65 Pleural Disease and Pneumothorax
 Radiologic Signs of Pleural Disease in the Intensive Care Unit
Radiologic Signs of Pleural Disease in the Intensive Care Unit
Pleural Fluid
Standard Chest Radiograph
In healthy humans in the supine position, the radiolucency of the lung base is equal to or greater than that in the lung apex.1 Furthermore, when in the supine position, breast and pectoral tissue tend to fall laterally away from the lung base, so an effusion should be suspected if there is increased homogeneous density over the lower lung fields compared to the upper lung fields. As the pleural effusion increases, the increased radiodensity involves the upper hemithorax as well. However, failure of chest wall tissue to move laterally, cardiomegaly, prominent epicardial fat pad, and lung collapse or consolidation may obscure a pleural effusion on a supine radiograph. Patient rotation or an off-center x-ray beam can mimic a unilateral homogeneous density. An absent pectoral muscle, prior mastectomy, unilateral hyperlucent lung, scoliosis, previous lobectomy, hypoplastic pulmonary artery, or pleural or chest wall mass may lead to unilateral homogeneous increased density and mimic an effusion.
Approximately 175 to 525 mL of pleural fluid results in blunting of the costophrenic angle on an erect radiograph.2 This quantity of effusion can be detected on a supine radiograph as an increased density over the lower lung zone. Failure to visualize the hemidiaphragm, absence of the costophrenic angle meniscus, and apical capping are less likely to be seen with effusions of less than 500 mL.1 The major radiographic finding of a pleural effusion in a supine position is increased homogeneous density over the lower lung field that does not obliterate normal bronchovascular markings, does not show air bronchograms, and does not show hilar or mediastinal displacement until the effusion is massive. If a pleural effusion is suspected in the supine patient, ultrasonography should be performed.
Other Radiographic Imaging
Sonography
Ultrasonography (US) provides good characterization for pleural diseases and is a useful diagnostic modality for critically ill patients who cannot be transported for computed tomography (CT). US takes less time and is less expensive than CT, can be done at the bedside, and can be repeated serially. Disadvantages include hindrance of the ultrasonic wave by air, either in the lung or pleural space, a restricted field of view, inferior evaluation of the lung parenchyma compared to CT, and operator dependence. US was helpful in diagnosis in 27 (66%) of 41 patients and treatment in 37 (90%) of 41 patients, and had an important influence on treatment planning in 17 (41%) of 41 critically ill patients.3
US has also been demonstrated to be a useful modality to guide bedside thoracentesis in the mechanically ventilated patient, resulting in high success rate and excellent safety of the procedure.4
Pneumothorax
When supine, pneumothorax gas migrates along the anterior surface of the lung, making detection on the anteroposterior radiograph problematic. The base, lateral chest wall, and juxtacardiac area should be carefully visualized for evidence of pneumothorax. Accumulation of air along the mediastinal parietal pleura may simulate pneumomediastinum.5 An erect or decubitus (suspected hemithorax up) radiograph should be obtained to assess for the presence of a pneumothorax. US is sensitive for the detection of pneumothorax by determining the presence or absence of “lung sliding.”6 In individuals without pneumothorax, the lung–chest wall interface, which represents a to-and-fro movement synchronized with respiration, can be identified. US visualization of lung sliding is correlated with the absence of pneumothorax, and from this sign alone, at least anterior pneumothorax can be excluded rapidly at the bedside of a mechanically ventilated patient. However, absence of lung sliding may be caused by the presence of large bullae or pleural symphysis caused by previous pleurodesis or pleural adhesions due to previous pleural disease. Hence, the absence of lung sliding is not specific for pneumothorax, but detection of lung sliding reliably excludes the presence of pleural air in the examined area.
The most common radiographic signs of tension pneumothorax are contralateral mediastinal shift, ipsilateral diaphragmatic depression, and ipsilateral chest wall expansion. Underlying lung disease may prevent total lung collapse even if tension is present; in patients on mechanical ventilation, little or no midline mediastinal shift may result from the tension.7 In the latter, a depressed ipsilateral diaphragm is a more reliable sign of tension than mediastinal shift.
In patients with acute respiratory distress syndrome (ARDS), barotrauma can result in a localized tension pneumothorax with a subtle contralateral mediastinal shift, flattening of the cardiac contour, and depression of the ipsilateral hemidiaphragm.8 Pleural adhesions and relative compressibility and mobility of surrounding structures, in addition to the supine position, probably account for these loculated tension pneumothoraces.
In a study of 88 critically ill patients with 112 pneumothoraces, the anteromedial and subpulmonic recesses were involved in 64% of patients in the supine and semierect position.9 Furthermore, in 30% of the pneumothoraces in this study that were not initially detected by the clinician or radiologist, half the patients progressed to tension pneumothorax. Therefore, a high index of suspicion is necessary to avoid catastrophic situations.
Factors that may contribute to an improved ability to diagnose this potentially lethal problem include familiarity with atypical locations of pneumothoraces in critically ill patients, usually due to the supine or semierect position; the consequence of underlying cardiopulmonary disease; and knowledge of other risk factors contributing to misdiagnosis (e.g., mechanical ventilation, altered mental status, prolonged ICU stay, and development of pneumothorax after peak physician staffing hours).10
 Evaluation of Pleural Effusion in the Intensive Care Unit
Evaluation of Pleural Effusion in the Intensive Care Unit
Diagnostic Thoracentesis
Indications
Although disease of any organ system can cause a pleural effusion in critically ill patients, the diagnoses listed in Table 65-1 represent the majority of the causes seen in ICUs. The types of pleural effusions seen in medical and surgical ICUs are similar, but some causes related to surgical (coronary artery bypass grafting, chylothorax, abdominal surgery) and nonsurgical trauma (hemothorax) represent a substantial percentage of surgical ICU effusions.
| In the Medical ICU | In the Surgical ICU |
|---|---|
| Atelectasis | Atelectasis |
| Congestive heart failure | Congestive heart failure |
| Pneumonia | Pneumonia |
| Hypoalbuminemia | Pancreatitis |
| Pancreatitis | Hypoalbuminemia |
| ARDS | Coronary artery bypass surgery |
| Pulmonary embolism | ARDS |
| Hepatic hydrothorax | Pulmonary embolism |
| Esophageal sclerotherapy | Esophageal rupture |
| Postmyocardial infarction | Hemothorax |
| Iatrogenic | Chylothorax |
| Abdominal surgery | |
| Iatrogenic |
ARDS, Acute respiratory distress syndrome; ICU, intensive care unit.
When a pleural effusion is suspected on physical examination and confirmed radiologically, a diagnostic thoracentesis under ultrasonographic guidance should be performed in an attempt to establish the cause. Exceptions are patients with a secure clinical diagnosis and a small amount of pleural fluid, as in atelectasis, or patients with uncomplicated congestive heart failure (CHF).13 Observation may be warranted in these situations, but thoracentesis should be performed if there are adverse changes.11
The indications for diagnostic thoracentesis do not change simply because the patient is in the ICU or on mechanical ventilation. In fact, establishing the diagnosis quickly in these critically ill patients may be more important and life saving than in non–critically ill patients. It has been well documented that even in patients on mechanical ventilation, diagnostic thoracentesis is safe if there is strict adherence to the general principles of the procedure and ultrasonography is used.4,12 Pneumothorax, the most clinically important complication of thoracentesis,13 is no more likely to occur in the patient on mechanical ventilation than in the patient who is not; however, if a pneumothorax does develop, the patient on mechanical ventilation is likely to develop a tension pneumothorax.
Complications
Complications of diagnostic thoracentesis include pain at the needle insertion site, bleeding (local, intrapleural, or intraabdominal), pneumothorax, empyema, and spleen or liver puncture. Pneumothorax has been reported in prospective studies to occur in 4% to 30% of patients.13,14–16 However, when ultrasound-guided thoracentesis is performed by experienced physician sonographers, pneumothorax or other injuries due to organ puncture appear to be rare events.4 Liver or spleen puncture tends to occur when the patient is not sitting absolutely upright because movement toward recumbency causes cephalad migration of the abdominal viscera. The upward displacement of abdominal organs is readily detected by ultrasonography. However, even if the liver or spleen is punctured with a small-bore needle, generally the outcome is favorable if the patient is not receiving anticoagulants and does not have a bleeding diathesis.
Therapeutic Thoracentesis
Indications and Contraindications
The primary indication for therapeutic thoracentesis is relief of dyspnea. Contraindications to therapeutic thoracentesis are similar to those for diagnostic thoracentesis. However, there appears to be an increased risk of pneumothorax,13 making a therapeutic thoracentesis in patients on mechanical ventilation potentially hazardous.
The technique for therapeutic thoracentesis is essentially the same as for diagnostic thoracentesis, except that a blunt-tip needle or plastic catheter, rather than a sharp-tip needle, should be used. This reduces the risk of pneumothorax, which may occur as fluid is removed and the lung expands toward the chest wall. Again, the use of sonographic guidance is recommended.17
The amount of fluid that can be removed safely from the pleural space at one session is controversial. Ideally, monitoring pleural pressure should dictate the amount of fluid that can be removed. As long as intrapleural pressure does not fall to less than −20 cm H2O, fluid removal can continue.18 However, intrapleural pressure monitoring is not done routinely. In the patient with contralateral mediastinal shift on chest radiograph who tolerates thoracentesis without chest tightness, cough, or light-headedness, probably several liters of pleural fluid can be removed safely, but neither the patient nor the operator may be aware of a precipitous drop in pleural pressure. In patients without a contralateral mediastinal shift or with ipsilateral shift (suggesting an endobronchial obstruction), the likelihood of a precipitous drop in intrapleural pressure is increased, and pleural pressure should be monitored during thoracentesis. Alternatively, a small-bore catheter connected to a standard thoracostomy pleural drainage system may be temporarily inserted, thus avoiding excessively negative pleural pressure development during drainage. Simple gravity drainage or drainage using any system incorporating a non-return valve do not reliably guard against the development of excessively negative pressure.
Physiologic Effects and Complications
Improvement in lung volumes up to 24 hours after therapeutic thoracentesis does not correlate with the amount of fluid removed, despite relief of dyspnea in those patients.19–21 In some patients, however, maximum spirometric improvement may not occur for several days. Patients with initial negative pleural pressures and those with more precipitous falls in pleural pressure with thoracentesis tend to have the least improvement in pulmonary function after therapeutic thoracentesis because many have a trapped lung or endobronchial obstruction.18 The mechanism of dyspnea from a large pleural effusion probably is related to the increase in chest wall resting volume, resulting in shortening of the respiratory muscles’ resting length and consequent decrease in contractile efficiency.20 Drainage of moderately sized pleural effusions (1495 mL) does not appear to result in predictable changes in respiratory system compliance or resistances, although a systematic decrease in work performed by the ventilator as a consequence of thoracentesis has been reported.22
Complications of therapeutic thoracentesis are the same as those seen with diagnostic thoracentesis. Three complications unique to therapeutic thoracentesis are hypoxemia, unilateral pulmonary edema, and hypovolemia. After therapeutic thoracentesis, hypoxemia may occur despite relief of dyspnea23,24 from worsening ventilation/perfusion relationships in the ipsilateral lung or clinically occult unilateral pulmonary edema.
Some investigators have concluded that the change in partial pressure of arterial oxygen (PaO2) after therapeutic thoracentesis is unpredictable24; some have observed a characteristic increase in PaO2 within minutes to hours,19 and others suggest a systematic decrease in PaO2 that returns to prethoracentesis values by 24 hours.23 In the largest study including 33 patients with various causes of unilateral pleural effusions, a significant increase in PaO2 was found at 20 minutes, 2 hours, and 24 hours after therapeutic thoracentesis.25 This was in conjunction with a decrease in the alveolar-arterial oxygen gradient [P(A-a)O2] and was accompanied by a small but significant decrease in shunt, without a change in the ratio of dead space to tidal volume (VD/VT). Data suggest an improved ventilation/perfusion relationship after therapeutic thoracentesis, with an increase in ventilation of parts of the lung that were previously poorly ventilated but well perfused. The relief of dyspnea in these patients cannot be explained by improved arterial oxygen tension.
Improvement in lung volumes is a constant finding after therapeutic thoracentesis but may take days or even weeks to maximize; immediate changes are usually modest and highly variable. Therefore, the relief of dyspnea cannot be adequately explained by changes in lung volume or in the mechanics of breathing but may be the result of decreased stimulation of lung or chest wall receptors, or both.20
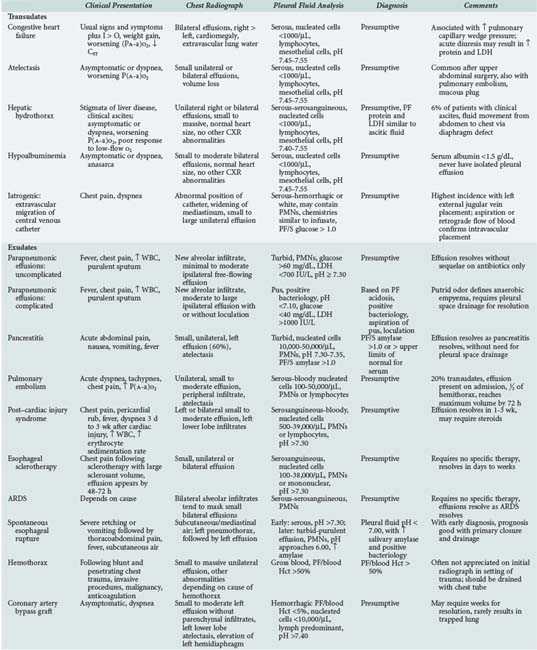
The differential diagnosis of pleural effusions in critically ill patients is outlined in Table 65-2. Brief discussions of the more common etiologies follow.
Atelectasis
Atelectasis is a common cause of small pleural effusions in comatose, immobile, pain-ridden patients in ICUs26 and after upper abdominal surgery.27,28 Other causes include major bronchial obstruction from lung cancer or a mucous plug. Atelectasis causes pleural fluid because of decreased pleural pressure. With alveolar collapse, the lung and chest wall separate further, creating local areas of increased negative pressure. This decrease in pleural pressure favors the movement of fluid into the pleural space, presumably from the parietal pleural surface. The fluid accumulates until the pleural or parietal-pleural interstitial pressure gradient reaches a steady state.
Congestive Heart Failure
CHF is the most common cause of transudative pleural effusions and a common cause of pleural effusions in ICUs. Pleural effusions due to CHF are associated with increases in pulmonary venous pressure.29 Most patients with subacute or chronic elevation in pulmonary venous pressure (pulmonary capillary wedge pressure of at least 24 mm Hg) have evidence of pleural effusion on US or lateral decubitus radiograph. Isolated increases in systemic venous pressure tend not to produce pleural effusions. Thus, patients with chronic obstructive pulmonary disease (COPD) and cor pulmonale rarely have pleural effusions, and the presence of pleural fluid implies another cause.
Most patients with pleural effusions secondary to CHF have the classic signs and symptoms. The chest radiograph shows cardiomegaly and bilateral small to moderate pleural effusions of similar size (right slightly greater than left). There is usually radiographic evidence of pulmonary congestion, with the severity of pulmonary edema correlating with the presence of pleural effusion.29
The effusion associated with CHF is a transudate, with mesothelial cells and lymphocytes accounting for the majority of the less than 1,000 cells per µL.12 Acute diuresis can raise the pleural fluid protein and lactate dehydrogenase into the range of an exudate.30,31 In the patient with secure clinical diagnosis of CHF, observation is appropriate. Thoracentesis should be performed if the patient is febrile, has pleural effusions of disparate size, has a unilateral pleural effusion, does not have cardiomegaly, has pleuritic chest pain, or has a PaO2 inappropriate for the degree of pulmonary edema.
Hepatic Hydrothorax
Pleural effusions occur in approximately 6% of patients with cirrhosis of the liver and clinical ascites. The effusions result from movement of ascitic fluid through congenital or acquired diaphragmatic defects.32–34
The patient usually has the classic stigmata of cirrhosis and clinically apparent ascites. The usual chest radiograph shows a normal cardiac silhouette and a right-sided pleural effusion, which can vary from small to massive; effusions are less likely isolated to the left pleural space or are bilateral.32–35 Rarely, a massive pleural effusion may be found without clinical ascites (demonstrated only by US), implying the presence of a large diaphragmatic defect. The pleural fluid is a serous transudate with a low nucleated cell count and a predominance of mononuclear cells, pH greater than 7.40, and a glucose level similar to that of serum.12 The fluid can be hemorrhagic due to an underlying coagulopathy or rupture of a diaphragmatic bleb. Demonstrating that pleural and ascitic fluids have similar protein and lactate dehydrogenase concentrations substantiates the diagnosis.32 If the diagnosis is problematic, injection of a radionuclide into the ascitic fluid, with detection on chest imaging within 1 to 2 hours, supports a pleuroperitoneal communication through a diaphragmatic defect36; delayed demonstration of the tracer suggests that the pathogenesis of the effusion is via convection through the mesothelium.
Hepatic hydrothorax may be complicated by spontaneous bacterial empyema (SBE), which is analogous to spontaneous bacterial peritonitis. The criteria for diagnosis of SBE are similar to those for the diagnosis of spontaneous bacterial peritonitis. SBE must be considered in the differential diagnosis of the infected cirrhotic patient, even in the absence of clinical ascites.37,38 The pleural fluid culture and analysis may reveal positive culture, a total neutrophil count of more than 500 cells per µL, and a serum to pleural fluid albumin gradient greater than 1.1. The chest radiograph should not show a pneumonic process. Treatment of SBE is conservative with antibiotics unless purulence is present, in which case tube thoracostomy must be considered.
Treatment of hepatic hydrothorax is directed at resolution of the ascites, using sodium restriction and diuresis. The effusion frequently persists unchanged until all ascites is mobilized. If the patient is acutely dyspneic or in respiratory failure, therapeutic thoracentesis should be done as a temporizing measure. Care should be exercised with paracentesis or thoracentesis, because hypovolemia can occur with rapid evacuation of fluid. Chest tube insertion should be avoided, as it can cause infection of the fluid, and prolonged drainage can lead to protein and lymphocyte depletion and renal failure. Chemical pleurodesis via a chest tube is often unsuccessful owing to rapid movement of ascitic fluid into the pleural space. Treatment options in hepatic hydrothorax refractory to medical management include transjugular intrahepatic portal systemic shunt and video-assisted thoracoscopy to patch the diaphragmatic defect, followed by pleural abrasion or talc poudrage in the properly selected patient.39,40
Hypoalbuminemia
Many patients admitted to a medical ICU have a chronic illness and associated hypoalbuminemia. When the serum albumin level falls below 1.8 g/dL, pleural effusions may be observed.41 Because the normal pleural space has an effective lymphatic drainage system, pleural fluid tends to be the last collection of extravascular fluid that occurs in patients with low oncotic pressure. Therefore, it is unusual to find a pleural effusion solely due to hypoalbuminemia in the absence of anasarca. Patients with hypoalbuminemic pleural effusions tend not to have pulmonary symptoms unless there is underlying lung disease, since the effusions are rarely large. Chest radiograph shows small to moderate bilateral effusions and a normal heart size. The pleural fluid is a serous transudate with less than 1000 nucleated cells per µL, predominantly lymphocytes and mesothelial cells. The pleural fluid glucose level is similar to that of serum, and the pH is in the range of 7.45 to 7.55. Diagnosis is presumptive if other causes of transudative effusions can be excluded. The effusions resolve when hypoalbuminemia is corrected.
Iatrogenic Causes
Extravascular migration of a central venous catheter can cause pneumothorax, hemothorax, chylothorax, or a transudative pleural effusion.42–44 Its incidence is estimated at less than 1% but may be considerably higher. Malposition of the catheter on placement should be suspected if there is absence of blood return or questionable central venous pressure measurements. The immediate postprocedure chest radiograph should be assessed for proper catheter placement; a catheter placed from the right side should not cross the midline. If the catheter is not in the appropriate vessel, phlebitis, perforation of a vein or the heart, or instillation of fluid into the mediastinum or pleural space can occur. In the alert patient, acute infusion of intravenous fluid into the mediastinum usually results in new-onset chest discomfort and dyspnea. Depending on the volume and the rate at which it is introduced into the mediastinum, tachypnea, worsening respiratory status, and cardiac tamponade may ensue. The chest radiograph shows the catheter tip in an abnormal position,45,46 a widened mediastinum, and evidence of unilateral or bilateral pleural effusions. The effusion can have characteristics similar to those of the infusate (milky if lipid is being given) and may be hemorrhagic and neutrophil-predominant due to trauma and inflammation. The pleural fluid to serum glucose ratio is greater than 1.0 if glucose is being infused.43 The pleural fluid glucose concentration can fall rapidly after glucose infusion into the pleural space, probably explaining the relatively low glucose concentrations in pleural fluid compared to the infusate.47 Extravascular migration of a central venous catheter appears to be more common with placement in the external jugular vein, particularly on the left side. Left-sided catheters appear to put the patient at increased risk of perforation because of the horizontal orientation of the left compared to the right brachiocephalic vein. When catheters are introduced from the left side, they should be of adequate length for the tip to rest in the superior vena cava.
Parapneumonic Effusions
Community-acquired or nosocomial pneumonia is common in critically ill patients. The classic presentation is fever, chest pain, leukocytosis, purulent sputum, and a new alveolar infiltrate on chest radiograph. In the elderly debilitated patient, however, many of these findings may not be present. The chest radiograph commonly shows a small to large ipsilateral pleural effusion.4,8,48–50 When the effusion is free flowing and anechoic on ultrasound, and thoracentesis shows a nonpurulent, polymorphonuclear (PMN) predominant exudate with a pH of 7.30 or greater, it is highly likely that the effusion will resolve during 7 to 14 days without sequelae with antibiotics alone (uncomplicated effusion). If the chest radiograph or CT demonstrates loculation and pus is aspirated, the diagnosis of empyema is established and immediate drainage is needed. In the free-flowing nonpurulent fluid, if Gram stain or culture is positive or pH is less than 7.30, the likelihood of a poor outcome increases, and the pleural space should be drained.
Although a meta-analysis found that low-risk patients with fluid pH between 7.20 and 7.30 may be managed without tube drainage, the patient admitted to the ICU typically cannot be considered low risk, and pH values of less than 7.30 should prompt drainage in most cases.51–53 Drainage can be accomplished by standard chest tube or small-bore catheter. When loculations occur, pleural space drainage should be accomplished by placement of image-guided tubes or catheters with fibrinolytics or empyectomy and decortication.54,55 Most thoracic surgeons routinely begin with thoracoscopy and, if not successful, proceed directly to a standard thoracotomy for empyectomy and decortication.56–59
Pancreatitis
Pleuropulmonary abnormalities are commonly associated with pancreatitis, largely owing to the close proximity of the pancreas to the diaphragm. Approximately half of patients with pancreatitis have an abnormal chest radiograph, with pleural effusions in 3% to 17%.60,61 Mechanisms that may be involved in the pathogenesis of pancreatic pleural effusion include direct contact of pancreatic enzymes with the diaphragm (sympathetic effusion), transfer of ascitic fluid via diaphragmatic defects, communication of a fistulous tract between a pseudocyst and the pleural space, and retroperitoneal movement of fluid into the mediastinum with mediastinitis or rupture into the pleural space.60,62 Ascitic amylase moves into the pleural space via the previously mentioned mechanisms. The pleural fluid/serum amylase ratio is greater than one in pancreatitis because of slower lymphatic clearance from the pleural space compared with more rapid renal clearance.
The effusion associated with acute pancreatitis is usually small and left-sided (60%) but may be isolated to the right side (30%) or be bilateral (10%).60 The patient usually presents with abdominal symptoms of acute pancreatitis. Diagnosis is confirmed by an elevated pleural fluid amylase concentration greater than that in serum. A normal pleural fluid amylase may be found early in acute pancreatitis but increases on serial measurements. The fluid is a polymorphonuclear (PMN)-predominant exudate with glucose values approximating those of serum. Leukocyte counts may reach 50,000 cells per µL. The pleural fluid pH is usually 7.30 to 7.35.
Pulmonary Embolism
The presence of a unilateral pleural effusion may suggest pulmonary embolism or obscure the diagnosis by directing attention to a primary lung or cardiac process. Pleural effusions occur in approximately 40% of patients with pulmonary embolism.63 These effusions result from several different mechanisms including increased pleural capillary permeability, imbalance in microvascular and pleural space hydrostatic pressures, and pleuropulmonary hemorrhage.63,64 Ischemia from pulmonary vascular obstruction, in addition to release of inflammatory mediators from platelet-rich thrombi, can cause capillary leak into the lung and, subsequently, the pleural space, explaining the usual finding of an exudative effusion. Transudates, described in approximately 20% of patients with pulmonary embolism, result from atelectasis.64
With pulmonary infarction, necrosis and hemorrhage into the lung and pleural space may result. More than 80% of patients with infarction have bloody pleural effusions, but more than 35% of patients with pulmonary embolism without radiographic infarction also have hemorrhagic fluid.63 The presence of a pleural effusion does not alter the signs or symptoms in patients with pulmonary embolism. Chest pain, usually pleuritic, occurs in most patients with pleural effusions complicating pulmonary embolism and is invariably ipsilateral.63 The chest radiograph virtually always shows a unilateral effusion that occupies less than one-third of the hemithorax.63 An associated pulmonary infiltrate (infarction) is seen in approximately half of patients with pulmonary embolism and effusion.
Pleural fluid analysis is variable and nondiagnostic.64 The pleural fluid is hemorrhagic in two-thirds of patients, but the number of red blood cells exceeds 100,000 per µL in less than 20%.64 The nucleated cell count ranges from less than 100 (atelectatic transudates) to greater than 50,000 per µL (pulmonary infarction).64 There is a predominance of PMNs when a thoracentesis is performed near the time of the acute injury and of lymphocytes with later thoracentesis. The effusion due to pulmonary embolism is usually (92%) apparent on the initial chest radiograph and reaches a maximum volume during the first 72 hours.63 Patients with pleural effusions that progress with therapy should be evaluated for recurrent embolism, hemothorax secondary to anticoagulation, an infected infarction, or an alternate diagnosis. When consolidation is absent on chest radiograph, effusions usually resolve in 7 to 10 days; with consolidation, the resolution time is 2 to 3 weeks.64
The association of pleural effusion with pulmonary embolism does not alter therapy. Furthermore, the presence of a bloody effusion is not a contraindication to full-dose anticoagulation because hemothorax is a rare complication of heparin therapy.65 An enlarging pleural effusion on therapy necessitates thoracentesis to exclude hemothorax, empyema, or another cause. Active pleural space hemorrhage necessitates discontinuation of anticoagulation, tube thoracostomy, and placement of a vena cava filter.
Post–Cardiac Injury Syndrome
Post–cardiac injury syndrome (PCIS) is characterized by fever, pleuropericarditis, and parenchymal infiltrates 3 weeks (2 to 86 days) after injury to the myocardium or pericardium.66–68 PCIS has been described after myocardial infarction, cardiac surgery, blunt chest trauma, percutaneous left ventricular puncture, and pacemaker implantation. The incidence after myocardial infarction has been estimated at up to 4% of cases,66 but with more extensive myocardial and pericardial involvement, it may be higher. It occurs with greater frequency (up to 30%) after cardiac surgery.69 The pathogenesis of PCIS remains obscure; an autoimmune response in patients with myocardial or pericardial injury and possibly concomitant viral illness has been speculated.70
Pleuropulmonary manifestations are the hallmark of PCIS. The most common presenting symptoms are pleuritic chest pain, found in virtually all patients, and fever, pericardial rub, dyspnea, and rales, which occur in half of patients.68 Rarely, hemoptysis occurs, an important differential point when pulmonary embolism with infarction is in the differential diagnosis. Fifty percent of patients have leukocytosis, and almost all have an elevated erythrocyte sedimentation rate (average, 62 mm per hour).68
The chest radiograph is abnormal in virtually all patients, with the most common abnormality being left-sided and bilateral pleural effusions; a unilateral right effusion is unusual.68 Pulmonary infiltrates are present in 75% of patients and are most commonly seen in the left lower lobe.66 The pleural fluid is a serosanguineous or bloody exudate with a glucose level above 60 mg per dL and pleural fluid pH above 7.30. Nucleated cell counts range from 500 to 39,000 per µL, with a predominance of PMNs early in the course.68 Pericardial fluid on echocardiogram is an important finding suggesting PCIS. The pleural fluid characteristics should help differentiate PCIS from a parapneumonic effusion and CHF, but do not exclude pulmonary embolism.
Esophageal Sclerotherapy
Pleural effusions are found in approximately 50% of patients 48 to 72 hours after esophageal sclerotherapy with sodium morrhuate and in 19% of patients after absolute alcohol sclerotherapy.71–73 Effusions may be unilateral or bilateral, with no predilection for side. Effusion appears more likely with larger total volumes of sclerosant injected and larger volume injected per site.71–72 The effusions tend to be small, serous exudates with variable nucleated (90 to 38,000 per µL) and red cell counts (126 to 160,000 per µL) and glucose concentration similar to that of serum.71 These effusions probably result from an intensive inflammatory reaction after extravasation of the sclerosant into the esophageal mucosa, resulting in mediastinal and pleural inflammation. The effusion not associated with fever, chest pain, or evidence of perforation is of little consequence, requires no specific therapy, and resolves during several days to weeks.71,72 However, late perforation may evolve in patients with apparent innocuous effusions. In patients with symptomatic effusions for 24 to 48 hours, diagnostic thoracentesis should be done and an esophagram considered.
Acute Respiratory Distress Syndrome
The presence of pleural effusions in ARDS has not been well appreciated. In a retrospective study of 25 patients with ARDS, a 36% incidence of pleural effusions was found, a percentage similar to that found with hydrostatic pulmonary edema.74 All patients had extensive alveolar pulmonary edema and endotracheal tube fluid that was compatible with increased permeability edema. Several experimental models of increased permeability pulmonary edema, including α-naphthyl thiourea, oleic acid, and ethchlorvynol, have been shown to produce pleural effusions. In the oleic acid and ethchlorvynol models, the development of pleural effusions lagged behind interstitial and alveolar edema by several hours. In the oleic acid model, 35% of the excess lung water collected in the pleural spaces. It appears that the pleura act as a reservoir for excess lung water in increased permeability and hydrostatic pulmonary edema. These effusions tend to be underdiagnosed clinically because the patient has bilateral alveolar infiltrates and the radiograph is taken with the patient in a supine position. Experimentally, the effusion is serous to serosanguineous, with a predominance of PMNs. These effusions usually require no specific therapy and resolve as ARDS resolves. However, in a series of positive end-expiratory pressure (PEEP)-unresponsive patients with ARDS, drainage of pleural effusion via tube thoracostomy has been shown to result in improved oxygenation.75 The decision to proceed to pleural space drainage in ARDS should be approached on a case-by-case basis and is not generally recommended.
Spontaneous Esophageal Rupture
Esophageal rupture, a potentially life-threatening event, requires immediate diagnosis and therapy. The history in spontaneous esophageal rupture is usually severe retching or vomiting or a conscious effort to resist vomiting. In some patients, the perforation may be silent. Early recognition of spontaneous rupture depends on interpretation of the chest radiograph. Several factors influence chest radiograph findings: the time between perforation and chest radiograph examination, site of perforation, and mediastinal pleural integrity.76 A chest radiograph taken within minutes of the acute injury is usually unremarkable. Mediastinal emphysema probably requires at least 1 to 2 hours to be demonstrated radiographically and is present in less than half of patients; mediastinal widening may take several hours.77 Pneumothorax, present in 75% of patients with spontaneous rupture, indicates violation of the mediastinal pleura; 70% of pneumothoraces are on the left, 20% are on the right, and 10% are bilateral.77 Mediastinal air is seen early if pleural integrity is maintained, whereas pleural effusion secondary to mediastinitis tends to occur later. Pleural fluid, with or without associated pneumothorax, occurs in 75% of patients. A presumptive diagnosis should immediately be confirmed radiographically. Esophagrams are positive in approximately 90% of patients.78 In the upright patient, rapid passage of the contrast material may not demonstrate a small rent; therefore, the study should be done with the patient in the appropriate lateral decubitus position.79
Pleural fluid findings depend on the degree of perforation and the timing of thoracentesis from injury. Early thoracentesis without mediastinal perforation shows a sterile, serous exudate with a predominance of PMNs, a pleural fluid amylase less than serum, and pH greater than 7.30.80 Once the mediastinal pleura tears, amylase of salivary origin appears in the fluid in high concentration.81 As the pleural space is seeded with anaerobic organisms from the mouth, the pH falls rapidly and progressively to approach 6.0.80,82 Other pleural fluid findings suggestive of esophageal rupture include the presence of squamous epithelial cells and food particles. The diagnosis of spontaneous esophageal rupture dictates immediate operative intervention. If diagnosed and treated appropriately within the first 24 hours with primary closure and drainage, survival is greater than 90%.77 Delay from the time of initial symptoms to diagnosis results in a reduced survival with any form of therapy.
Hemothorax
Hemothorax (blood in the pleural space) should be differentiated from a hemorrhagic pleural effusion, because the latter can be the result of only a few drops of blood in pleural fluid. An arbitrary but practical definition of a hemothorax with regard to therapy is a pleural fluid/blood hematocrit ratio greater than 30%. The majority of hemothoraces result from penetrating or blunt chest trauma.83 Hemothorax can also result from invasive procedures such as placement of central venous catheters, thoracentesis, and pleural biopsy, as well as from pulmonary infarction, malignancy, or ruptured aortic aneurysm. Bleeding can occur from vessels of the chest wall, lung, diaphragm, or mediastinum. Blood that enters the pleural space clots, rapidly undergoes fibrinolysis, and becomes defibrinogenated; thus, it rarely causes significant pleural fibrosis.
Hemothorax should be suspected in any patient with blunt or penetrating chest trauma. If a pleural effusion is found on the admitting chest radiograph, thoracentesis should be performed immediately and the hematocrit measured on the fluid. The hemothorax may not be apparent on the initial chest radiograph, which may be due to the supine position of the patient. Because bleeding may be slow and not appear for several hours, it is imperative that serial radiographs be obtained in these patients. The incidence of concomitant pneumothorax is high (approximately 60%).83 Patients with traumatic hemothorax should be treated with immediate tube thoracostomy.83–85 Large-diameter chest tube drainage evacuates the pleural space, may tamponade the bleeding (especially if the origin is from a pleural laceration), allows monitoring of the bleeding, and decreases the likelihood of subsequent fibrothorax.85,86 If bleeding continues without signs of slowing, thoracotomy should be performed, depending on the individual circumstance.85 Pleural effusions occasionally occur after removal of the chest tube from traumatic hemothoraces.87 A diagnostic thoracentesis is indicated to exclude empyema. If empyema is excluded, the pleural effusion usually resolves without specific treatment and without residual pleural fibrosis.
Coronary Artery Bypass Surgery
A small, left pleural effusion is virtually always present after coronary artery bypass surgery. This is associated with left lower lobe atelectasis and elevation of the left hemidiaphragm on chest radiograph. Left diaphragm dysfunction is secondary to intraoperative phrenic nerve injury from cold cardioplegia, stretch injury, or surgical trauma.88–90 The larger and grossly bloody effusions tend to be associated with internal mammary artery grafting, which causes marked exudation from the bed where the internal mammary artery was harvested.91
The pleural fluid is a hemorrhagic exudate with a low nucleated cell count, a glucose level similar to that of serum, and a pH greater than 7.40. Rarely, a loculated hemothorax may develop with trapped lung, resulting in clinically significant restriction.92 If there is a large effusion that qualifies as a hemothorax (see previous section), the fluid should be drained by tube thoracostomy. It is also prudent to drain moderately large, bloody effusions to avoid later necessity for decortication.
Abdominal Surgery
Approximately half of the patients who undergo abdominal surgery develop small unilateral or bilateral pleural effusions within 48 to 72 hours of surgery.27,28 The incidence is higher after upper abdominal surgery, in patients with postoperative atelectasis, and in patients who have free ascitic fluid at the time of surgery.27 Larger left-sided pleural effusions are common after splenectomy.27 The effusion is usually an exudate with less than 10,000 nucleated cells per µL. The glucose level is similar to that of serum, and pH is usually greater than 7.40.27 The effusion usually is related to diaphragmatic irritation or atelectasis. Small effusions generally do not require diagnostic thoracentesis, are of no clinical significance, and resolve spontaneously. Pleural effusion from subphrenic abscess or pulmonary embolism is unlikely to occur within 2 to 3 days of surgery. The only indication for diagnostic thoracentesis would be to exclude infection if the effusion is relatively large or loculated.
Chylothorax
Trauma from surgery accounts for approximately 25% of cases of chylothorax, second only to lymphoma. Most series estimate an incidence of chylothorax of less than 1% after thoracic surgery,93 but a 3% incidence has been reported after esophagectomy.94 Virtually all intrathoracic procedures, including lobectomy, pneumonectomy, and coronary artery bypass grafting, have been reported to cause chylothorax. Other iatrogenic chylothoraces can be caused by complications of prolonged central vein catheterization. Nonsurgical trauma, such as penetrating and nonpenetrating neck, thoracic, and upper abdominal injuries, also has been associated with chylothorax.
When the thoracic duct is torn by stretching during surgery, chyle leaks into the mediastinum and subsequently ruptures through the mediastinal pleura. In the nonsurgical setting, penetrating injuries and fractures may directly tear the thoracic duct. Chylothorax from a central venous catheter usually involves venous thrombosis. Other rare causes of chylothorax include sclerotherapy of esophageal varices and translumbar aortography.95–97
The patient may be asymptomatic if the effusion is small and unilateral, or may present with dyspnea with a large unilateral effusion or bilateral effusions. The pleural fluid is usually milky, but 12% can be serous or serosanguineous,98 with less than 7000 nucleated cells per µL, virtually all lymphocytes. The pleural fluid pH is alkaline (7.40–7.80), and triglyceride levels are greater than plasma levels. Finding a pleural fluid triglyceride concentration of greater than 110 mg/dL makes the diagnosis of chylothorax highly likely.98 If the triglyceride level is less than 50 mg/dL, chylothorax is highly unlikely. Triglyceride levels of 50 to 110 mg/dL indicate the need for lipoprotein electrophoresis98; the presence of chylomicrons confirms a chylothorax. The thoracic duct defect after trauma usually closes spontaneously within 10 to 14 days, with chest tube drainage as well as bed rest and total parenteral nutrition to minimize chyle formation. A pleuroperitoneal shunt relieves dyspnea, recirculates chyle, and prevents malnutrition and immunocompromise.
Duropleural Fistula
Disruption of the dura and parietal pleura by surgical and nonsurgical trauma may result in a duropleural fistula with subsequent development of a pleural effusion.99–102 The pleural fluid characteristics depend on the severity of the trauma and the delay between the trauma and the pleural fluid analysis. Pleural fluid due to a chronic duropleural fistula is usually a colorless transudate with low mononuclear cell count; a duropleural fistula associated with recent trauma may be a transudate or an exudate.101,102 The diagnosis may even be delayed because of a coexisting process such as hemothorax. The diagnosis of duropleural fistula is established by the detection of β2-transferrin in the pleural fluid.103 Confirmation of the fistula by conventional or radionuclide myelography is recommended if surgical management is contemplated.
 Pneumothorax
Pneumothorax
Pathophysiology
When there are rapid fluctuations in intrathoracic pressure, however, a large transpulmonary pressure gradient occurs transiently. Bronchial and bronchiolar obstruction, resulting in air trapping, can significantly increase the transpulmonary pressure gradient. The alveolar walls and visceral pleura maintain the pressure gradient between the airways and pleural space. When the pressure gradient is transiently increased, alveolar rupture may occur; air enters the interstitial tissues of the lung and may enter the pleural space, resulting in a pneumothorax. If the visceral pleura remain intact, the interstitial air moves toward the hilum, resulting in pneumomediastinum.104–105 Because mean pressure within the mediastinum is always less than in the periphery of the lung, air moves proximally along the bronchovascular sheaths to the hilum and mediastinal soft tissues.
The development of pneumomediastinum after alveolar rupture requires continual cyclic respiratory efforts, which result in slow movement of air from the ruptured alveolus along a pressure gradient to the mediastinum.105 Mediastinal air may decompress into the cervical and subcutaneous tissues or the retroperitoneum. With abrupt rise in mediastinal pressure or insufficient decompression to subcutaneous tissue, the mediastinal pleura may rupture, causing pneumothorax. Inadequate decompression of the mediastinum, rather than direct rupture of subpleural blebs into the pleural space, may be the major cause of pneumothorax.104
When pneumothorax occurs, the elasticity of the lung causes it to collapse. Lung collapse continues until the pleural defect seals or pleural and alveolar pressures equalize. When a ball-valve effect occurs at the site of communication between the pleural space and the alveolus, permitting only egress of air from the lung, there is a progressive accumulation of air within the pleural space, which can result in markedly increased positive pleural pressure, producing a tension pneumothorax. Tension pneumothorax compresses mediastinal structures, resulting in impaired venous return to the heart, decreased cardiac output, and at times, fatal cardiovascular collapse.106–107 Rarely, tension along the bronchovascular sheaths and in the mediastinum can cause collapse of the pulmonary arteries and veins, resulting in cardiovascular collapse.104
Patients with primary spontaneous pneumothorax have a decrease in vital capacity and an increase in the P(A-a)O2 gradient; they usually present with hypoxemia due predominantly to the development of an intrapulmonary shunt and areas of low ventilation/perfusion in the atelectatic lung.108,109 Hypercapnia does not occur because there is adequate function in the uninvolved lung to maintain necessary alveolar ventilation. Patients with secondary spontaneous pneumothorax, in contrast, commonly develop hypercapnia because the gas exchange abnormality caused by the pneumothorax is superimposed on lungs with preexisting abnormal pulmonary gas exchange.
Pneumothorax in the Intensive Care Unit
Iatrogenic Pneumothorax
Central Venous Catheters
Central venous catheters are used routinely in critically ill patients for volume resuscitation, parenteral nutrition, and drug administration. Approximately 3 million central venous catheters are placed annually in the United States, and this procedure continues to be associated with clinically relevant morbidity and some mortality. The morbidity and mortality associated with central venous catheter use are most commonly physician related.42 Pleural complications of acquisition of venous access and the indwelling phase of central venous catheters include pneumothorax, hydrothorax, hemothorax, and chylothorax. In a recent study of mechanical complications of central venous catheters, 1.1% of 534 patients had pneumothorax.110 This translates into approximately 33,000 pneumothoraces per year from central venous catheter insertions in critically ill patients in the United States. In the same study, none of the 405 patients developed pneumothorax when the central venous catheter was replaced over a guidewire.
The subclavian and internal jugular routes have been associated with pneumothorax, hemothorax, chylothorax, and catheter placement into the pleural space. Cannulation of the subclavian vein is associated with a higher risk of pneumothorax (less than 5%)111 than cannulation of the internal jugular vein (less than 0.2%)112; with the external jugular venous approach, pneumothorax is avoided. There is a greater risk of pneumothorax with the infraclavicular compared to the supraclavicular approach to the subclavian vein. All complications of insertion, regardless of approach, can be reduced by appropriate physician training and experience. Operator inexperience appears to increase the number of complications with the internal jugular approach. It probably does not have as much impact on the incidence of pneumothorax with the subclavian vein approach, which accounts for 25% to 50% of all complications.113
Most pneumothoraces occur at the time of the procedure from direct lung puncture, but delayed pneumothoraces have been noted; therefore, it is prudent to view a chest radiograph 12 to 24 hours after the procedure. Up to half of patients with needle-puncture pneumothorax may be managed expectantly without the need for tube drainage. Bilateral pneumothoraces have been reported to occur from unilateral attempts,113 and death can occur when there is a delay in the diagnosis of pneumothorax. As stated previously, a pneumothorax may be more difficult to detect while the patient is supine. Additional views should be taken, especially if the venous cannulation does not proceed as anticipated. With any newly placed central venous catheter, a postprocedure chest radiograph should be obtained, regardless of the site cannulated, to assure that the catheter tip is properly positioned. If a small pneumothorax is diagnosed by chest radiograph and the patient is asymptomatic and not on mechanical ventilation, the patient can be followed expectantly with repeat chest radiographs to assure that the leak has ceased. If the patient is on mechanical ventilation or the pneumothorax is large or has caused significant symptoms or gas exchange abnormalities, then tube thoracostomy should be performed as soon as possible.
Barotrauma
Pulmonary barotrauma is an important clinical problem because of the widespread use of mechanical ventilation. Barotrauma occurs in approximately 3% to 10% of patients on mechanical ventilation and includes parenchymal interstitial gas, pneumomediastinum, subcutaneous emphysema, pneumoperitoneum, and pneumothorax.7,114–118 The most clinically important form is pneumothorax, occurring in 1% to 15% of all patients on mechanical ventilation. In patients with ARDS, rates of 6.5% to 87% have been reported.117,118 The number of ventilation days, underlying disease (ARDS, COPD, necrotizing pneumonia), and use of PEEP have an impact on the incidence of pneumothorax.114–116,119,120 When a pneumothorax develops in the setting of mechanical ventilation, 30% to 97% of patients develop tension pneumothorax.7,115,119,120 The reported incidence of barotrauma varies widely between the studies, with the lowest incidences reported in the most recent large series.118 This may be partly explained by the adoption of less aggressive ventilation strategies over time.
The initial radiographic sign of barotrauma is often pulmonary interstitial gas or emphysema.117,121 In the early stages, however, interstitial gas may be difficult to detect radiographically. This harbinger of pneumothorax may be detected as distinct subpleural air cysts, linear air streaks emanating from the hilum, and perivascular air halos. Subpleural air cysts, most commonly seen in ARDS, tend to appear abruptly on the chest radiograph as single or multiple thin-walled, round lucencies, and are most often visualized at the lung bases, medially or diaphragmatically.122 The cysts, which may expand rapidly, are usually 3 to 5 cm in diameter. Differentiating between peripheral subpleural air cysts and a localized basilar pneumothorax may be problematic. Pleural air cysts appear to be more common in younger patients, possibly because connective tissue planes of the lung are looser in younger patients than in older patients.123 The risk of tension pneumothorax is substantial in patients who have developed subpleural lung cysts with continued mechanical ventilation. When mechanical ventilation is discontinued, the cyst may resolve spontaneously or become secondarily infected.
Ultrasonography has emerged as a bedside modality for the detection of pneumothorax. The absence of lung sliding is the finding associated with pneumothorax.6 False-positive results may occur and are due to bullous lung disease or preexisting pleural symphysis.6,124,125 The disappearance of lung sliding that was present previously may be more specific for the development of pneumothorax—for example, after line placement. However, this subject awaits further study.
When evidence of barotrauma without pneumothorax is observed in any patient requiring continued mechanical ventilation, immediate attempts should be made to lower the plateau airway pressure. In ARDS, tidal volumes126,127 and inspiratory flow rates should be lowered, an attempt should be made to reduce or remove PEEP, and neuromuscular blockers and sedation should be considered.128 In status asthmaticus, in addition to the aforementioned maneuvers, controlled hypoventilation should be accomplished.129,130 There is no evidence supporting the use of prophylactic chest tubes. However, the patient should be monitored closely for tension pneumothorax and provisions made for emergency bedside tube thoracostomy.
Tension Pneumothorax
Pneumothorax in the mechanically ventilated patient usually presents as an acute cardiopulmonary emergency, beginning with respiratory distress and, if unrecognized and untreated, progressing to cardiovascular collapse. In one report of 74 patients, the diagnosis of pneumothorax was made clinically in 45 (61%) patients based on hypotension, hyperresonance, diminished breath sounds, and tachycardia.120 The mortality rate was 7% in these patients diagnosed clinically. In the remaining 29 patients, diagnosis was delayed between 30 minutes and 8 hours, and 31% of these patients died of pneumothorax. Other series of barotrauma in the setting of mechanical ventilation have reported mortality rates from 58% to 77%.7,116
When the clinical signs and symptoms are noted in mechanically ventilated patients, treatment should not be delayed to obtain radiographic confirmation. If a chest tube is not immediately available, placement of a large-bore needle into the anterior second intercostal space on the suspected side is life saving and confirms the diagnosis, as a rush of air is noted on entering the pleural space. An appropriately large chest tube can then be placed and connected to an adequate drainage system that can accommodate the large air leak that may develop in mechanically ventilated patients.130
On relief of the tension, there is a rapid improvement in oxygenation, increase in blood pressure, decrease in heart rate, and fall in airway pressures. In experimental tension pneumothorax, it has been observed that the inability to raise cardiac output in response to hypoxemia leads to a reduction in systemic oxygen transport and a decrease in mixed venous partial pressure of oxygen (PO2), partially explaining the cardiovascular collapse seen in these patients.107 In mechanically ventilated patients, a decrease in cardiac output is an inevitable consequence of tension pneumothorax.
 Bronchopleural Fistula
Bronchopleural Fistula
Definition and Causes
Communication between the bronchial tree and the pleural space is a dreaded complication of mechanical ventilation.131,132 There are three presentations of bronchopleural fistula (BPF): (1) failure to reinflate the lung despite chest tube drainage, or continued air leak after evacuation of pneumothorax in the setting of chest trauma; (2) complication of a diagnostic or therapeutic procedure such as thoracic surgery; and (3) complication of mechanical ventilation, usually for ARDS.106 In ARDS, often a pneumothorax occurs under tension and is later associated with empyema, multiple sites of leakage, and a poor prognosis. A large air leak through a BPF can result in failure of lung reexpansion, loss of a significant amount of each delivered tidal volume, loss of the ability to apply PEEP, inappropriate cycling of the ventilator,133 and inability to maintain alveolar ventilation (Table 65-3).
Management
Given the frequency of barotrauma in BPF in mechanically ventilated patients, intensivists are called to give advice on the management of these difficult patients. Definitive therapy of BPF frequently involves invasive surgical approaches that include thoracoplasty, mobilization of the pectoralis or intercostal muscles, bronchial stump stapling, and decortication.134–139 Although some of these techniques are still used today, there is a trend toward more conservative management of acute and chronic BPF, using innovations of standard techniques and new modalities that include chest tube management, drainage systems, ventilatory support, and definitive nonoperative therapy (Table 65-4). Even insertion of an endobronchial valve designed for the treatment of emphysema may be considered in selected patients.140 Nonoperative therapy provides an alternative to the surgical approaches in patients who are poor operative candidates. Each patient with a BPF is unique and requires individual management based on the specific clinical setting. Attention to the basics of medical care of patients with BPF should not be neglected in the face of the potentially dramatic events related to the BPF. Nutritional status must be maintained, appropriate antibiotics used for the infected pleural space, and the space adequately drained.
TABLE 65-4 Management of Bronchopleural Fistula in Patients Requiring Mechanical Ventilation
| Conservative |
Chest Tubes
The initial therapy for pneumothorax in a patient on mechanical ventilation is placement of a chest tube in an attempt to reexpand the lung. The chest tube is initially necessary, can be detrimental later, and may play a role more important than that of a passive conduit. Air leaks in the setting of BPF range from less than 1 to 16 L per minute141; therefore, a chest tube that permits prompt and efficient drainage of this level of airflow is required. Gas moves through a tube in a laminar fashion and is governed by Poiseuille’s law (v = [π r4 P/8lV]t). In the clinical setting, the gas moving through a chest tube is moist; therefore, it is subject to turbulent flow and governed by the Fanning equation (v = [π r2 r5 P/fl]).141–143 Therefore, both the length (l) and, even more so, the radius (r) are important when choosing a chest tube and connecting tubing to evacuate a BPF adequately (as flow varies exponentially to the fifth power of the radius of the tube). The smallest internal diameter that allows a maximum flow of 15.1 L per minute at −10 cm H2O suction is 6 mm141,142 (a 32 French chest tube has an internal diameter of 9 mm). A chest tube with a diameter adequate to convey the potentially large airflow of the BPF must be considered. A chest tube with too small a diameter can lead to lung collapse and tension pneumothorax in the setting of a mobile mediastinum.
Not only can the chest tube be used to drain pleural air, it can also be used to limit the air leak in certain situations. One modality is the application of intrapleural pressure equivalent to the level of PEEP during the expiratory phase of ventilation.144–146 With positive intrapleural pressure applied through the chest tube, the air leak persists during the inspiratory phase of ventilation but decreases during expiration, allowing maintenance of PEEP in patients in whom it is necessary for adequate oxygenation. Synchronized closure of the chest tube during the inspiratory phase has also been used to control the air leak.147,148 A combination of these techniques has been suggested for patients with significant BPF air leaks during both the inspiratory and expiratory phases of mechanical ventilation.131,148 These techniques pose potential hazards, including increased pneumothorax and tension pneumothorax,131,147 necessitating extremely close patient monitoring when such manipulations are used.
Instillation of chemical agents through the chest tube may potentially help close the BPF if the anatomic defect is small and single, but it is unlikely to be successful if the fistula is large or if there are multiple fistulas. Various agents have been successful in preventing recurrent pneumothoraces in patients who are not on mechanical ventilation,149–152 but BPF in the setting of mechanical ventilation is a different situation. One study compared the recurrence of pneumothorax in 39 patients with BPF randomized to intrapleural tetracycline or placebo groups.153 There was no evidence that intrapleural tetracycline facilitated closure of the BPF. No adverse effects were encountered from the instillation of tetracycline in patients with persistent air leaks.
The chest tube may be associated with adverse effects in patients with BPF. The gas escaping through the chest tube represents part of the minute ventilation delivered to the patient and makes maintenance of an effective tidal volume problematic.154,155 Maintenance of a specific level of ventilation is not only affected by the amount of gas escaping through the fistula. The escaping gas does not passively flow from the airways into the BPF but is involved in physiologic gas exchange.154,155 Approximately 25% of the minute ventilation has been found to escape via the BPF in patients with ARDS, with more than 20% of CO2 excretion occurring by this route in half of the patients.155 The role of the BPF in active CO2 exchange is complex; proposed mechanisms include drainage of gas from alveoli in the area of the BPF and removal of gas from remote alveolar areas by pressure gradients created by the BPF.156
Carbon dioxide excretion and a reduction in minute ventilation occur to a lesser extent in BPF trauma victims.154 In these patients, variable CO2 excretion and loss of minute ventilation were dynamic and dependent on the level of chest tube suction. The difference between trauma and ARDS patients may have been due to the variability of lung compliance and the use of different ventilators.155 Also, BPF may affect oxygen use, which generally decreases the use of inspired oxygen before it escapes through the fistula.154 This relationship is variable but requires consideration in patients with oxygenation problems.
Negative pressure applied to the chest tube may be transmitted beyond the pleural space and into the airways, creating inappropriate cycling of the ventilator.133,156 The increased flow through a BPF can occur with increased negative pleural pressure and may interfere with closure and healing of the fistulous site.131 Therefore, the least amount of chest tube suction that keeps the lung inflated should be maintained in patients with BPF. The chest tube is a potential source of infection, both at the insertion site and within the pleural space.
Drainage Systems
As with the chest tube, the resistance of flow of gases is a consideration in the choice of the drainage system for the patient with a BPF.141 The size of the air leak and the flow the drainage system can accommodate are necessary considerations. In an experimental model of BPF that simulated the type of air leak seen clinically (mean maximal flow, 5 L per minute), four pleural drainage units (PDU)—Emerson Post-Operative Pump (Emerson), Pleur-Evac (Teleflex Medical), Sentinel Seal (Tyco), and Thora-Klex (Avilor)—were tested at water seal, −20 cm H2O, and −40 cm H2O suction.141 Compared to the water seal, −20 cm H2O suction significantly increased the ability of all four PDUs to evacuate air via the chest tube, but an increase in suction to −40 cm H2O did not significantly alter flow. When the air leak reached 4 to 5 L per minute, use of the Thora-Klex or Sentinel Seal became clinically impractical. The Pleur-Evac can handle flow rates up to 34 L per minute, but its use with rates over 28 L per minute is impractical owing to intense bubbling in the suction control chamber.112 Air leaks of this magnitude are infrequent clinically in BPF and are likely to be seen only with major airway disruption or diffuse parenchymal leak secondary to ARDS with severe barotraumas.156 In the latter situations, the low-pressure, high-volume Emerson suction pump remains the only PDU capable of handling the air leak.141 The choice of PDU should be influenced by its physiologic capabilities and the type of BPF air leak encountered.
Mechanical Ventilation
Conventional Ventilation
The dilemma with a BPF in a mechanically ventilated patient is achieving adequate ventilation and oxygenation while allowing repair of the BPF to occur. Because air flow escaping through a BPF theoretically delays healing of the fistulous site, reducing flow through the fistula has been a major goal in promoting repair. The BPF provides an area of low resistance to flow and acts as a conduit for the escape of a variable percentage of delivered tidal volume during conventional positive-pressure mechanical ventilation. Thus the goal of management is to maintain adequate ventilation and oxygenation while reducing the fistula flow.131 Using the lowest possible tidal volume, fewest mechanical breaths per minute, lowest level of PEEP, and shortest inspiratory time can do this. Avoidance of expiratory retard also reduces airway pressures. Using the greatest number of spontaneous breaths per minute, thereby reducing use of positive pressure, may also be advantageous. Intermittent mandatory ventilation may have an advantage over assist-control ventilation in BPF.
In a retrospective study of 39 patients with BPF who were maintained on conventional ventilation, only two patients developed a pH less than 7.30, despite air leaks of up to 900 mL per breath.156 Overall, mortality was higher when the BPF developed late in the illness and was higher with larger leaks (more than 500 mL per breath).
High-Frequency Ventilation
Despite anecdotal reports, experimental data, and clinical studies involving high-frequency ventilation (HFV) in the setting of BPF, controversy exists. However, there appear to be subgroups of patients with BPF in whom HFV may be beneficial. Both animal157 and human158 studies suggest that HFV is superior to conventional ventilation in controlling PO2 and partial pressure of carbon dioxide (PCO2) when there is a proximal (tracheal or bronchial) unilateral or bilateral fistula in the presence of normal lung parenchyma.
The use of HFV in BPF in patients with parenchymal lung disease such as ARDS is more controversial. Although some studies have shown that HFV improves or stabilizes gas exchange in patients with extensive parenchymal lung disease others have not shown a beneficial effect on gas exchange or a reduction in fistula outflow.159,160 A trial of HFV appears reasonable in the patient with a proximal BPF and normal lung parenchyma; however, it is unclear whether HFV should be considered the primary mode of ventilation in this setting. Despite discrepancies in clinical results, a trial of HFV in a critically ill patient with a BPF and diffuse parenchymal disease who fails conventional ventilation appears justified. Caution must be exercised, however, with close monitoring of gas exchange parameters and fistula flow whenever HFV is used.
Other Modes of Ventilation
Other maneuvers during both conventional ventilation and HFV can be potentially helpful in patients with BPF. Selective intubation and conventional ventilation of the unaffected lung in patients with unilateral BPF may be useful but predisposes to the collapse of the nonintubated lung.161–163 The use of differential lung ventilation with conventional ventilation may be of benefit in some patients.159 Positioning of the patient such that the BPF is dependent has been shown to decrease fistula flow.163
Case reports and animal studies suggest other potential applications of HFV in BPF, including the use of independent lung ventilation with HFV applied to the BPF lung and conventional ventilation to the normal lung.164 Another mode of HFV, ultra high-frequency jet ventilation, is being explored and has been used with some success in reducing BPF in humans165 and animal models.166 Independent lung ventilation with ultra high-frequency lung ventilation applied to the BPF lung and conventional ventilation to the normal lung led to rapid BPF closure in two of three patients.165
Flexible Bronchoscopy
The flexible bronchoscope can be valuable in the diagnosis of BPF.167–169 Bronchoscopic therapy of BPF has several potential advantages, including low cost, shortened hospital stay, and relative noninvasiveness, particularly in poor operative candidates167–169 (see Chapter 9). Proximal fistulas, such as those associated with lobectomy or pneumonectomy or stump breakdown, can be directly visualized through the bronchoscope. Distal fistulas cannot be visualized directly and require bronchoscopic passage of an occluding balloon to localize the bronchial segment leading to the fistula.170–172 A balloon is systematically passed through the working channel of the bronchoscope and into each bronchial segment in question and then inflated; a reduction in air leak indicates localization of a bronchial segment communicating with the BPF. Once the fistula has been localized, various materials can be passed through a catheter in the working channel of the bronchoscope and into the area of the fistula.167–176 Direct application of a sealant through the working-channel catheter onto the fistula site is the method generally used for directly visualized proximal fistulas. For distal fistulas, a multiple-lumen Swan-Ganz catheter has been used to localize the BPF and pass the occluding material of choice.170
Several agents have been used through the bronchoscope in an attempt to occlude BPF. These include fibrin agents,169–170 cyanoacrylate-based agents,167 absorbable gelatin sponge (Gelfoam [Pfizer]), blood-tetracycline,171 and lead shot.172 The reports on all of these agents are limited to only a few patients. The cyanoacrylate-based and fibrin agents have received the most attention but still have had less than 20 total cases reported. These patients have had at least a 50% reduction of fistula flow, and most had closure of the fistula subsequent to sealant application, although multiple applications were necessary in some patients. These agents appear to work in two phases, with the agent initially sealing the leak by acting as a plug and subsequently inducing an inflammatory process with fibrosis and mucosal proliferation permanently sealing the area.167 They are not useful with large proximal tracheal or bronchial ruptures or multiple distal parenchymal defects.170
Anzueto A, Frutos-Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30(4):612-619.
Doelken P, Abreu R, Sahn SA, Mayo PH. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest. 2006;130(5):1354-1361.
Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184.
Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348.
Pneumothorax in the ICU. Lung sliding reliably rules out pneumothorax.
1 Woodring JH. Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR Am J Roentgenol. 1984;142(1):59-64.
2 Collins JD, Burwell D, Furmanski S, et al. Minimum detectable pleural effusions: a roentgen pathology model. Radiology. 1975;105:51.
3 Yu C-J, Yang P-C, Chang D-B, et al. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. AJR Am J Roentgenol. 1992;159:695.
4 Mayo PH, Goltz HR, Tafreshi M, et al. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125:1059.
5 Moskowitz PS, Griscom NT. The medial pneumothorax. Radiology. 1976;120:143.
6 Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108:1345.
7 Rohlfing BM, Webb WR, Schlobohm RM. Ventilator-related extra-alveolar air in adults. Radiology. 1976;121:25.
8 Gobien RP, Reines HD, Schabel SI. Localized tension pneumothorax: unrecognized form of barotrauma in adult respiratory distress syndrome. Radiology. 1982;142:15.
9 Tocino IM, Miller MH, Fairfax WR. Distribution of pneumothorax in the supine and semirecumbent critically ill adult. AJR Am J Roentgenol. 1985;144:901.
10 Kollef MH. Risk factors for the misdiagnosis of pneumothorax in the intensive care unit. Crit Care Med. 1991;19:906.
11 Lipscomb DJ, Flower CDR, Hadfield JW. Ultrasound of the pleura: an assessment of its clinical value. Clin Radiol. 1981;32:289.
12 Godwin JE, Sahn SA. Thoracentesis: a safe procedure in mechanically ventilated patients. Ann Intern Med. 1990;113:800.
13 Collins TR, Sahn SA. Thoracentesis: clinical value, complications, technical problems, and patient experience. Chest. 1987;91:817.
14 Bartter T, Mayo PD, Pratter MR, et al. Lower risk and higher yield for thoracentesis when performed by experienced operators. Chest. 1993;103:1873.
15 Seneff MG, Corwin W, Gold LH, et al. Complications associated with thoracentesis. Chest. 1986;89:97.
16 Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. Arch Intern Med. 1990;150:873.
17 Heidecker J, Huggins JT, Sahn SA, et al. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130:1173.
18 Light RW, Jenkinson SG, Minh V, et al. Observations on pleural pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis. 1980;121:799.
19 Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540.
20 Estenne M, Yernault J-C, Detroyer A. Mechanism of relief of dyspnea after thoracentesis in patients with large effusions. Am J Med. 1983;74:813.
21 Light RW, Stansbury DW, Brown SE. Changes in pulmonary function following therapeutic thoracentesis. Chest. 1981;80:375.
22 Doelken P, Abreu R, Sahn SA. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest. 2006;130:1354.
23 Brandstetter RD, Cohen RP. Hypoxemia after thoracentesis: a predictable and treatable condition. JAMA. 1979;242:1060.
24 Karetzky M, Kothari GA, Fourre JA, et al. The effect of thoracentesis on arterial oxygen tension. Respiration. 1978;36:96.
25 Perpina M, Benlloch E, Marco V, et al. The effect of thoracentesis on pulmonary gas exchange. Thorax. 1983;38:747.
26 Mattison L, Coppage L, Alderman D, et al. Pleural effusions in the medical intensive care unit: prevalence, causes and clinical implications. Chest. 1997;111:1018.
27 Light RW, George RB. Incidence and significance of pleural effusion after abdominal surgery. Chest. 1976;69:621.
28 Nielsen PH, Jepsan SB, Olsen AD. Postoperative pleural effusion following upper abdominal surgery. Chest. 1989;96:1133.
29 Wiener-Kronish JP, Matthay MA, Callen PW, et al. Relationship of pleural effusions to pulmonary hemodynamics in patients with congestive heart failure. Am Rev Respir Dis. 1987;132:1253.
30 Shinto RA, Light RW. Effects of diuresis on the characteristics of pleural fluid in patients with congestive heart failure. Am J Med. 1990;88:230.
31 Chakko SC, Caldwell SH, Sforza PP. Treatment of congestive heart failure: its effect on pleural fluid chemistry. Chest. 1989;95:798.
32 Lieberman FL, Hidemura R, Peters RL, et al. Pathogenesis and treatment of hydrothorax complicating cirrhosis with ascites. Ann Intern Med. 1966;64:341.
33 Sadler TW. Langman’s Medical Embryology, 7th ed. Baltimore: Williams & Wilkins; 1995. p 176
34 Johnson RF, Loo RB. Hepatic hydrothorax: studies to determine the source of the fluid and report of 13 cases. Ann Intern Med. 1964;61:385.
35 Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis. 1997;17:227.
36 Frazer IH, Lichtenstein M, Andrews JT. Pleuroperitoneal effusion without ascites. Med J Aust. 1983;2:520.
37 Xiol X, Castellvi JM, Guardiola J, et al. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719.
38 Abba AA, Laajam MA, Zargar SA. Spontaneous neutrocytic hepatic hydrothorax without ascites. Respir Med. 1996;90:631.
39 Strauss RM, Martin LG, Kaufman SL, et al. Transjugular intraheaptic portal systemic shunt for the management of symptomatic cirrhotic hydrothorax. Am J Gastroenterol. 1994;89:1520.
40 Mouroux J, Perrin C, Venissac N, et al. Management of pleural effusion of cirrhotic origin. Chest. 1996;109:1093.
41 Eid AA, Keddissi JI, Kinasewitz GT. Hypoalbuminemia as a cause of pleural effusions. Chest. 1999;115:1066.
42 Scott WL. Complications associated with central venous catheters: a survey. Chest. 1988;94:1221.
43 Duntley P, Siever J, Korwes ML, et al. Vascular erosion by central venous catheters: clinical features and outcome. Chest. 1992;101:1633.
44 Ellis LM, Vogel SBIII, Copeland EM. Central venous catheter vascular erosions. Ann Surg. 1989;209:475.
45 Aslamy Z, Dewald LL, Heffner JE. MRI of central venous anatomy: Implications for central venous catheter insertion. Chest. 1998;114:820.
46 Wechsler RJ, Byrne KJ, Steiner RM. The misplaced thoracic venous catheter: detailed anatomical consideration. Crit Rev Diagn Imaging. 1982;21:289.
47 Ball GV, Whitfield CL. Studies on rheumatoid disease pleural fluid. Arthritis Rheum. 1966;9:846.
48 Heffner JE, McDonald J, Bareberi C, et al. Management of parapneumonic effusions: an analysis of physician practice patterns. Arch Surg. 1995;130:433.
49 Taryle DA, Potts DE, Sahn SA. The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74:170.
50 Light RW, Girard EM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med. 1980;69:507.
51 Heffner JE. Indications for draining a parapneumonic effusion: an evidence-based approach. Semin Respir Infect. 1999;14:48.
52 Heffner JE, Brown LK, Barberi C, et al. Pleural fluid chemical analysis in parapneumonic effusions: a meta-analysis. Am J Respir Crit Care Med. 1995;151:1700.
53 Lesho EP, Roth BJ. Is pH paper an acceptable, low-cost alternate to the blood gas analyzer for determining pleural fluid pH? Chest. 1997;112:1291.
54 Ashbaugh DG. Empyema thoracis: factors influencing morbidity and mortality. Chest. 1991;99:1162.
55 Sahn SA. Use of fibrinolytic agents in the management of complicated parapneumonic effusions. Thorax. 1998;53(Suppl):S65.
56 Ridley PD, Brainbridge MV. Thoracoscopic débridement and pleural irrigation in the management of empyema thoracis. Ann Thorac Surg. 1991;51:461.
57 Landreneau RJ, Keenan RJ, Hazelrigg SR, et al. Thoracoscopy for empyema and hemothorax. Chest. 1995;109:18.
58 Podbielski FJ, Maniar HS, Rodriguez HE, et al. Surgical strategy of complex empyema thoracis. JSLS. 2000;4:287.
59 Cunniffe MG, Maguire D, McAnena OJ, et al. Video-assisted thoracoscopic surgery in the management of loculated empyema. Surg Endosc. 2000;14:175.
60 Kaye MD. Pleuropulmonary complications of pancreatitis. Thorax. 1968;23:297.
61 Maringhini A, Ciambra M, Patti R, et al. Ascites, pleural, and pericardial effusions in acute pancreatitis: a prospective study of the incidence, natural history, and prognostic role. Dig Dis Sci. 1996;41:848.
62 Anderson WJ, Skinner DB, Zuidema GD, et al. Chronic pancreatic pleural effusions. Surg Gynecol Obstet. 1973;137:827.
63 Bynum LJ, Wilson JEIII. Radiographic features of pleural effusions in pulmonary embolism. Am Rev Respir Dis. 1978;117:829.
64 Bynum LJ, Wilson JEIII. Characteristics of pleural effusions associated with pulmonary embolism. Arch Intern Med. 1976;136:159.
65 Simon HB, Daggett WN, DeSanctis RW. Hemothorax as a complication of anticoagulant therapy in the presence of pulmonary infarction. JAMA. 1969;208:1830.
66 Dressler W. The post-myocardial infarction syndrome: a report of 44 cases. Arch Intern Med. 1959;103:28.
67 Engle MA, Ito T. The post-pericardiotomy syndrome. Am J Cardiol. 1961;7:73.
68 Stelzner TJ, King TEJr, Antony VB, et al. The pleuro-pulmonary manifestations of the postcardiac injury syndrome. Chest. 1983;84:383.
69 Kaminsky ME, Rodan BA, Osborne DR, et al. Post-pericardiotomy syndrome. AJR Am J Roentgenol. 1982;138:503.
70 Kim S, Sahn SA. Postcardiac injury syndrome: an immunologic pleural fluid analysis. Chest. 1996;109:570.
71 Bacon BR, Bailey-Newton RS, Connors AFJr. Pleural effusions after endoscopic variceal sclerotherapy. Gastroenterology. 1985;88:1910.
72 Saks BJ, Kilby AE, Dietrich PA. Pleural and mediastinal changes following endoscopic injection sclerotherapy of esophageal varices. Radiology. 1983;149:639.
73 Parikh SS, Amarapurkar DN, Dhawan PS, et al. Development of pleural effusion after sclerotherapy with absolute alcohol. Gastrointest Endosc. 1993;39:404.
74 Aberle DR, Wiener-Kronish JP, Webb WR, et al. Hydrostatic versus increased permeability pulmonary edema: diagnosis based on radiographic criteria in critically ill patients. Radiology. 1988;168:73.
75 Talmor M, Hydo L, Gershenwald JG, et al. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery. 1998;123:137.
76 Parkin GJS. The radiology of perforated esophagus. Clin Radiol. 1973;24:324.
77 O’Connell ND. Spontaneous rupture of the esophagus. AJR Am J Roentgenol. 1967;99:186.
78 Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of esophageal perforation and rupture. Ann Thorac Surg. 1986;42:235.
79 DeMeester TR. Perforation of the esophagus. Ann Thorac Surg. 1986;42:231.
80 Maulitz RM, Good JTJr, Kaplan RL, et al. The pleuropulmonary consequences of esophageal rupture: an experimental model. Am Rev Respir Dis. 1979;120:363.
81 Sherr HP, Light RW, Merson MH, et al. Origin of pleural fluid amylase in esophageal rupture. Ann Intern Med. 1972;76:985.
82 Abbott OA, Mansour KA, Logan WDJr, et al. Atraumatic so-called “spontaneous” rupture of the esophagus. J Thorac Cardiovasc Surg. 1970;59:67.
83 Graham JM, Mattox KL, Beall ACJr. Penetrating trauma of the lung. J Trauma. 1979;19:665.
84 Beall ACJr, Crawford HW, DeBakey ME. Considerations in the management of acute traumatic hemothorax. J Thorac Cardiovasc Surg. 1966;52:351.
85 Weil PH, Margolis IB. Systematic approach to traumatic hemothorax. Am J Surg. 1981;142:692.
86 Griffith GL, Todd EP, McMillin RD, et al. Acute traumatic hemothorax. Ann Thorac Surg. 1978;26:204.
87 Wilson JM, Boren CH, Peterson SR, et al. Traumatic hemothorax: is decortication necessary? J Thorac Cardiovasc Surg. 1979;77:489.
88 Iverson L, Mittal A, Dugan D, et al. Injuries to the phrenic nerve resulting in diaphragmatic paralysis with special reference to stretch trauma. Am J Surg. 1976;132:263.
89 Marco J, Hahn J, Barner H. Topical cardiac hypothermia and phrenic nerve injury. Ann Thorac Surg. 1977;23:235.
90 Wheeler W, Rubis L, Jones C, et al. Etiology and prevention of topical cardiac hypothermia-induced phrenic nerve injury and left lower lobe atelectasis during cardiac surgery. Chest. 1985;88:680.
91 Landymore RW, Howell F. Pulmonary complications following myocardial revascularization with the internal mammary artery graft. Eur J Cardiothorac Surg. 1990;4:156.
92 Kollef MH. Trapped-lung syndrome after cardiac surgery: a potentially preventable complication of pleural injury. Heart Lung. 1990;19:671.
93 Ferguson MK, Little AG, Skinner DB. Current concepts in the management of postoperative chylothorax. Ann Thorac Surg. 1985;45:542.
94 Orringer MB, Bluett M, Deeb GM. Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery. 1988;104:720.
95 Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J. 1997;10:1157.
96 Nygaard SD, Berger HA, Fick RB. Chylothorax as a complication of oesophageal sclerotherapy. Thorax. 1992;47:134.
97 Weidner WA, Steiner RM. Roentgenographic demonstration of intrapulmonary and pleural lymphatics during lymphangiography. Radiology. 1971;100:533.
98 Staats BA, Ellefson RD, Budhan LL, et al. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55:700.
99 Monla-Hassan J, Eichenhorn M, Spickler E, et al. Duro-pleural fistula manifested as a large pleural transudate. Chest. 1998;114:1786.
100 D’Souza R, Doshi A, Bhojraj S, et al. Massive pleural effusion as the presenting feature of a subarachnoid-pleural fistula. Respiration. 2002;69:96.
101 Pollack II, Pang D, Hall W. Subarachnoid-pleural and subarachnoid mediastinal fistulae. Neurosurgery. 1990;26:519.
102 Assietti R, Kibble MB, Bakay R. Iatrogenic cerebrospinal fluid fistula to the pleural cavity: case report and literature review. Neurosurgery. 1993;33:1004.
103 Skedros DG, Cass SP, Hirsch BE, et al. Beta-2 transferrin assay in clinical management of cerebral spinal fluid and perilymphatic fluid leaks. J Otolaryngol. 1993;22:341.
104 Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiments. Medicine. 1944;23:281.
105 Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med. 1939;64:913.
106 Gustman P, Yerger L, Wanner A. Immediate cardiovascular effects of tension pneumothorax. Am Rev Respir Dis. 1983;127:171.
107 Hurewitz AN, Sidhu U, Bergofsky B, et al. Cardiovascular and respiratory consequence of tension pneumothorax. Bull Eur Physiopathol Respir. 1986;22:545.
108 Norris RM, Jones JG, Bishop JM. Respiratory gas exchange in patients with spontaneous pneumothorax. Thorax. 1968;23:427.
109 Moran JF, Jones RH, Wolfe WG. Regional pulmonary function during experimental unilateral pneumothorax in the awake state. J Thorac Cardiovasc Surg. 1977;74:394.
110 Hagley MT, Martin B, Gast P, et al. Infectious and mechanical complications of central venous catheters placed by percutaneous venipuncture and over guide wires. Crit Care Med. 1992;20:1426.
111 Eerola R, Kaukinen L, Kaukinen S. Analysis of 13,800 subclavian catheterizations. Acta Anaesthesiol Scand. 1985;29:193.
112 Tyden H. Cannulation of the internal jugular vein: 500 cases. Acta Anaesthesiol Scand. 1982;26:485.
113 Weiner P, Sznajder I, Plavnick L, et al. Unusual complications of subclavian vein catheterization. Crit Care Med. 1984;12:538.
114 Kumar A, Pontoppidan H, Falke KJ, et al. Pulmonary barotrauma during mechanical ventilation. Crit Care Med. 1973;1:1.
115 Zimmerman JE, Dunbar BS, Klingenmaier CH. Management of subcutaneous emphysema, pneumomediastinum, and pneumothorax during respirator therapy. Crit Care Med. 1975;3:69.
116 Cullen DJ, Caldera DL. The incidence of ventilator-induced pulmonary barotrauma in critically ill patients. Anesthesiology. 1979;50:185.
117 Tocino I, Westcott JL. Barotrauma. Radiol Clin North Am. 1996;34:59.
118 Anzueto A, Frutos-Vivar F, Esteban A. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30:612.
119 Zwillich CW, Pierson DJ, Creagh CE, et al. Complications of assisted ventilation: a prospective study of 354 consecutive episodes. Am J Med. 1974;57:161.
120 Steier M, Ching N, Roberts EB, et al. Pneumothorax complicating continuous ventilatory support. J Thorac Cardiovasc Surg. 1979;67:17.
121 Johnson TH, Altman AR. Pulmonary interstitial gas: first sign of barotrauma due to PEEP therapy. Crit Care Med. 1979;7:532.
122 Albelda SM, Gefter WB, Kelley MA, et al. Ventilator-induced subpleural air cysts: clinical, radiographic, and pathologic significance. Am Rev Respir Dis. 1983;127:360.
123 Westcott JL, Cole SR. Interstitial pulmonary emphysema in children and adults: roentgenographic features. Radiology. 1974;111:367.
124 Kirkpatrick AW, Ng AK, Dulchavsky SA, et al. Sonographic diagnosis of pneumothorax inapparent on plain radiography: confirmation by computed tomography. J Trauma. 2001;50:750.
125 Dulchavsky SA, Hamilton DR, Diebel LN, et al. Thoracic ultrasound diagnosis of pneumothorax. J Trauma. 1999;47:970.
126 Snyder J, Carrol G, Schuster DP, et al. Mechanical ventilation: physiology and application. Curr Probl Surg. 1984;21:1.
127 Suter PM, Fairley HP, Isenberg MD. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest. 1978;73:158.
128 Willetts SM. Paralysis of ventilated patients: yes or no? Intensive Care Med. 1985;11:2.
129 Darioli E, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129:385.
130 Baumann MH, Sahn SA. Tension pneumothorax: diagnostic and therapeutic pitfalls. Crit Care Med. 1993;21:177.
131 Powner DJ, Grenvik A. Ventilatory management of life-threatening bronchopleural fistulae: a summary. Crit Care Med. 1981;9:54.
132 Ratliff JL, Hill JD, Fallat RJ, et al. Complications associated with membrane lung support by venoarterial perfusion. Ann Thorac Surg. 1975;19:537.
133 Tilles RB, Don HF. Complications of high pleural suction in bronchopleural fistulas. Anesthesiology. 1975;43:486.
134 Steiger Z, Wilson RF. Management of bronchopleural fistulas. Surgery. 1984;158:267.
135 Shenstone NS. The use of intercostal muscle in the closure of bronchopleural fistulae. Ann Surg. 1936;4:560.
136 Beltrami V. Surgical transsternal treatment of bronchopleural fistula postpneumonectomy. Chest. 1989;95:379.
137 Barker WL, Faber LP, Ostermiller WE, et al. Management of persistent bronchopleural fistulas. J Thorac Cardiovasc Surg. 1971;62:393.
138 Demos NJ, Timmes JJ. Myoplasty for closure of tracheal bronchofistula. Ann Thorac Surg. 1973;15:88.
139 Hankins JR, Miller JE, McLaughlin JS. The use of chest wall muscle flaps to close bronchopleural fistulas: experience with 21 patients. Ann Thorac Surg. 1978;6:491.
140 Ferguson JS, Sprenger K, VanNatta T. Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest. 2006;129:479.
141 Rusch VW, Capps JS, Tyler ML, et al. The performance of four pleural drainage systems in an animal model of bronchopleural fistula. Chest. 1988;4:859.
142 Batchelder TL, Morris KA. Critical factors in determining adequate pleural drainage in both the operated and nonoperated chest. Am Surg. 1962;28:296.
143 Swensen EW, Birath G, Ahbeck A. Resistance to airflow in bronchospirometric catheters. J Thorac Surg. 1957;33:275.
144 Downes JB, Chapman RL. Treatment of bronchopleural fistula during continuous positive pressure ventilation. Chest. 1976;69:363.
145 Phillips YY, Lonigan RM, Joyner LR. A simple technique for managing a bronchopleural fistula while maintaining positive pressure ventilation. Crit Care Med. 1979;7:351.
146 Weksler N, Ovadia L. The challenge of bilateral bronchopleural fistula. Chest. 1989;95:938.
147 Gallagher TJ, Smith RA, Kirby RR, et al. Intermittent inspiratory chest tube occlusion to limit bronchopleural cutaneous air leaks. Crit Care Med. 1976;4:328.
148 Bevelaqua FA, Kay S. A modified technique for the management of bronchopleural fistula in ventilator-dependent patients: a report of 2 cases. Respir Care. 1986;31:904.
149 Larrieu AJ, Tyers FO, Williams EH, et al. Intrapleural instillation of quinacrine for treatment of recurrent spontaneous pneumothorax. Ann Thorac Surg. 1979;28:146.
150 Goldszer RC, Bennett J, VanCampen J, et al. Intrapleural tetracycline for spontaneous pneumothorax. JAMA. 1979;241:724.
151 Macoviak JA, Stephenson LW, Ochs R, et al. Tetracycline pleurodesis during active pulmonary-pleural air leak for prevention of recurrent pneumothorax. Chest. 1982;81:78.
152 Verschoof AC, Vende T, Greve LH, et al. Thoracoscopic pleurodesis in the management of spontaneous pneumothorax. Respiration. 1988;53:197.
153 Light RW, O’Hara VS, Moritz TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax. JAMA. 1990;264:2224.
154 Powner DJ, Cline CD, Rodman GH. Effect of chest-tube suction on gas flow through a bronchopleural fistula. Crit Care Med. 1985;13:99.
155 Bishop MJ, Benson MS, Pierson DJ. Carbon dioxide excretion via bronchopleural fistulas in adult respiratory distress syndrome. Chest. 1987;91:400.
156 Pierson DJ, Horton CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation: a review of 39 cases. Chest. 1986;90:321.
157 Kuwik RJ, Glass D, Coombs DW. Evaluation of high-frequency positive pressure ventilation for experimental bronchopleural fistula. Crit Care Med. 1981;9:164.
158 Turnbull AD, Carlon GC, Howland WS, et al. High-frequency jet ventilation in major airway or pulmonary disruption. Ann Thorac Surg. 1981;32:468.
159 Albeda SM, Hansen-Flaschen JH, Taylor E, et al. Evaluation of high-frequency jet ventilation in patients with bronchopleural fistulas by quantitation of the airleak. Anesthesiology. 1985;63:551.
160 Bishop MJ, Benson MS, Sato P, et al. Comparison of high-frequency jet ventilation with conventional mechanical ventilation for bronchopleural fistula. Anesth Analg. 1987;66:833.
161 Rafferty TD, Palma J, Motoyama EK, et al. Management of a bronchopleural fistula with differential lung ventilation and positive end-expiratory pressure. Respir Care. 1980;25:654.
162 Brown CR. Postpneumonectomy empyema and bronchopleural fistula: use of prolonged endobronchial intubation: a case report. Anesth Analg. 1973;52:439.
163 Lau K. Postural management of bronchopleural fistula. Chest. 1988;94:1122.
164 Feeley TW, Keating D, Nishimura T. Independent lung ventilation using high-frequency ventilation in the management of a bronchopleural fistula. Anesthesiology. 1988;69:420.
165 Crimi G, Candiani A, Conti G, et al. Clinical applications of independent lung ventilation with unilateral high-frequency jet ventilation (ILV-UHFJV). Intensive Care Med. 1986;12:90.
166 Orlando R, Gluck EH, Cohen M, et al. Ultra-high-frequency jet ventilation in a bronchopleural fistula model. Arch Surg. 1988;123:591.
167 Torre M, Chiesa G, Ravine M, et al. Endoscopic gluing of bronchopleural fistula. Ann Thorac Surg. 1987;43:295.
168 Hoier-Madsen K, Schulze S, Pedersen VM, et al. Management of bronchopleural fistula following pneumonectomy. Scand J Thorac Cardiovasc Surg. 1984;18:263.
169 Glover W, Chavis TV, Daniel TM, et al. Fibrin glue application through the flexible fiberoptic bronchoscope: closure of bronchopleural fistula. J Thorac Cardiovasc Surg. 1987;93:470.
170 Regel G, Sturm JA, Neumann C, et al. Occlusion of bronchopleural fistula after lung injury: a new treatment by bronchoscopy. J Trauma. 1989;29:223.
171 Lan R, Lee C, Tsai Y, et al. Fiberoptic bronchial blockade in a small bronchopleural fistula. Chest. 1987;92:944.
172 Ratliff JL, Hill JD, Tucker H, et al. Endobronchial control of bronchopleural fistulae. Chest. 1971;71:98.
173 Ellis JH, Sequeira FW, Weber TR, et al. Balloon catheter occlusion of bronchopleural fistulae. AJR Am J Roentgenol. 1982;138:157.
174 Roksvaag H, Skalleberg L, Nordberg C, et al. Endoscopic closure of bronchial fistula. Thorax. 1983;38:696.
175 Menard JW, Prejean CA, Tucker YW. Endoscopic closure of bronchopleural fistulas using a tissue adhesive. Am J Surg. 1980;155:415.
176 Jones DP, David I. Gelfoam occlusion of peripheral bronchopleural fistulas. Ann Thorac Surg. 1986;42:334.