97 Pleural effusion
Salient features
Examination
• Decreased movement on the affected side
• Tracheal deviation to the opposite side
• Stony dull note on the affected side
• Decreased vocal resonance and diminished breath sounds on the affected side.
• Percuss for the upper level of effusion in the axilla
• Listen for bronchial breath sounds
• Listen for aegophony at the upper level of the effusion
• It is important to elicit any evidence of an underlying cause, such as clubbing, tar staining, lymph nodes, radiation burns and mastectomy, raised JVP, rheumatoid hands or butterfly rash.
For clinical detection, 500 ml of pleural fluid should be present.
Questions
How would you investigate this patient?
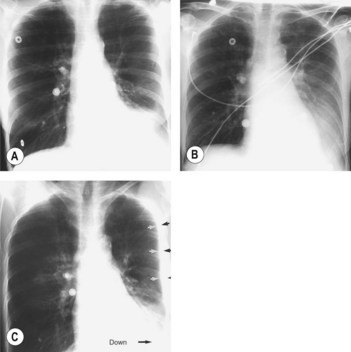
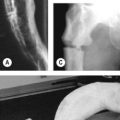
• Chest radiography: standard posteroanterior and lateral films detect pleural fluid in excess of 175 ml. Obliteration of costophrenic angle to hemithorax suggest fluid. Subpulmonic effusion can simulate an elevated diaphragm (Fig. 97.1).
• Pleural biopsy: the biopsy specimen is sent for histopathological examination and mycobacterial culture.
How would you differentiate between the above?
• Pleural effusion: stony dull note; trachea may be deviated to the opposite side in large effusions
• Pleural thickening: trachea not deviated; breath sounds will be heard
• Consolidation: vocal resonance increased; bronchial breath sounds and associated crackles
• Collapse: trachea deviated to the affected side; absent breath sounds.
How would you differentiate between an exudate and a transudate?
• The protein content of an exudate is >3 g/l. However, if this criterion alone is applied, about 10% of the exudates and 15% of the transudates will be wrongly classified. A more accurate diagnosis is made when Light’s criteria (Ann Intern Med 1972;77:507–13) for an exudate are applied: (1) the ratio of the pleural fluid to serum protein is greater than 0.5; (2) the ratio of pleural fluid to serum LDH is greater than 0.6; (3) pleural fluid LDH is greater than two-thirds the upper normal limit for blood LDH. Light’s criteria have been shown to have a sensitivity of 100% but a low specificity of 72% (Chest 1990;98:546–9). This was because many patients with effusion caused by chronic cardiac failure have protein values in the exudate range, particularly when on chronic diuretic therapy. Serum–effusion albumin gradient (i.e. serum albumin minus pleural fluid albumin) was 95% sensitive but a more specific (100%) marker of exduative effusion. A gradient of <12 g/l indicates an exudative effusion whereas a gradient >12 g/l indicates a transudative effusion. Measuring the difference between the serum and the pleural-fluid albumin levels is useful in patients with heart failure who are taking diuretics since a difference >12 g/l is consistent with a transudative effusion, even though other Light’s criteria for an exudative effusion have been met.
• The pleural fluid cholesterol level is <600 mg/l in transudates. All malignant effusions have a pleural cholesterol level greater than this value, and therefore this test is useful to separate these two categories of effusion (Chest 1987;92:296–302, Chest 1991;99:1097–102).
Advanced-level questions
Mention a few conditions in which the pleural fluid pH and glucose levels are low but lactate dehydrogenase is raised
Empyema, malignancy, TB, rheumatoid arthritis, SLE and oesophageal rupture.
What is the role of pleural fluid cytology in the diagnosis of pleural effusion?
• Pleural fluid usually contains about 1.5 × 109 cells/l (predominantly mononuclear cells). Counts above 50 × 109 cells/l are seen in parapneumonic effusions, whereas transudates usually have counts of <1× 109 cells/l.
• Pleural fluid eosinophilia (i.e. greater than 10%) suggests a benign disease, including pneumothorax, asbestos-related effusions and post-haemothorax, although malignancy cannot be excluded.
• Pleural fluid lymphocytosis is seen in about one-third of transudates, in malignancy, TB, lymphoma, collagen vascular diseases and sarcoidosis.
• Computed interactive morphometry (analyses the size and nuclei of cells in a stained centrifuged specimen) differentiates between malignant cells and reactive lymphocytosis. This method is particularly useful when differentiating between benign reactive mesothelial cells from malignancy.
What characteristics of the pleural fluid in a parapneumonic effusion indicate a need for closed-tube drainage?
What are the causes of an exudate with negative cytology findings and pleural fluid lymphocytosis?
Possible causes include TB, collagen vascular diseases and tumours, including lymphoma.
In such patients what other tests could you perform on the pleural fluid to determine the underlying cause?
• Pleural fluid adenosine deaminase concentration (an enzyme involved in purine metabolism and found in T lymphocytes) is markedly raised in tuberculous and rheumatoid effusions compared with malignant effusions.
• Increased pleural fluid lysozyme concentration (muramidase) is used to differentiate TB, rheumatoid arthritis and empyema from malignant effusions.
• Combined use of these two tests yields a sensitivity and specificity of 100% for tuberculous effusions if empyema is excluded.
• Gamma-interferon and soluble interleukin 2 receptor levels are also raised in tuberculous effusions compared with malignant effusions.
• Estimation of pleural fluid rheumatoid factor and anti-nuclear antibodies is useful in confirming the diagnosis of rheumatoid and lupus erythematosus, respectively.
In which conditions is the pleural fluid bloody?
Haemorrhagic fluid is seen in malignancy, pulmonary embolus, TB and trauma to the chest.
What are the earliest radiological signs of pleural fluid?
The earliest radiological signs are blunting of the costophrenic angle on the anterior–posterior view or loss of clear definition of the diaphragm posteriorly on the lateral view (Fig. 97.1).
What are the mechanisms for abnormal accumulation of pleural fluid?
There are three main mechanisms:
• An abnormality of the pleura itself, such as neoplasm or inflammatory process, usually associated with increased permeability (e.g. increased vascular permeability in pneumonia)
• Disruption of the integrity of a fluid-containing structure within the pleural cavity, such as the thoracic duct, oesophagus, major blood vessels or tracheobronchial tree, with leakage of the contents into the pleural space (e.g. decreased lymphatic drainage as in mediastinal carcinomatosis)
• Abnormal hydrostatic or osmotic forces operating on an otherwise normal pleural surface and producing a transudate (e.g. increased hydrostatic pressure in heart failure, decreased osmotic pressure in nephrotic syndrome, increased intrapleural pressure as in atelectasis).