectomy (EPP), the removal of the visceral and parietal pleura, ipsilateral lung, hemidiaphragm, and part of the pericardium, is the surgical treatment of choice in the 10% to 15% of patients who present with resectable disease and is reported to prolong survival (74% 2-year survival and 39% 5-year survival).4 The greatest survival benefit in patients with MPM after EPP is seen in those with epithelial histology, a primary tumor that is limited in extent, and no nodal metastases. Conversely, patients with sarcomatoid histology and nodal metastases have a poor survival benefit after EPP and are typically primarily treated with palliative chemotherapy.5
Epidemiology and Risk Factors
MPM occurs more frequently in men than in women with a ratio of 4:1; however, the incidence in women is increasing.6 Peak incidence occurs in the sixth to seventh decades of life and is associated with a history of occupational exposure to asbestos in 40% to 80% of patients.7 In asbestos workers, the incidence of MPM is 10%.8 In contrast, the incidence of MPM in the general population is lower, estimated at 0.01% to 0.24%.7,8
Asbestos, a collective term for a group of complex hydrated silicates, has varying degrees of carcinogenicity. MPM develops after a latent period of up to 50 years from exposure to asbestos. There are two principal forms of asbestos: long, thin fibers known as amphiboles (amosite and crocidolite) and serpentine fibers known as chrysotile. The risk is low if exposed to chrysotile asbestos only. There is a dose-response relationship between crocidolite asbestos exposure and MPM. The exposure-specific risk of MPM from the three principal commercial asbestos types is approximately 1:100:500 for chrysotile, amosite, and crocidolite.9 Chrysotile accounts for approximately 80% of the asbestos used in the Western world. Occupations at highest risk include insulation work, asbestos production and manufacture, heating industry, shipyard work, construction, and automotive brake-lining manufacture and repair.7 Based on regulation by environmental regulatory bodies and the latency period, predictions for a MPM peak in the United States was 2004; Australia, 2015; Europe, 2020; and Japan, 2025.10 However, these predictions did not include many unanticipated factors such as the World Trade Center attack in 2001 in which an estimated 10 million New Yorkers were exposed to asbestos dust.11,12
Because 20% of MPM patients do not have an exposure to asbestos, alternative factors are presumed to be involved. Simian virus 40 (SV40), a DNA virus, has been implicated as a cofactor in the cause of MPM. SV40 nucleic acids have been documented in a proportion of MPM cases.13 This virus blocks tumor suppressor genes and is a potent oncogenic virus in human and rodent cells.
Molecular biologic features of angiogenesis in MPM are important in the development of novel therapeutic strategies. MPM cells produce many growth factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF08-β).14–16 MPM expresses the highest known levels of vascular endothelial growth factor (VEGF) of any solid tumors. VEGF expression in MPM is associated with poor survival and is now considered to be an independent prognostic factor in MPM. There is a positive correlation between VEGF expression and tumor stage (P < .05).17 VEGF inhibitors have been shown to reduce MPM growth in animal models. Studies on the use of antiangiogenesis agents to target the VEGF pathway are ongoing. These agents include PTK787, an inhibitor of the PDGF/VEGF pathway, and bevacizumab, a recombinant human anti-VEGF monoclonal antibody.16 Genetic alterations in tumor-suppressor genes such as p16, p14, and NF2 are common, and the activity of the antiapoptosis molecule Bcl-xL is elevated in MPM.12,18 Furthermore, MPM cells usually express telomerase, enabling cells to be resistant. In addition, interleukin-8, a potent chemokine with proangiogenesis function, has been shown to be an autocrine growth factor in MPM cell lines.16
Anatomy and Pathology
The anatomy of the pleura is complex. The inferior margins of the pleura in the posterior costodiaphragmatic recesses of the hemithorax extend considerably lower than the corresponding border of the lung, to the level of the T12 vertebra. The diaphragm extends more inferiorly and arises from the anterolateral surfaces of the upper three lumbar vertebrae. Macroscopically, the affected lung is covered by a thick layer of soft, gelatinous, grayish-pink tumor. Microscopically, MPM is classified into three histologic categories that provide a foundation for prognosis and therapy, forming a critical basis for epidemiologic and clinical studies. These categories are epithelial (55-65%), sarcomatoid (10-15%), and mixed or biphasic (20-35%).19 The desmoplastic variant is considered a subtype of sarcomatous diffuse MPM. The epithelial type consists of cuboidal or polygonal cells with abundant pink cytoplasm and uniform round nuclei forming a tubular and papillary structure. The sarcomatoid or mesenchymal type of MPM consists of sheets of spindle cells of variable size, cellularity, and pleomorphism. The mixed type of MPM contains both epithelial and sarcomatoid patterns. The World Health Organization (WHO) classification requires that 10% or more of each component be present to fit the classification of biphasic.20 Special features of MPM include positive staining for acid mucopolysaccharide, strong staining for keratin proteins, and on electron microscopy, the presence of long microvilli and abundant tonofilaments but absence of microvillous rootlets and lamellar bodies. To differentiate epithelial MPM from adenocarcinoma, immunohistochemistry panels are useful. Epithelial MPM cells are positive for certain keratin proteins (AE1/AE3, CK5/6, CK7), calretinin, WT-1, D2-40, HBMe1, mesothelin, and thrombomodulin and negative for many markers including pCEA, TTF1, CD15(Leu-M1), BerEp4, B72.3, BG-8, and MOC-31.20 In contrast, immunohistochemistry is less helpful in sarcomatoid diffuse MPM.
Cytologic evaluation of pleural fluid (26% sensitivity) and needle aspiration biopsy (20.7% sensitivity) are inadequate to diagnose MPM.21 If tumor cells are present, distinguishing MPM from metastatic adenocarcinoma or severe atypia can be difficult. In contrast, image-guided core needle biopsy to obtain larger tissue samples has been shown to improve diagnostic accuracy (77% with ultrasound guidance and 83% with CT guidance).22 When a larger diagnostic specimen is needed, a Cope needle biopsy, video-assisted thoracoscopic surgery (VATS), or open biopsy is performed. VATS has a diagnostic rate of 98% and is becoming the preferred method of diagnosis. However, this procedure has two disadvantages: the visceral and parietal layers of the pleura must not be adherent and chest wall seeding occurs in up to one half of the patients.6,21 In contrast, chest wall seeding occurs in 22% of image-guided biopsies.22 To prevent tumor growth within biopsy sites, trocar ports, thoracoscopic tracts, and chest tube tracts, patients undergoing EPP typically have these tracts resected. In addition, local radiation therapy can be used to prevent chest wall seeding.
Key Points Anatomy and pathology
• MPM involves parietal and visceral pleural surfaces and extends into the interlobar fissures, along the diaphragm, mediastinum, and pericardium.
• Tumor can invade lung and peritoneum.
• MPM is divided into three histologic categories: epithelial (55-65%), sarcomatoid (10-15%), and mixed or biphasic (20-35%).
Patterns of Tumor Spread
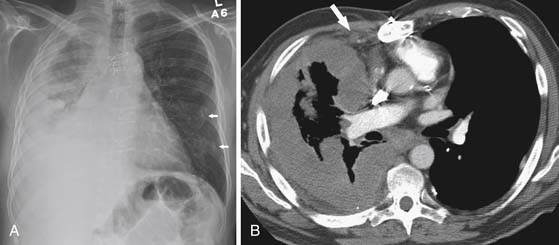
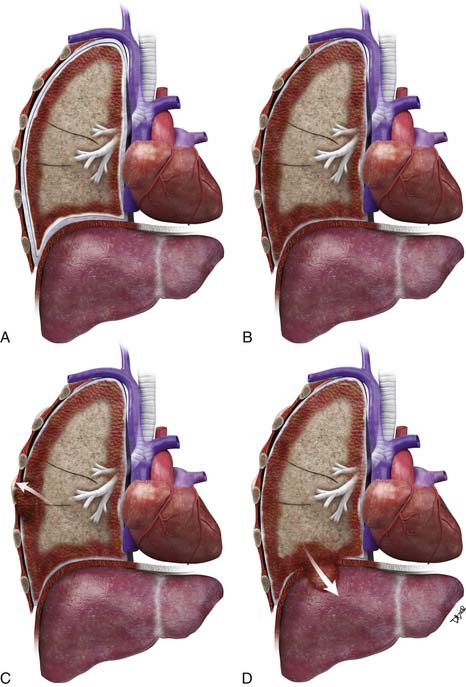
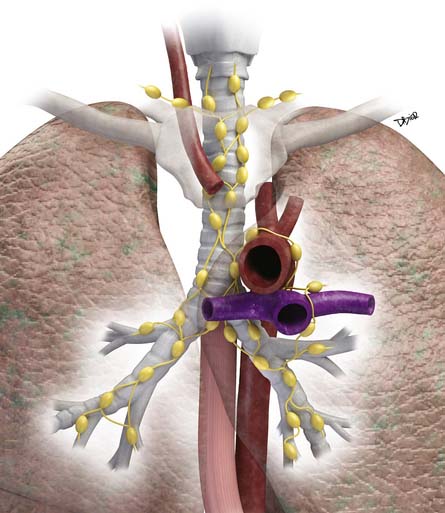
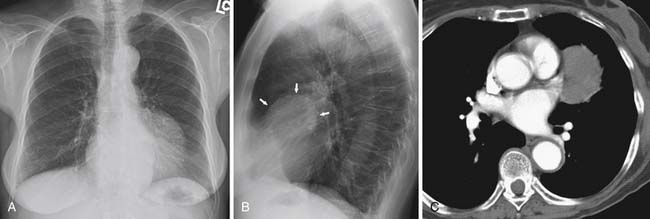
Lymphatic dissemination is common and mediastinal nodes are involved in 50% of cases. To understand the lymphatic spread of MPM, it is essential to examine the complex lymphatic drainage system of the pleura. The visceral pleural lymphatics follow the same drainage pattern as the lungs. However, the parietal pleural lymphatic drainage system is different. The anterior parietal pleura drains into the internal mammary lymph nodes (Figure 8-1). The posterior parietal pleura drains into the extrapleural/intercostal lymph nodes, which are located in the paraspinal fat adjacent to the heads of the ribs (Figure 8-2). The anterior and lateral diaphragmatic lymphatics drain into the internal mammary and anterior diaphragmatic lymph nodes. The posterior diaphragm drains into the para-aortic and posterior mediastinal lymph nodes. There are free anastomoses between lymphatics on both surfaces of the diaphragm, including the retrocrural, inferior phrenic and gastrohepatic space, and the region of the celiac axis.
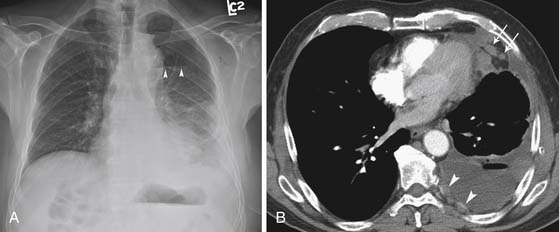
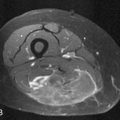
Distant hematogeneous metastases are common and can involve the lungs, liver, spleen, adrenals, lymph nodes, bones, and brain (Figure 8-3). Extrathoracic metastatic disease has been documented at autopsy in 50% to 80% of cases.23
Key Points Tumor spread
• Local spread involves the parietal and visceral pleura, extends to interlobar fissures, and along the diaphragm, mediastinum, and pericardium.
• Owing to the complex drainage system of the pleura, evaluation of nodal disease in the extrapleural/intercostal, internal mammary, diaphragmatic, and upper abdominal regions is essential.
• Mediastinal nodal disease is seen in 50% of cases.
• Transdiaphragmatic invasion can result in spread to the peritoneum, liver, and spleen.
• Hematogeneous dissemination occurs in 50% to 80% at autopsy.
Staging Evaluation
Multiple staging systems have been proposed for MPM.24,25 In an attempt to distinguish patients who would benefit from surgical resection from those needing palliative treatment, the International Mesothelioma Interest Group (IMIG) staging system for MPM was proposed and is gaining universal acceptance (Tables 8-1 and 8-2).26 This system describes the extent of tumor according to a traditional tumor-node-metastasis (TNM) classification: local extent of the primary tumor (T descriptor), the presence and location of lymph node involvement (N descriptor), and the presence or absence of distant metastatic disease (M descriptor) (Figures 8-4 and 8-5). This system stratifies patients into categories with similar prognoses in an effort to select homogeneous groups of patients for entry into clinical trials to better assess new treatment options. Primarily to identify patients who are potentially resectable, this staging system uses criteria to determine the extent of local tumor and regional lymph node status, two factors that have been shown to be related to overall survival rate.26,27 The presence of advanced locoregional primary tumor (T4), N2-N3 disease (mediastinal, internal mammary, and supraclavicular lymph nodes), and M1 disease preclude surgery. However, staging using imaging modalities such as CT, MRI, and PET has limitations. This limitation together with the morbidity and mortality associated with EPP has resulted in the need for extended surgical staging (ESS) in patients being evaluated for resection. In our institution, cervical mediastinoscopy or endobronchial ultrasound-guided lymph node biopsy, laparoscopy, and peritoneal lavage are routinely performed in MPM patients undergoing preoperative evaluation. Rice and coworkers28 reported that ESS precluded 15 of the 118 patients (12.7%) assessed by clinical staging alone to be candidates for EPP.
Table 8-1 Tumor-Node-Metastasis International Staging System for Diffuse Malignant Pleural Mesothelioma
| T—Primary Tumor | |
| T1a | Tumor limited to ipsilateral parietal pleural, including mediastinal and diaphragmatic pleura |
| No involvement of visceral pleura | |
| T1b | Tumor involving ipsilateral parietal pleura, including mediastinal and diaphragmatic pleura |
| Scattered foci of tumor also involving visceral pleura | |
| T2 | Tumor involving each ipsilateral pleural surface with at least one of the following features: |
Tumor involving all of ipsilateral pleural surfaces with at least one of the following:
Tumor involving all of ipsilateral pleural surfaces with at least one of the following:
• Diffuse extension or multifocal masses of tumor in chest wall, with or without associated rib destruction
• Direct transdiaphragmatic extension of tumor to peritoneum
• Direct extension of tumor to contralateral pleura
• Direct extension of tumor to one or more mediastinal organs
• Direct extension of tumor into spine
• Tumor extending through to internal surface of pericardium with or without pericardial effusion or tumor involving myocardium
Table 8-2 Staging Classification of Stage by Tumor-Node-Metastasis Description
| STAGE | DESCRIPTION |
|---|---|
| Ia | T1aN0M0 |
| Ib | T1bN0M0 |
| II | T2N0M0 |
| III | Any T3M0 |
| Any N1M0 Any N2M0 |
|
| IV | Any T4 |
| Any N3 Any M1 |
It is important to note that imaging is inaccurate in determining the true extent of MPM. When compared with surgical staging, CT has been shown to underestimate the extent of disease in patients with early chest wall involvement, small positive lymph nodes, transdiaphragmatic extension, peritoneal implants, and abdominal organ metastases less than 2 mm in size.29 Despite these limitations, CT with its easy accessibility and cost-effectiveness remains the imaging modality of choice in the initial staging and follow-up of patients with MPM.
T Staging
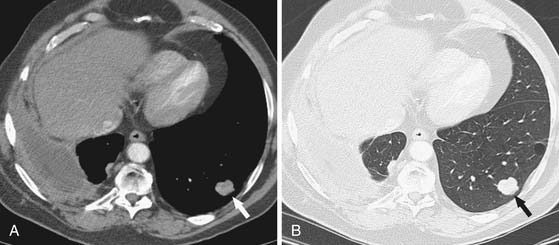
Accurate T staging is emphasized by the IMIG primarily to determine resectability.26 In patients with locally advanced tumors, radiologic imaging is usually directed at distinguishing T3 disease (a solitary focus of chest wall involvement, involvement of the endothoracic fascia, mediastinal fat extension, or nontransmural pericardial involvement) from nonresectable (T4) disease (diffuse tumor extension or multiple chest wall foci; direct extension to the mediastinal organs, spine, internal pericardial surface, or contralateral pleura; and transdiaphragmatic invasion) (Figure 8-6). However, the parameters for T staging are pathologic descriptors that are often difficult to determine by CT and MRI.
In locally advanced (T4) disease, the poor accuracy of older CT in assessing transdiaphragmatic extension of MPM is due to its inability to detect small volume and microscopic invasion as well as the inherent limitation of axial imaging to delineate the diaphragm from the primary pleural tumor. With the use of multidetector row computed tomography (MDCT), PET/CT imaging allows high-resolution multiplanar reconstruction to better evaluate the diaphragm. However, the accuracy of PET/CT is also suboptimal in detecting subtle transdiaphragmatic extension. Because of the limitation of imaging, preoperative laparoscopy is routinely performed in our institution in patients being evaluated for EPP. In the study by Rice and coworkers,28 laparoscopy identified 10/109 patients (9%) with transdiaphragmatic invasion or peritoneal metastases compared with 3/109 patients identified by cross-sectional imaging. Importantly, laparoscopy even identified transdiaphragmatic extension in patients with minimal peridiaphragmatic tumor on CT.
N Staging
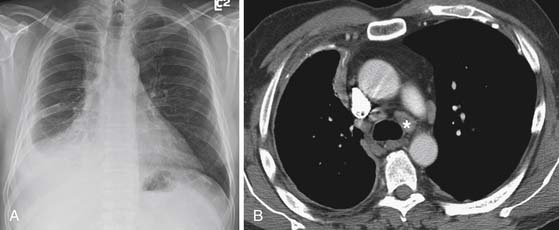
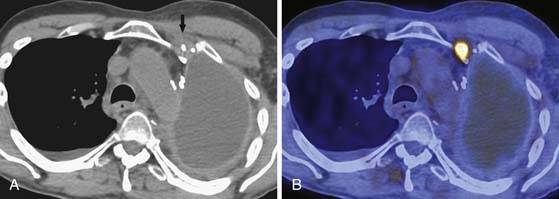
The N descriptor defines the presence and location of nodal metastases (see Table 8-1). Large retrospective studies have shown that up to 50% of patients with MPM who undergo EPP have intrathoracic nodal metastases.30 The accurate detection of intrathoracic nodal metastases is important because survival is poor in patients with mediastinal, supraclavicular, and internal mammary nodal metastases and the presence of N2-N3 disease would preclude curative resection. CT is almost uniformly used to evaluate for the presence or absence of hilar and mediastinal nodal metastases. However, although CT is accurate in demonstrating enlarged nodes, the specificity for metastases is less than optimal because metastases can be present in small nodes and enlarged lymph nodes can be hyperplastic (Figures 8-7 and 8-8). In addition, when compared with other intrathoracic malignancies, the lymphatic pattern of spread of MPM is complex with multiple drainage systems and detection of nodal metastases is suboptimal. Because the survival of patients with extrapleural nodal involvement has been reported to be poor, assessment by invasive sampling prior to EPP has been suggested to be important in patient selection.5 Complete nodal evaluation by mediastinoscopy or endobronchial ultrasound-guided lymph node biopsy, although important, lacks the required sensitivity and specificity to determine appropriate management of MPM. In a study at our institution, mediastinoscopy had a sensitivity of only 36% for intrathoracic (N2) nodal metastases detected at surgery.28 Schouwink and colleagues31 performed mediastinoscopy in 43 patients with MPM and compared the staging accuracy with that of CT. Sensitivity, specificity, and accuracy were 80%, 100%, and 93% for mediastinoscopy compared with 60%, 71%, and 67% for CT. Furthermore, it is important to note that not all nodal stations are accessible at mediastinoscopy.5
The role of PET in the detection of mediastinal nodal metastases, particularly for nodal stations not accessible by mediastinoscopy, can aid in the preoperative evaluation of patients considered for EPP.32–34 However, Flores and associates35 reported a sensitivity of only 11% for PET imaging in the detection of nodal metastases in patients with MPM. The low sensitivity of PET in their study may be due in part to PET findings not being correlated with CT. However, in a study at our institution, integrated PET/CT imaging was also found to be inaccurate in the evaluation of nodal MPM metastases. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET/CT in lymph node staging in patients with N2 disease was 38%, 78%, 60%, 58%, and 59%, respectively.36
In the detection of nodal disease, PET/CT is limited not only by the false-negative results in patients with microscopic disease below the resolution of PET but also by the false-positive results in patients with fluoro-2-deoxy-D-glucose (FDG)–avid inflammatory/infectious etiologies. These potential pitfalls can lead to misinterpretation and have implications for management. Thus, we advocate sampling of all FDG-avid nodes in patients with MPM being considered for EPP.
M Staging
Distant metastases have historically been considered to be an uncommon late manifestation of MPM.37 The poor prognosis and rapid demise of patients together with the lack of an effective medical therapeutic option or potentially curative surgical resection in the past negated the need for accurate determination of the presence or absence of distant metastases. These distant metastases are now considered to occur more commonly than previously reported and can be solitary or diffuse with involvement of brain, lung, bone, adrenal, peritoneum, abdominal nodes, and abdominal wall.
There are few reports of the use of PET in detecting extrathoracic metastases in patients with MPM.33,35,36 In one study, PET identified occult extrathoracic metastases in 2 of 18 patients (11%), precluding surgical resection.33 In a study at our institution, integrated PET/CT detected extrathoracic metastases in approximately 25% of patients being evaluated for EPP.36 Importantly, in more than half of these patients, extrathoracic metastases were not identified by routine clinical and conventional radiologic evaluation. Interestingly, others have reported that distant metastasis can be the initial site of relapse after EPP, and this could reflect limitations in conventional staging with CT.38 Our experience suggests that distant MPM metastases that develop soon after EPP may have been present at the time of surgery but were not detected by conventional staging. Improvement in the accuracy of M staging with PET/CT can lead to more appropriate selection of patients for EPP and decrease the number of patients with early recurrence of MPM. In addition, co-registration of PET/CT data allows precise anatomic localization of areas of increased FDG uptake and can be useful in guiding biopsy of these sites.
Imaging
Chest Radiography
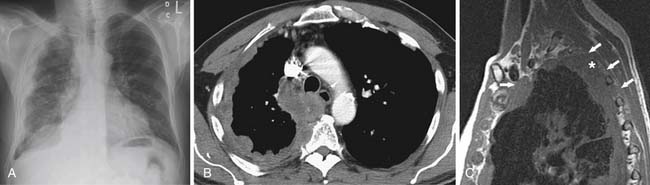
Radiographic evaluation of patients with MPM typically shows a unilateral pleural abnormality. A pleural effusion is seen in 30% to 80% of patients with MPM.39 In 45% to 60% of patients, a smooth lobular pleural mass is demonstrated.39 Diffuse unilateral pleural thickening occurs in up to 60% of patients with MPM.39 The pleural thickening can form a rind and grow into the fissures. As the tumor grows, there is encasement of the lung with signs of volume loss on the affected side, including ipsilateral shift of the mediastinum, elevation of the hemidiaphragm, and narrowing of the intercostal spaces. If the pleural tumor is bulky, there can be contralateral shift of the mediastinum. It is important to be aware that calcified pleural plaques occur in only 20% of patients, and consequently, the absence of pleural plaques should not be used to exclude MPM in patients presenting with a pleural abnormality.40
Radiographic evaluation of local extent of disease and identification of metastases is not sensitive or specific. For instance, although periosteal reaction along the ribs, rib erosion, or destruction has been described as a manifestation of chest wall invasion, these findings are not common (20%).39 In addition, hilar enlargement and mediastinal widening can be due to direct tumor invasion or metastatic adenopathy. Because findings in the lungs can be obscured by the pleural disease on chest radiography, CT better evaluates pulmonary fibrosis and metastases.
Computed Tomography
CT is more sensitive than radiography in the detection of early abnormalities in patients with MPM. CT features of MPM include unilateral pleural effusion (74%) and nodular pleural thickening (92%), which can be discrete or diffuse with involvement of the fissures.41 In one study, pleural fluid filled up to one third of the hemithorax in 50% of patients with MPM, up to two thirds of the hemithorax in 40%, and more than two thirds of the hemithorax in 10%.41 As the tumor grows to form a pleural rind, circumferential encasement of the lung results in volume loss in the ipsilateral hemithorax in 42% of patients.41 The signs of volume loss include ipsilateral mediastinal shift, elevation of the ipsilateral hemidiaphragm, and narrowing of the intercostal spaces. However, contralateral shift of the mediastinum has been described in 14% of patients due to bulky disease and/or large pleural effusions.41
CT is valuable in evaluating the extent of disease at initial staging. CT can assess for involvement of the chest wall, diaphragm, and mediastinum. CT features of local chest wall invasion include obliteration of extrapleural fat planes, invasion of intercostal muscles, displacement of ribs by tumor, and bone destruction. The finding of irregularity of the interface between the chest wall and the tumor has not been found to be a reliable indicator of chest wall invasion.42
Diaphragmatic invasion is suspected when a soft tissue mass encases the hemidiaphragm. Conversely, a clear fat plane between the inferior diaphragmatic surface and the adjacent abdominal organs and a smooth diaphragmatic contour suggest that the disease is limited to the thorax and does not extend through the diaphragm.42 Scanning in the axial plane has inherent limitation in the assessment of the inferior surface of the diaphragm. However, the advent of MDCT and the capability for multiplanar re-formation has improved evaluation of the diaphragm.
Mediastinal involvement includes local invasion of vascular structures and mediastinal organs. Direct mediastinal extension results in obliteration of mediastinal fat planes. CT evidence of invasion of vascular structures and mediastinal organs such as the great vessels, esophagus, and trachea is suggested when a soft tissue mass surrounds more than 50% of the structure.42 Pericardial invasion is characterized by nodular pericardial thickening with or without a pericardial effusion.
Mediastinal lymph node involvement can be due to direct invasion or metastatic spread. Intrathoracic nodal disease is reported in 34% to 50% of patients with MPM.29,43,44 Although CT is the most frequently used modality to evaluate thoracic lymph nodes, it can be difficult or impossible to distinguish hilar or mediastinal lymph nodes as separate structures from the pleural tumor. Similarly, irregular pleural thickening along the mediastinal surface can obscure enlarged mediastinal lymph nodes. The accuracy of CT in the assessment of mediastinal nodal disease remains low45 and mediastinoscopy is indicated when patients are considered for surgical resection in our institution. In this regard, the diagnostic accuracy of cervical mediastinoscopy is 93% compared with 67% for CT.31
CT is also useful in evaluation of the lungs, often obscured by pleural masses or effusions in patients with MPM on chest radiographs. Hematogenous metastases to the lungs can manifest as nodules, masses, and rarely, diffuse military disease. Lymphangitic spread of tumor presents as focal or diffuse nodular interlobular septal thickening. In addition to revealing pulmonary neoplastic involvement, CT can also demonstrate pulmonary fibrosis caused by prior exposure to asbestos. The pulmonary fibrosis adjacent to the heart produces the “shaggy heart” appearance of asbestosis.46
Magnetic Resonance Imaging
MRI has been shown to be superior to CT in staging evaluation of areas of local invasion in two sites: the endothoracic fascia/single chest wall focus (accuracy 69% vs. 46%) and the diaphragm (accuracy 82% vs. 55%).45 Thus, MRI can be used to assess diaphragmatic involvement when CT findings are equivocal. However, in our institution, patients do not undergo routine MRI evaluation of the diaphragm. This is because the accuracy of MRI is not optimal for diagnosing subtle transdiaphragmatic extension. Instead, laparoscopy is performed in patients considered for EPP to evaluate for transdiaphragmatic extension and peritoneal disease. The rationale for performing laparoscopy is that direct visualization of the undersurface of the diaphragm can detect small volume disease. In addition, peritoneal lavage performed concurrently can detect unsuspected peritoneal metastases. This is particularly important in view of the significant morbidity and mortality rate of extrapleural pneumonectomy.
Positron Emission Tomography
Another potentially valuable tool in the preoperative assessment of patients with MPM is PET. PET imaging of malignancies is typically performed with the radiopharmaceutical FDG, a D-glucose analogue. Increased glucose metabolism by malignant cells results in increased uptake and accumulation of FDG, allowing diagnosis, staging, and assessment of treatment response. However, the role of FDG-PET in the staging of MPM has not been fully elucidated. In our experience, integrated PET/CT (the integration of functional PET data with anatomic CT data) has improved diagnostic accuracy in the staging of patients with MPM. A small study comparing FDG-PET imaging with CT performed in 18 patients with MPM showed that PET detected occult metastases in 2 patients being considered for surgical resection.33 In addition, because FDG-PET provides information on metabolically active sites of disease, this modality can be used in conjunction with anatomic imaging to select the most appropriate area for biopsy.47 Integrated PET/CT allows more precise anatomic localization of disease and is useful in detecting nodal and systemic metastatic disease.36,48 However, the ability of PET/CT to correctly stage locoregional disease is suboptimal.48 In a study performed at our institution, T staging was accurately determined in 63% of patients undergoing EPP, and 29% of the patients had understaged T disease secondary to locoregional disease not detected by preoperative imaging.36 The strength of PET/CT in staging patients with MPM is in the detection of extrathoracic metastases. In one recent study, integrated PET/CT identified occult metastases in 25% of the patients being evaluated for EPP.36 Importantly, in more than half of these patients, extrathoracic metastases were not identified by routine clinical and conventional radiologic evaluation. In addition, co-registration of PET/CT data allows precise anatomic localization of areas of increased FDG uptake and can be useful in guiding biopsy of these sites.
Novel imaging agents are expected to have an important role in the management of MPM. The use of molecular bioprobes, such as 99mTechnetium-labeled mAb K1 antibodies that bind the mesothelin antigen, can prove useful for imaging MPM in the posttherapy setting. These bioprobes target tumor based on biochemical and physiologic properties rather than structural properties.49
Differential Diagnosis
MPM typically manifests radiologically as a unilateral pleural effusion, moderate to large in size, with or without a pleural mass, or diffuse pleural thickening with or without a pleural effusion. The differential diagnosis of a unilateral pleural effusion is extensive and includes congestive heart failure, infection, subdiaphragmatic disease, pulmonary embolism, and collagen vascular disease. In contradistinction, the differential diagnosis of diffuse nodular pleural thickening is limited and includes MPM, metastatic disease, and in cases with a mediastinal mass, thymic malignancy with pleural metastases. CT features that aid in differentiating malignant from benign pleural disease include pleural thickening with a circumferential distribution encasing the lung (sensitivity 100%, specificity 41%), pleural thickening of greater than 1 cm in thickness (sensitivity 94%, specificity 36%), and nodular morphology (sensitivity 94%, specificity 51%).40
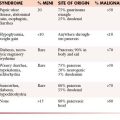
Patients with LFTP range broadly in age from 5 to 87 years with most between 45 and 65 years of age and there is no significant sex predilection. In about half of the cases, patients are asymptomatic and LFTP is detected incidentally at chest radiography. The most common symptoms are cough, chest pain, and dyspnea. Other symptoms can include chills, fever, weight loss, debility, and a sensation of something flopping around in the chest. Symptomatic hypoglycemia is seen in up to 6% of patients. Hypertrophic osteoarthropathy is seen in 17% to 35% of cases.50
Benign and malignant subtypes of LFTP have been described. On histologic examination, the lesion consists of ovoid or spindle-shaped cells with round to oval nuclei, an evenly distributed fine chromatin, inconspicuous nucleoli, and bipolar faintly eosinophilic cytoplasm with indistinct cell borders separated by collagen. Based on the presence of more than four mitotic figures per 10 high-power-fields, these tumors are classified as malignant. On macroscopic examination, the tumor arises from the visceral pleura. Pedunculation is present in approximately 50%, and owing to the presence of the stalk, which can be up to 9 cm in length, LFTP can be mobile. Radiologically, LFTP has classic features of an extraparenchymal mass (Figure 8-9). On cross-sectional imaging, they have a well-defined lobular contour with heterogeneous attenuation. Surgical resection is curative in the majority of patients, although a small number can recur, undergo malignant transformation, or metastasize. The prognosis for patients with LFTP is generally favorable. The majority of lesions behave in a benign manner (88%), but approximately 12% of patients die of extensive intrathoracic tumor growth or unresectable recurrence.50
Key Points The radiology report
• For resectability, it is important to distinguish T3 disease (a solitary focus of chest wall involvement, involvement of the endothoracic fascia, mediastinal fat extension, or nontransmural pericardial involvement) from nonresectable (T4) disease.
• Presence of N3 disease (contralateral mediastinal, contralateral internal mammary, and supraclavicular lymph nodes) and metastasis M1 precludes surgery.
• N2 disease also precludes curative resection because survival is poor in patients who undergo EPP.
Treatment
Single-modality approaches to treating malignant pleural mesothelioma (i.e., surgery, chemotherapy, or radiation) alone failed to effectively extend survival.51 Thus, an aggressive, multimodality treatment strategy has been developed that combines complete macroscopic resection with some form of additional therapy to prevent local recurrence by addressing the microscopic residual disease.5 This strategy remains the only treatment option for prolonging survival beyond the current median of 7 months without treatment, and the only means of producing long-term survivors among select patients with favorable prognostic factors.
Currently, the standard of care for first-line systemic therapy is cisplatin plus pemetrexed. Pemetrexed, a multitargeted antifolate, in combination with cisplatin has been reported in a multicenter phase III study of 448 patients to have an objective response rate of 41% and improve overall survival by 3 months.3 In addition, both gemcitabine and vinorelbine have demonstrated activity in this disease, alone or in combination with cisplatin. Newer agents aimed at the inhibition of targets, such as VEGF or histone deacetylase (HDAC), are undergoing further investigation. A phase II trial that randomized previously untreated patients to receive cisplatin plus gemcitabine, with or without bevacizumab, a humanized monoclonal antibody directed against VEGF, did not result in significant improvements in either response (25% vs. 22%), median progression-free survival (PFS) (6.9 vs. 6.0 mo), or median overall survival (15.6 vs. 14.7 mo) compared with chemotherapy alone.52 However, subset analysis did show a correlation between higher baseline plasma VEGF levels and shorter PFS and overall survival, suggesting a basis for further investigation in a more selected population.52
Surgical options include EPP and pleurectomy and decortication (P/D). EPP is the radical en bloc resection of the lung, pleura, diaphragm, and pericardium. Fusion of the pleura at the central tendon of the diaphragm and the lateral portion of the pericardium mandates resection and subsequent reconstruction with a prosthetic patch. P/D is a lung-sparing operation in which the diseased pleural envelope that encases and constricts the lung is mobilized off the chest wall, mediastinum, diaphragm, and pericardium and then meticulously stripped from the surface of the lung. P/D is generally well tolerated with low morbidity. The mortality rate is approximately 1.8% when the procedure is performed at a high-volume center.30 Reports of median survival in the literature range from 9 to 20 months. However, the technical challenge of separating tumor and visceral pleura from the lung parenchyma can result in suboptimal cytoreduction.
The relatively low incidence of the MPM and, therefore, difficulty in patient accrual for clinical trials, particularly large, randomized, prospective trials, pose a challenge in the establishment of standardized treatment protocols. Given this limitation, it is known that, for patients with MPM of epithelial histology, early-stage disease, negative nodes, and adequate pulmonary function, EPP and adjuvant therapy are most likely to prolong survival (68% 2-yr survival and 46% 5-yr survival).5 Patients who undergo an EPP for definitive surgical management of MPM receive radiation therapy to the entire ipsilateral hemithorax for curative intent. All scars and drain sites are delineated at the time of simulation, because these will be targeted in the radiation field as well. The treatment field is generally defined by the following borders: superiorly, the thoracic inlet; inferiorly, the insertion of the diaphragm (generally, the bottom of L2); laterally, flashing the skin; and medially, the contralateral edge of the vertebral body if no mediastinal disease or 2.0 cm medial to the contralateral edge if mediastinal disease is present.
With these general treatment fields in mind, a novel technique to treat the entire hemithorax after EPP utilizes intensity-modulated radiation therapy (IMRT), which has been developed at M. D. Anderson Cancer Center, with good outcomes.53 In this technique, the region of the removed pleura is contoured by the radiation oncologist in consultation with the surgeon to carefully delineate appropriate target volumes, which include all preoperative pleural surfaces, ipsilateral mediastinal lymph nodes, the retrocrural space, and the deep margin of the thoracotomy incision. Multiple beams and inverse planning are then utilized to treat the region at risk to the same dose of 4500 cGy in 25 fractions while placing dose constraints on normal structures such as the heart, kidney, spinal cord, and stomach (Figure 8-10). However, caution is warranted to limit the contralateral lung to very low doses to prevent the development of severe pneumonitis, because prior retrospective studies have shown that fatal complications can occur.54 Typically, the contralateral lung is constrained to a mean lung dose of less than 800 cGy and restrict the amount of lung receiving 20 Gy or higher to less than 7% (V20 < 7%).
The surgical procedure of P/D has historically been thought to be palliative in nature, but the technique is increasingly utilized for the treatment of this disease owing to recent studies that have shown similar survival and decreased morbidity as EPP in well-selected patients.30 In the past, the radiation treatment field after P/D consisted of targeting high-risk postoperative regions, as determined by postoperative imaging and discussion with the treating surgeon. However, with the advent of IMRT, the possibility of whole pleura radiation in this setting is being explored, such that patients that are not candidates for EPP could still be considered for definitive treatment. A phase I protocol is currently being opened at our institution exploring whole pleura radiation using IMRT after P/D.
Key Points Therapies
• First-line systemic chemotherapy is cisplatin plus pemetrexed.
• EPP and adjuvant therapy are most likely to prolong survival in patients with MPM of epithelial histology, early-stage disease, negative nodes, and adequate pulmonary function.
• IMRT uses multiple beams and inverse planning to treat the region at risk to the same dose of 4500 cGy in 25 fractions while placing dose constraints on normal structures such as the heart, kidney, spinal cord, and stomach.
Treatment Response and Prognosis
Surveillance
In terms of anatomic imaging assessment of treatment response, bidimensional measurement of the tumor based on guidelines from WHO has been replaced by unidimensional longest-diameter measurement of the tumor in a single CT slice that showed the greatest tumor extent based on the Response Evaluation Criteria In Solid Tumors (RECIST) approach.55 The RECIST response criteria categorizes change in tumor diameter between two CT scans as progressive disease if this change reflects an increase in diameter of at least 20%, partial response if this change reflects a decrease in diameter of at least 30%, and stable disease if the change is between these two thresholds. However, because of the unique morphology of the pleural rind of MPM, shortcomings of this approach have led to the proposal of an alternative measurement protocol.56,57 “Modified RECIST” has become standard in MPM, with unidimensional tumor thickness measurements perpendicular to the chest wall or mediastinum measured in two sites at three different levels on CT.56 Axial CT slices used for measurement must be at least 1 cm apart and related to anatomic landmarks in the thorax, preferably above the level of division of the main bronchi. Nodal, subcutaneous, and other measurable lesions are measured unidimensionally as per the RECIST criteria. Unidimensional measurements are added to produce the total tumor measurement with the sum of six pleural thickness measurements forming one univariate diameter.
Alternatively, volumetric tumor analysis can be done by serial segmentation.58 Computerized techniques that quantify tumor volume on CT before, during, and after therapy can aid in the evaluation of tumor regression/progression and assessment of therapeutic response using full three-dimensional volumetric tumor analysis.
In terms of functional imaging, the semiquantitative evaluation of FDG uptake on PET as measured by the standardized uptake value (SUV) has been used as an indicator of prognosis and in the assessment of treatment response.59,60 Low SUV and epithelial histology indicate the best survival whereas high SUV and nonepithelial histology indicate the worst survival.59 In a multivariate analysis of 65 patients with MPM, median survival was 14 and 24 months for the high- and low-SUV groups, respectively. High-SUV tumors were associated with a 3.3 times greater risk of death than low-SUV tumors (P = .03).59 Mixed histology carried a 3.2 times greater risk of death than epithelial histology (P = 0.03).59 Gerbaudo and coworkers61 reported that the intensity of FDG uptake by the primary malignancy had a poor correlation with histologic grade but a good correlation with surgical stage. Furthermore, in this study, the increment of FDG lesion uptake over time was a better predictor of disease aggressiveness than was the histologic grade. The findings from these two small studies suggest that PET can have a role in the stratification of patients with MPM for treatment and clinical trials.
In the assessment of treatment response, PET using the semiquantitative measurement of FDG uptake has been evaluated with direct comparison between the pretreatment and the posttreatment scans.62 The predictive value of PET to assess treatment efficacy after two cycles of single-agent pemetrexed or pemetrexed in combination with carboplatin was evaluated in 20 patients with MPM.62 Ceresoli and colleagues62 showed a significant correlation (P < .05) between early metabolic response and median time-to-tumor progression: 14 months for metabolic responders compared with 7 months for nonresponders. Patients showing metabolic response also had a trend toward longer overall survival. Interestingly, no correlation was found between radiologic response assessed by CT and time-to-tumor progression.
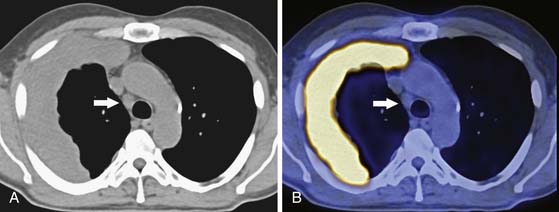
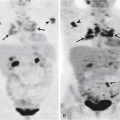
In terms of surveillance, recurrence and/or progressive metastatic disease are usually evaluated by CT scan. Patterns of recurrence include a soft tissue lesion along the resection margin, pericardial effusion/thickening, ascites, peritoneal fat stranding, new pulmonary nodules, and mediastinal adenopathy (Figure 8-11). The emerging role for PET/CT is in the restaging of MPM, in evaluating response to therapy, and as an independent metabolic indicator of prognosis.63,64
Complications of Therapy
Chest radiographs and CT are typically the modalities used to monitor patients for complications due to chemotherapy, radiation therapy, and surgery. Chemotherapy-induced drug toxicity to the lungs is discussed in Chapter 39. In terms of complications of radiation therapy, although IMRT following EPP limits the contralateral lung to very low doses, radiation pneumonitis remains a concern because fatal complications can occur.54
Chest radiographs are usually used to evaluate for complications following surgery for MPM. Typically, the pneumonectomy space begins to fill with fluid generally at the rate of one intercostal space per week. Whereas a sudden increase in fluid can indicate hemothorax or a chylous leak, a decrease in the amount of fluid in the pneumonectomy space can signify the presence of a bronchopleural fistula or leakage of fluid into the abdomen via the diaphragmatic reconstruction. MDCT with the capability for multiplanar re-formats and three-dimensional imaging can help delineate a bronchopleural fistula.
A rare but serious complication after left pneumonectomy is gastric herniation, which can lead to gastric strangulation. This complication can be detected on chest radiographs with the gastric bubble located above the reconstructed left hemidiaphragm. Another rare complication is the postpneumonectomy syndrome.65 This rare syndrome is caused by extreme rotation and shift of the mediastinum after pneumonectomy resulting in symptomatic central airway compression and obstruction.
Following radical pleurectomy, PET/CT is helpful in differentiating the granulation tissue from recurrent tumor because both entities can present as irregular and nodular tissue along the resection margins. Using semiquantitative evaluation of tracer uptake, serial PET/CT can distinguish tumor, which manifests as progressive increase in FDG uptake, from granulation tissue, which remains stable or decreases in FDG avidity over time.66
Key Points Therapy complications
• Chest x-ray and CT are typically used to monitor patients for complications due to therapy: drug toxicity and radiation pneumonitis.
• After EPP, the pneumonectomy space fills with fluid generally at the rate of one intercostal space per week.
• An increase in fluid can indicate hemothorax or a chylous leak.
• Decrease in fluid can indicate bronchopleural fistula or leakage of fluid into the abdomen via the diaphragmatic reconstruction. MDCT can help delineate a bronchopleural fistula.
1. Sugarbaker D.J. Multimodality management of malignant pleural mesothelioma: introduction. Semin Thorac Cardiovasc Surg. 2009;21:95-96.
2. Tsao A.S., Wistuba I., Roth J.A., et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081-2090.
3. Vogelzang N.J., Rusthoven J.J., Symanowski J., et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644.
4. Sugarbaker D.J., Garcia J.P. Multimodality therapy for malignant pleural mesothelioma. Chest. 1997;112:272S-275S.
5. Sugarbaker D.J., Flores R.M., Jaklitsch M.T., et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54-63. discussion 63-65
6. Pisani R.J., Colby T.V., Williams D.E. Malignant mesothelioma of the pleura. Mayo Clin Proc. 1988;63:1234-1244.
7. McDonald A.D., McDonald J.C. Malignant mesothelioma in North America. Cancer. 1980;46:1650-1656.
8. Ribak J., Lilis R., Suzuki Y., et al. Malignant mesothelioma in a cohort of asbestos insulation workers: clinical presentation, diagnosis, and causes of death. Br J Ind Med. 1988;45:182-187.
9. Hodgson J.T., Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg. 2000;44:565-601.
10. Bianchi C., Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379-387.
11. Ismail-Khan R., Robinson L.A., Williams C.C.Jr., et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13:255-263.
12. Robinson B.W., Lake R.A. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591-1603.
13. Gazdar A.F., Butel J.S., Carbone M. SV40 and human tumours: myth, association or causality? Nat Rev Cancer. 2002;2:957-964.
14. Dazzi H., Hasleton P.S., Thatcher N., et al. Malignant pleural mesothelioma and epidermal growth factor receptor (EGF-R). Relationship of EGF-R with histology and survival using fixed paraffin embedded tissue and the F4, monoclonal antibody. Br J Cancer. 1990;61:924-926.
15. Marzo A.L., Fitzpatrick D.R., Robinson B.W., et al. Antisense oligonucleotides specific for transforming growth factor beta2 inhibit the growth of malignant mesothelioma both in vitro and in vivo. Cancer Res. 1997;57:3200-3207.
16. Zucali P.A., Giaccone G. Biology and management of malignant pleural mesothelioma. Eur J Cancer. 2006;42:2706-2714.
17. Demirag F., Unsal E., Yilmaz A., et al. Prognostic significance of vascular endothelial growth factor, tumor necrosis, and mitotic activity index in malignant pleural mesothelioma. Chest. 2005;128:3382-3387.
18. Robinson B.W., Creaney J., Lake R., et al. Soluble mesothelin-related protein—a blood test for mesothelioma. Lung Cancer. 2005;49(Suppl 1):S109-S111.
19. Attanoos R.L., Gibbs A.R. Pathology of malignant mesothelioma. Histopathology. 1997;30:403-418.
20. Chirieac L.R., Corson J.M. Pathologic evaluation of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:121-124.
21. Boutin C., Rey F., Gouvernet J., et al. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: prognosis and staging. Cancer. 1993;72:394-404.
22. Metintas M., Ozdemir N., Isiksoy S., et al. CT-guided pleural needle biopsy in the diagnosis of malignant mesothelioma. J Comput Assist Tomogr. 1995;19:370-374.
23. Pass H.I., Kranda K., Temeck B.K., et al. Surgically debulked malignant pleural mesothelioma: results and prognostic factors. Ann Surg Oncol. 1997;4:215-222.
24. Butchart E.G., Ashcroft T., Barnsley W.C., et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax. 1976;31:15-24.
25. Sugarbaker D.J., Strauss G.M., Lynch T.J., et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol. 1993;11:1172-1178.
26. Rusch V.W. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest. 1995;108:1122-1128.
27. Tammilehto L., Kivisaari L., Salminen U.S., et al. Evaluation of the clinical TNM staging system for malignant pleural mesothelioma: an assessment in 88 patients. Lung Cancer. 1995;12:25-34.
28. Rice D.C., Erasmus J.J., Stevens C.W., et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg. 2005;80:1988-1992. discussion 1992-1993
29. Rusch V.W., Godwin J.D., Shuman W.P. The role of computed tomography scanning in the initial assessment and the follow-up of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1988;96:171-177.
30. Wolf A., Daniel J., Sugarbaker D.J. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg. 2009;21:132-148.
31. Schouwink J.H., Kool L.S., Rutgers E.J., et al. The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg. 2003;75:1715-1718. discussion 1718-1719
32. Nanni C., Castellucci P., Farsad M., et al. Role of 18F-FDG PET for evaluating malignant pleural mesothelioma. Cancer Biother Radiopharm. 2004;19:149-154.
33. Schneider D.B., Clary-Macy C., Challa S., et al. Positron emission tomography with F18-fluorodeoxyglucose in the staging and preoperative evaluation of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2000;120:128-133.
34. Benard F., Sterman D., Smith R.J., et al. Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest. 1998;114:713-722.
35. Flores R.M., Akhurst T., Gonen M., et al. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2003;126:11-16.
36. Erasmus J.J., Truong M.T., Smythe W.R., et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: staging implications. J Thorac Cardiovasc Surg. 2005;129:1364-1370.
37. Antman K.H. Natural history and staging of malignant mesothelioma. Chest. 1989;96:93S-95S.
38. Rusch V.W., Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996;111:815-825. discussion 825-826
39. Wechsler R.J., Rao V.M., Steiner R.M. The radiology of thoracic malignant mesothelioma. Crit Rev Diagn Imaging. 1984;20:283-310.
40. Leung A.N., Muller N.L., Miller R.R. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990;154:487-492.
41. Kawashima A., Libshitz H.I. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol. 1990;155:965-969.
42. Patz E.F.Jr., Shaffer K., Piwnica-Worms D.R., et al. Malignant pleural mesothelioma: value of CT and MR imaging in predicting resectability. AJR Am J Roentgenol. 1992;159:961-966.
43. Grondin S.C., Sugarbaker D.J. Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest. 1999;116:450S-454S.
44. Davis J.M. The pathology of asbestos related disease. Thorax. 1984;39:801-808.
45. Heelan R.T., Rusch V.W., Begg C.B., et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol. 1999;172:1039-1047.
46. Miller B.H., Rosado-de-Christenson M.L., Mason A.C., et al. From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics. 1996;16:613-644.
47. Zubeldia J., Abou-Zied M., Nabi H. Evaluation of patients with known mesothelioma with 18F-fluorodeoxyglucose and PET. Comparison with computed tomography. Clin Positron Imaging. 2000;3:165.
48. Wilcox B.E., Subramaniam R.M., Peller P.J., et al. Utility of integrated computed tomography-positron emission tomography for selection of operable malignant pleural mesothelioma. Clin Lung Cancer. 2009;10:244-248.
49. Lindmo T., Boven E., Cuttitta F., et al. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77-89.
50. Rosado-de-Christenson M.L., Abbott G.F., McAdams H.P., et al. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics. 2003;23:759-783.
51. Boutin C., Schlesser M., Frenay C., et al. Malignant pleural mesothelioma. Eur Respir J. 1998;12:972-981.
52. Karrison T., Kindler H.L., Gandara D.R., et al. Final analysis of a multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients with malignant mesothelioma (MM). Proc Am Soc Clin Oncol. 2007;18:5391.
53. Rice D.C., Stevens C.W., Correa A.M., et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685-1692. discussion 1692-1693
54. Allen A.M., Czerminska M., Janne P.A., et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640-645.
55. James K., Eisenhauer E., Christian M., et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523-528.
56. Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-260.
57. van Klaveren R.J., Aerts J.G., de Bruin H., et al. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;43:63-69.
58. Pass H.I., Temeck B.K., Kranda K., et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1998;115:310-317. discussion 317-318
59. Flores R.M. The role of PET in the surgical management of malignant pleural mesothelioma. Lung Cancer. 2005;49(Suppl 1):S27-S32.
60. Steinert H.C., Santos Dellea M.M., Burger C., et al. Therapy response evaluation in malignant pleural mesothelioma with integrated PET-CT imaging. Lung Cancer. 2005;49(Suppl 1):S33-S35.
61. Gerbaudo V.H., Britz-Cunningham S., Sugarbaker D.J., et al. Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax. 2003;58:1077-1082.
62. Ceresoli G.L., Chiti A., Zucali P.A., et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F]fluorodeoxyglucose. J Clin Oncol. 2006;24:4587-4593.
63. Gerbaudo V.H., Sugarbaker D.J., Britz-Cunningham S., et al. Assessment of malignant pleural mesothelioma with (18)F-FDG dual-head gamma-camera coincidence imaging: comparison with histopathology. J Nucl Med. 2002;43:1144-1149.
64. Flores R.M., Akhurst T., Gonen M., et al. Positron emission tomography predicts survival in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2006;132:763-768.
65. Shen K.R., Wain J.C., Wright C.D., et al. Postpneumonectomy syndrome: surgical management and long-term results. J Thorac Cardiovasc Surg. 2008;135:1210-1216. discussion 1216-1219
66. Gill R.R., Gerbaudo V.H., Sugarbaker D.J., et al. Current trends in radiologic management of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:111-120.