Placental and fetal growth and development
Early placental development
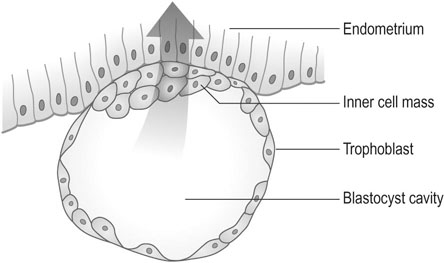
The outer layer of the blastocyst consists of primitive cytotrophoblast and, by day 7, the blastocyst penetrates the endometrium as a result of trophoblastic invasion (Fig. 4.1). The outer layer of trophoblast becomes a syncytium. In response to contact with the syncytiotrophoblast, the endometrial stromal cells become large and pale, a process known as the decidual reaction. Some endometrial cells are phagocytosed by the trophoblastic cells.
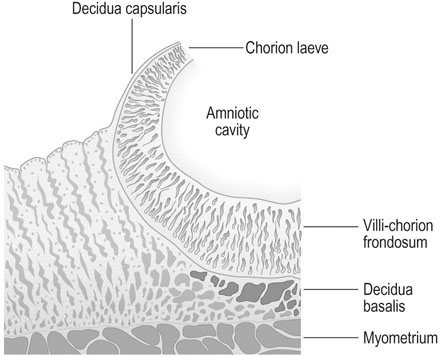
Although trophoblastic cells surround the original blastocyst, the area that develops into the placenta becomes thickened and extensively branched and is known as the chorion frondosum. However, in the area that subsequently expands to form the outer layer of the fetal membranes or chorion laeve, the villi become atrophic and the surface becomes smooth (Fig. 4.2). The decidua underlying the placenta is known as the decidua basalis and the decidua between the membranes and the myometrium as the decidua capsularis.
Further placental development
By 6 weeks after ovulation, the trophoblast has invaded some 40–60 spiral arterioles. Blood from the maternal vasculature pushes the free-floating secondary and tertiary capillaries into a tent-shaped maternal cotyledon. The tents are held down to the basal plate of the decidua by anchoring villi, and the blood from arterioles spurts towards the chorionic plate and then returns to drain through maternal veins in the basal plate. There are eventually about 12 large maternal cotyledons and 40–50 smaller ones (Fig. 4.3).
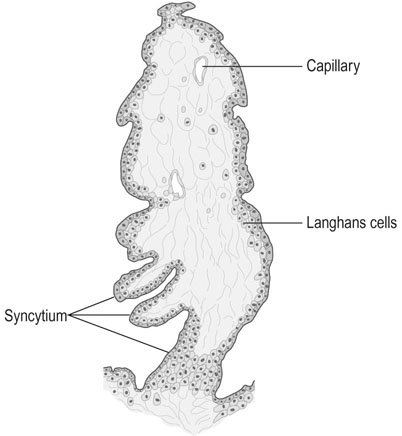
The villus
Despite the arrangement of villi into maternal cotyledons, the functional unit of the placenta remains the stem villus or fetal cotyledon. The end unit of the stem villus, sometimes known as the terminal or chorionic villus is shown in Figure 4.4. There are initially about 200 stem villi arising from the chorion frondosum. About 150 of these structures are compressed at the periphery of the maternal cotyledons and become relatively functionless, leaving a dozen or so large cotyledons and 40–50 smaller ones as the active units of placental function.
Structure of the umbilical cord
The umbilical cord contains two arteries and one vein (Fig. 4.5). The two arteries carry deoxygenated blood from the fetus to the placenta and the oxygenated blood returns to the fetus via the umbilical vein. Absence of one artery occurs in about 1 in 200 deliveries and is associated with a 10–15% incidence of cardiovascular anomalies. The vessels are surrounded by a hydrophilic mucopolysaccharide known as Wharton’s jelly and the outer layer covering the cord consists of amniotic epithelium. The cord length varies between 30 and 90 cm.
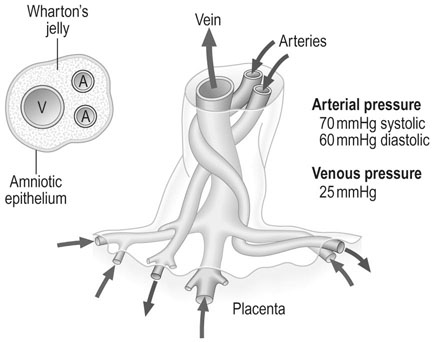
In the full-term fetus, the blood flow in the cord is approximately 350 mL/min.
Uteroplacental blood flow
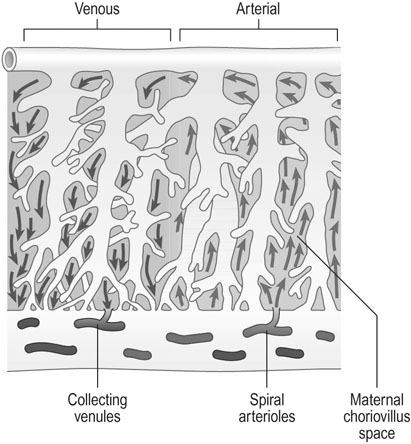
Trophoblastic cells invade the spiral arterioles within the first 10 weeks of pregnancy and destroy some of the smooth muscle in the wall of the vessels which then become flaccid dilated vessels. Maternal blood enters the intervillous space and, during maternal systole, blood spurts from the arteries towards the chorionic plate of the placenta and returns to the venous openings in the placental bed. The intervillous space is characterized by low pressures, with a mean pressure estimated at 10 mmHg and high flow. Assessments of uterine blood flow at term indicate values of 500–750 mL/min (Fig. 4.6).
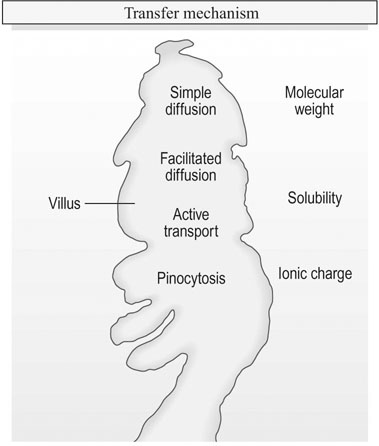
Placental transfer
Transfer of materials across the placental membrane is governed by molecular mass, solubility and the ionic charge of the substrate involved. Actual transfer is achieved by simple diffusion, facilitated diffusion, active transport and pinocytosis (Fig. 4.7).
Simple diffusion
< ?xml:namespace prefix = "mml" />
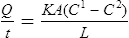
where 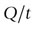 is the quantity transferred per unit of time, K is a diffusion constant for the particular substance, A is the total surface area available, C1 and C2 indicate the difference in concentrations of solute and L represents the thickness of the membrane.
is the quantity transferred per unit of time, K is a diffusion constant for the particular substance, A is the total surface area available, C1 and C2 indicate the difference in concentrations of solute and L represents the thickness of the membrane.
Placental function
The placenta has three major functions:
Gaseous exchange
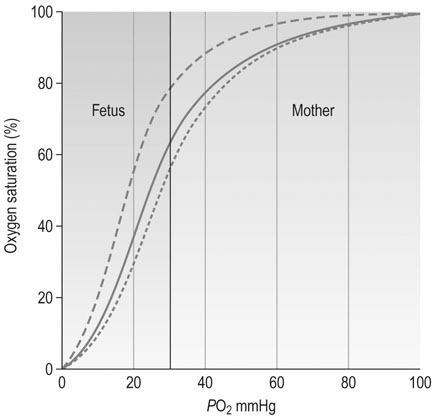
Oxygen transfer
The average oxygen saturation of maternal blood entering the intervillous space is 90–100% at a PO2 of 90–100 mmHg and these high levels of oxygen favour transfer to the fetal circulation. After the placenta itself has utilized some of this oxygen, the remainder is available to the fetal circulation. Fetal haemoglobin has a higher affinity for oxygen than does adult haemoglobin and haemoglobin concentration is higher in the fetus. All of these factors favour the rapid uptake of oxygen by the fetus at PO2 levels as low as 30–40 mmHg. The extent to which haemoglobin can be saturated by oxygen is affected by hydrogen ion concentration. The increase that occurs in deoxygenated blood arriving in the placental circulation from the fetus favours the release of maternal oxygen in the fetoplacental bed. The oxygen dissociation curve is shifted to the right by the increase in H+ concentration, PCO2 and temperature and this is known as the Bohr effect (Fig. 4.8). Oxygen is predominantly transported in the form of oxyhaemoglobin as there is little free oxygen in solution.
Placental hormone production
Protein hormones
Chorionic gonadotrophin
The hormone is measured by agglutination inhibition techniques using coated red cells or latex particles and this forms the basis for the standard modern pregnancy test (see Chapter 18). This will be positive in urine by 2 weeks after the period is missed in 97% of pregnant women. Home pregnancy test kits are able to detect 25-50iu/L of βhCG.
Fetal development
Growth
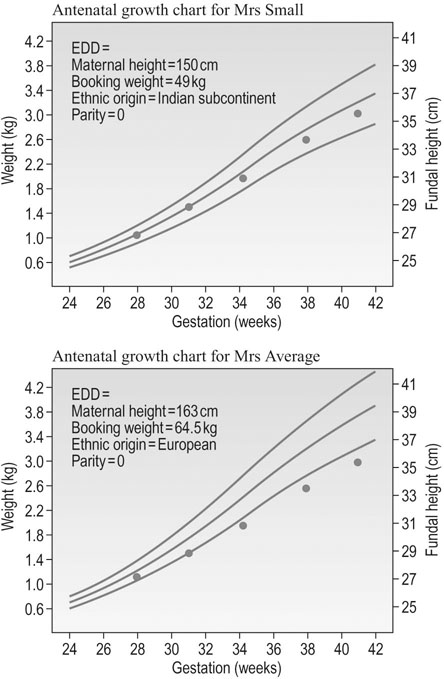
Fetal birth weight is determined by gestational age, race, maternal height and weight and parity. Thus, the projected normal birth weight for an infant is determined by a combination of all of these factors (Fig. 4.9). The normal growth curve therefore varies in each infant and can only be determined by taking into account the history of each individual mother. From all these factors, a nomogram for growth can be calculated. Figure 4.9 has the nomogram constructed for Mrs Small that shows the 5th and 95th centile to be between 35 and 39 cm in fundal height at 41 to 42 weeks, whilst it is 37 to 42 cm for Mrs Average. Hence the same growth trajectory plotted in the Mrs Small’s nomogram shows the fetus to be growing within normal limits and the last plot shows the estimated fetal weight to be about 3.0 kg, but the same plots in Mrs Average’s nomogram results in a fetus that shows growth restriction with progress of gestation.
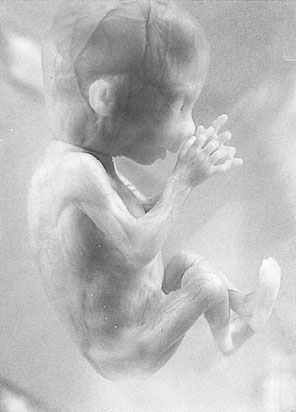
The characteristic appearance of the fetus at 12 weeks gestation is shown in Figure 4.10. The skin is translucent and there is virtually no subcutaneous fat so that the blood vessels in the skin are easily seen, but even at this stage the fetus reacts to stimuli. The upper limbs have already reached their final relative length and the external genitals are distinguishable externally but remain undifferentiated.
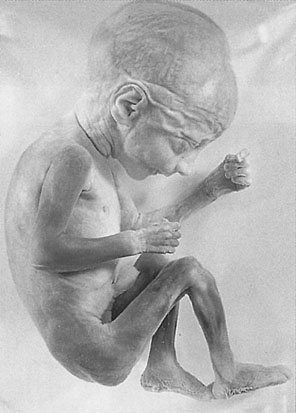
By 16 weeks gestation (Fig. 4.11), the crown–rump length is 122 mm and the lower limbs have achieved their final relative length. The external genitalia can now be differentiated.

By 24 weeks gestation (Fig. 4.12), the crown–rump length is 210 mm. The eyelids are separated, the skin is opaque but wrinkled because of the lack of subcutaneous fat, and there is fine hair covering the body. By 28 weeks, the eyes are open and the scalp is growing hair.
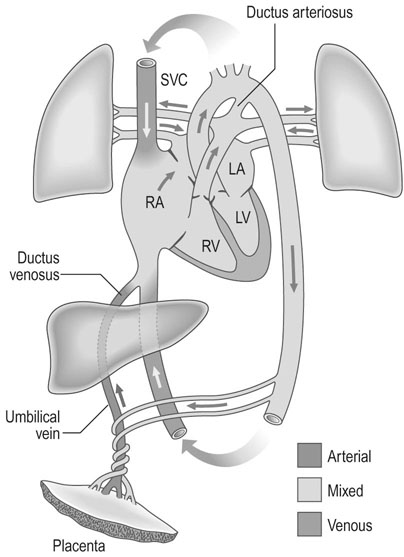
The cardiovascular system
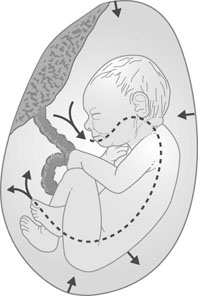
The heart develops initially as a single tube and, by 4–5 weeks gestation, a heartbeat is present at a rate of 65 beats/min. The definitive circulation has developed by 11 weeks gestation and the heart rate increases to around 140 beats/min. In the mature fetal circulation, about 40% of the venous return entering the right atrium flows directly into the left atrium through the foramen ovale (Fig. 4.13). Blood pumped from the right atrium into the right ventricle is expelled into the pulmonary artery, where it passes either into the aorta via the ductus arteriosus or into the pulmonary vessels.
The special senses
Visual perception is much more difficult to assess but it seems likely that some perception to light through the maternal abdominal wall does develop in late pregnancy. Certainly, fetal eye movements can be observed during pregnancy and form an important part of the observations made concerning various fetal behavioural states, a subject that is discussed in Chapter 10.
Amniotic fluid
Formation
The role of the fetus in the regulation of amniotic fluid volume in normal pregnancy is poorly understood but the fetus swallows amniotic fluid, absorbs it in the gut and, in later pregnancy, excretes urine into the amniotic sac (Fig. 4.14).
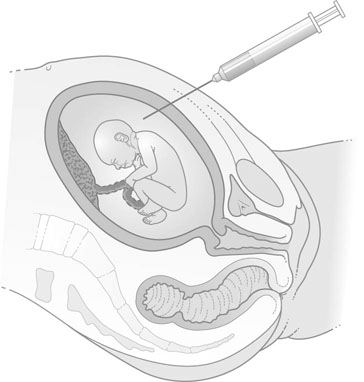
Amniocentesis
Amniotic fluid is obtained by the procedure of amniocentesis. This procedure involves inserting a fine-gauge needle under aseptic conditions through the anterior abdominal wall of the mother under local anaesthesia. The procedure, when used for diagnostic testing for chromosomal abnormalities, is commonly performed at 14–16 weeks gestation but can be performed as early as 12 weeks in some circumstances. The procedure must be performed under ultrasound control in order to identify the best and most accessible pool of amniotic fluid and, where possible, to avoid the placenta and the fetus. Up to 10 mL of fluid is withdrawn and the presence of a fetal heart beat is checked both before and after the procedure (Fig. 4.15).