Chapter 14 Physical management of altered tone and movement
Introduction
The term muscle tone describes the normal resistance felt when moving a limb passively through range (Burke, 1988). Muscle tone is an integral part of movement and posture but following neurological insult may present in a variety of altered states (Table 14.1). The importance of tone alterations remains the subject of debate, at both a physiological level and within the wider discussion of its relevance to physiotherapists treating neurological patients (Boyd & Ada, 2008). Furthermore, secondary changes within the peripheral tissues are known to contribute to and complicate the clincial picture (O’Dwyer & Ada, 1996). Irrespective of the underlying cause, physiotherapists manage the effects of tone alterations using their skills of clinical reasoning to judge whether tone changes enable or inhibit an individual’s function. For example, patients may use extensor spasticity to stand and transfer; conversely, muscle spasms may be so disabling that they hinder comfortable seating (Thompson et al., 2005). Movement disorders that include dysfunction of muscle tone are complex and challenge the interpretation and identification of which component primarily contributes to the movement dysfunction. Currently within clinical practice there is inconsistent use of terminology to describe tonal alterations and peripheral adaptations, which hinders effective decision-making and communication between health-care professionals. Drawing upon recent literature, this chapter defines the most commonly used terms (Tables 14.1–14.4) and places the physical management of these manifestations within the International Classification of Functioning, Disability and Health (ICF) (World Health Organization (WHO), 2001).
| Normal muscle tone | ‘The resistance of the limb to passive stretch determined by the physical inertia of the limb as well as the passive mechanical properties of the soft tissues because in a normal, relaxed muscle, there is no neural response to the stretch’ (Burke, 1988). |
| Hypertonia | ‘An increase in stiffness with resistance to stretch in one direction’ (Ada & Canning, 2009, p. 81). There may be neural (e.g. spastic dystonia) and non-neural (e.g. contracture) contributory impairments. The term hypertonia should not be used interchangeably with spasticity because it is inaccurate and confusing (Ada & Canning, 2009). |
| Hypotonia | ‘Less than normal resistance to passive movement’ (Ada & Canning, 2009). |
Table 14.2 Features of upper motor neurone syndrome (Sheean, 2002, 2008)
| Positive features | Afferent drive: in response to peripheral stimulation | |
| (additional or exaggerated phenomena: muscle overactivity) | Proprioceptive stretch reflexes: | |
| Spasticity Clonus Tendon hyperreflexia with irradiation Positive support reaction |
||
| Nociceptive reflexes: | ||
| Flexor spasms | ||
| Cutaneous reflexes: | ||
| Extensor spasms | ||
| Extensor plantar response (positive Babinski) | ||
| Efferent drive: supraspinal activity, observed during movement | ||
| Spastic dystonia | ||
| Associated reactions | ||
| Disordered control of voluntary movement: | ||
| Reduced reciprocal inhibition leading to pathological (spastic) co-contraction | ||
| Excessive reciprocal inhibition leading to apparent weakness | ||
| Negative features | ||
| (loss or reduction of phenomena) | Weakness | |
| Fatiguability | ||
| Loss of dexterity | ||
| Acute hypotonia | ||
| Adaptive features | ||
| (soft tissue) | Non-neural contributions (biomechanical component) | Stiffness and shortening of peripheral soft tissue structures |
| Neural contributions (overactive muscle contraction) | ||
| Spasticity | ||
| Spastic dystonia | ||
| Hyperreflexia | ||
Table 14.3 Definitions of the positive features of upper motor neurone syndrome
| Positive features | |
| Spasticity | ‘A motor disorder characterised by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks resulting from hyperexcitability of the stretch reflex as one component of the upper motor neurone syndrome’ (Lance, 1980, 485). |
| Clasp knife phenomenon | The response of a muscle with spasticity to passive stretch – a rapid stretch to the muscle elicits a velocity dependent tonic stretch reflex. The resistance produced by the reflex contraction of the muscle slows the movement thereby reducing the stimulus eliciting the stretch reflex to below threshold and the resistance to the passive movement melts away. As the stretch continues, a second mechanism comes into play which suggests that as the muscle continues to lengthen the sensitivity to stretch reduces, indicating that the tonic stretch reflex is length – as well as velocity – dependent (Burke, 1988; Sheean, 2008). |
| Clonus | A rhythmical contraction of a muscle in response to a brisk stretch, maintained. Often seen in the gastrocnemius/soleus as the heel hangs off the footplate of a wheelchair and stretches the back of the calf. The stretch of the calf elicits a stretch reflex causing gastrocnemius/soleus to contract, plantarflexing the ankle and eliminating the stretch. If the relaxation is rapid and the stretch is maintained, another stretch reflex will be elicited and the ankle will plantarflex, again setting up the cycle for a sustained rhythmic contraction. This will continue as long as the stretch is maintained (Burke, 1988; Sheean, 2008). |
| Hyperreflexia | A greater than normal reflex response (e.g. the presence of reflex responses when a relaxed muscle is stretched at the speed of normal movement (Boyd & Ada, 2008, p. 80). |
| Positive support reaction | A response of the lower limb, evoked by the foot coming into contact with the ground and eliciting a proprioceptive stretch of the intrinsic foot muscles and an exteroceptive stimulus, caused by pressure on the sole of the foot during attempted weight bearing. It produces plantar flexion and inversion of the ankle, and sometimes knee extension (Bobath, 1990). |
| Flexor spasms
Extensor spasms |
Usually lower limb spasms that are probably distorted flexor withdrawal reflexes. Caused by nociceptive (e.g. pressure sores), cutaneous (e.g. bed sheets moving across the legs) or visceral (e.g. distended bladder or bowel) stimuli (Sheean, 2008). Extension of the lower limb usually evoked by cutaneous stimulation of the groin, buttock, or back of the leg or following a sudden stretch to the iliopsoas and thus evoking the extensor component of the crossed extensor reflex (Burke, 1988; Sheean, 2008). |
| Extensor plantar response (Babinski sign) | Upward movement (dorsiflexion/extension) of the great toe with ankle dorsiflexion in response to a non-painful cutaneous stimulation on the plantar aspect of the foot moving from a lateral to medial direction; a disinhibited flexor withdrawal reflex. A normal Babinski test would produce flexion of the great toe and plantar flexion of the ankle (Burke, 1988; Sheean, 2008). |
| Spastic dystonia | ‘Continuous muscle contractions that occur in the apparent absence of voluntary contraction and of any sensory feedback from the periphery (proprioceptive, cutaneous or nociceptive)’ (Sheean, 2002, p. 7). Thought to be due to tonic supraspinal drive to the alpha motor neurones, e.g. the hemiplegic posture (Sheean, 2008). Spastic dystonia can be altered by changes in posture (Denny-Brown (1966) cited in Sheean, 2008) and through stretch modulation via proprioceptive or vestibular mechanisms (Burke, 1988). |
| Associated reactions | An involuntary activation of muscles remote from those normally engaged in the task, e.g. upper limb flexion during sit to stand. The amount of activation and movement is usually in proportion to the effort expended in executing the task. Thought to be due to tonic efferent drive of alpha motor neurones and may be due to a failure to inhibit spread of motor activity through propriospinal pathways in addition to soft tissue adaptation (Sheean, 2002, 2008). |
| Reciprocal innervation (coordination): Task dependent control of agonists and antagonists minimising the number of commands sent to individual muscles mediated by the Ia inhibitory interneurone (Gordon, 1991). Reciprocal inhibition: Inhibition of antagonists which would otherwise inhibit voluntary movement; enhances speed and efficiency of movement by making sure that prime movers are not opposed by antagonists (Gordon, 1991). Co-contraction: The simultaneous contraction of agonist and agonist, e.g. around the wrist during a gripping activity (Gordon, 1991). |
|
| Disordered reciprocal inhibition | Disruption to the descending excitatory and inhibitory signals from the major descending pathways converging on the Ia inhibitory interneurone, causing difficulty with reciprocal co-ordination and shifting between co-contraction and reciprocal inhibition and impairing reciprocal innervation and the task-dependent coordination of agonists and antagonists. The clinical picture may be complicated by soft tissue adaptation and remains a topic of debate in the literature (Sheean, 2008). |
| Reduced reciprocal inhibition | Pathological (spastic) co-contraction, e.g. elbow flexors are not inhibited as the elbow extends due to simultaneous activation of the flexors and extensors (Sheean, 2008). |
| Excessive reciprocal inhibition | Sustained activation of one muscle group causing inability to activate the antagonist when required, e.g. activation of soleus during the gait cycle which is sustained during swing phase and results in inhibition of tibialis anterior; often attributed to weakness in tibialis anterior (Sheean, 2008). |
Table 14.4 Definitions of the negative features of upper motor neurone syndrome
| Negative features | |
| Weakness | An inability to generate, sustain and synchronize the necessary voluntary force for effective motor behaviour (Landau, 1988) causing disorganization of motor behaviour inappropriate to the task and context (Carr & Shepherd, 2003). Mechanisms involve: (a) disrupted descending input onto the motor neurone pool; (b) decreased number of motor units activated; (c) decreased motor unit discharge rate; and (d) disrupted motor unit recruitment (Gemperline, et al., 1995; Rosenfalck & Andreassen, 1980). |
| Fatiguability | Combined influence of disuse and deconditioning, as well as an increase in the proportion of functionally slow motor units which result in poor endurance for sustained voluntary force output (Carr & Shepherd, 1998). |
| Loss of dexterity | An inability or difficulty in performing actions quickly and skilfully using independent movements of any part of the body (particularly loss of fractionation – independent movement of individual fingers, e.g. typing or manipulating objects), spatial and/or temporal inaccuracies due to slow production of force and an inability to rapidly alter the degree of contraction in specific muscles or muscle groups during task performance, resulting in a loss of flexibility of motor control with respect to the changing environment or task demands (Ada & Canning, 2009). |
| Acute hypotonia (neural shock) |
Suppression of spinal reflexes for a variable amount of time (depending on the site of the lesion), the return of spinal reflex behaviour suggests that mechanisms of neuronal plasticity may be involved (Sheean, 2002). |
Overview of upper motor neurone syndrome
The upper motor neurone syndrome (UMNS) occurs following a lesion affecting part or all of the long descending tracts that control tone and movement, and which have a direct or indirect influence on the excitability of the motor neurone pool (Barnes, 2008). The clinical picture following an upper motor neurone (UMN) lesion depends on the site and size of the lesion and how much neural adaptation has taken place since the lesion occurred (Sheean, 2002).
Impairments arising from nervous system lesions were originally classified into two groups described as either ‘positive’ or ‘negative’ (Jackson, 1958). This classification is still used, particularly with reference to UMNS (see Table 14.2). This system has good clinical utility and provides a helpful structure for the assessment of the underlying causes of activity limitations and their relative contribution to the overall clinical picture (Ada & Canning, 2009; Carr & Shepherd, 2003).
Positive features
Positive features describe exaggerations of normally occurring motor activity, e.g. hyperreflexia. These impairments are further classified into two groups (Sheean, 2008):
Negative features
Negative features refer to a loss or reduction in normal activity, e.g. weakness. The functional significance of the negative features in terms of their contribution to disability cannot be underestimated (Ada & Canning, 2009; Barnes, 2008; Canning et al, 2004; Landau, 1980). For example, most of the rehabilitation effort for recovery of functional movement for people with cerebral lesions (e.g. stroke) is usually directed towards the negative features because of their proportionally greater contribution to the prevailing activity limitations and participation restrictions (Carr & Shepherd, 2003). This is not to say that the positive features do not have functional consequences, but that simply reducing impairment like spasticity will not necessarily improve function unless the negative and adaptive features are also addressed (Boyd & Ada, 2008).
Adaptive features
Adaptive features are secondary impairments that develop as adaptations to the primary impairments (the positive and negative features); their combined effects have a profound influence on functional performance (e.g. the effect of shortening of gastrocnemius/soleus on walking and sit to stand) such that the adaptive features are usually considered alongside the positive and negative features as part of the clinical assessment (Carr & Shepherd, 2003). Adaptive changes result in an increased resistance to passive movement termed hypertonia. In UMNS, hypertonia should be understood simply as an increased resistance to passive movement. Further assessment should help determine whether the resistance is principally due to neural influences, e.g. hyperreflexia, or non-neural influences (local or peripheral adaptation of soft tissue structures) or a combination of the two. This is an important distinction because stiffness and contracture may exist in the absence of neural influences (O’Dwyer et al., 1996) or may be primarily due to neural activity or a combination of both (O’Dwyer & Ada, 1996). As the treatment for the neural component differs from that of the non-neural component, further clinical investigation will be required to identify the most appropriate intervention (O’Dwyer & Ada, 1996).
The characteristic features of UNMS are outlined in Table 14.2. Tables 14.3 and 14.4 provide definitions for each of the impairments.
The primary impairments of UMNS result from disruption of the supraspinal control of descending pathways that normally control excitatory and inhibitory influences on proprioceptive, cutaneous and nociceptive spinal reflexes (Sheean, 2008). These reflexes become hyperactive and account for the majority of the positive features of UMNS (Sheean, 2008). It is likely that the positive features emerge due to a combination of diminished descending cortical control in addition to plastic reorganization at spinal cord and cortical level (Sheean, 2008).
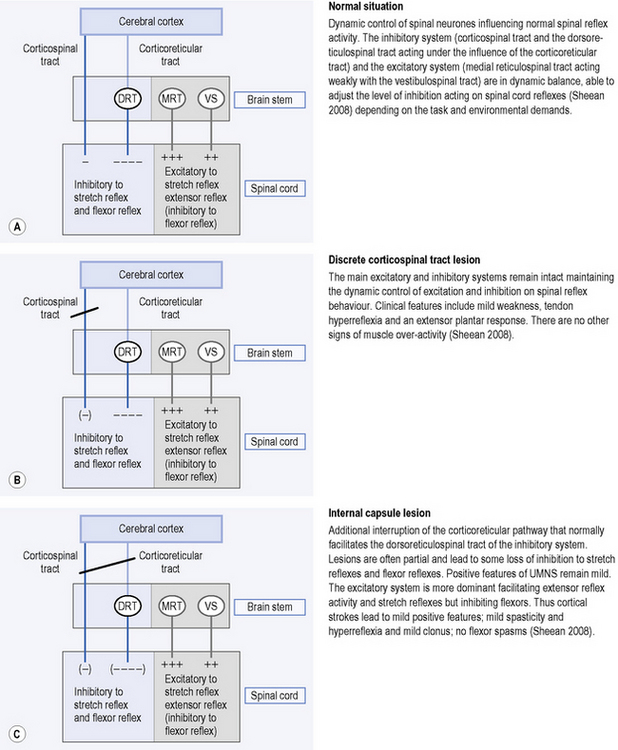
The inhibitory system
Corticoreticular fibres, which travel with, but are separate from, the corticospinal tract, facilitate an inhibitory area in the medulla called the ventromedial reticular formation. This area gives rise to the dorsal reticulospinal tract which is located in the dorsolateral funiculus (a column of fibres in the spinal cord). The main influence of the dorsal reticulospinal tract, which acts weakly with the corticospinal tract, is inhibitory to stretch reflexes and flexor reflexes (Sheean, 2008) (see Figure 14.1A).
The excitatory system
This system is slightly more diffusely organized in the brain stem and is not under tight cortical control; the bulbopontine tegmentum is the most important area because it gives rise to the medial reticulospinal tract. This tract, acting weakly with the vestibulospinal tract, is excitatory to stretch reflexes and extensor reflexes but, like the inhibitory system, is also inhibitory to flexor reflexes (Sheean, 2008) (see Figure 14.1A).
It is already possible to see from the above how disruption to one or all of the descending pathways will disrupt the balance of the excitatory and inhibitory inputs to the spinal motor neurones. The critical features of the descending system are therefore identified by Sheean (2008) as:
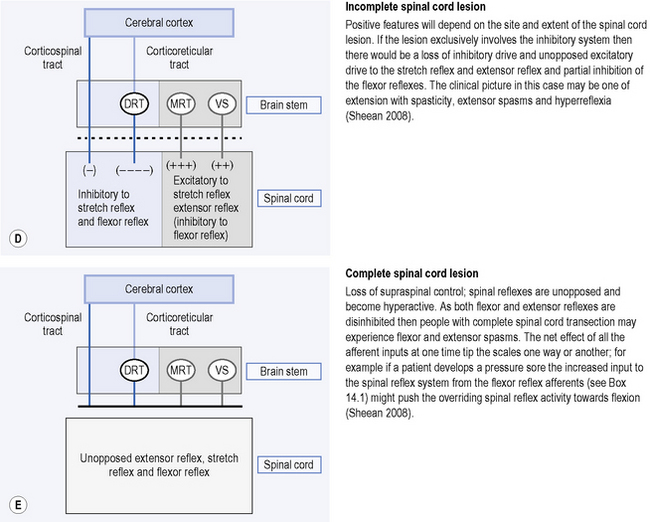
Figure 14.1 A–E explains the origin of the clinical presentations resulting from lesions to different anatomical locations of the descending tracts, after Sheean (1998, 2008). Figure 14.1E refers to flexor reflex afferent (FRA) reflexes, which are described in more detail in Box 14.1.
Box 14.1 What are flexor reflex afferent (FRA) reflexes?
FRA reflexes are multi-connected spinal reflex pathways that are normally tightly controlled by supraspinal activity in both the excitatory and inhibitory systems (Sheean, 2008). FRA reflexes carry information from the skin, fascia, muscle, bone, capsule, bursa and gastrointestinal tract. Their activity can result in either facilitation of flexor activity, flexor inhibition or excitation of extensor activity (Sheean, 2008). It is thought that under normal conditions supraspinal control determines which pathways are activated according to the functional task (Burke, 1988). In UMNS with extensive spinal lesions (e.g. multiple sclerosis) the activity of the FRAs is no longer controlled by descending input. Therefore, constipation or pressures sores for example, may increase the input from flexor reflex afferents to the spinal cord and tip the reflex activity towards flexor dominance reflected clinically as flexor spasms or paraplegia in flexion (Sheean, 2008).
Physical management of positive, negative and adaptive features
Limitations in activities and restrictions in participation from loss of movement can arise from the combined effect of positive (i.e. spasticity), negative (i.e. weakness) and adaptive features (i.e. contractures) (Ada et al., 2006b). Interventions include manual (hands on), specific adjuncts to treatment and education.
Use of movement
Manual techniques are amongst the principle means available to the physiotherapists in the treatment and management of the altered tone seen post neurological insult. The importance of afferent inputs and their effects on muscle tone and postural/biomechanical alignment have been well documented in the literature (Rothwell, 1994; Shumway-Cook & Woollacott, 2007). Changes in cortical representations have been shown to accompany functional gains after rehabilitation (Johansson, 2000; Richards et al., 2008). Such studies help to provide direct evidence that altering passive or active sensory input can drive motor output; therapeutic movement and handling is a means of altering afferent input to the patient. An understanding of the basic principles that affect neural plasticity is key to enhancing motor recovery after neurological insult (Kleim & Jones, 2008).
The main aims of physical interventions are:
Maintenance of soft tissue length
The need for prevention of soft tissue changes in people with neurological impairments as a prerequisite to the prevention of contractures is widely acknowledged (Bovend’Eerdt et al., 2008; Katalinic et al., 2008). Without full range of motion, peripheral changes cause muscle imbalance and can compound any central motor dysfunction (Fitzgerald & Stokes, 2004). The physiotherapist must be able to make an in-depth analysis of posture and movement patterns as a basis for clinical reasoning and deciding upon primary problems and secondary compensations (Meadows & Williams, 2009). Stretching is widely used as an intervention to prevent or minimize contracture formation.
Stretching, assisted and passive movements
When handling a patient, the therapist must be ready to adapt to any changes in the response of the muscle to the afferent input from the movement. The aim of stretch interventions is to maintain or increase range of movement in soft tissues and can be carried out by the therapist or by the patient. Stretching can have immediate and lasting effects; the former being related to changes in the viscous properties whereas the mechanism behind longer lasting effects is still not fully understood but thought to be connected to changes in sarcomeres; Katalinic et al. (2008) and Bovend’Eerdt et al. (2008) provide detailed overviews. Changes in the muscle itself include thixotrophy (Vattanaslip et al., 2000) and even where there is no neurological damage, the normal resistance to movement is the result of such things as muscle, tendon and connective tissue inherent stiffness.
The length of time required to prevent contracture formation also remains an area of debate; the literature suggests intervals from 30 minutes to 6 hours (Tardieu et al, 1988; Williams, 1990). Discussion continues in clinical practice as to the effectiveness of applying stretch and remains an active area of research (Katalinic et al., 2008).
Changes in the motor and sensory systems can be expected even when movement is passive (Lotze et al., 2003) or, indeed, if physical movements are cognitively rehearsed (mental practice; Page et al., 2009). The importance of carrying out active/assisted or passive movements through their full range is paramount and attention should be paid to muscles that cross two or more joints. Movements should be performed with care, confidence and variety, and the patient should be taken out of his or her preferred posture. Movement should not be vigorous, and never forced, as this could be a causative factor in heterotopic ossification (HO), although the underlying mechanism remains poorly understood (Chua & Kong, 2003). However, movement can prevent the development of HO and should be carefully encouraged even when HO is present (Knight et al., 2003).
Modulation of muscle tone
Therapeutic movement and alteration in alignment of body parts are thought to be able to influence muscle tone in other areas indirectly. The trunk, head and shoulder and pelvic girdles have been proposed to be particularly influential in altering muscle tone (Meadows et al., 2009). Schmit et al. (2000) found that repeated joint movements had beneficial, albeit short-term, effects on spastic hypertonia at the elbow. This alteration of muscle tone may be augmented by presynaptic inhibition from the periphery, leading to neuroplastic adaptation (Rothwell, 1994). Additional preliminary findings around therapeutic touch, such as slow stroking on hypertonic muscles in multiple sclerosis, has indicated a reduction in alpha-motor neuron excitability (Brouwer & de Andrade, 1995). The use of rotation is also thought to be important in modulating tone (Proske et al., 2000) and the additional components of traction and compression can likewise be used (Rosche et al., 1996). Mayston (2002) also suggests that therapists alter tone at a non-neural level by affecting muscle length and range, which gives an improved alignment as a prerequisite for better movement.
Re-education of movement
As movement dysfunction arises from a combination of positive, negative and adaptive impairments, the clinical reasoning process and interventions subsequently selected should reflect this balance. Many therapists believe that a higher quality of movement translates directly to greater functional ability (Davidson & Waters, 2000) but this relationship has yet to be proven empirically. Reduction of tone should be only part of a treatment so as not to affect function inadvertently for those who rely upon positive features for the achievement of motor goals, e.g. transfers. Other factors such as practice, specificity of training, transfer of training and feedback need to be considered as part of the overall framework of therapeutic practice (Lennon & Bassile, 2009). Treatment can be based on one or more of the various models of motor control and some of these treatment approaches are discussed in Chapter 12, as well as in the chapters on specific conditons (Chs 2–10).
Weight-bearing
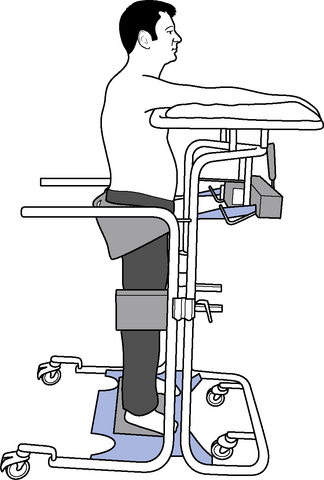
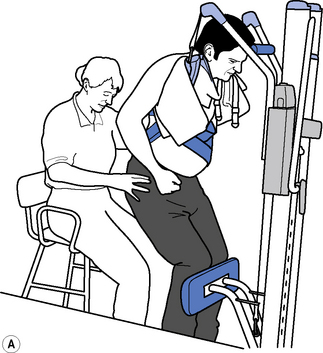
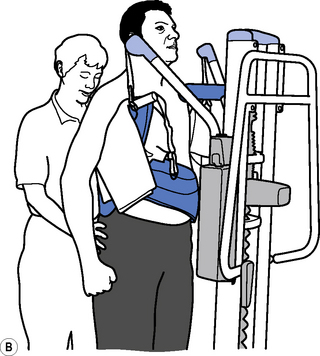
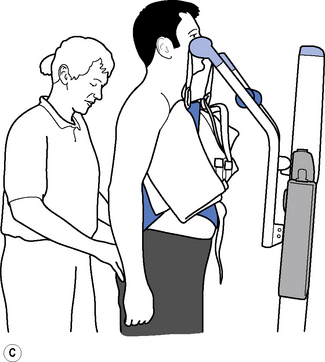
Therapeutic standing is a way of maintaining length in the soft tissues, modulating tone and encouraging extensor activity. To be most effective it should be dynamic to promote tonal changes (Massion, 1994), activating the extensor muscles whilst reciprocally inhibiting the flexors via the vestibuar system (Markham, 1987; Brown, 1994). Standing can be effective in modulating tone and reducing the frequency of spasms, whilst maintaining joint range (Bohannon, 1993). A pilot study looking at the efficacy and feasibility of a therapeutic standing programme for people with multiple sclerosis showed increased range of movement in wheelchair-dependent subjects and a downward trend in spasticity and spasms was noted (Baker et al., 2007). A decrease in motor neuron excitability in patients with spastic hemiparesis was also noted after a single session of standing on a tilt table for 30 minutes (Tsai et al., 2001). Tilt tables can be used to stand medically stable patients in the early stages of rehabilitation, even if they are still unconscious; wedges under the feet can be used as required to increase the stretch on the calf musculature. Alternatively, in the more awake patient, backslabs (Carter & Edwards, 2002) or electrical standing frames may be used (Figure 14.2). With the current emphasis on risk management it is recommended that, where possible and practicable, equipment should be used to minimize the manual handling risk (Chartered Society of Physiotherapy (CSP), 2008). The use of standing hoists can allow patients to weight-bear regularly in a normal functional task and so should be encouraged (Figure 14.3).
Weight-bearing with specific alignment can also be achieved through the upper limbs to maintain length and influence tone, but must be performed with extreme care. Normal biomechanical alignment is maintained by external rotation at the shoulder and the wrist joint should not be overstretched (Champion & Barber, 2009).
Adjuncts to physical management
Positioning and seating
Therapeutic positioning and seating is underpinned by the premise of providing sufficient external support to enable a person to cope better with the effects of gravity, and to maintain postural alignment without using undue muscular activity; the reader is referred to Pope (2007) for a detailed review of posture and seating.
The relationship between positioning and outcome is still the subject of debate, with research evidence, whilst not conclusive, showing limited effectiveness (de Jong et al., 2006; Fox et al., 2000; Gustafsson & McKenna, 2006). However, there remains a consensus view in the literature advocating the importance of positioning and the need for multidisciplinary team involvement (Pope, 2007; Intercollegiate Stroke Working Party (ISWP), 2008). Where possible, positioning should be integrated into functional activities in a normal daily routine (Figure 14.4) and appropriate manual handling equipment and techniques always considered (CSP, 2008). Standard positioning charts are useful to promote consistency across the team and can be personalized, for example drawing on additional pillows to illustrate adjustment for specific postural needs; alternatively, photographs obtained with informed consent can be used.
Positioning in bed
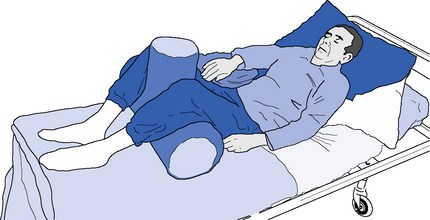
Optimal positioning of a patient in supine can present a variety of challenges for the therapist. For example, patients with predominantly negative impairments may all too readily accept the supportive surface and be at risk of loss of range, whereas those with positive or indeed adaptive impairments may not be able to adapt their posture to the base provided. The use of pillows, wedges and T-rolls can assist in altering limb and body postures and help provide appropriate support (Figure 14.5). Profiling electrical beds can also be utilized to help maintain different postural alignment, likewise side-lying can be used for introducing change and breaking up any predominant tonal patterns or preferred postures. Positioning a patient in prone can be beneficial for some but its use must be considered carefully, for instance those with tracheotomies or severe contractures may be unable to achieve this position. As the head, shoulders, thighs and knees are the main load-bearing areas in prone, the introduction of a wedge can facilitate function by moving the fulcrum of movement to the pelvis (Pope,2007) or a commercially produced beanbag can also be tried to encourage head extension and shoulder protraction (Figure 14.6).
Mattresses
All patients should have a mattress that matches his or her pressure and positoning needs; a recent Cochrane Review by McInnes et al. (2008) provides comprehensive evaluation of the different support surfaces for pressure ulcer prevention. Physiotherapists should collaborate with nursing staff to facilitate optimal prescription for function. For example, the use of overlays on a standard mattress may be sufficient for pressure care yet affect transfers, as it increases the overall bed height so only a standing hoist can be used with taller patients. A replacement mattress that is the same height as a standard mattress but has higher pressure relieving properties may be preferable in terms of rehabilitation, although it is more costly. Overhead hoists may have to be used for air-filled mattresses. Findings from the PRESSURE Trial Group (Nixon et al., 2006) indicated that more than one-third of patients reported difficulties associated with movement in bed, and getting in and out of bed with overlays.
For acute head injuries where intracranial pressure (ICP) is a concern, electronic pressure beds can offer an effective way to achieve weight transference and positive effects on respiratory function. For patients with uncontrolled movements (e.g. Huntington’s disease, see Ch. 7), reduced sensation, or cognitive or perceptual deficits, the use of cot sides, or placing the mattress on the floor, should be considered to reduce the risk of injury to the patient from falling out of bed.
Sitting
Being able to sit well not only is central to an effective posture management system, but it can also help with respiration, eating and communication whilst promoting social interaction. The position of the pelvis is the keystone for building a framework of postural support, whereas hips, knees and feet should be at 90°, and consideration given to the provision of head and arm support (Pope, 2007). Ideally hospitals and rehabilitation units should have a range of wheelchairs and seating systems, e.g. tilt in space, available for short-term loan where the needs of the acute patient may change rapidly. Pressure care and postural support cushions should be considered in conjunction with MDT colleagues. Collins (2007) details a practical guide to cushions, however, in practice, ward chairs often have to be adapted with everyday items such as towels and pillows to help achieve optimum postures. If patients have severe prolonged difficulties with seating a referral to a specialist seating service should be considered.
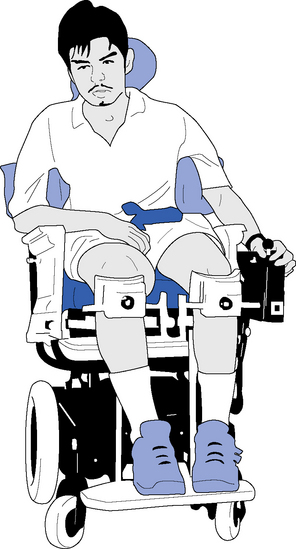
Electric and self-propelled wheelchairs
Appropriate wheelchair provision can promote independence and improve quality of life (Devitt et al., 2004). However, debate remains in clinical practice over encouraging self-propulsion in the early stages of rehabilitation with Cornall (1991) stating it increases spasticity, whereas more recent studies have shown no adverse effects (Barrett et al., 2001; Pomeroy et al., 2003). A wide variety of manual or electric wheelchairs, including indoor/outdoor electrically powered chairs (EPIOC) are available through either the NHS or privately and the introduction of a voucher scheme has helped to increase choice (www.direct.gov.uk). However, findings from a qualitative study of people with severe disability from multiple sclerosis found that whilst tilt-in-space wheelchairs were more comfortable than standard wheelchairs, reducing spasms and improving posture, they had negative aspects such as their bulky size and lack of manoeuvrability (Dewey et al., 2004).
Whilst electric wheelchairs provide an alternative to manual chairs, cognitive and perceptual difficulties can limit a person’s ability to use one, but it should nonetheless not be ruled out automatically before the individual is assessed (Dawson & Thornton, 2003). Some wheelchairs offer the facility of helping the person into a near-standing position, which is helpful for maintaining range of movement in joints and for pressure care (O’Connor & Smith, 2008).
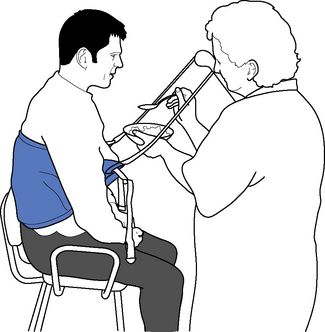
Splinting/casting*
The best approach to contractures remains prevention yet in some patients, despite proactive intervention, adaptive impairments still occur. Altered tone has long been implicated in the development of contractures and without intervention affected areas are at risk of losing range from soft-tissue changes (Goldspink & Williams, 1990). Results from a recent study showed for the first time that spasticity can cause contracture in the first 4 months post stroke, but authors noted that thereafter muscle weakness became the main reason for limitations in activities (Ada et al., 2006b). Patients who show no functional recovery in muscle activity 2 to 4 weeks after stroke have been shown to be at high risk of developing contractures (Pandyan et al., 2003).
Whilst the mechanism behind this intervention remains not fully understood it is thought to have a neurophysiological and biomechanical basis (Lannin et al., 2007b; Mortenson & Eng, 2003; Moseley et al., 2008).
Taking the decision to cast a limb requires careful clinical reasoning and discussion with colleagues in the MDT. Splinting may be precluded if the condition of the patient’s skin or vascular system is poor as there is a risk of pressure sores and tissue damage. If the patient is demonstrating challenging behaviour the splint may present a danger to the patient or others. Incontinence will need to be managed in conjunction with the nursing staff; if the cast becomes wet or soiled it will require removal and replacing. The use of a synthetic/fibreglass-based material instead of plaster is preferable in these types of situations as it is more durable, lighter and dries more rapidly. The development of new materials has given rise to more techniques, e.g. the ability to combine ‘soft and hard’ sections means that areas can be selectively reinforced as required. Details about application procedures are outside the scope of this text and the reader are referred to Edwards and Charlton (2002), although, as pointed out by Lannin et al. (2007b) in a recent systematic review, there is little consensus in practice for casting protocols.
Main forms of splinting
Preventive splinting
Preventive splinting (Stoeckmann, 2001) may be necessary for patients who exhibit a number of the identified risk factors. Mortenson and Eng (2003) carried out a systematic review of the use of casts in adults with joint hypomobility and hypertonia following brain injury and a grade B on the hierarchy of evidence scale was given to the use of casting to increase range of motion or to prevent its loss. Reduction of spasticity is cited as a goal of casting in neurological patients (Fess et al., 2005). Barnard et al. (1984) reported a decrease in this positive impairment after using plaster boots, whereas King (1982) noted less spasticity in the elbow flexors following casting. Robichaud and Agostinucci (1996) hypothesized that this effect might be due to the reduction in input to tactile, proprioceptive and temperature receptors from wearing the cast. They suggested that the cast promotes total contact, even pressure and warmth, thus decreasing the excitability of the alpha or gamma motor neurones in the spinal cord. This hypothesis was further supported by Childers et al. (1999), who used electromyography (EMG) to look at the use of inhibitory casting to decrease spasticity in the upper limb and found a reduction in the vibratory inhibition index. This correlated with a decrease in motor neurone excitability in the hypertonic upper limb. However, in the absence of activity and function, any gains in movement achieved may be difficult to maintain, particularly in the presence of persistent tonal changes (Hill, 1994). In early rehabilitation after stroke, a night splint has been found to be as effective as standing on a tilt table for 30 minutes in maintaining range of movement at the ankle joint (Robinson et al., 2008).
An alternative to casting is pressure splints (Johnstone, 1995) which may be used for short periods of the day. Pizzi et al. (2005) examined the effects of reflex inhibitory splinting (using a volar static splint) in 40 people with post stroke spasticity of the upper limb. The authors found that during the 3 months of the study the splint, which was worn for 90 minutes a day, was well tolerated with a reduction in elbow spasticity and significant gains in wrist passive range of movement (extension more than flexion). Its use was recommended as part of an integrative approach to maintenance.
Corrective splinting or serial casting
Corrective splinting is used to increase range of movement in the presence of contracture, although the lasting effects of any gains achieved may be transient and difficult to maintain (Moseley et al., 2008). However, there is good evidence (level 1b) supporting the use of casting to gain range of elbow extension in adults following brain injury (Lannin et al., 2007b).
Two common methods of serial casting are cyclinders or drop out casts. The advantage of drop out casts is that active movement can still be encouraged and function is less compromised. Electrical stimulation can also be applied to the appropriate muscle (Stoeckmann, 2001). Frequency of changing corrective casts and the amount of stretch applied varies within clinical practice (Edwards & Charlton, 2002; Lannin et al., 2007b). With lower limb casting a platform should be built under the toes to prevent clawing and shortening of the toe flexors. Cyclinders made from synthetic material can be ‘zipped’ with a cut down the length of the splint to effectively bivalve it. This approach has a number of benefits; it is easy to remove to check the skin with high-risk patients; it can be held in place with bandages for prolonged periods, but removed for therapy or personal hygeine.
Cast braces with adjustable hinges can be used for slowly correcting contracted joints, especially at the elbow or knee when the contracture is 90° or more and serial casting is difficult. A risk factor may be swelling around the elbow or knee. Orthoses with a sprung hinge can assist in stretching out contractures (Farmer & James, 2001). Interesting results have been noted in five patients, who were described as having chronic spastic hemiparesis secondary to stroke, and who wore simple wrist splints made of mesh material during the daytime for 8 weeks. They had significant increases in the active range of shoulder flexion and finger extension with a decrease in muscle tone (Fujiwara et al., 2004). Contrary to these findings, Lannin et al. (2007a) in a study of 63 adults post stroke concluded that splints that held the wrist in either neutral or extension and were worn overnight for 4 weeks did not reduce wrist contracture. The authors concluded, therefore, that the practice of routine wrist splinting after stroke should be stopped, a claim that has been countered in the literature by Marossezky et al. (2008) who suggest the conclusion could be restated as ‘splinting does not prevent contracture in patients at minimal risk of contracture’. Clinicians thus need to consider the merits of each individual case and not routinely carry out any intervention, including splinting, without clinical reasoning.
Dynamic splinting
Dynamic or functional splinting aims to facilitate recovery and assist stability for improved function (Fess et al., 2005). Examples are ankle–foot orthoses (de Wit et al., 2004; Pohl & Mehrholz, 2006) and hinged ankle-foot orthoses (Tyson & Thornton, 2001). A recent study by Tyson and Rogerson (2009) describes how non-ambulant patients undergoing rehabilitation felt their walking, confidence and safety improved with the use of assistive walking devices. They were also satisfied with the appearance and comfort of the splints; walking impairments remained unchanged.
Strapping is an alternative short-term method, can be applied to virtually any joint and is particularly useful for the ankle and shoulder joints. Strapping is gaining in popularity in conjunction with musculoskeletal muscle imbalance treatment approaches (see Ch. 12). In the presence of mild hypertonia, strapping may suffice, in particular for the prevention of hemiplegic shoulder pain (Griffin & Bernhardt, 2006). Hangar et al. (2000) found that where patients had their shoulders strapped they had a trend towards less pain and better arm and hand function on final assessment. The short-term effect of using dynamic Lycra splints on the upper limb with neurological impairments suggests some benefits in function (Gracies et al., 2000; Watson et al., 2007).
Orthotic insoles
The use of orthotic insoles should be explored with the podiatry department. A primary goal of foot orthoses is to aid the maintenance and redistribution of weight-bearing patterns (Edwards & Charlton, 2002). In cases where the alignment is good and there is minimal shortening, the authors have clinical experience of using orthotic insoles to ‘walk out’ contracture.
Targeted strength training
Targeted strength training is an important aspect of neurological rehabilitation (Ch. 18). Previous concerns that such intervention would lead to an increase of positive features has not been bourne out by the research evidence (Flansbjer et al., 2008; ISWP, 2008). Indeed, the increasing recognition of negative impairments such as muscle weakness, which is a cardinal feature post stroke (Cramp et al., 2006), has translated to changes in clinical practice, with therapists incorporating task-specific muscle strengthening exercises into treatment programmes (Ada et al., 2006a; Bale & Strand, 2008). However, the wider effects of strengthening on improving participation in societal roles remain unknown (Morris et al., 2004). Chapter 18 discusses the role of muscle strengthening in different neurological disorders and outlines the need for further clinical research.
Exercise
Evidence suggests that exercise does not result in increased positive features and indeed can help to address negative and adaptive changes (see Ch. 18). Nevertheless, neurological impairments can bring challenges to maintaining an active lifestyle in keeping with health-related fitness, but exercise programmes can be designed to increase cardiovascular conditioning (Kilbreath & Davis, 2005; Pang et al., 2005; White & Dressendorfer, 2004). For example, people with moderate disability post stroke have been involved in fitness programmes including aerobic and strength components lasting from 30 to 90 minutes (Duncan et al., 2003). Dawes et al. (2000) found that high-intensity cycling did not lead to any increase in tone. This finding was further supported by Lee et al. (2008) who looked at the effects of aerobic cycle training and progressive resistance training on walking ability post stroke, and found that both musculoskeletal and cardiovascular impairments were still modifiable some years after stroke with selected robust exercise. The use of partial-body-weight support treadmill training has likewise produced positive outcomes on functional mobility (Hesse, 2008) and aerobic treadmill training has been noted for its potential to reduce effort and fatigue in people with multiple sclerosis (Newman et al., 2007).
Hydrotherapy and swimming
Hydrotherapy and swimming can provide freedom and challenge of movement to people with neurological impairments that are often not available to them on land (Cook, 2007). During hydrotherapy the water has a dual role, providing support and warmth, and has a global effect on muscle activity; it can be utilized to alter tone and joint range prior to treatment on dry land. Cook (2007) provides a comprehensive overview of the muscular, cardiovascular, haematological, renal and respiratory effects of immersion. Although the published evidence for hydrotherapy in neurological conditions is sparse, beneficial effects have been noted in people with spasticity following spinal injury (Kesiktas et al., 2004), stroke (Taylor et al., 1993) and multiple sclerosis (Peterson, 2001). The Halliwick concept is one well recognized approach for people of all ages which emphasizes ability rather than disability (Skinner & Thomson, 2008).
Electrical stimulation
Electrical stimulation is used in a number of different formats by physiotherapists in the treatment of positive and negative impairments with mixed results. A systematic review of electrical stimulation for shoulder pain found no significant effect on upper limb spasticity, but some improved range of passive lateral rotation noted (Price & Pandyan, 2001), whereas electrical stimulation combined with Bobath therapy in a randomized controlled trial (RCT; n=40) helped reduce plantarflexor spasticity in a cohort of stroke patients (Bakhtiary & Fatemy, 2008). Another study using a home programme of neuromuscular and sensory amplitude electrical stimulation on 10 people with chronic stroke had mixed results; four showed a reduction in spasticity yet others had an increase in tone that Sullivan and Headman (2007) attributed to subjects having to travel to the testing site in extreme cold weather. Krause et al. (2008) found that functional electrical stimulation along with cycling interventions in five people with spinal cord injury led to a reduction in spastic muscle tone. Results from a randomized single blind crossover trial of 32 people with multiple sclerosis that looked at the effect of transcutaneous electrical nerve stimulation (TENS) on spasticity suggest it does not appear to be effective with spasticity, but that longer applications (8 hours per day) may be useful with pain and muscle spasm (Miller et al., 2007).
Electrical stimulation has also been recommended by the National Institute for Health and Clinical Excellence (NICE) (2009) for use with drop foot, a negative impairment of central neurological origin. EMG triggered FES has been studied in the rehabilitation of wrist and finger extensors in later stage stroke and has shown positive changes in the ability to grasp small objects and maintain muscle activity (Hara et al., 2006).
Biofeedback
Evidence regarding the use of biofeedback in neurological rehabilitation is mixed. Hiraoka (2001) reported an increased functional ability in the hemiplegic upper limb following the use of EMG biofeedback, whereas a Cochrane Review by Woodford and Price (2007) on standard physiotherapy plus EMG feedback only indicated possible positive effects at the shoulder. Moreland et al. (1998) found it had a beneficial effect on improving the strength of ankle dorsiflexors. Van Peppen et al. (2006) reported no significant difference with the use of visual feedback in balance or walking ability, yet an earlier study by Sackley and Lincoln (1997) found visual feedback seemed linked to recovery of balance. Cheng et al. (2004) described improved weight transference with biofeedback.
Medication
The therapist needs a basic understanding of the pharmacological means of controlling altered tone (see chapters on specific neurological conditions). Physiotherapists’ skills in assessing tone, over time, can also play a valid role in evaluating the effectiveness of drugs and when they should be administered. Medication may be appropriate where physical means are inadequate, but consideration needs to be given to their use (Thompson et al., 2005). Pain increases spasticity and so the use of analgesia should be considered; however, not all patients can communicate effectively to request analgesia.
It is essential to establish if the presentation of spasticity is generalized, regional or focal. The treatment options are then clearer. For generalized spasticity, oral medication may be a first approach. For focal spasticity, injections of botulinum toxin may be a useful adjunct to therapy or, where more permanent paralysis is required, phenol nerve blocks can be used. Guidelines for the use of botulinum toxin have been produced and emphasize the need for a multidisciplinary team approach (Royal College of Physicians, 2009). Intrathecal baclofen may be used where spasticity is a major problem; functional benefit may be gained if it unmasks underlying motor control previously hidden by the positive impairment or alleviates pain from muscle spasms (Kofler et al., 2009).
Continuous passive movement
Whilst continuous passive movement (CPM) machines are common in orthopaedics they can also be beneficial in neurological physiotherapy. For example, CPM can be used to help improve range of movement in head-injured patients with contractures (Macfarlane & Thornton, 1997); it is thought to assist in the breakdown of abnormal cross-bridge attachments, which have been shown to contribute to abnormal stiffness during lengthening (Carey & Burghardt, 1993). However, unless combined with active movement, it is likely to require repeated use to prevent the reformation of anomalous attachments (Singer et al., 2001). Furthermore, Lynch et al. (2005) compared the use of CPM with self-range motion exercise in 32 stroke patients and found a positive trend for CPM towards improved shoulder joint stability but no differences in motor impairment, disability, pain or tone.
Thermal treatments
Ice can have a temporary effect in reducing tone, although it is usually applied as an adjunct and only in a specific area. The application of ice to spastic quadriceps muscle has been demonstrated to lead to an objectively measured reduction in spasticity (Brown et al., 1993). Cooling has also been shown to have some benefits to patients with an essential tremor (Cooper et al., 2000). Two studies (Feys et al., 2005; Quintern et al., 1999) reported significant reductions in tremor following cooling of the upper limb in people with MS that could have important functional implications when performing discrete functional activities such as intermittent self-catheterization or taking a meal. However, the effect of cold in people with MS can be variable, exacerbating symptoms in some people (see Ch. 5).
The application of heat packs can help to increase range of motion (Funk et al., 2001) by altering the properties of connective tissue that can be affected in movement dysfunction (Hardy & Woodall, 1998).
Acupuncture
The use of acupuncture in the treatment of neurological conditions remains the subject of debate. The National Clinical Guideline for Stroke (ISWP, 2008), in drawing upon key evidence from two Cochrane Systematic Reviews (SR) by Wu et al. (2006) and Zhang et al. (2005), recommends acupuncture should only be used in the context of ongoing clinical trials. Results of these SRs show acupuncture to be safe in the stroke population post 30 days, but evidence of benefit remains lacking, requiring more methodologically sound research to be carried out. Yet in a small RCT (n=7) conducted by Mukherjee et al. (2007), a significant decrease in spasticity in wrist flexors was noted when using electroacupuncture combined with strengthening exercises. Sonde et al. (2000) likewise reported a decrease in spasticity in the lower limb following the use of high-frequency TENS to stimulate acupuncture points. Donnellan and Shanley (2008) examined the use of acupuncture in people with secondary progressive multiple sclerosis and raised the possibility it may provoke lower limb muscle spasms in those with spasticity. Overall, they concluded that there was preliminary evidence to suggest acupuncture is safe in this population, but advised that the risk of increasing spasms should be raised with individuals.
Education of patients and carers
Management of altered tone requires a 24-hour approach and education is an essential component. Education needs to be tailored to the patient’s abilities and desire to know and must be given in a way that is understandable. Allison et al. (2008) found that health professionals’ use of language was often a barrier to patient and carer understanding. The physiotherapist must try to judge what information to provide and when to provide it, but it is the patient who decides when or if he/she uses this information. Research indicates that information is best delivered in a way that actively involves patients and carers and allows them to have repeated opportunities to ask questions and to feel empowered about making decisions (Reynolds, 2005a; Smith et al, 2008; also see Ch. 11).
Patients need education to be able to identify possible triggers of increased spasms. Nociceptive stimuli, such as those from skin, bladder and bowel (even tight clothing and wrinkled seat cushions), can exacerbate spasticity through stimulating flexor reflex afferent (FRA) activity (Sheean, 2008). With patients who have difficulty communicating or who are in a comatose state, increasing spasticity can be a sign that there is another problem, such as infection or constipation.
Patients may be able to learn to exhale and breathe through spasms to help prevent further tensing and worsening the spasm (Livingstone, 1998). Some patients will be able to gain further cognitive control over their spasms. If clonus is a problem, patients can be taught to push down through the long axis of the lower leg via the knee, giving a prolonged stretch to inhibit overactivity in the affected muscles.
A home programme may be incorporated into the overall management of tone, and compliance may be increased if exercises are included as part of everyday life, e.g. standing up against a wall whilst brushing teeth. Even if the person is unable to carry out all the exercises or stretches independently, he or she should direct a carer and thus retain responsibility. In some cases the spouse or partner may not see this as part of his or her role and the wish to maintain the partner and not the carer role should be respected (Courts et al., 2005). All carers should have their needs assessed separately (ISWP, 2008). Relevant information about support groups or networks (see Appendix) can also be provided.
Factors influencing decision-making in the management of altered muscle tone
Factors that influence decision-making include:
A holistic view of the patient should be taken when making decisions about the management of tonal difficulties; Thompson et al. (2005) and Barnes (2008) provide useful algorithms to assist clinical decision-making. Details of the patient’s previous lifestyle should be considered, as well as his or her aspirations for the future. Quality of life is both personal and subjective; the individual must be consulted and goals set jointly (see next section and Ch. 11). The views of family and friends should be sought if it is not possible to communicate with the patient.
Diagnosis and prognosis
Prediction of outcome is multifactorial and individual, although there are some common factors linked to a more positive prognosis (Counsell et al., 2004; Jiang et al., 2002). In acute head injury where the prognosis is difficult to predict and the patient is at particular risk of developing contractures, emphasis is often placed on respiratory problems and insufficient attention given to potential adaptive impairments. The physiotherapist should advocate on behalf of the patient and be proactive in their approach to rehabilitation until factors indicate otherwise.
The ability to learn
The response to physical rehabilitation cannot be seen in isolation from perception, cognition, motivation, premorbid ability, behavioural difficulties and communication dysfunction. Motor learning is described as a complex perception–cognition–action process (Shumway-Cook & Woollacott, 2007). This area is covered in more detail in Chapter 17 on psychological management. Motivation is described by the WHO as a global mental function that can be both a conscious or unconscious drive that leads to an incentive to act (WHO, 2001) and is key in rehabilitation.
Carryover and teamwork
Carryover can be described as the extent to which treatment gains are maintained and used functionally between treatment sessions. Management of physical impairments requires a 24-hour approach for maximum effectiveness and it is essential the whole rehabilitation team works towards common goals and has the knowledge and skills necessary to provide a coordinated approach (Thompson et al., 2005). For example, the speech and language therapist could undertake treatment while the patient is in a standing frame, or a perching stool could be used to encourage a more extended posture during activities in other therapies. Regular communication between staff including joint goals and treatment sessions, meetings, multidisciplinary notes and training may assist professions to work together effectively (Kilbride et al., 2005; Reynolds, 2005b).
Measuring effects of interventions
Altered tone and movement patterns from the singular or combined effects of positive, negative or adaptive impairments can affect the individual at the various levels of body function/structure, activity and participation (WHO, 2001). When selecting an outcome measure to assess the impact of an intervention consideration should be given as to what level is affected; see Table 14.5 for examples of different scales that can be utilized. Ideally, selected outcome measures should be a combination of impairment and activity to ensure interventions remain patient centred.
| ICF level | Examples of outcome measures |
|---|---|
| Body functions and structure | Ashworth Scale, Modified Ashwoth Scale, Tardieu Scale, Glasgow Coma Scale, Fugl Meyer, Visual Assessment Scale, Stroke Impact Scale (SIS) (impairment section), Motor Assessment Scale (MAS), Nine Hole Peg Test, MRC Measure of Power, Timed Walk |
| Activity | Motor Assessment Scale, FIM, FAM, Nottingham ADL, Barthel Index, SIS activity section, 10 metre walk, 6 minute walk, Action Research Arm Test, EDSS (Kurtzke Expanded Disability Scale), Timed Up & Go, Berg Balance scale |
| Participation | SIS partcipation section, Quality of Life SF 36, Nottingham Health Profile, Stroke-Adated Sickness Impact profile (SA-SIP-30) |
(From Wade, DT, 1992. Measurement in neurological rehabilitation. By permission of Oxford University Press.)
Please note there can be some areas of overlap between categories; issues of reliability and validity should also be taken into account (Stokes, 2009). Nevertheless, it is recognized that the measurement of tone at the level of impairment in the practice setting is challenging. Measurement is often based on the assessment of resistance to passive movement of the limb, but the limitation in movement may be from a combination of impairments (Ada et al., 2006b). Levin et al. (2009) also raised an important point that many outcome measures are not sensitive enough to differentiate how a task is accomplished, for example the quality of movement; without attention to quality it is not possible to tell if compensatory movements have been utilized or if there has been recovery of motor patterns. The authors (ibid) propose a solution within the framework of the ICF model (WHO, 2001).
Commonly used scales are the Ashworth Scale (AS) (Ashworth, 1964), the Modified Ashworth Scale (MAS) (Bohannon & Smith, 1987), which has greater reliability in the upper limb (Pandyan et al., 1999) and the Tardieu Scale (Tardieu et al., 1954). The Tardieu Scale is said to be able to identify the presence of spasticity in upper and lower limb muscles more effectively than the AS and distinguish between spasticity and contracture (Patrick & Ada, 2006). The Modified Tardieu Scale has also been shown to be more reliable than the MAS in patients with severe brain injury (Mehrholz et al., 2005). The Motor Assessment Scale (Carr et al., 1985) also includes a measurement of tone as well as activity items. There is yet to be developed a widely available clinical tool with good validity and reliability that can distinguish between neural and non-neural components, although some early work on a novel approach to objectively measuring tone and biomechanical properties in the clinical setting using a non-invasive hand-held device called a Myoton®, (www.myoton.com) has been documented (Ianieri et al., 2008).
Other clinical measures
A paper walkway (Tyson & Thornton, 2001) can give useful information in the absence of a gait laboratory (i.e. stride length, step width and length). Painted footprints allow changes in weight distribution to be recorded and are a useful way of documenting the realignment of the foot complex. Similarly, periodic photographs allow comparison over time (Tyson & DeSouza, 2003). The procedure needs to be standardized to ensure that the same perspective and distance are used each time. Video recordings are an excellent means of documenting change in posture and movement (Wiles et al., 2003). It is possible to use frequency of events, e.g. spasms (Snow et al., 1990) to assess the outcome of intervention.
Goal setting and goal attainment scaling
Goal setting is the key to good practice in rehabilitation and should actively involve patients in the process wherever possible; if the patient is not able to participate, appropriate family members or a patient advocate should be included (ISWP, 2008). However, in practice, goal setting varies and a recent postal survey found that patients need to be given more opportunities to be engaged in the process but ‘the shift from the patient role to one of partnership in care is developing slowly’ (Holliday et al., 2005, p.231).
The goal attainment scale (GAS), a mathematical way to quantify achievement of goals, was first described in the 1960s (Kiresuk & Sherman, 1968); Turner-Stokes (2009) provides up to date practical guidance for its use in rehabilitation. For patients with severe and complex disabilities, goals may be divided into passive or active function, where passive is related to ease of caring for the individual and active represents a gain in independent activity (Ashford & Turner-Stokes, 2006).
Movement disorders and tonal changes
Parkinson’s disease
Physiotherapy is unlikely to influence the cardinal signs of Parkinson’s disease, therefore rehabilitation is primarily concerned with the treatment and management of the functional consequences of these primary impairments (Stack, 2009), see Chapter 6 and Table 14.6.
| Bradykinesia | Slowness of movement (Berardelli, et al., 2001) |
| Akinesia | Inability to initiate or prolonged initiation of movement due to difficulty selecting and/or activating motor pathways in the central nervous system (Berardelli, et al., 2001), may result in ‘freezing’ episodes (motor blocks) during the execution of complex movement (Morris, 2000), e.g. turning may provoke freezing; it is worse during the simultaneous performance of more than one task, e.g. walking and talking (Bloem, et al., 2001). Akinesia also describes the poverty of movement seen in Parkinson’s disease, e.g. in facial expression or arm swing during walking (Berardelli, et al., 2001). |
| Hypokinesia | Small amplitude movements, e.g. producing micrographia, i.e. small writing (Berardelli, et al., 2001) or slowed movement. |
| Rigidity | Increased resistance to passive movement, bidirectional, not velocity dependent and does not involve hyperexcitable tendon reflexes. Resistance felt throughout movement is referred to as ‘lead pipe’ rigidity. When a tremor is superimposed on rigidity it is termed ‘cog-wheel’ rigidity (Sanger, et al., 2003). |
| Resting tremor | See Table 14.9 |
Ataxia
Ataxia is a general term used to describe the decomposition of movement (Rothwell, 1994). Ataxia is characterized by errors in the rate, range, direction and force of movement (Ada & Canning, 2009) and, in general terms, movement is characterized as being uncoordinated. Ataxia is the principle symptom of cerebellar disorder, but is also noted in sensory ataxia (Lindsay & Bone, 2004). Ataxia presents with various movement disorders, see Table 14.7 and Chapter 5 on MS.
| Dysmetria | Excessive movement or overshooting (hypermetria) or a deficient extent of movement or undershooting (hypometria). Seen as an inaccurate amplitude of movement and misplaced force, reflecting impaired timing of force generation. |
| Rebound | Problem with braking of movement. Demonstrated by asking the individual to flex the elbow isometrically against the examiner’s resistance. When resistance is suddenly released, the person is unable to stop the resultant movement, the limb overshoots and rebounds excessively. |
| Dysdiadochokinesia | Difficulty performing rapidly alternating movements, e.g. pronation and supination, which worsens with repeated attempts. |
| Tremor | See Table 14.9 intention tremor |
| Dyssynergia | Lack of coordination between agonists and antagonists and other synergists resulting in absence of smooth sequential performance of muscle action, e.g. heel to shin test. |
| Hypotonia | Diminished resistance to passive movement, although the mechanism behind this remains unclear. |
(From Carr J, Shepherd R. Neurological Rehabilitation: Optimising Motor Performance. Oxford, Butterworth Heinemann, 1998. Reprinted with permission of Elsevier Ltd.)
A systematic review of physiotherapy for people with cerebellar ataxia (Martin et al., 2009) concluded there is modest evidence for the effectiveness of physiotherapy to improve gait, trunk control and activity limitations. Further guidance by Cassidy et al. (2009) on physiotherapy interventions can be found at www.ataxia.org.uk. In vestibular ataxia, habituation exercises should be used, and can lead to a decrease in symptoms (Herdman & Whitney, 2000; see also Ch. 13). In sensory ataxia, the use of compensation strategies for function (Figure 14.7) and advice are essential to prevent injury or skin damage.
Other movement disorders and alterations in muscle tone
This section describes and provides definitions for other movement disorders and alterations in muscle tone that the physiotherapist may see infrequently, but nonetheless will need to be aware of and be able to identify (Tables 14.8, 14.9). Physiotherapy will have no direct effect on these impairments, but interventions should focus on preventing secondary complications.
| Dyskinesia | An umbrella term that means difficulty moving (see Appendix 1, Glossary) |
| Myoclonus | Brief, shock like jerks of a limb or body parts that may be restricted to one part of the body (focal myclonus) or generalized (generalized myoclonus) (Delgado & Albright, 2003) |
| Ballismus | From the Greek meaning jumping about. Violent, large amplitude involuntary, flailing movement of the limbs, sometimes affecting one side of the body (hemiballismus) (Delgado & Albright, 2003) |
| Chorea | From the Greek khoreia meaning dance. An involuntary hyperkinetic movement disorder characterized by random, quick jerks that move from one joint to another (Delgado & Albright, 2003). A feature of Huntington’s disease (see Ch. 7). |
| Dystonia | Sustained contraction of muscle groups, resulting in twisting movements and unusual postures (Albanese, et al., 2006). Classification of dystonia is made by: (a) cause, e.g. whether dystonia is the only clinical sign or combined with other neurological features; (b) age at onset (early or late); and (c) distribution (e.g. focal, generalized). Treatment is primarily pharmacological (e.g. Botulinum toxin) or surgical (e.g. deep brain stimulation) (Albanese, et al., 2006) |
| Tremor | See Table 14.9 for detailed definitions. |
Table 14.9 Definitions of tremor (Deuschl, et al., 1998)
| Tremor | A rhythmic, involuntary movement of a limb or body part, classified into the following categories. |
| Resting tremor | Occurs at rest when the limb is relaxed and fully supported, e.g. Parkinson’s disease and is known as ‘pill-rolling’. |
| Action tremors: occur during movement, i.e. produced by voluntary contraction of muscle | Postural tremor: occurs when voluntarily maintaining a position against gravity, e.g. holding an arm out straight. |
| Isometric tremor: tremor as a result of muscle contraction against a solid stationary object, e.g. making a fist. | |
| Kinetic tremor: occurs during any type of voluntary movement, further subdivided into: |
Reprinted with permission of John Wiley & Sons.
Case histories
Ada L., Canning C.G. Common motor impairments and their impact on activity. In: Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone, Elsevier; 2009:73-93.
Ada L., Dorsch S., Canning C. Strengthening interventions increase and improve activity after stroke: a systematic review. Aust. J. Physiother.. 2006;52:241-248.
Ada L., O’Dwyer N., O’Neill E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: an observational study. Disabil. Rehabil.. 2006;28:891-897.
Albanese A., Barnes M.P., Bhatia K.P., et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur. J. Neurol.. 2006;13:433-444.
Allison R., Evans P.H., Kilbride C. Secondary prevention of stroke: using the experiences of patients and carers to inform the development of an educational resource. Fam. Pract.. 2008;25:355-361.
Ashford S., Turner-Stokes L. Goal attainment for spasticity management using botulinum toxin. Physiother. Res. Int.. 2006;11:24-34.
Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practioner. 1964;192:540-542.
Baker K., Cassidy E., Rone-Adams S. Therapeutic standing for people with multiple sclerosis: Eficacy and feasibility. Int. J. Ther. Rehabil.. 2007;14:104-109.
Bale M., Strand L.I. Does functional strength training of the leg in subacute stroke improve physical performance? A pilot randomized controlled trial. Clin. Rehabil.. 2008;22:911-921.
Bakhtiary A.H., Fatemy E. Does electrical stimulation reduce spasticty after stroke? A randomized controlled study. Clin. Rehabil.. 2008;22:418-425.
Barnard P., Dill H., Eldredge P., et al. Reduction of hypertonicity by early casting in a comatose head-injured individual. A case report. Phys. Ther.. 1984;64:1540-1542.
Barnes M.P. An overview of the clinical management of spasticity. In: Barnes M.P., Johnson G.R., editors. Upper motor neurone syndrome and spasticity: clinical management and neurophysiology. Cambridge: Cambridge University Press; 2008:1-8.
Barrett J.A., Watkins C., Plant R., et al. The COSTAR wheelchair study: a two-centred pilot study of self-propulsion in a wheelchair in early stroke rehabilitation. Clin. Rehabil.. 2001;15:32-41.
Berardelli A., Rothwell J.C., Thompson P.D., Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131-2146.
Bloem B.R., Valkenburg V.V., Slabbekoorn M., van Dijk J.G. The multiple task test. Strategies in Parkinson’s disease. Exp. Brain Res.. 2001;137:478-486.
Bobath B. Adult Hemiplegia Evaluation and Treatment. Oxford: Butterworth Heinemann, 1990.
Bohannon R.W. Tilt table standing for reducing spasticity after spinal cord injury. Arch. Phys. Med. Rehabil.. 1993;74:1121-1122.
Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther.. 1987;67:206-207.
Bovend’Eerdt T.J., Newman M., Barker K., et al. The Effects of Stretching in Spasticity: A Systematic Review. Arch. Phys. Med. Rehabil.. 2008;89:1395-1406.
Boyd R.N., Ada L. Physiotherapy management of spasticity. In: Barnes M.P., Johnson G.R., editors. Upper motor neurone syndrome and spasticity: clinical management and neurophysiology. Cambridge: Cambridge University Press; 2008:79-98.
Brouwer B., de Andrade V.S. The effects of slow stroking on spasticity in patients with multiple sclerosis; a pilot study. Physiother. Theory Pract.. 1995;11:13-21.
Brown P. Pathophysiology of spasticity – editorial. J. Neurol. Neurosurg. Psychiatry. 1994;57:773-777.
Brown R.A., Holdsworth L., Leslie G.C., et al. The effects of time after stroke and selected therapeutic techniques on quadriceps muscle tone in stroke patients. Physiother. Theory Pract.. 1993;9:131-142.
Burke D. Spasticity as an adaptation to pyramidal tract injury. Waxman S.G., editor. Functional Recovery in Neurological Disease, vol. 47. New York: Ravenpress. 1988:401-423. Advances in Neurology
Canning C.G., Ada L., Adams R., O’Dwyer N.J. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin. Rehabil.. 2004;18:300-308.
Carey J.R., Burghardt T.P. Movement dysfunction following central nervous system lesions: a problem of neurologic or muscular impairment? Phys. Ther.. 1993;73:538-547.
Carr J.H., Shepherd R.B., Nordholm L., et al. Investigation of a new motor assessment scale for stroke patients. Phys. Ther.. 1985;65:175-176.
Carr J., Shepherd R. Neurological Rehabilitation: Optimising Motor Performance. Oxford: Butterworth Heinemann, 1998.
Carr J., Shepherd R. Stroke Rehabilitation: guidelines for exercise and training to optimise motor skill. Oxford: Butterworth Heinemann, 2003.
Carter P., Edwards S. General principles of treatment. In: Edwards S., editor. Neurological Physiotherapy. London: Churchill Livingstone; 2002:121-153.
Cassidy E., Kilbride C., Holland A. Guidance for Physiotherapy Intervention for People with Ataxia. 2009. Available from www.ataxia.org.uk
Champion J., Barber C. Recovery of the upper limb. In: Meadows L., Raine S., Lynch-Ellerington M., editors. Bobath Concept: Theory and Clinical Practice in Neurological Rehabilitation. Oxford: Wiley-Blackwell, 2009.
Chartered Society of Physiotherapy. Guidance on Manual Handling in Physiotherapy, third ed. London: The Chartered Society of Physiotherapy, 2008.
Cheng P.T., Chin-Man W., Chai-Ying C. Effects of visual feedback rhythmic weight-shift training on hemiplegic stroke patients. Clin. Rehabil.. 2004;18:747-753.
Childers M.K., Biswass S.S., Petroski G., et al. Inhibitory casting decreases a vibratory inhibition index of the h reflex in the upper limb. Arch. Phys. Med. Rehabil.. 1999;80:714-716.
Chua K.S.G., Kong K.H. Acquired heterotopic ossification in the settings of cerebral anoxia and alternative therapy: two cases. Brain Inj.. 2003;17:535-544.
Collins F. A practical guide to wheelchair cushions. Int. J. Ther. Rehabil.. 2007;14:557-561.
Cook B. Hydrotherapy. In: Pope P.M., editor. Severe and Complex Neurological Disability. Management of the Physical Condition. Edinburgh: Butterworth Heinemann Elsevier; 2007:216-229.
Cooper C., Evidente V.G.H., Hentz J.G., et al. The effect of temperature on hand function in patients with tremor. J. Hand Ther.. 2000;13:276-288.
Cornall C. Self-propelling wheelchairs: the effects on spasticity in hemiplegic patients. Physiother. Theory Pract.. 1991;7:13-21.
Cott C., Finch E. Goal setting in physical therapy practice. Physiother. Canada. 1990;43:19-22.
Counsell C., Dennis M., McDowall M. Prediciting fucntional outcome in acute stroke: comparison of a simple six variable model with other predictive systems and informal clinical prediciton. J. Neurol. Neurosurg. Psychiatry. 2004;75:401-405.
Courts N.F., Newton A.N., McNeal L.J. Husbands and wives living with multiple sclerosis. J. Neurosci. Nurs.. 2005;37:20-27.
Cramp M.C., Greenwood R.J., Gill C., et al. Low intensity strength training for ambulatory stroke patients. Disabil. Rehabil.. 2006;28:883-889.
Davidson I., Waters K. Physiotherapists working with stroke patients: a national survey. Physiotherapy. 2000;86:69-80.
Dawes H., Bateman A., Wade D., et al. High intensity cycling exercise after stroke: a single case study. Clin. Rehabil.. 2000;14:570-573.
Dawson J., Thornton H. Can Patients with Unilateral Neglect following Stroke drive Electrical Powered Wheelchairs? Br. J. Occup. Ther.. 2003;66:49-54.
De Jong L.D., Nieuwboer A., Aufdemkampe G. Contracture preventive positioning of the hemiplegic arm in subacute stroke patients: a pilot randomized controlled trial. Clin. Rehabil.. 2006;20:656-667.
Delagdo R.M., Albright A.L. Movement disorders in children: definitions, classifications and grading systems. J. Child Neurol.. 2003;18:S1-S8.
Denny Brown D. The Cerebral Control of Movement. Liverpool: Liverpool University Press. 1998.Sheean G., editor. Spasticity Rehabilitation. London: Churchill Communications Europe Limited, 1966;287-295. cited in Sheean, G. 1998 Pathophysology of Spasticity.
Deuschl G., Bain P., Brin M., an Ad Hoc Scientific Committee. Consensus statement of the movement disorder society on tremor. Mov. Disord.. 1998;13(Suppl. 3):2-23.
Devitt R., Chau B., Jutai J.W. The Effect of Wheelchair Use on the Quality of Life of Persons with Multiple Sclerosis. Occup. Ther. Health Care. 2004;17:63-79.
Dewey A., Rice-Oxley M., Dean T. A Qualitative Study Comparing the Experiences of Tilt-in-Space Wheelchair Use and Conventional Wheelchair Use by Clients Severely Disabled with Multiple Sclerosis. Br. J. Occup. Ther.. 2004;67:65-74.
de Wit D.C., Buurke J.H., Nijlant, et al. The effect of an ankle-foot orthosis on walking ability in chronic stroke patients: a randomized controlled trial. Clin. Rehabil.. 2004;18:550-557.
Donnellan C.P., Shanley J. Comparison of the effect of two types of acupuncture on quality of life in secondary progressive multiple sclerosis: a preliminary single randomised controlled trial. Clin. Rehabil.. 2008;22:195-205.
Duncan P., Studenski S., Richards L., et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173-2180.
Edwards S., Charlton P. Splinting and the use of orthoses in the management of patients with neurological disorder. In: Edwards S., editor. Neurological Physiotherapy. London: Churchill Livingstone; 2002:219-254.
Fess E., Gettle K.S., Philips C.A., et al. Splinting for Patients with Upper Extremity Spasticity. In: Fess E., Gettle K.S., Philips C.A., et al, editors. Hand and Upper Extremity Splinting. Principles and Methods. third ed. Missouri: Elsevier Mosby; 2005:517-536.
Farmer S., James M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil. and Rehabil.. 2001;23:549-558.
Feys P., Helsen W., Liu X., et al. Effects of peripheral cooling on intention tremor in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2005;76:373-379.
Fitzferald D., Stokes M. Muscle imbalance in neurological conditions. In: Stokes M., editor. Physical Management in Neurological Rehabilitation. second ed. Edinburgh: Elsevier Mosby; 2004:501-516.
Flansbjer U.B., Miller M., Downham D., et al. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J. Rehabil. Med.. 2008;40:42-48.
Fox P., Richardson J., McInnes B., et al. Effectiveness of a Bed Positioning Program for Treating Older Adults With Knee Contractures Who Are Institutionalised. Phys. Ther.. 2000;80:363-372.
Fujiwara T., Liu M., Hase K., et al. Electrophysiological and clinical assessment of a simple wrist-hand splint for patients with chronic spastic hemiparesis secondary to stroke. Electromyogr. Clin. Neurophysiol.. 2004;44:423-429.
Funk D., Swank A.M., Adams K.J., et al. Effects of moist heat pack application over static stretching on hamstring flexibility. J. Strength Cond. Res.. 2001;15:123-126.
Gemperline J.J., Allen S., Walk D., Rymer W.Z. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101-1114.
Goldspink G., Williams P.E. Muscle fibre and connective tissue changes associated with use and disuse. In: Ada L., Canning C., editors. Key Issues in Neurological Physiotherapy. London: Butterworth Heinnemann; 1990:20-50.
Gordon J. Spinal Mechanisms of Motor Coordination. In: Kendel E.R., Schwartz J.H., Jessell T.M., editors. Principles of Neuroscience. Connecticut: Appleton Lange Norwalk; 1991:581-595.
Gracies J.M., Fitzpatrick R., Wilson L., et al. Short term effects of dynamic Lycra splints on upper limb in hemiplegic patients. Arch. Phys. Med. Rehabil.. 2000;81:1547-1555.
Griffin A., Bernhardt J. Strapping the hemiplegic shoulder prevents development of pain during rehabilitation: a randomized controlled trial. Clin. Rehabil.. 2006;20:287-295.
Gustafsson L., McKenna K. A programme of static positional stretches does not reduce hemiplegic shoulder pain or maintain shoulder range of motion – a randomized controlled trial. Clin. Rehabil.. 2006;20:277-286.
Hanger H.C., Whitewood P., Brown G., et al. A randomised controlled trial of strapping to prevent post stroke shoulder pain. Clin. Rehabil.. 2000;14:370-380.
Hara Y., Ogawa S., Muraoka Y. Hybride power-assisted functional electrical stimulation to improve hemiparetic upper-extremity function. Am. J. Phys. Med. Rehabil.. 2006;85:977-985.
Hardy M., Woodall W. Therapeutic effects of heat, cold and stretch on connective tissue. J. Hand Ther.. 1998;11:148-156.
Herdman S.J., Whitney S.L. Assessment and management of central vestibular disorders. In Herdman S., editor: Vestibular Rehabilitation, second ed, Philadelphia: FA Davis, 2000.
Hesse S. Treadmill training with partial body weight support after stroke. A review. NeuroRehabilitation. 2008;23:55-65.
Hill J. The effects of casting on upper extremity motor disorders after brain injury. Am. J. Occup. Ther.. 1994;48:219-223.
Hiraoka K. Rehabilitation effort to improve upper limb extremity function in post-stroke patients: a meta-analysis. J. Phys. Ther.. 2001;13:5-9.
Holliday R.C., Antoun M., Playford D. A Survey of Goal-Setting Methods Used in Rehabilitation. Neurorehabil. Neural Repair. 2005;19:227-231.
Ianieri G., Ranieri M., Marvulli R., et al. The approach to rehabilitation in post-traumatic spasticity. Abstracts Toxins. 2008;51:29.
Intercollegiate Stroke Working Party. National clinical guideline for stroke, third ed. London: Royal College of Physicians, 2008.
Jackson H.J. Evolution and dissolution of the nervous system speech and various papers, addresses and lectures. Taylor J., Holmes G., Walshe F.M.R., editors. John Hughlings Jackson Selected Writings, vol. 2. Arts and Boeve Nijmegen, 1958.
Jiang J.-Y., Gao G.-Y., Li W.-P., et al. Early Indicators of Prognosis in 846 Cases of Severe Traumatic Brain Injury. J. Neurotrauma. 2002;19:869-874.
Johansson B.B. Brain plasticity and stroke rehabilitation: the Willis lecture. Stroke. 2000;31:223-230.
Johnstone M. Restoration of Normal Movement after Stroke. Edinburgh: Churchill Livingstone, 1995.
Katalinic O.M., Harvey L.A., Herbert R.D., et al. Stretch interventions for contractures. Cochrane Database Syst. Rev.. (4):2008. CD 007455
Kesiktas N., Erdogan N., Gulsen G., et al. The Use of Hydrotherapy for the Management of Spasticity. Neurorehabil. Neural Repair. 2004;18:268-273.
Kilbreath S.L., Davis G.M. Cardiorespiratory fitness after stroke. In: Refshauge K., Ada L., Ellis E., editors. Science-Based Rehabilitation. Theories into Practice. Edinburgh: Elsevier Butterworth Heinemann; 2005:131-158.
Kilbride C., Meyer J., Flatley M., et al. Stroke Units: the implementation of a complex intervention. Educational Action Research. 2005;13:479-503.
King T.I. Plaster splinting as a means of reducing elbow flexor spasticity: a case study. Am. J. Occup. Ther.. 1982;36:671-673.
Kiresuk T., Sherman R.. Goal attainment scaling: a general method of evaluating comprehensive mental health programmes. Community Ment. Health J., 4. 1968: 443-453. cited in Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin. Rehabil.. 2009;23:362-370.
Kleim J.A., Jones T.A. Principles of experience-dependent neural plasticity: implications for rehabilitation. American Journal of Speech Hearing and Language Research. 2008;51:225-239.
Knight L.A., Thornton H.A., Turner-Stokes L. Management of neurogenic heterotrophic ossification. Physiotherapy. 2003;89:471-477.
Kofler M., Quirbach E., Schauer R., et al. Limitations of Intrathecal Baclofen for Spastic Hemiparesis Following Stroke. Neurorehabil. Neural Repair. 2009;23:26-31.
Krause P., Szecsi A., Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin. Rehabil.. 2008;22:627-634.
Lance J.W. Symposium synopsis. In: Feldman R.G., Young R.R., Koella W.P., editors. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 1980:485-494.
Landau W.M. Spasticity: what is it? What is it not? In: Feldman R.G., Young R.R., Koella W.P., editors. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 1980:17-24.
Landau W.M. Clinical Neuromythology II, parables of palsy pills and PT pedagogy. A spastic dialectic. Neurology. 1988;38:1496-1499.
Lannin N.A., Cusick A., McCluskey A., et al. Effects of splinting on wrist contracture after stroke: a randomized controlled trial. Stroke. 2007;38:111-116.
Lannin N.A., Novak I., Cusick A. A systematic review of the upper extremity casting for children and adults with central nervous system motor disorders. Clin. Rehabil.. 2007;21:963-976.
Lee M.J., Kilbreath S.L., Singh M.F., et al. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise-controlled trial. J. Am. Geriatr. Soc.. 2008;56:976-985.
Lennon S., Bassile C. Guiding principles for neurological physiotherapy. In: Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone Elsevier; 2009:97-111.
Levin M.F., Kleim J.A., Wolf S.L. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? Neurorehabil. Neural Repair. 2009;23:313-319.
Lindsay K.W., Bone I. Neurology and Neurosurgery Illustrated, fourth ed. Edinburgh: Churchill Livingstone, 2004.
Livingstone L. Coping with labour: what are the options? In: Sapsford R., Bullock-Saxton J., Markwell S., editors. Women’s Health. A Textbook for Physiotherapists. London: WB Saunders, 1998.
Lotze M., Braun C., Birbaumer N., et al. Motor learning elicited by voluntary drive. Brain. 2003;126:866-872.
Lynch D., Ferraro M., Krol J., et al. Continuous passive motion improves shoulder joint integrity following stroke. Clin. Rehabil.. 2005;19:594-599.
Macfarlane A., Thornton H.A. Solving the problem of contractures – throw out the recipe book? Physiother. Res. Int.. 1997;2:1-6.
Markham C.H. Vestibular control of muscular tone and posture. Can. J. Neurol. Sci.. 1987;14:493-496.
Marossezky J.E., Gurka J.A., Baguley I.J. Splinting Poststroke: the Jury Is Still Out. Stroke. 2008;39:e46.
Martin C.L., Tan D., Bragge P. Effectiveness of physiotherapy for adults with cerebellar dysfunction: a systematic review. Clin. Rehabil.. 2009;23:15-26.
Massion J. Postural control system. Curr. Opin. Neurobiol.. 1994;4:877-887.
Mayston M. Problem solving in neurological physiotherapy – setting the scene. In: Edwards S., editor. Neurological Physiotherapy. London: Churchill Livingstone; 2002:3-19.
McInnes E., Bell-Syer S.E., Dumville J.C., et al. Support surfaces for pressure ulcer prevention. Cochrane Database Syst. Rev.. 4, 2008. CD001735
Meadows L., Raine S., Lynch-Ellerington M., editors. Bobath Concept: Theory and Clinical Practice in Neurological Rehabilitation. Oxford: Wiley-Blackwell, 2009.
Meadows L., Williams J. An Understanding of Functional Movement as a Basis for Clinical Reasoning. In: Meadows L., Raine S., Lynch-Ellerington M., editors. Bobath Concept: Theory and Clinical Practice in Neurological Rehabilitation. Oxford: Wiley-Blackwell, 2009.
Mehrholz J., Wagner K., Meiβner M., et al. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin. Rehabil.. 2005;19:751-759.
Miller L., Mattison P., Paul L., et al. The effects of transcutaneous electrical nerve stimulation (TENS) on spasticity in multiple sclerosis. Clin. Rehabil.. 2007;13:527-533.
Moreland J.D., Thompson M.A., Fuoco A.R. Electromyographic biofeedback to improve lower extremity function after stroke; a meta-analysis. Arch. Phys. Med. Rehabil.. 1998;79:134-140.
Morris M. Movement disorders in peoplw with Parkinson’s disease: a model for physical therapy. Phys. Ther.. 2000;80:578-597.
Morris S.L., Dodd K.J., Morris M.E. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin. Rehabil.. 2004;18:27-39.
Mortenson P.A., Eng J. The Use of Casts in the Management of Joint Mobility and Hypertonia Following Brain Injury in Adults. A Systematic Review. Phys. Ther.. 2003;83:648-658.
Moseley A., Hassett L.M., Leung J., et al. Serial casting versus positioning for the treatment of elbow contractures in adults with traumatic brain injury: a randomized controlled trial. Clin. Rehabil.. 2008;22:406-417.
Mukherjee M., McPeak L.K., Redford J.B., et al. The effect of electro-acupuncture on spasticity of the wrist joint in chronic stroke survivors. Arch. Phys. Med. Rehabil.. 2007;88:159-166.
National Institute for Health and Clinical Excellence. Functional electrical stimulation for drop foot of central origin. 2009. Accessed 22.04.09 from http://www.nice.org.uk/nicemedia/pdf/IPG278Guidance.pdf
Newman M.A., Dawes H., van den Berg M., et al. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: a pilot study. Mult. Scler.. 2007;13:113-119.
Nixon J., Nelson E.A., Cranny G., et al. Pressure relieving support surfaces: a randomised evaluation. Health Technol. Assess.. 2006;10:iii-iiv. ix–ix, 1–163
O’Connor R., Smith R. Wheelchair and Special Seating for Neurological Conditions. Advances in Clinical Neurosciences and Rehabilitation. 2008;8:18-22.
O’Dwyer N., Ada L. Reflex hyperexcitability and muscle contracture in relation to spastic hypertonia. Curr. Opin. Neurol.. 1996;9:451-455.
O’Dwyer N., Ada L., Neilson P. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737-1749.
Page S.J., Szaflarski J.P., Eliassen J.C., et al. Cortical Plasticity Following Motor Skill Learning During mental Practice in Stroke. Neurorehabil. Neural Repair. 2009;23:382-388.
Pandyan A.D., Cameron M., Powell J., et al. Contractures in the post-stroke wrist: a pilot study of its time course of development and its association with upper limb recovery. Clin. Rehabil.. 2003;17:88-95.
Pandyan A.D., Johnson G.R., Price C.I.M., et al. A review of the properties and limitations of the Ashworth and modified Ashworth scales as measures of spasticity. Clin. Rehabil.. 1999;13:373-383.
Pang M.Y.C., Eng J.J., Dawson A.S., et al. A community-based Fitness and Mobility Exercise (FAME) program for older adults with chronic stroke: A randomized controlled trial. J. Am. Geriatr. Soc.. 2005;53:416-423.
Patrick E., Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin. Rehabil.. 2006;20:173-182.
Peterson C. Exercise in 94 degree F water for a patient with multiple sclerosis. Phys. Ther.. 2001;81:1049-1058.
Pizzi A., Carlucci G., Falsini C., et al. Application of a volar splint in poststroke spasticity of the upper limb. Arch. Phys. Med. Rehabil.. 2005;86:1855-1859.
Pohl M., Mehrholz J. Immediate effects of an individually designed functional ankle-foot orthosis on stance and gait in hemiparetic patients. Clin. Rehabil.. 2006;20:324-330.
Pomeroy V., Mickelborough J., Hill E., et al. A hypothesis: self propulsion in a wheelchair early after stroke might not be harmful. Clin. Rehabil.. 2003;17:174-180.
Pope P.M. Severe and Complex Neurological Disability. Management of the Physical Condition. Edinburgh: Butterworth Heinemann Elsevier, 2007.
Price C.I.M., Pandyan A.D. Electrical stimulation for preventing and treating post stroke shoulder pain: a systematic Cochrane review. Clin. Rehabil.. 2001;15:5-19.
Proske U., Wise A.K., Gregory J.E. The role of muscle receptors in the detection of movements. Prog. Neurobiol.. 2000;60:85-96.
Qunitern J., Immisch I., Albrecht H., et al. Influence of visual and propriocpetive afferences on upper limb ataxian patients with mulitple sclerosis. J. Neurol. Sci.. 1999;163:61-69.
Reynolds F. Patient education and empowerment. In: Reynolds F., editor. Communication and Clinical Effectiveness in Rehabilitation. Edinburgh: Elsevier Butterworth Heinemann; 2005:169-201.
Reynolds F. Teamwork in the rehabilitation setting. In: Reynolds F., editor. Communication and Clinical Effectiveness in Rehabilitation. Edinburgh: Elsevier Butterworth Heinemann; 2005:205-225.
Richards L.G., Stewart K.C., Woodbury M.L., et al. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMA and fMRI evidence. Neuropsychologia. 2008;46:3-11.
Robichaud J.A., Agostinucci J. Air–splint pressure effect on soleus muscle alpha motoneuron reflex excitability in subjects with spinal cord injury. Arch. Phys. Med. Rehabil.. 1996;77:778-782.
Robinson W., Smith R., Aung O., et al. No difference between wearing a night splint and standing on a tilt table I preventing ankle contracture early after stroke: a randomised trial. Aust. J. Physiother.. 2008;54:33-38.
Rosche J., Rub B., Niemann-Delius B., et al. Effects of physiotherapy on F-wave amplitudes in spasticity. Electromyogr. Clin. Neurophysiol.. 1996;36:509-511.
Rosenfalck A., Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J. Neurol. Neurosurg. Psychiatry. 1980;43:907-916.
Rothwell J.C. Control of Human Voluntary Movement, second ed. London: Chapman & Hall, 1994.
Royal College of Physicians. British Society of Rehabilitation Medicine. Chartered Society of Physiotherapy et al. Spasticity in adults: management using botulinum toxin. National Guideline. London: Royal College of Physicians, 2009.
Sackley C.M., Lincoln N.B. Single-blind randomised controlled trial of visual feedback after stroke: effects on stance and symmetry and function. Disabil. Rehabil.. 1997;19:536-546.
Sanger T.D., Delgardo M.R., Gaebler-Spira D., et al. Classification and definition of disorders casuing hypertonia in childhood. Paediatrics. 2003;111:e89-e97.
Schmit B.D., Dewald J.P., Rymer W.Z. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch. Phys. Med. Rehabil.. 2000;81:269-278.
Sheean G. Pathophysiology of Spasticity. In: Sheean G., editor. Spasticity Rehabilitation. London: Churchill Communications Europe Limited; 1998:287-295.
Sheean G. The Pathophysiology of Spasticity. Eur. J. Neurol.. 2002;9(Suppl. 1):3-9.
Sheean G. Neurophysiology of Spasticity. In: Barnes M.P., Johnson G.R., editors. Upper Motor Neurone Syndrome and Spasticity: Clinical Management and Neurophysiology. Cambridge: Cambridge University Press; 2008:9-63.
Shumway-Cook A., Woollacott M. Motor control: translating research into clinical practice, third ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
Singer B., Dunne J., Allison G. Clinical evaluation of hypertonia in the triceps surae muscles. Phys. Ther. Rev.. 2001;6:71-80.
Skinner A., Thomson A. Aquatics Therapy and the Halliwick Concept. Exceptional Parent. 2008;38:76-77.
Smith J., Forster A., House A., et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst. Rev.. (2):2008. CD001919
Snow B.J., Tsui J.K.C., Bhatt M.H., et al. Treatment of spasticity with Botulinum toxin: a double blind study. Ann. Neurol.. 1990;28:512-515.
Sonde L., Kalimo H., Viitanen M. Stimulation with high frequency TENS – effects on lower limb spasticity after stroke. Advances in Physiotherapy. 2000;2:183-187.
Stack E. The patient with degenerative disease: Parkinson’s disease. In: Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone, Elsevier; 2009:175-190.
Stoeckmann T. Casting for the person with spasticity. Top. Stroke Rehabil.. 2001;8:27-35.
Stokes E. Outcome measurement. In: Stokes M., Lennon S., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone Elsevier; 2009:191-201.
Sullivan J.E., Headman L.D. Effects of home-based sensory and motor amplitude electrical stimulation on arm dysfunction in chronic stroke. Clin. Rehabil.. 2007;21:142-150.
Tardieu C., Lespargot A., Tabary C., et al. How long must the soleus muscle be stretched each day to prevent contracture? Dev. Med. Child Neurol.. 1988;30:3-10.
Tardieu G., Shentoub S., Delarue R. A la recherche d’une technique de mesure de la spasticite. Review Neurol. 1954;91:143-144.
Taylor E.W., Morris D., Shaddeau S., et al. Effects of water walking on hemiplegic gait. Aquatic physical therapy. Journal of the Aquatic Section of the American Physical Therapy Association. 1993;1:10-13.
Thompson A.J., Jarrett L., Lockley L., et al. Clinical management of spasticity. J. Neurol. Neurosurg. Psychiatry. 2005;76:459-463.
Thornton H., Kilbride C. Physical management of abnormal tone and movement. In: Stokes M., editor. Physical Management in Neurological Rehabilitation. second ed. Edinburgh: Elsevier Mosby; 2004:431-450.
Tsai K.H., Chun-Yu Y., Chang H.Y., et al. Effects of a Single Session of Prolonged Muscle Stretch on Spastic Muscle of Stroke Patients. Proc. Natl. Sci. Counc. Repub. China B. 2001;25:76-81.
Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin. Rehabil.. 2009;23:362-370.
Tyson S.F., DeSouza L.H. A clinical model for the assessment of posture and balance in people with stroke. Disabil. Rehabil.. 2003;25:120-126.
Tyson S.F., Rogerson L. Assistive walking devices in nonambulant patients undergoing rehabilitation after stroke: the effects on functional mobility, walking impairments and patients’ opinion. Arch. Phys. Med. Rehabil.. 2009;90:475-479.
Tyson S.F., Thornton H.A. The effect of a hinged ankle foot orthosis on hemiplegic gait: objective measures and users’ opinions. Clin. Rehabil.. 2001;15:53-58.
Van Peppen R.P.S., Kortsmit M., Lindeman E., et al. Effects of Visual Feedback Therapy on Postural Control in Bilateral Standing after Stroke: A Systematic Review. J. Rehabil. Med.. 2006;38:3-9.
Vattanaslip W., Ada L., Crosbie J. Contribution of thixotrophy, spasticity, and contracture to ankle stiffness after stroke. J. Neurol. Neurosurg. Psychiatry. 2000;69:34-39.
Wade D.T. Measurement in neurological rehabilitation. Oxford: Oxford University Press, 1992.
Watson M.J., Crosby P., Matthew M. An evaluation of the effects of a dynamic lycra splint orthoses on arm function in a late stage patient with acquired brain injury. Brain Inj.. 2007;21:753-761.
White L.J., Dressendorfer R.H. Exercise and Multiple Sclerosis. Sports Med.. 2004;34:1077-1100.
Wiles C.M., Newcombe R.G., Fuller K.J., et al. Use of videotape to assess mobility in a controlled randomized crossover trial of physiotherapy in chronic multiple sclerosis. Clin. Rehabil.. 2003;17:256-263.
Williams P.E. Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann. Rheum. Dis.. 1990;49:3-16.
Woodford H., Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst. Rev.. (2):2007. CD004585
World Health Organization. International Classification of Functioning and Disability. Geneva: WHO, 2001. Available online at http:/www.who.int/classification/icf
Wu H.M., Tang J.L., Lin X.P., et al. Acupuncture for stroke rehabilitation. Cochrane Database Syst. Rev.. (3):2006. CD004131
Zhang S.H., Liu M., Asplund K., et al. Acupuncture for acute stroke. Cochrane Database Syst. Rev.. (2):2005. CD003317