19 Platysma and the Nefertiti Lift®
Summary and Key Features
• The platysma acts as a major depressor in the lower face
• Anterior and posterior platysma bands can be treated with a total of 10–30 units of onabotulinum toxin A (BoNT-A) in women and with a total of 10–40 units in men (3–5 injection sites per band)
• The Nefertiti Lift® uses BoNT-A to redefine and sharpen the jawline whilst helping to lift the corners of the mouth. Dosage is 15–20 units onabotulinum toxin A per side injected intradermally
• Complications such as dysphagia and dysphonia can be avoided if injections are intradermal, there is no excessive toxin migration, and the total dose per session does not exceed 40 units
• Asymmetric smile, a rare adverse event associated with the Nefertiti Lift®, can be prevented through precise dosing (maximum dose of 20 units onabotulinum toxin A or equivalent per side, per session) and accurate intradermal injection
• Poor candidates for the Nefertiti Lift® include: patients with excessive submental fat, heavy jowls, or poor downward pull of the platysma with no prominent posterior platysma band
• Excellent candidates for the Nefertiti Lift® include: patients with prominent posterior platysma band and disappearing jawline with platysma contraction
The platysma: anatomy and dynamic aesthetic clinical implications
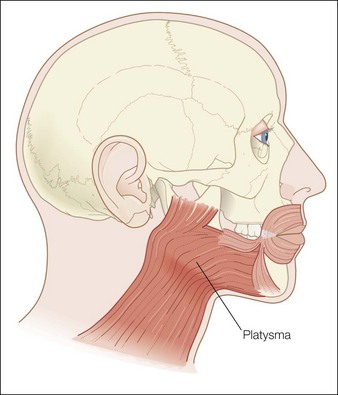
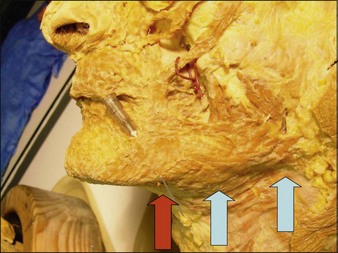
The platysma is a large, flat cutaneous muscle stretching from the upper décolleté to the mid cheek. It originates inferiorly from the pectoralis and deltoid fascia. The fibers ascend upwards and at the mandible some fibers insert directly into bone under the mandible, its only bony insertion. The other fibers blend into the modiolus, the depressor anguli oris, the lower lip, the depressor labialis inferioris and mostly continue to ascend above the mandible where they blend into the SMAS (superficial muscular aponoretic system). In the midline of the neck the platysma is absent except when the fibers from each side decussate (intertwine) with the other side under the chin (Fig. 19.1).
Injection techniques for the neck
Vertical bands
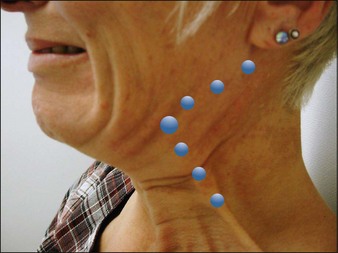
After asking the patient to contract their platysma the practitioner should hold the band to be injected between the thumb and index finger. Deep intradermal injections of 2.5–5 units should be injected equidistantly in 3–7 sites per band. A maximum of 30 units for women and 40 units for men should be respected per session (Fig. 19.2).
The Nefertiti Lift®
Nefertiti was an Egyptian queen famed for her beauty. A statue of her bust can be seen in the Neues Museum in Berlin. It is one of the most recognizable works from Ancient Egypt thanks to her perfect facial proportions, elegant neck, and sharp jawline and angle (Fig. 19.3).
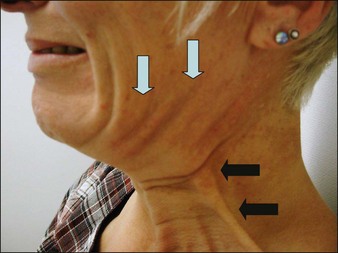
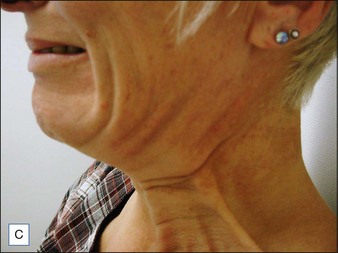
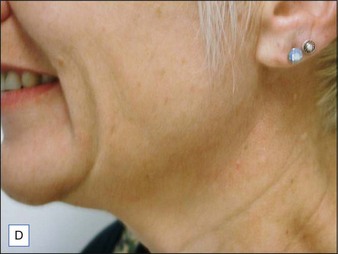
Patients should be carefully assessed for their suitability prior to treatment. To do this patients should be in a sitting position then asked to pull down hard on their platysma muscle to make the posterior vertical platysma band clearly visible. If this action causes the mandibular border to disappear it is a good indication that treatment is likely to be successful (Fig. 19.4).
Prior to treatment, injection points under the mandible going posteriorly and into the contracted vertical posterior platysma band should be marked on the skin. Injection of 2–2.5 units of onabotulinum toxin A should be administered intradermally and tangentially in four points, 1–2 cm apart, under each mandible and into the upper half of the posterior vertical platysma band for a total of 15–20 units per side. In this way a double dose equating to 4–5 units should be administered at the point where the horizontal and vertical injection points meet (see Fig. 19.5).
Case Study 1
‘I don’t want cosmetic surgery!’
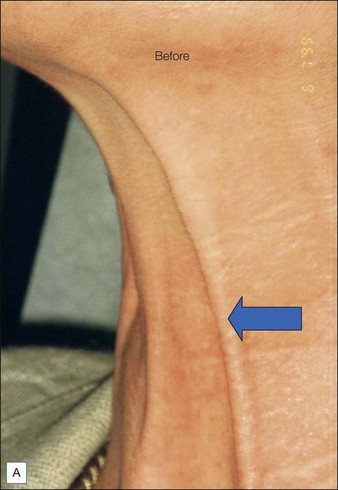
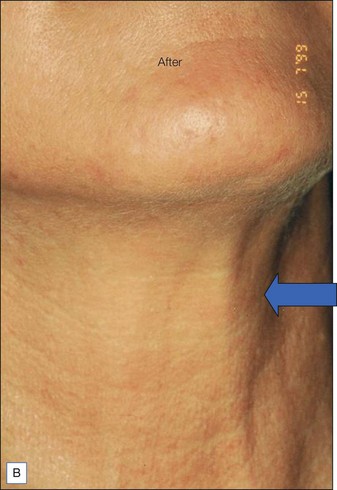
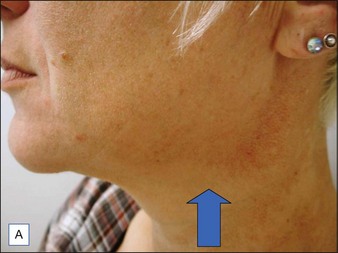
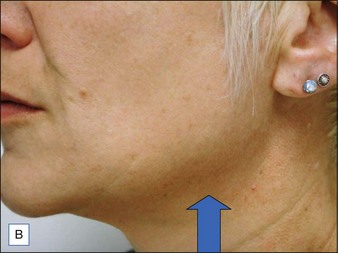
BoNT-A was used to treat the upper face according to standard protocols. For the lower face 20 units of BoNT-A were injected under each mandible and into the top part of the posterior platysma band on each side of the neck (Fig. 19.5).
The before and after photographs clearly illustrate the effect of the Nefertiti Lift® in redefining the jawline, reducing jowling, and improving skin laxity (Fig. 19.6).
Potential complications of the Nefertiti Lift®
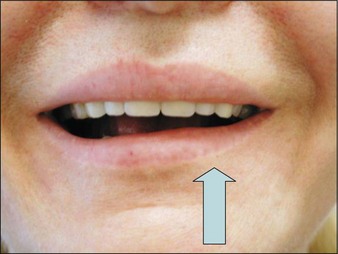
There is a risk of modifying the smile if BoNT-A is inadvertently injected into the depressor labialis inferior (DLI). Unless rectified by injecting the contralateral DLI, effects will last approximately 4 weeks (Fig. 19.7).
This risk of modified smile can be avoided by:
 starting the mandibular injections at a point 1 cm posterior to the junction of nasolabial folds and mandible
starting the mandibular injections at a point 1 cm posterior to the junction of nasolabial folds and mandible
 always pointing the syringe towards the mandibular angle posteriorly
always pointing the syringe towards the mandibular angle posteriorly
Brandt FS, Bellman B. Cosmetic use of botulinum A exotoxin for the aging neck. Dermatologic Surgery. 1998;24:1232–1234.
Brandt FS, Boker A. Botulinum toxin for the rejuvenation of the neck. Clinics in Dermatology. 2003;21:513–520.
Carruthers J, Fagien S, Matarasso SL, the Botox Consensus Group. Consensus recommendations on the use of botulinum toxin type A in facial aesthetics. Plastic and Reconstructive Surgery. 2004;114(6 suppl):S1–S22.
Carruthers J, Carruthers A. Aesthetic botulinum A toxin in the mid and lower face and neck. Dermatologic Surgery. 2003;29:468–476.
Gassia V, Beylot C, Béchaux S, et al. Botulinum toxin injection techniques in the lower third and middle of the face, the neck and the décolleté: the ‘Nefertiti lift’. Annals of Dermatology and Venereology. 2009;136(suppl 4):S111–S118.
Hoefflin SM. Anatomy of the platysma and lip depressor muscles. Dermatologic Surgery. 1998;24:1225–1231.
Levy PM. The ‘Nefertiti Lift’: a new technique for specific recontouring of the jawline. Journal of Cosmetic and Laser Therapy. 2007;9(4):249–252.
Park MY, Ahn KY, Jung DS. Botulinum toxin type A treatment for contouring of the lower face. Dermatologic Surgery. 2003;29:477–483.
Rohen JW, Yokochi C, Lütjen-Drecoll E. Color atlas of anatomy, 4th edn. Philadelphia: Lippincott Williams & Wilkins; 1998.