Pleural Effusion and Empyema
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with pleural diseases.
• Describe the causes of pleural diseases.
• List the cardiopulmonary clinical manifestations associated with pleural diseases.
• Describe the general management of pleural diseases.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs
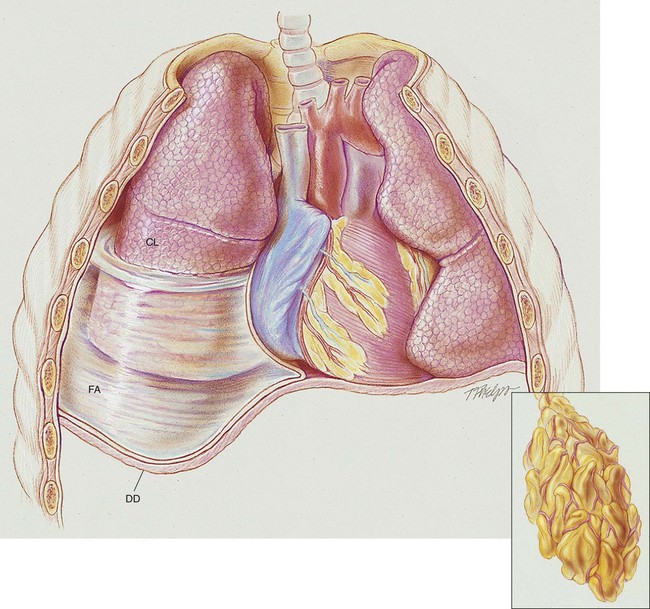
A number of pleural diseases can cause fluid to accumulate in the pleural space; this fluid is called a pleural effusion, or if infected, an empyema (see Figure 23-1). Similar to free air in the pleural space, fluid accumulation separates the visceral and parietal pleura and compresses the lungs. In severe cases, atelectasis will develop, the great veins may be compressed, and cardiac venous return may be diminished. Pleural effusion and empyema produce a restrictive lung disorder.
Etiology and Epidemiology
• Pleural fluid protein >2.9 g/dL (29 g/L)
• Pleural fluid cholesterol >45 mg/dL (1.16 mmol/L)
• Pleural fluid lactate dehydrogenase (LDH) >60% of upper limit for serum
Common Causes of Exudative Pleural Effusion
Malignant Pleural Effusions
Tuberculosis
Pleural effusion may develop from extension of a caseous tubercle into the pleural cavity. It also is possible that the inflammatory reaction that develops in tuberculosis obstructs the lymphatic pores in the parietal pleura. This in turn leads to an accumulation of protein and fluid in the pleural space. Pleural effusion caused by tuberculosis is generally unilateral and small to moderate in size (see Chapter 17).
General Management of Pleural Effusion
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. The hypoxemia that develops in pleural effusion is mostly caused by the atelectasis and pulmonary shunting associated with the disorder. Hypoxemia caused by capillary shunting is often refractory to oxygen therapy (see Oxygen Therapy Protocol, Protocol 9-1).
Mechanical Ventilation Protocol
Because acute ventilatory failure and hypoxemia may be seen in severe pleural effusions, continuous mechanical ventilation may be required to maintain an adequate ventilatory status. Continuous mechanical ventilation is justified when the acute ventilatory failure is thought to be reversible (see Mechanical Ventilation Protocols, Protocol 9-5, Protocol 9-6, and Protocol 9-7).
CASE STUDY
Pleural Effusion and Empyema
Admitting History
Respiratory Assessment and Plan
S “I can’t take a deep breath.”
O Malnourished appearance with poor personal hygiene; cyanosis with an occasional hacking, nonproductive cough; vital signs: BP 130/60, HR 112, RR 36 and shallow, T 37.7° C (99.8° F); trachea slightly shifted to the left; dull percussion notes over the right middle and right lower lobes; normal vesicular breath sounds over the left lung fields and right upper lobe; no breath sounds over the right middle and right lower lobes; CXR: large, right-sided pleural effusion, right middle and right lower lobes partially collapsed and consolidated; about 2 L of yellow fluid obtained via thoracentesis; ABGs (on 3 L/min O2 by nasal cannula): pH 7.48, Paco2 24, 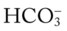 17, Pao2 37, Spo2 72%
17, Pao2 37, Spo2 72%
• Right-sided pneumonia and pleural effusion (CXR)
• Partially collapsed right middle and lower lobes; atelectasis versus pneumonia (CXR)
• Respiratory distress (vital signs, ABGs)
• Acute alveolar hyperventilation with severe hypoxemia (ABGs)
• Metabolic (lactic) acidosis likely (ABGs compared with Pco2/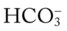 /pH relationship nomogram)
/pH relationship nomogram)
P Begin Lung Expansion Therapy Protocol (incentive spirometry q2h) and Oxygen Therapy Protocol (Fio2 = 0.50 per HAFOE mask). Monitor vital signs carefully and reevaluate.
Respiratory Assessment and Plan
S “I’m feeling better but not great yet.”
O Cyanotic and pale appearance; occasional dry, nonproductive cough; vital signs: BP 135/85, HR 100, RR 24, T normal; dull percussion notes over right middle and right lower lobes; normal vesicular breath sounds over left lung and over right upper lobe; bronchial breath sounds over right middle and lower lobes; CXR: small right-sided pleural effusion; right middle and right lower lobe consolidation; ABGs: pH 7.52, Paco2 29, 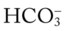 22, Pao2 57; Spo2 92% on an Fio2 of 0.50.
22, Pao2 57; Spo2 92% on an Fio2 of 0.50.
• Small right-sided pneumonia and pleural effusion, greatly improved (CXR)
• Atelectasis and consolidation in right middle and lower lung lobes (CXR)
• Continued respiratory distress, but improving (vital signs, ABGs)
• Acute alveolar hyperventilation with moderate hypoxemia, improved (ABGs)
P Up-regulate Lung Expansion Therapy Protocol (CPAP mask at 10 cm H2O q2h for 15 minutes). Up-regulate Oxygen Therapy Protocol (Fio2 = 0.60 per HAFOE mask). Monitor and reevaluate.
Respiratory Assessment and Plan
S “I’ve finally caught my breath.”
O Relaxed, alert appearance, in semi-Fowler’s position; paleness but no cyanosis; no spontaneous cough; vital signs: BP 128/79, HR 88, RR 16, T normal; dull percussion notes in right middle and right lower lung lobes; normal vesicular breath sounds over left lung and right upper lobe; bronchial breath sounds over right middle and right lower lobes; ABGs: pH 7.45, Paco2 36, 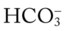 24, Pao2 77; Spo2 95%
24, Pao2 77; Spo2 95%
• Small, right-sided pneumonia and pleural effusion, greatly improved (previous CXR)
• Atelectasis and consolidation in right middle and right lower lung lobes (previous CXR)
P Maintain present level of Lung Expansion Therapy Protocol and Oxygen Therapy Protocols. Monitor and reevaluate each shift.
Discussion
During the first assessment, the respiratory care practitioner recognizes that the patient has significant respiratory morbidity. Indeed, the patient has an extensive right-sided pneumonia and pleural effusion and partially collapsed right middle and lower lobes. Clearly the patient is in respiratory distress. The patient’s acute alveolar hyperventilation and severe hypoxemia are a direct result of the partial collapse of the lung lobes. Because of the extremely low Pao2 noted on the initial arterial blood gas, the presence of lactic acid is very likely. In fact, this was confirmed by the respiratory practitioner with the Pco2/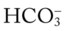 /pH nomogram. Understanding that Atelectasis is the main pathophysiologic mechanism operating in this case (see Figure 9-8), the practitioner correctly assesses the situation as one that requires careful monitoring and begins the Lung Expansion Therapy Protocol (Protocol 9-3) (with incentive spirometry) and the Oxygen Therapy Protocol (Protocol 9-1) (with a high concentration of oxygen).
/pH nomogram. Understanding that Atelectasis is the main pathophysiologic mechanism operating in this case (see Figure 9-8), the practitioner correctly assesses the situation as one that requires careful monitoring and begins the Lung Expansion Therapy Protocol (Protocol 9-3) (with incentive spirometry) and the Oxygen Therapy Protocol (Protocol 9-1) (with a high concentration of oxygen).
At the time of the second assessment, the patient was beginning to improve, although she still had signs of right middle and lower lobe Consolidation (Figure 9-9). Good breath sounds were heard over the left lung and upper right lung, although bronchial breath sounds reflecting consolidation were still noted on the right. The respiratory care practitioner was appropriately concerned that atelectasis was still present, and in such a case he or she should increase the Lung Expansion Therapy Protocol (Protocol 9-3). In this case, the practitioner selected a continuous positive airway pressure (CPAP) mask at 10 cm H2O every 2 hours for 15 minutes. The practitioner could have also intensified use of incentive spirometry, carefully used intermittent positive-pressure breathing (IPPB) or extended the amount of time the patient was using the CPAP mask.

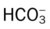

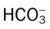
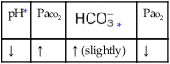
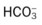 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa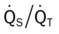
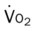
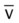 )
)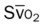

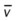 )O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
)O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 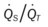 , pulmonary shunt fraction;
, pulmonary shunt fraction; 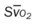 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 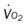 , oxygen consumption.
, oxygen consumption.
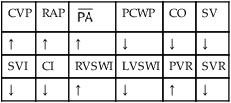
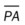 , mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.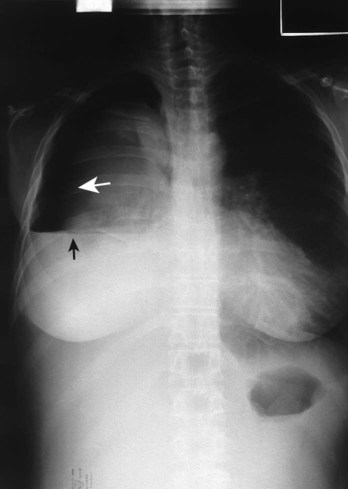
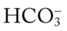 17, and Pa
17, and Pa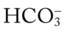 22, and Pa
22, and Pa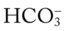 24, and Pa
24, and Pa