40 Pleural Effusions and Pneumothorax
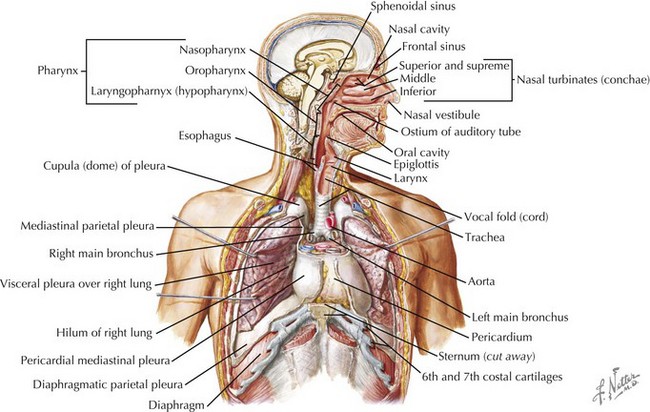
Pleural effusions and pneumothoraces occur as a result of structural and mechanical abnormalities of the pleural space. Abnormalities of the pleural space are an important cause of morbidity and mortality in infants and children worldwide, and the number of children who develop clinically significant pleural effusions is increasing. Pleural effusions are the result of excessive fluid accumulation in the pleural space, and pneumothoraces occur as a result of the accumulation of air within the pleural space. To better understand the pathophysiology of pleural effusions and pneumothoraces, it is essential to understand the anatomy of the pleural space. The pleural space is a potential anatomic space, approximately 10 to 20 µm wide, located between the visceral and parietal pleurae. The visceral pleura lines the surface of the lung parenchyma, including the interlobar fissures, and the parietal pleura lines the inner surface of the chest wall, mediastinum, and diaphragm (Figure 40-1). The pleural space contains a small amount of fluid (0.3 mL/kg body weight) that is in equilibrium between the amount of fluid formed (filtered) and the amount removed (absorbed).
Pleural Effusions
Evaluation and Management
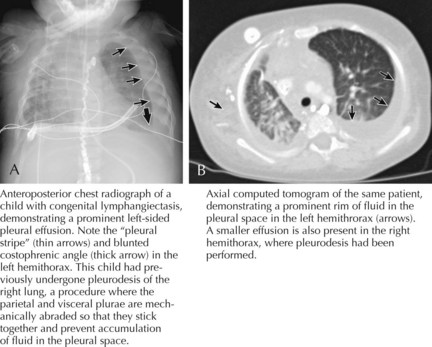
Children with the clinical history and findings suggestive of a pleural effusion should be evaluated with an upright chest radiograph and a lateral decubitus view (Figure 40-2). Performing radiographic examinations with the child in multiple positions helps to demonstrate a shift in the effusion with position changes. These radiographic images can help in making the diagnosis of pleural effusion and in determining the need for thoracocentesis or chest tube placement.
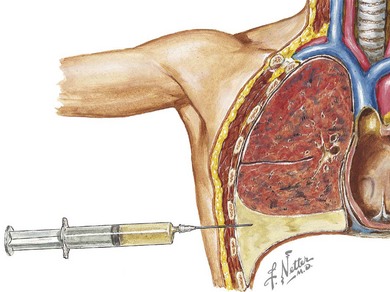
When infection is in the differential diagnosis, pleural fluid aspiration via thoracocentesis should be performed if at least 1 cm of fluid is seen on decubitus radiographs to determine the type of effusion (i.e., infectious exudate, empyema, hydrothorax, hemothorax, or chylothorax) before starting therapy (Figure 40-3). Certain laboratory studies should always be performed on the pleural fluid aspirate. Additional laboratory studies should also be obtained during the evaluation of a child with a parapneumonic effusion or empyema (Table 40-1). Microbiologic studies of the pleural fluid can identify a causative organism, and the analysis of pleural fluid is helpful in guiding therapeutic options. Pleural fluid can be obtained through thoracocentesis, aspiration under ultrasonographic guidance, or through video-assisted thoracoscopic surgery (VATS), depending on the presence of loculations or the availability of pediatric surgeons trained in VATS.
Table 40-1 Laboratory Studies Obtained at Time of Pleural Fluid Aspiration
| Pleural fluid | Protein, pH, glucose, LDH, cholesterol Differential cell count Gram stain and cultures (bacterial, fungal, and mycobacterial) Latex agglutination studies (especially if antibiotics have been administered before pleural fluid aspiration) Specific or broad-range PCR studies Cytology (if malignancy is suspected) |
| Serum | Complete blood cell count with differential Serum LDH (pleural fluid : serum LDH ratio >0.6 is indicative of an exudate) Serum protein (pleural fluid : serum protein >0.5 is indicative of an exudate) Glucose Blood culture (in children with a parapneumonic effusion) CRP (useful in monitoring therapeutic progress) |
| Miscellaneous | Sputum culture Mantoux testing and sputum (or gastric aspirates) for AFB in patients with risk factors for tuberculosis |
AFB, acid-fast bacilli; CRP, C-reactive protein; LDH, lactic dehydrogenase; PCR, polymerase chain reaction.
Pneumothorax
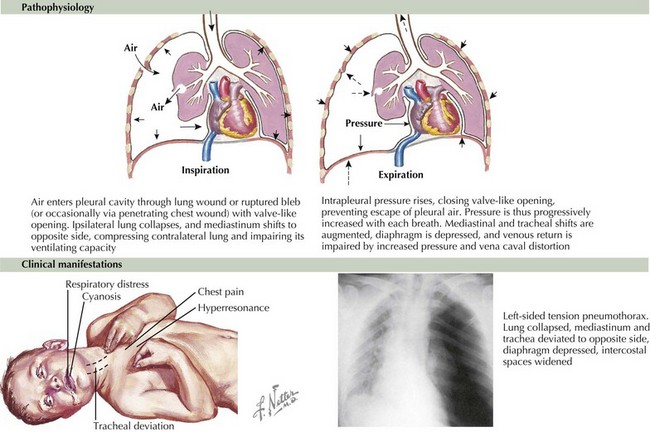
Pneumothorax is the accumulation of extrapulmonary air in the pleural space. A pneumothorax typically results from leakage of air from within the lung through the visceral pleura, but air can also enter the pleural space from a defect in the parietal pleura (Figure 40-4). Pneumothoraces are uncommon in children but can be life threatening.
Etiology and Pathogenesis
Secondary Spontaneous Pneumothorax
A small pneumothorax can be well tolerated, and the child will frequently be asymptomatic; in contrast, a large pneumothorax usually causes physiologic abnormalities. When air enters the pleural space as the result of a pneumothorax, the lung collapses because the outward pull of the chest wall is uncoupled from the inward recoil of the lung. The intrapleural pressure may remain atmospheric with a small pneumothorax. However, when air enters the pleural space during inspiration but cannot exit during exhalation (tension pneumothorax), the continuing air leak causes increased positive pressure in the pleural space that is higher than atmospheric pressure. This results in further compression of the lung, a shift of the mediastinal structures toward the contralateral side, decreased venous return, and decreased cardiac output (see Figure 40-4).
Evaluation and Management
The diagnosis of a pneumothorax is verified radiographically (see Figure 40-4). Lateral and anteroposterior views of the chest can confirm the presence of intrapleural air that appears to outline the visceral pleura. Expiratory views accentuate the contrast between lung markings and the hyperlucency of the air in the pleural space. The trachea and mediastinum shift away from the pneumothorax, especially in the case of a tension pneumothorax. However, if both lungs are poorly compliant, as in patients with CF or respiratory distress syndrome, the unaffected lung may not collapse easily, and the shift may not occur.
The aspiration of pleural air via a needle thoracostomy is necessary for pneumothoraces that occupy more than 15% of the involved hemithorax; if a tension pneumothorax is suspected; or if the child has severe dyspnea, hypoxemia, or significant pain (see Figure 40-4). Needle aspiration is associated with a recurrence risk of 20% to 50%; therefore, close follow-up is required. If a child has failed a needle aspiration, has a large pneumothorax, or is having recurrent spontaneous pneumothoraces, a thoracostomy tube should be placed; pigtail catheters are frequently used. A water seal device or one-way Heimlich valve should be used to prevent reaccumulation of the air.
Avansino JR, Goldman B, Sawin RS, et al. Primary operative versus nonoperative therapy for pediatric empyema: a meta-analysis. Pediatrics. 2005;115:1652-1659.
Balfour-Lynn IM, Abrahamson E, Cohen G, et al. BTS guidelines for the management of pleural infection in children. Thorax. 2005;60(Suppl 1):i1-i21.
Baumann MH. Management of spontaneous pneumothorax. Clin Chest Med. 2006;27:369-381.
Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602.
Jaffé A, Balfour-Lynn IM. Management of empyema in children. Pediatr Pulmonol. 2005;40:148-156.
Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med. 2000;342:868-874.
Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113:1735-1740.
Shaw KS, Prasil P, Nguyen LT, et al. Pediatric spontaneous pneumothorax. Semin Pediatr Surg. 2003;12:55-61.