Plastic Repair of the Perineum (Perineorrhaphy)
Perineal reconstruction is indicated for patients who have dyspareunia associated with any number of causes, including, but not limited to, scar formation secondary to lichen sclerosus; tearing secondary to childbirth; excessively tight closure of an episiotomy; scar formation due to episiotomy breakdown, secondary infection, or suture reactive inflammation; faulty attempts at perineal repair; trauma; ulcer formation secondary to poor blood supply; burn scar formation due to electrosurgery, laser, or chemicals; chronic infection; and atrophy (Figs. 76–1 through 76–3).
Despite a long-held belief that tightening of the perineum and introitus will improve a woman’s sexual response, invariably this action leads to dyspareunia. The anatomy of the vulva and vagina was described earlier; however, a few points should be considered here. First, the levator ani muscles do not cross the midline beneath (posterior to) the vagina. These muscles insert laterally into the wall of the lower vagina under the bulb. The levators insert lateral to and into the anterior anal sphincter. Second, the superficial muscles of the perineum are very thin structures and add little mass to the ill-defined perineal body. The bulk of that structure consists of the anterior external sphincter ani. Third, no well-defined fascial plane exists in the area of the perineum with the exception of Colles’ fascia and the investment fascia overlying the external sphincter ani. Fourth, picking up muscle mass and plicating across the midline posterior to the lower vagina creates an unnatural hump; additionally, placement of a large number of absorbable sutures into the same tissue produces an inflammatory response, diminishes the blood supply to the overlying epithelium, and results in gross scar formation. These factors will result in painful intercourse because the normal anatomy is distorted. All of the procedures listed under the fourth point therefore should be avoided. Finally, unless the patient is symptomatic, surgery in this area should not be done. Even though the physical attributes of an individual woman’s perineum may not be pleasing to an examiner’s eyes, this is not an indication to perform surgery to “improve” it. Similarly, surgery to “tighten things up” based on the mindset of better sex for the patient’s partner is unjustified. Perineal plastic and reconstructive surgery is based on the provision of easy vaginal ingress and the limitations of gross scar formation. Preservation or reconstruction of normal anatomy to restore physiologic function is the goal of perineal surgery.
The area of the vestibule or lower vagina that preoperatively has objectively demonstrated hypersensitivity and production of pain when provoked will be removed. Patients who have atrophy should be pretreated with topical and systemic estrogen at least 1 to 2 months before surgery. Testosterone topically applied does nothing whatsoever to nourish or improve the epithelium. More often, application of testosterone ointment produces an uncomfortable burning sensation.
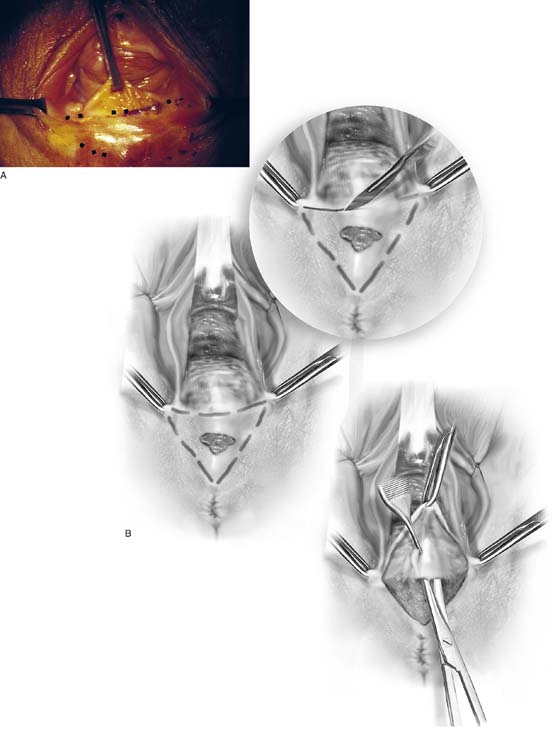
The patient is placed in the dorsal lithotomy position, prepared, and draped. The area of the vagina, vestibule, or perineum to be excised is traced with a marking pen (Fig. 76–4A, B). The surgeon should check vaginal mobility with Allis clamps before excising the perineal tissue. Because the vagina will be advanced, the surgeon will need to estimate the distance between the advanced vagina and the perineal edge to avoid excessive tension during suture line closure. When the markings have satisfied the surgeon’s eye, a 1 : 100 vasopressin solution is injected subdermally with a 1½, 27-gauge needle. A transverse incision is made across the posterior vaginal wall with a No. 15 scalpel blade. This line will form the base of a triangle (see Figs. 76–4B, 76–5). The left and right lines of the triangle are equidistant and intersect at a central point on the perineum, as drawn previously by the surgeon. The skin and mucosa within the triangle are resected with the use of Adson-Brown forceps and Allis clamps for traction (Fig. 76–6). Cutting is done with Stevens or Metzenbaum scissors (see Figs. 76–4B, 76–7). The plane of dissection is the fascia between the anus and the vagina and the fascia underlying the vestibule and perineum. The triangular piece of tissue is dissected from apex to base until it is completely free; it is then removed with the accompanying cicatrix (Fig. 76–8). A second glove is placed over the surgeon’s first glove, and a rectal examination is done to determine the position of the anus/rectum in relation to the plane of dissection. The removed tissue is placed into fixative and sent to pathology. The fascial edge underlying the vagina is grasped with forceps and elevated. Another 5 to 10 mL of vasopressin mixture is injected into it (Fig. 76–9). Next, the fascia is undermined with Stevens scissors for 5 to 10 mm superiorly (Fig. 76–10). The fascia is sutured transversely to the fascia underlying the perineal skin with interrupted 3-0 Vicryl sutures. Bleeding vessels are clamped with mosquito clamps and suture-ligated with 4-0 Vicryl. The vaginal mucosa is advanced without tension to the perineal skin edge (Fig. 76–11). The vaginal mucosa is sutured to perineal skin transversely along the line of the initial base incision with interrupted 3-0 Vicryl sutures (Fig. 76–12A through D). The wound is irrigated with normal saline. Ease of entry into the vagina is checked by inserting loosely approximated index and center fingers through the introitus (Fig. 76–13). The wound is covered with silver sulfadiazine (Silvadene). The final rectal examination is done to check the integrity of the bowel. Postoperatively, the patient takes daily salt water tub baths and applies Silvadene to the wound 3 times daily and at bedtime. A stool softener is prescribed because the patient is instructed to avoid bearing down. Nothing is placed in the vagina for 6 weeks. The final result of this operation is the excision of scar tissue, removal of poorly vascularized skin, and expansion of the vaginal opening.
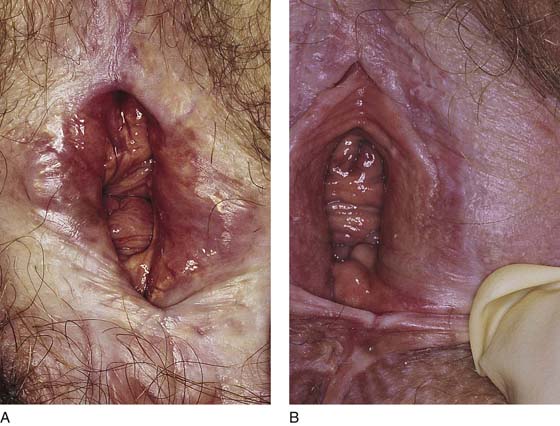
FIGURE 76–1 A. Severe and undertreated lichen sclerosus that has led to significant skin thickening and vulvar scarring. Elasticity in the affected areas has disappeared. B. This patient subsequently responded to serial, subdermal dexamethasone (Decadron) injections (see Chapter 79). Nevertheless, permanent scar formation at the posterior commissure and perineum led to significant dyspareunia.
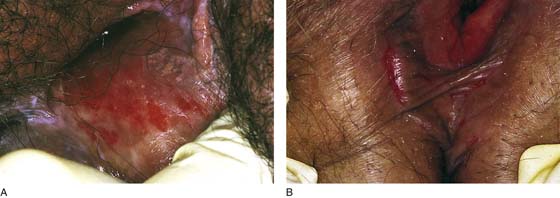
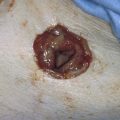
FIGURE 76–2 A. Chronic ulcer formation is evident in the fossa navicularis and posterior vestibule. The past history revealed prior excisions and laser vaporization, which were performed as attempts to “cure” the recurrent ulcers. The basic problem in this instance is atrophy secondary to a poor blood supply. Subsequent trauma results in ulcers that heal poorly and slowly. B. Scarring of the vulva secondary to prior injury. The inelastic tissue forms fissures when stretched.
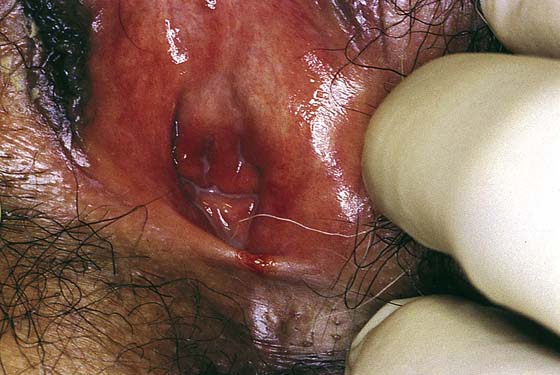
FIGURE 76–3 The posterior commissure tear in this atrophic vulva occurred acutely during examination.
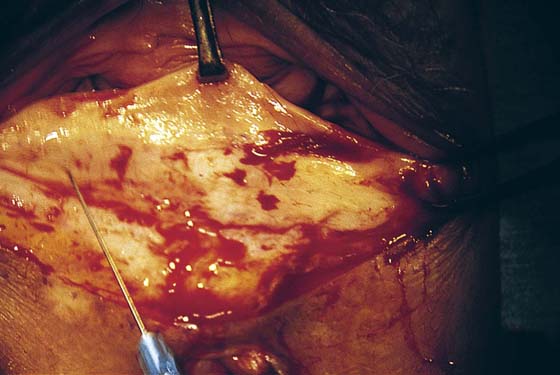
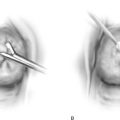
FIGURE 76–4 A. The posterior vagina is elevated with an Allis clamp. The incision lines are traced with a sterile marking pen (emphasized by dots). B. The drawing shows a scarred perineum with an ulcer secondary to a poor vascular supply. Incision lines have been traced. The initial cut is made at the base of the inverted triangle. The skin and scar tissue are dissected with Metzenbaum scissors.
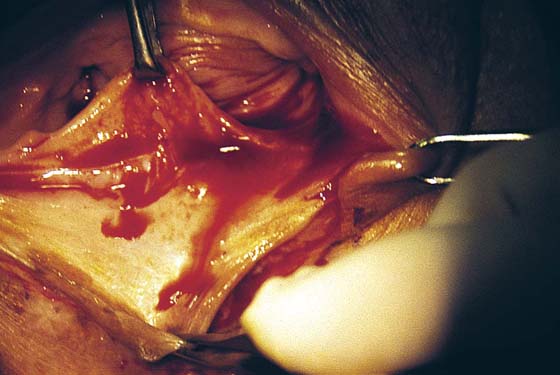
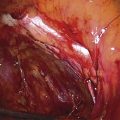
FIGURE 76–5 The incision is made across the posterior, lower vagina. The area has been previously injected with a 1 : 100 vasopressin solution.
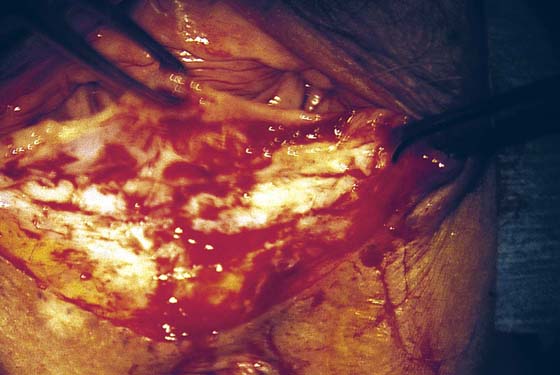
FIGURE 76–6 Beginning at the apex of the triangle located on the perineum, the skin and underlying connective tissue are sharply dissected in a superior direction.
FIGURE 76–7 Scar tissue is sharply dissected with the surrounding connective tissue with the use of Stevens scissors.
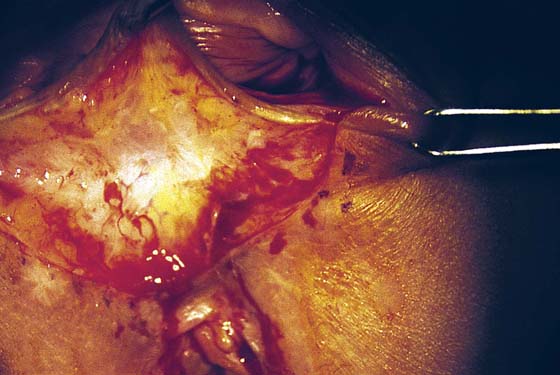
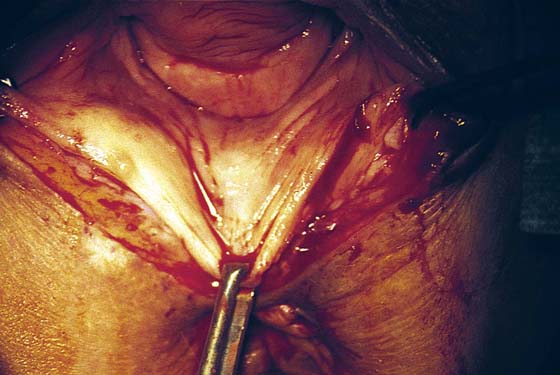
FIGURE 76–8 The triangle of skin, connective tissue, and hard scar has been excised. Hemostasis is excellent. Any small bleeding points are suture-ligated with 4-0 Vicryl.
FIGURE 76–9 A second injection of 1 : 100 vasopressin (10 mL) is made into the vaginal margin and into the submucosa of the vagina.
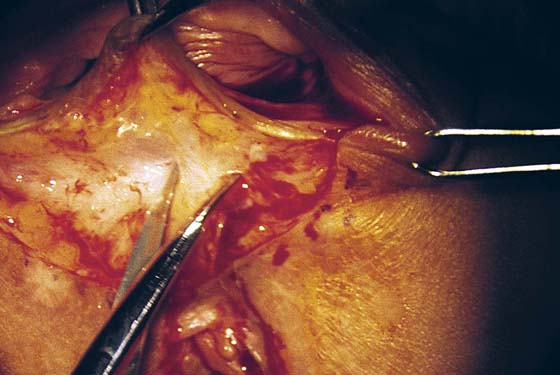
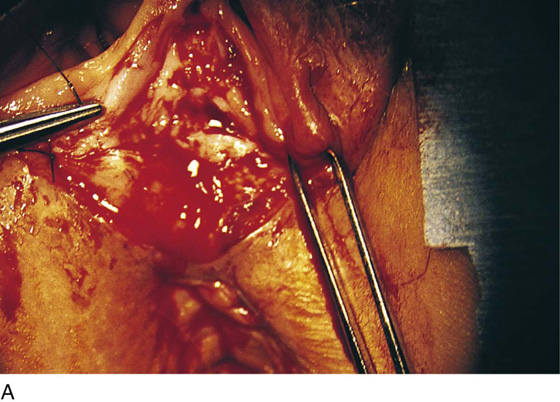
FIGURE 76–10 The vaginal margin is undermined with the use of Stevens scissors.
FIGURE 76–11 The mobility of the vagina is tested by applying an Allis clamp and advancing the vagina to the perineum. No tension should be required to mobilize the vagina.
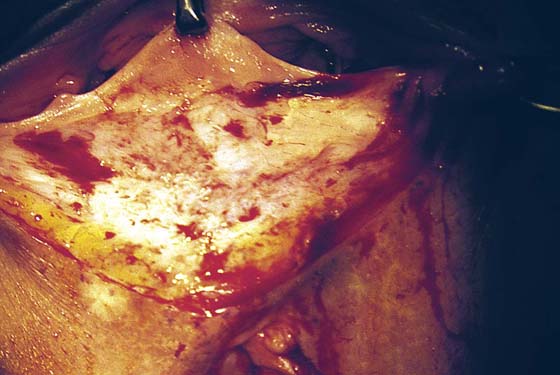
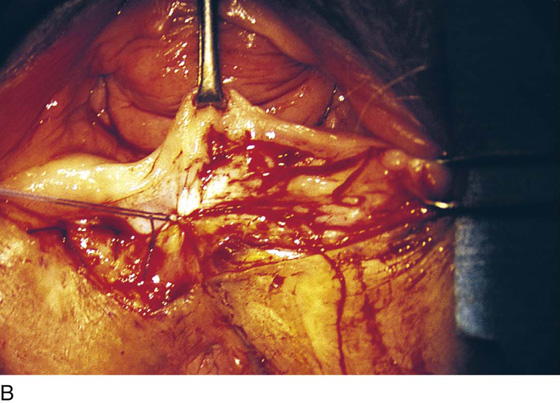
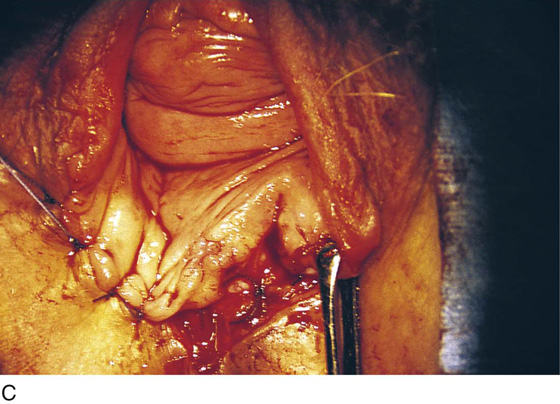
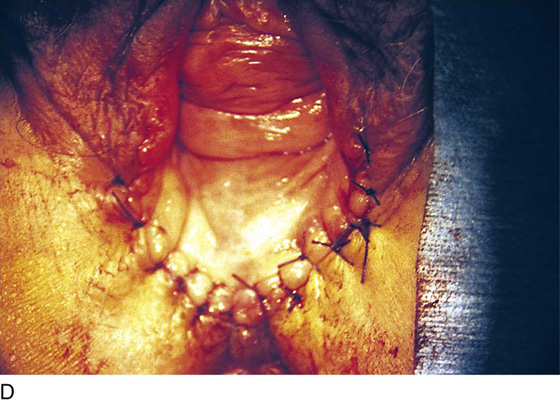
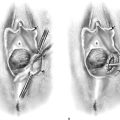
FIGURE 76–12 A. A layer of interrupted 3-0 Vicryl is placed through the perineal fascia and the vaginal submucosa. Closure is always transverse. B. When vaginal and perineal stromal sutures have been completed (i.e., extending across from right to left margin, side-to-side transverse closure), the wound is irrigated with sterile saline. C. Next, the vaginal mucosa is sutured to the perineal skin margin. Again, the orientation is similarly a transverse approximation without tension on the suture line. D. Final closure of the defect creates a smooth and wide entry into the vagina. The blood supply to this area is excellent (i.e., via advanced vaginal tissue).
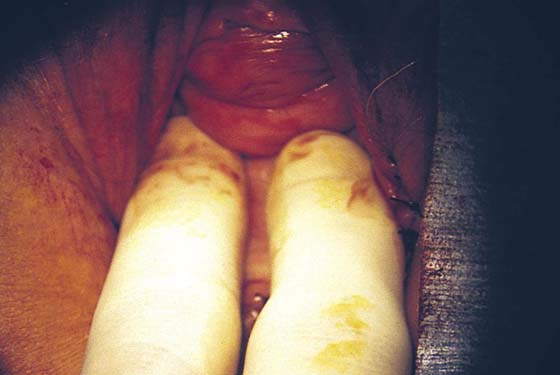
FIGURE 76–13 The adequacy of the vaginal opening is checked at the end of the operative procedure.