Physiological changes in pregnancy
From a teleological point of view, there are two main reasons for these changes:
The uterus
The non-pregnant uterus weighs ~40–100 g, increasing during pregnancy to 300–400 g at 20 weeks and 800–1000 g at term. Involution is rapid over the first 2 weeks after delivery, but slows thereafter and is not complete by 2 months. The uterus consists of bundles of smooth muscle cells separated by thin sheets of connective tissue composed of collagen, elastic fibres and fibroblasts. All hypertrophy during pregnancy. The muscle cells are arranged as an innermost longitudinal layer, a middle layer with bundles running in all directions and an outermost layer of both circular and longitudinal fibres partly continuous with the ligamentous supports of the uterus (Fig. 3.1). Myometrial growth is almost entirely due to muscle hypertrophy and elongation of the cells from 50 µm in the non-pregnant state to 200–600 µm at term, although some hyperplasia may occur during early pregnancy. The stimulus for myometrial growth and development is the effect of the growing conceptus and oestrogens and progesterone.
The cervix
The cervix is predominantly a fibrous organ with only 10% of uterine muscle cells in the substance of the cervix. Eighty percent of the total protein in the non-pregnant state consists of collagen, but by the end of pregnancy the concentration of collagen is reduced to one-third of the amount present in the non-pregnant state. The principal function of the cervix is to retain the conceptus (Fig. 3.2).
The characteristic changes in the cervix during pregnancy are:
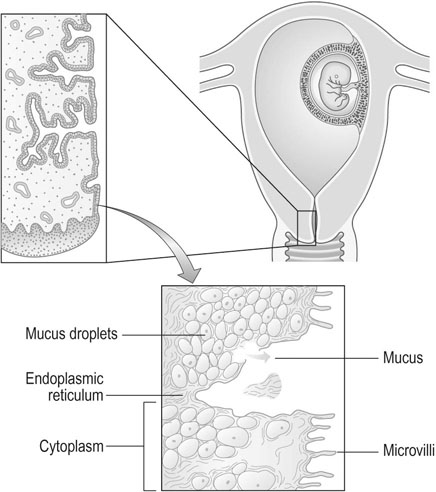
• Hypertrophy of the cervical glands producing the appearance of a cervical erosion; an increase in mucous secretory tissue in the cervix during pregnancy leads to a thick mucus discharge and the development of an antibacterial plug of mucus in the cervix.
• Reduced collagen in the cervix in the third trimester and the accumulation of glycosaminoglycans and water, leading to the characteristic changes of cervical ripening. The lower section shortens as the upper section expands, while during labour there is further stretching and dilatation of the cervix.
The corpus uteri
• As progesterone concentrations rise in the mid-secretory phase of an ovulatory menstrual cycle, endometrial epithelial and stromal cells stop proliferating and begin to differentiate, with an accumulation of maternal leukocytes, mainly NK cells (see above: Immunology). This decidualization is essential for successful pregnancy.
• The uterus changes in size, shape, position and consistency. In later pregnancy, the enlargement occurs predominantly in the uterine fundus so that the round ligaments tend to emerge from a relatively caudal point in the uterus. The uterus changes from a pear shape in early pregnancy to a more globular and ovoid shape in the second and third trimesters. The cavity expands from some 4 mL to 4000 mL at full term. The myometrium must remain relatively quiescent until the onset of labour.
• All the vessels supplying the uterus undergo massive hypertrophy. The uterine arteries dilate so that the diameters are 1.5 times those seen outside pregnancy. The arcuate arteries, supplying the placental bed, become 10 times larger and the spiral arterioles reach 30 times the prepregnancy diameter (see below). Uterine blood flow increases from 50 mL/min at 10 weeks gestation to 500–600 mL/min at term.
In the non-pregnant uterus, blood supply is almost entirely through the uterine arteries, but in pregnancy 20–30% is contributed through the ovarian vessels. A small contribution is made by the superior vesical arteries. The uterine and radial arteries are subject to regulation by the autonomic nervous system and by direct effects from vasodilator and vasoconstrictor humoral agents.
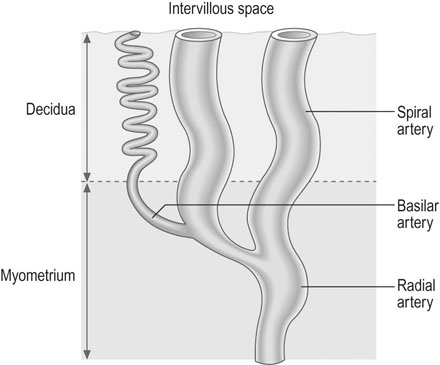
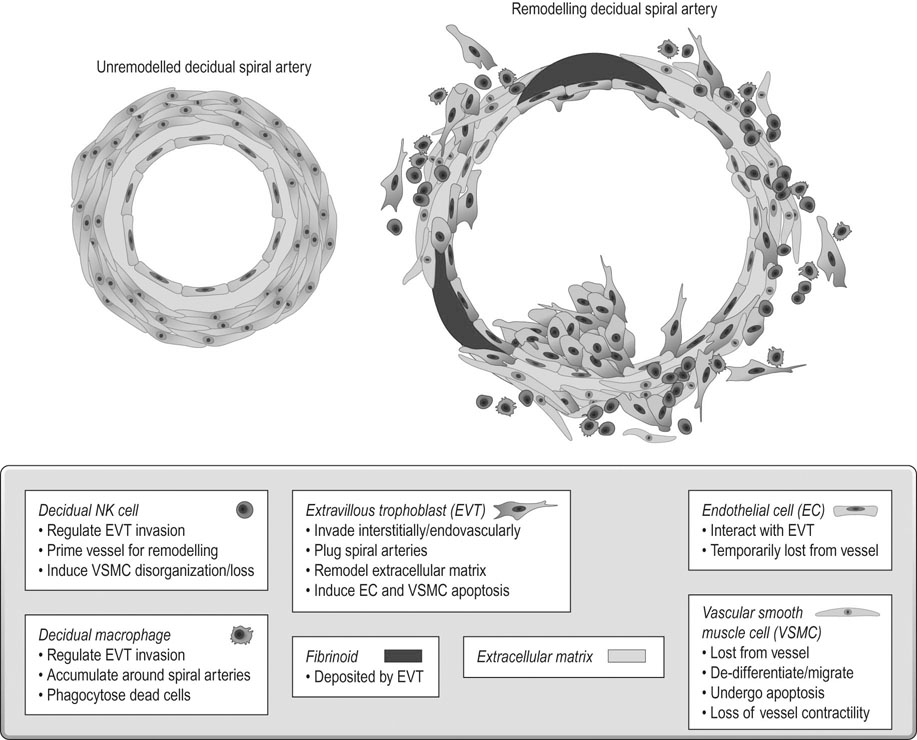
The final vessels delivering blood to the intervillous space (Fig. 3.3) are the 100–150 spiral arterioles. Two or three spiral arterioles arise from each radial artery and each placental cotyledon is provided with one or two. The remodelling of these spiral arteries is very important for successful pregnancy. Cytotrophoblast differentiates into villous or EVT. The latter can differentiate further into invasive EVT, which in turn is interstitial, migrating into the decidua and later differentiating into myometrial giant cells, or endovascular that invade the lumen of the spiral arteries. The intrauterine oxygen tension is very low in the first trimester, stimulating EVT invasion.
In the first 10 weeks of normal pregnancy, EVT invades the decidua and the walls of the spiral arterioles, destroying the smooth muscle in the wall of the vessels, which then become inert channels unresponsive to humoral and neurological control (Fig. 3.4). From 10–16 weeks, a further wave of invasion occurs, extending down the lumen of the decidual portion of the vessel; from 16–24 weeks this invasion extends to involve the myometrial portion of the spiral arterioles. The net effect of these changes is to turn the spiral arterioles into flaccid sinusoidal channels.
The development of myometrial activity
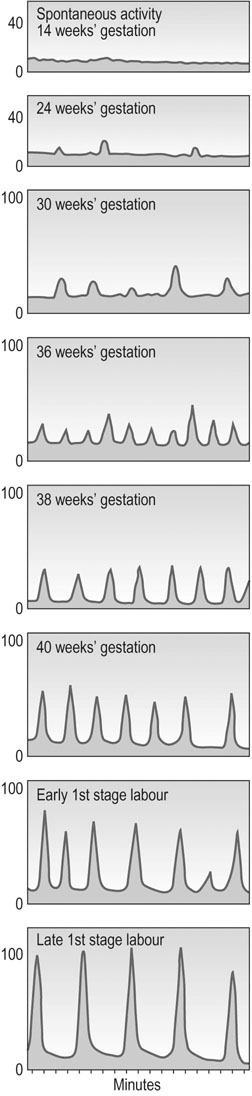
In late gestation, the fetus continues to grow, but the uterus stops growing, so tension across the uterine wall increases. This stimulates expression of a variety of gene products such as oxytocin and prostaglandin F2α receptors, sodium channels and the gap junction protein. Pro-inflammatory cytokine expression also increases. Once labour has begun, the contractions in the late first stage may reach pressures up to 100 mmHg and occur every 2–3 minutes (Fig. 3.5). See Chapter 11 for a discussion of labour and delivery.
The cardiovascular system
The cardiovascular system is one of those that shows proactive adaptations for a potential pregnancy during the luteal phase of every ovulatory menstrual cycle, long before there is any physiological ‘need’ for them. Many of these changes are almost complete by 12–16 weeks gestation (Fig. 3.6 and Table 3.1).
Table 3.1
Percentage change in some cardiovascular variables during pregnancy
| First trimester | Second trimester | Third trimester | |
| Heart rate | +11 | +13 | +16 |
| Stroke volume (mL) | +31 | +29 | +27 |
| Cardiac output (L/min) | +45 | +47 | +48 |
| Systolic BP (mm/Hg) | −1 | +1 | +6 |
| Diastolic BP (mmHg) | −6 | −3 | +7 |
| MPAP (mmHg) | +5 | +5 | +5 |
| Total peripheral resistance (resistance units) | −27 | −27 | −29 |
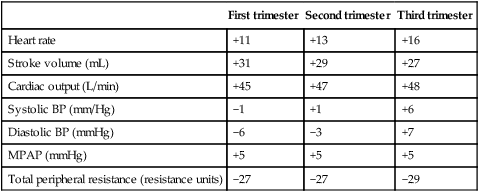
BP, blood pressure; MPAP, mean pulmonary artery pressure.
Data are derived from studies in which pre-conception values were determined. The mean values shown are those at the end of each trimester, and are thus not necessarily the maxima. Note that the changes are near maximal by the end of the first trimester.
(Data from Robson S, Robson SC, Hunter S, et al. (1989) Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989; 256:H1060. Table reproduced from Broughton Pipkin F (2001) Maternal physiology. In: Chamberlain GV, Steer P (eds) Turnbull’s Obstetrics, 3rd edn. Churchill Livingstone, London; with permission from Elsevier.)
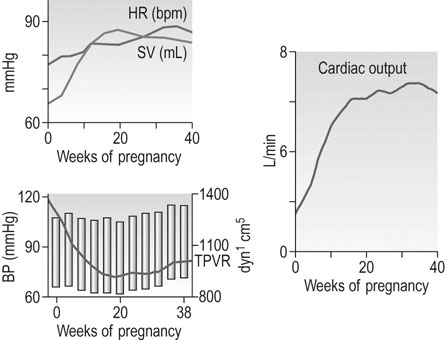
Total peripheral resistance
Total peripheral resistance (TPR) is not measured directly, but is calculated from the mean arterial pressure divided by cardiac output. The total peripheral resistance has fallen by 6 weeks gestation, so afterload falls. This is ‘perceived’ as circulatory underfilling, which is thought to be one of the primary stimuli to the mother’s circulatory adaptations. It activates the renin–angiotensin–aldosterone system and allows the necessary expansion of the plasma volume (PV; see below: Renal function). In a normotensive non-pregnant woman the TPR is around 1700 dyn/s/cm; this falls to a nadir of 40–50% by mid-gestation, rising slowly thereafter towards term, reaching 1200–1300 dyn/s/cm in late pregnancy. The fall in systemic TPR is partly associated with the expansion of the vascular space in the uteroplacental bed and the renal vasculature in particular; blood flow to the skin is also greatly increased in pregnancy as a result of vasodilatation.
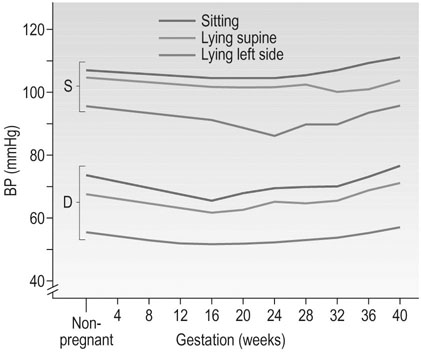
Arterial blood pressure
Posture has a significant effect on blood pressure in pregnancy; pressure is lowest with the woman lying supine on her left side. The pressure falls during gestation in a similar way whether the pressure is recorded sitting, lying supine or in the left lateral supine position, but the levels are significantly different (Fig. 3.7). This means that mothers attending for antenatal visits must have their blood pressure recorded in the same position at each visit if the pressures are to be comparable. Special care must be taken to use an appropriate cuff size for the measurement of brachial pressures. This is especially important with the increasing incidence of obesity among young women. The gap between the fourth and fifth Korotkoff sounds widens in pregnancy, and the fifth Korotkoff sound may be difficult to define. Both these factors may cause discrepancies in the measurement of diastolic pressure in pregnancy. Although most published studies of blood pressure are based on the use of Korotkoff fourth sound, it is now recommended to use the fifth sound where it is clear and the fourth sound only where the point of disappearance is unclear. Automated sphygmomanometers are unsuitable for use in pregnancy when the blood pressure is raised, as in pre-eclampsia.
The blood
Blood volume is a measurement of plasma volume and red cell mass. The indices are under separate control mechanisms. Plasma volume changes are considered below (see: Renal function).
Erythrocytes
Haemoglobin concentration, haematocrit and red cell count fall during pregnancy because the plasma volume rises proportionately more than the red cell mass (‘physiological anaemia’, see Table 9.1). However, in normal pregnancy the mean corpuscular haemoglobin concentration remains constant. Serum iron concentration falls but the absorption of iron from the gut rises and iron-binding capacity rises in a normal pregnancy, since there is increased synthesis of the β1-globulin, transferrin. Maternal dietary iron requirements more than double. Plasma folate concentration halves by term, because of greater renal clearance, although red cell folate concentrations fall less. In the late 1990s, 20% of the female population aged 16–64 years in the UK was estimated to have serum ferritin levels below 15 µ g/L, indicating low iron stores; no similar survey appears to have been undertaken since then. Pregnant adolescents seem to be at particular risk of iron deficiency. Even relatively mild maternal anaemia is associated with increased placental : birth weight ratios and decreased birth weight.
The white cells
The total white cell count rises during pregnancy. This increase is mainly due to an increase in neutrophil polymorphonuclear leukocytes that peaks at 30 weeks’ gestation (Fig. 3.8). A further massive neutrophilia normally occurs during labour and immediately after delivery, with a fourfold increase in the number of polymorphs.
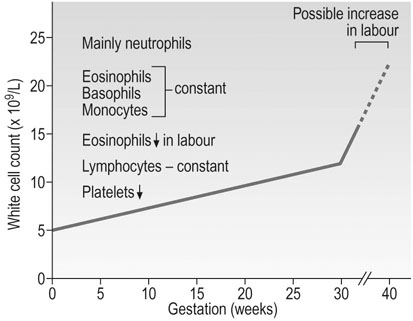
There is also an increase in the metabolic activity of granulocytes during pregnancy, which may result from the action of oestrogens. This can be seen in the normal menstrual cycle where the neutrophil count rises with the oestrogen peak in mid-cycle. Eosinophils, basophils and monocytes remain relatively constant during pregnancy, but there is a profound fall in eosinophils during labour and they are virtually absent at delivery. The lymphocyte count remains constant and the numbers of T and B cells do not alter, but lymphocyte function and cell-mediated immunity in particular are depressed, possibly by the increase in concentrations of glycoproteins coating the surface of the lymphocytes, reducing the response to stimuli. There is, however, no evidence of suppression of humoral immunity or the production of immunoglobulins.
Clotting factors
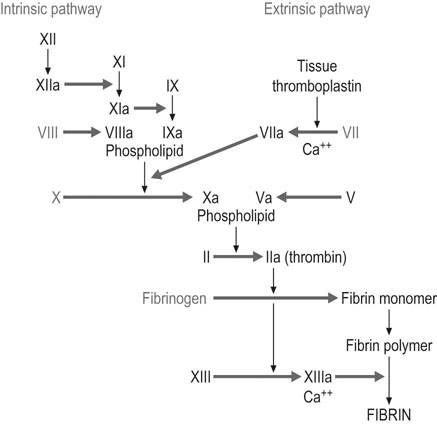
There are major changes in the coagulation system in pregnancy, with an increased tendency towards clotting (Box 3.1). In a situation where haemorrhage from the uterine vascular bed may be sudden, profuse and life-threatening, the increase in coagulability may play a life-saving role. On the other hand, it increases the risk of thrombotic disease.
Many clotting factors remain constant in pregnancy but there are notable and important exceptions (Fig. 3.9). Factors VII, VIII, VIII:C, X and IX (Christmas factor) all increase during pregnancy, whereas factors II and V tend to remain constant. Factor XI falls to 60–70% of the non-pregnant values and concentrations of factor XIII fall by 50%. Protein C, which inactivates factors V and VIII, is probably unchanged in pregnancy, but concentrations of protein S, one of its co-factors, fall during the first two trimesters.
Respiratory function
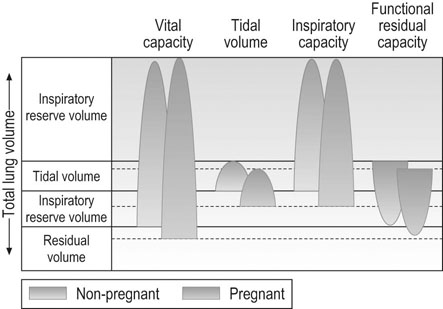
Vital capacity describes the maximum amount of gas that can be expired after maximum inspiration. Since residual volume decreases slightly in pregnancy (Fig. 3.10), vital capacity increases slightly. Vital capacity is related to body weight and is reduced by obesity. Inspiratory capacity measures tidal volume plus inspiratory reserve volume. It increases progressively during pregnancy by ~300 mL while residual volume decreases by about 300 mL. This improves gas mixing. Forced expiratory volume in 1 second (FEV1) and peak expiratory flow remain constant in pregnancy and women with asthma do not appear to be affected by pregnancy.
Renal function
Anatomy
Physiology
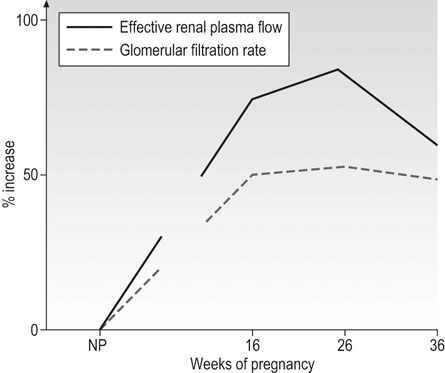
Both renal blood flow (RBF) and glomerular filtration rate (GFR) increase during an ovulatory menstrual cycle, and this increase is maintained should conception occur. Renal blood flow increases by 50–80% in the first trimester, is maintained at these levels during the second trimester, and falls by ~15% thereafter (Fig. 3.11). Creatinine clearance is a useful indicator of GFR but gives values that are significantly less than those obtained by inulin clearance (gold standard). The 24-hour creatinine clearance has increased by 25% 4 weeks after the last menstrual period and by 45% at 9 weeks. In the third trimester, there is some decrease towards non-pregnant values, but less than the fall in RBF. The filtration fraction thus falls in the first trimester, is stable in the second, and rises towards non-pregnant values towards term.
The filtered load of sodium increases by 5000–10 000 mmol/day because of the increase in the GFR. Tubular reabsorption increases in parallel with the GFR (see: Renin–angiotensin system, below), with the retention of 3–5 mmol of sodium per day into the fetal and maternal stores. The total net sodium gain amounts to 950 mmol mainly stored in the maternal compartment. However, the plasma concentration of sodium falls slightly in pregnancy, because of the marked rise in plasma volume. A similar change occurs with potassium ions, with a net gain of approximately 350 mmol.
The tubular reabsorption of calcium is enhanced, presumably under the influence of the increased concentrations of 1,25-dihydroxyvitamin D. Even so, urinary calcium excretion is two- to threefold higher in normal pregnancy than in the non-pregnant woman. Renal bicarbonate reabsorption and hydrogen ion excretion appear to be unaltered during pregnancy. Although pregnant women can acidify their urine, it is usually mildly alkaline.
Nutrients in blood
Maternal carbohydrate metabolism
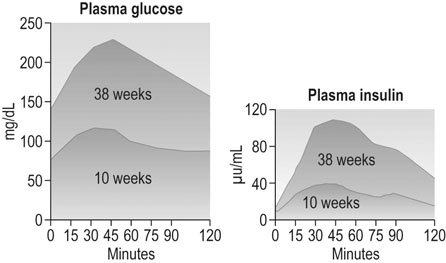
Glucose is the major substrate for fetal growth and nutrition, so carbohydrate metabolism in pregnancy is very important for fetal development. Neither the absorption of glucose from the gut nor the half-life of insulin seem to change. However, by 6–12 weeks gestation, fasting plasma glucose concentrations have fallen to about 0.5–1 mmol/L lower than non-pregnant values; fetal concentrations run ~20% lower than this. The mother’s plasma insulin concentrations rise. By the end of the first trimester the increase in blood glucose following a carbohydrate load is less than outside pregnancy (Fig. 3.12). Pregnant women develop insulin resistance, so any given glucose challenge will produce extra insulin, which does not reduce the blood glucose levels as quickly as the response in non-pregnant women. The insulin resistance is hormonally driven, possibly via human placental lactogen or cortisol. The management of the pregnant woman with diabetes is discussed in Chapter 9.
Maternal weight gain
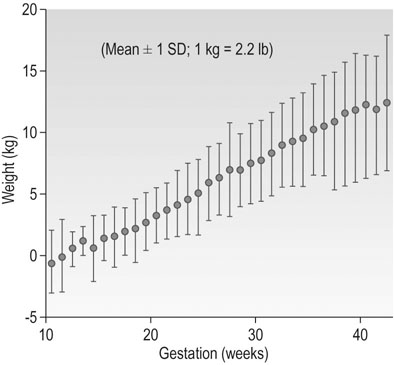
Pregnancy is an anabolic state. The average weight gain over pregnancy in a woman of normal BMI is ~12.5 kg. Many women during the first trimester do not gain any weight because of reduced food intake associated with loss of appetite and morning sickness. However, in normal pregnancy, the average weight gain is 0.3 kg/week up to 18 weeks, 0.5 kg/week from 18 to 28 weeks and thereafter a slight reduction with a rate of ~0.4 kg/week until term (Fig. 3.13). The range of maternal weight gain in normal pregnancy may vary from near zero to twice the mean weight gain as a result of variation in the multiple contributory factors. The basal metabolic rate rises by ~5% by the end of pregnancy in a woman of normal weight. Figure 3.14 summarizes the relative maternal and fetal contributions to weight gain at term.
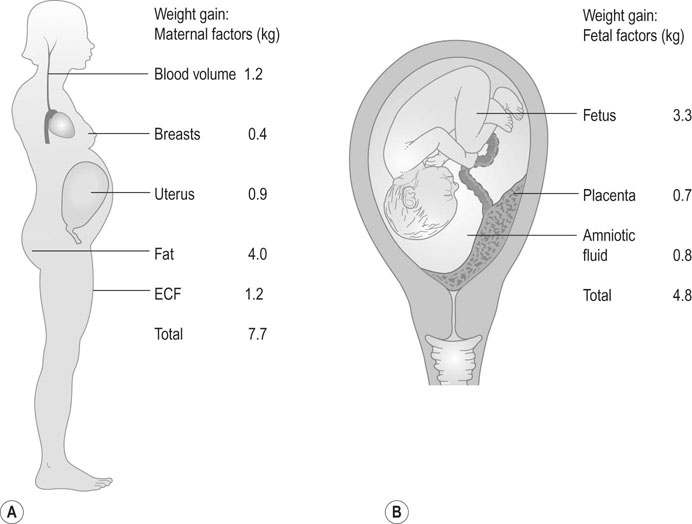
No more protein is laid down than can be accounted for by fetal and placental growth and by the increase in size in specific target organs such as the uterus and the breasts.
The breasts
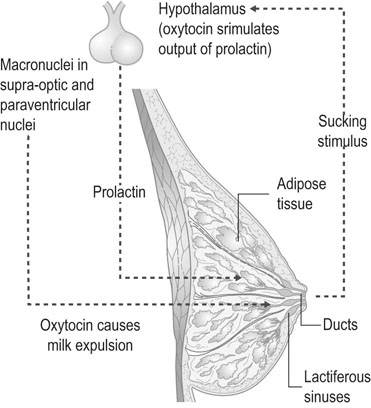
Breast development during pregnancy
High oestrogen concentrations, with growth hormone and glucocorticoids, stimulate ductal proliferation during pregnancy (Fig. 3.15). Alveolar growth is stimulated in the oestrogen-primed breast by progesterone and prolactin. Secretory activity is initiated during pregnancy and is promoted by prolactin and placental lactogen so that from 3 to 4 months onwards and for the first 30 hours after delivery, a thick, glossy, protein-rich fluid known as colostrum can be expressed from the breast. However, full lactation is inhibited during pregnancy by the high levels of oestrogen and progesterone that block the alveolar transcription of α-lactalbumin.
Endocrine changes
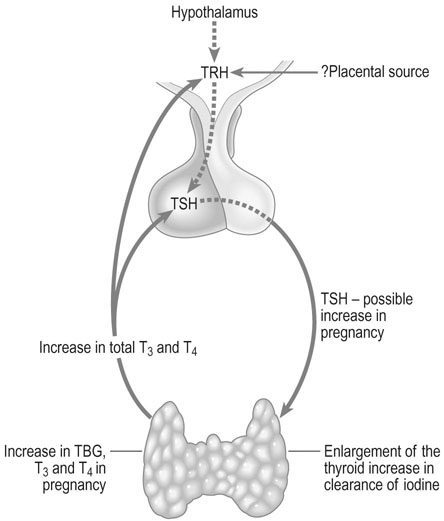
The thyroid
The thyroid gland enlarges in up to 70% of pregnant women, the percentage varying depending on iodine intake. In normal pregnancy, there is increased urinary excretion of iodine and transfer of iodothyronines to the fetus. This in turn results in a fall of plasma inorganic iodide levels in the mother. At the same time, the thyroid gland triples its uptake of iodide from the blood, creating a relative iodine deficiency which is probably responsible for the compensatory follicular enlargement of the gland (Fig. 3.16).
The adrenal gland
The adrenal glands remain constant in size but exhibit changes in function.
Plasma aldosterone from the zona glomerulosa rises progressively throughout pregnancy (see above: The renin–angiotensin system); there is also a substantial increase in the weak mineralocorticoid deoxycorticosterone that is apparent by 8 weeks gestation and may reflect production by the fetoplacental unit.