Chapter 18 Physical activity and exercise in neurological rehabilitation
Terminology and definitions
 Performance-related fitness: Components of fitness necessary for optimal performance at work or sport (Bouchard et al., 2007).
Performance-related fitness: Components of fitness necessary for optimal performance at work or sport (Bouchard et al., 2007). Health-related fitness: Components of fitness that benefit from a physically active lifestyle and relate to health (Bouchard et al., 2007). These include cardiovascular endurance, muscular strength and endurance, flexibility and body composition (American College of Sports Medicine (ACSM), 2006).
Health-related fitness: Components of fitness that benefit from a physically active lifestyle and relate to health (Bouchard et al., 2007). These include cardiovascular endurance, muscular strength and endurance, flexibility and body composition (American College of Sports Medicine (ACSM), 2006).Introduction and context
Engaging in exercise and being physically active has shown substantial health benefits in the general population. There is now overwhelming evidence that active lifestyles reduce rates of hypertension, obesity, stroke, coronary artery disease, some cancers, diabetes and osteoporosis (Blair, 2007; Cluve, 2008). A change in lifestyle from inactivityto activity and participation in exercise is associated with a reduction in disease and reduced premature mortality. This suggests that for most of us it is never too late to start being active and change exercise behaviour because the benefits are achievable at any age (ACSM, 2006).
Individuals with neurological conditions are often challenged by movement difficulties and therefore adopt a more sedentary lifestyle with increasing muscle weakness and cardiovascular deconditioning. This exposes them to all the risk factors associated with inactivity, in addition to the risk factors that contributed to their conditions in the first place. A basic level of fitness is essential for carrying out activities of daily living. For example, light housework and carrying shopping requires approximately 40–50% of peak VO2. Housework of lower workload intensity requires a minimum of 32% of peak VO2 (Arnett et al., 2008). This chapter proposes that, just like the general population, individuals with neurological conditions also benefit from staying active and taking regular exercise. This chapter therefore provides an overview of activity and exercise in these patient groups and outlines the current evidence to support this aspect of patient management. Wherever possible, guidance for exercise prescription will be presented. Aerobic training, resisted muscle strengthening, constraint-induced movement therapy and treadmill training will be discussed in more detail. Also see ‘Exercise and Movement’ in Chapter 12.
Being active with a neurological condition
Most people know that being active has health benefits and yet the majority of the general population is not adequately active to benefit (Marcus et al., 2000). Levels of exercise adoption suggest that this is also true for people with neurological conditions. For example, peak oxygen consumption following stroke has been found to be as low as low as 50% of the capacity of age- and sex-matched sedentary controls (Pang et al., 2006). In many cases it may be the fact that a person has an impairment or disability which contributes to their inactivity. Serious life events, such as being diagnosed with a long-term condition, make it more difficult to adopt or maintain an adequate level of exercise (Oman & King, 2000). It is probably unrealistic to expect our patients to change typical behaviours of exercise after a stroke, acquired brain injury, or being diagnosed with Parkinson’s disease. However, it appears that it is exactly this type of behaviour change that is required in most of our patients if they are to experience what we know are the benefits of exercise. Therefore, we are seriously challenged to identify the barriers to exercise adoption (also see ‘Engagement with falls prevention’ in Ch. 20). We will also need to be able to design more appropriate exercise service models and prescribe specific exercises to meet the goals of the patient, or adapt more general exercise guidance to fit specific conditions.
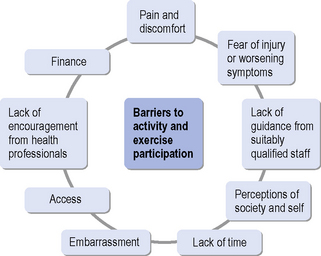
Kang (2007) found that young people with disabilities face many barriers to participating in or maintaining exercise behaviour. Barriers most frequently cited were a lack of time and actual pain and discomfort during exercise. Others were lack of facilities to exercise with peers, adverse weather and misconceptions by non-disabled people about the ability of the patient to take part in exercise. A better understanding of exercise ‘adherence models’ therefore may help service provision in the long term. It has recently been suggested that it is predominantly the intentions from the outset, described as the ‘Theory of Planned Behaviour’ (Yardley & Donovan-Hall, 2007), that determine exercise adherence and, to a lesser extent, symptom experience during exercise and rehabilitation. Very specific support in the form of counselling has also improved exercise adherence (van der Ploeg et al., 2006). A recent study (Elsworth et al., 2009) reported that people with neurological conditions would enjoy being active and taking part in exercise, but that they are mostly put off by embarrassment and the lack of well-educated staff with detailed knowledge of individual conditions. Group participation with people with similar disabilities was preferred and supervision by professionals with appropriate expertise was seen as a priority. This chapter therefore outlines the barriers that can relate to the individual and their personal concerns, the professional and support staff and their expertise, the facilities and transport and the overall service provision (see Figure 18.1).
Overcoming barriers to leading an active life and participating in exercise is a key challenge for rehabilitation therapists and knowledge of reported barriers is the first step in rising to this challenge. The reader is at this stage also reminded that physical activity and participation inexercise fit into the ICF model and framework described by the World Health Organization (WHO, 2001). According to this classification model, barriers to being active and taking part in exercise may well be related to the impairments caused by a particular pathological process. In addition, attitudes of society, inappropriate environments and a lack of adequate transport may make it difficult for some people to take part in regular exercise.
Opening opportunities for participation in exercise is therefore a specific challenge and some recent developments utilizing internet technology may offer alternatives to the more traditional fitness centre or rehabilitation facility. A range of small-scale studies has investigated the feasibility and potential outcome of home-based community exercise supported by internet technology. Often referred to as ‘telehealth’ or ‘telemedicine’, this approach seems appropriate in certain circumstances and may be of particular interest to individuals who live in more rural communities.Van den Berg et al. (2006) investigated the internet-based delivery of a home exercise programme for people with rheumatoid arthritis. Whilst the findings may not directly translate to people with neurological conditions, it is worth considering the lessons learned from this trial. The study compared two internet-based exercise programmes. One programme was individualized to the participant with tailored exercises and e-mail correspondence. The other only contained general exercise advice. The group receiving individualized exercise guidance was significantly better in achieving recommended exercise amounts.Finkelstein et al. (2008) evaluated the acceptability and effectiveness of a home-based telemedicine exercise programme in a small group of people with multiple sclerosis (MS). Support and guidance by a telehealth team over the 12-week exercise period showed significant improvements in walking ability and balance scores. Patients were also very satisfied with the service.
Hartley (2009) investigated the use of an exercise therapy model, largely based on patient self-management. The study showed that a self-management exercise programme for people with early MS was effective in improving gait and quality of life.
Measuring physical activity and fitness in neurological rehabilitation
Measurement and assessment of activity and physical fitness also fits into the ICF classification (WHO, 2001). Therapists are encouraged to choose appropriate assessment tools to capture the ability or difficulty of an individual to take part in physical activities. This may involve selecting either a global activity scale or, where available, a disease-specific measurement. Table 18.1 provides a selection of measurement scales to choose from. Guidance on a variety of rehabilitation measurements has been produced by Ainsworth (2009), Finch et al. (2002), Stokes (2009) and Bowling (2001).
| Activity scale/measurement | Reference | Comment |
|---|---|---|
| Barthel Index | Mahoney and Barthel, 1965 | Has been used in stroke, spinal cord injuries, MS and older people; good reliability in stroke |
| Functional Independence Measure (FIM) | Keith et al., 1987 | Has been used in MS, older people, stroke, head injury, Parkinson’s disease; reliability variable between conditions |
| Rivermead Mobility Index | Collen et al., 1991 | Developed for stroke patients and has shown reliability in this group |
| Baecke Activity Questionnaire | Voorips et al., 1991 | Designed for use in older people; has been used in Parkinson’s disease |
| Physical Activity Questionnaire (IPAQ) | Craig et al., 2003 | The questionnaire can be administered in different forms (e.g. telephone or interview) and has shown reliability |
More recently, a number of authors have evaluated the use of motion sensors in various patient groups, including some with neurological conditions. No comprehensive review on the use of these devices for people with neurological conditions currently exists and therefore there is yet no clear sense of the value of these instruments in neuro-rehabilitation. However, early signs suggest that these relatively simple and inexpensive devices may provide an objective measure of activity and therefore add to the often subjective nature of activity scales or patient recall. The group of motion sensors suggested for this purpose range from simple step counters to a variety of accelerometers. The technology has been evaluated in a group of older people by de Bruin et al. (2008)and their findings suggest that the technology is available and improving in terms of device reliability. Acceptability for the wearer varies between devices and must be considered carefully. Accelerometers have also been used in activity assessment in young people with cerebral palsy (Van der Slot et al., 2007), stroke patients (Hale et al., 2008) and in Parkinson’s disease (Skidmore et al., 2008). Common to all these studies was the relative ease of use and good reliability of measurements.
Measuring physical or health-related fitness requires a set of scales or tools, which will be different from activity measurements. Therapists or researchers are advised to consider carefully the nature of the impairment they may wish to evaluate and also the cost of measurement and the necessary skill required in conducting fitness measurements. A range of measurements and tools are available and Table 18.2 provides a selection of relevant fitness assessment tools. In many instances simple timed walking tests will provide a useful measure of walking-related fitness/ability which can be used to assess, review or screen patients. More complex measurements, particularly those that involve the measurement of metabolic systems, will of course provide an extensive range of information. However, conducting measurements of this nature requires specialist equipment and training, and may not add significantly to the patient assessment information. Therefore, therapists are advised to consider carefully what type of assessment information they require.
Table 18.2 Measurements and tests for physical and health-related fitness
| Exercise/ fitness measurement | Reference | Comment |
|---|---|---|
| Walking tests, e.g. |
Mudge and Stott, 2007
Effects of exercise and physical activity
Evidence suggests that staying active and taking regular exercise has clear health benefits (Blair, 2007). There is now also a growing body of evidence showing that people with neurological conditions can benefit from exercise. There are now growing recommendations to include aspects of exercise training as part of the overall rehabilitation for people following spinal cord injury (Janssen & Hopman, 2005), stroke (Royal College of Physicians, 2004), MS (Rietberg et al., 2004), cerebral palsy (Rogers et al., 2008) and Parkinson’s disease (Skidmore et al., 2008). The effects of exercise are usually described in terms of acute and chronic effects on the cardiorespiratory and neuromuscular system. Table 18.3 briefly summarizes some of these general effects of exercise. For further details, the reader is referred to exercise physiology textbooks, such as McArdle et al. (2006). It is important to note that the response to exercise in people with neurological conditions will vary and therapists are advised to take this into account when prescribing exercise.
| Exercise response and effect | Comment |
|---|---|
| Acute cardiorespiratory response |
HR, heart rate; VO2, maximal oxygen uptake
The effects on the nervous system of exercise and its response have not undergone extensive research. However, it appears that this area would be of particular interest to patients with neurological conditions. A small body of knowledge exists which suggests that there may be effects of exercise which go beyond conditioning of the cardiorespiratory and muscular system. These effects have so far been studied in animal models under a limited number of conditions. These studies suggest that taking part in regular aerobic exercise may have a neuroprotective effect.Smith & Zigmond (2003) investigated this possibility in an animal model of Parkinson’s disease. Their study showed that forced physical activity in rats had a neuroprotective effect, believed to be due to a reduction in oxidative stress. Exercise may be able to slow the cognitive decline seen in Parkinson’s disease and also in Alzheimer’s disease (Dishman et al., 2006). Exercise enhances the calcium transport to the brain which in turn enhances dopamine synthesis. This may explain the potential for neuroprotection or restoration in Parkinson’s disease and dementia (Sutoo & Akiyama, 2003). An intensive 12-week ‘running’ activity in rats after middle cerebral artery occlusion has resulted in reduced infarct volumes, mediated via increased nerve growth factor (Ang et al., 2003). The authors concluded that physical exercise may cause neuroprotection following stroke. These studies are restricted to animal models but they point towards an exciting potential role of exercise in rehabilitation. This role may go beyond increasing fitness and strength for the purpose of improving function and quality of life. Exercise may protect the brain from some chronic diseases, such as Parkinson’s disease. Exercise may also have potential to facilitate brain plasticity following stroke. Basic research and clinical studies suggest that high intensity (i.e. high repetition, velocity and complexity) is a characteristic of exercise that may be important in promoting activity-dependent neuroplasticity of the injured brain (Nudo & Milliken, 1996).
Aerobic exercise training in neurological rehabilitation
Aerobic exercise programmes
Walking, either overground or treadmill, is a task-orientated aerobic exercise programme that emerges from the literature as a firm favourite. Overground programmes have the additional advantage of being easily accessible and of low cost. Ada et al. (2003b) showed that a community-based programme incorporating treadmill training alongside an overground walking programme significantly improved walking speed and walking capacity, although effects on disability were less clear. Trials involving treadmills have also demonstrated improvements ranging from improved gait speed and capacity (Macko et al., 2005) to changes in corticomotor excitablity (Fisher et al., 2008). Further detail and guidance for the use of treadmill training can be seen later in this chapter.
Another common aerobic approach utilizes cycle ergometry with functional and health-related quality of life improvements being demonstrated in a range of conditions including MS (Kileff & Ashburn, 2005; Mostert & Kesselring, 2002); spinal cord injury (Ditor et al., 2003; Hicks et al., 2003); and stroke (Tang et al., 2009). Aquatic exercise programmes are also popular and research has shown improvements in peak workload and function (Chu et al., 2004; Pariser et al., 2006).
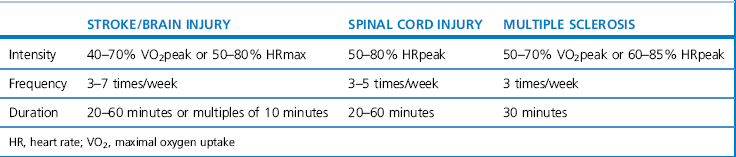
A combination of aerobic exercise, including walking, cycling and swimming, can result in positive treatment outcomes (Pang et al., 2006; Snook & Motl, 2009). Comprehensive guidance for prescribing aerobic exercise in neurological rehabilitation is currently incomplete and not fully based on high-quality evidence. Table 18.4 outlines some guidance for a number of conditions based on Gordon et al. (2004) and the ACSM (2003). The guidance is very broad and must be adapted to the individual after careful assessment.
Sports/exercise classes
People with spinal cord injury participating in sports have reported effects on aspects of health-related quality of life as well as facilitation of community reintegration by, for example, improved social reintegration networks or peer support (Labronici et al., 2000; Tasiemski et al., 2005). It is worth noting that Tasiemski et al. (2000) found that levels of participation in sporting activities decreased significantly after injury in people with spinal cord injury and that barriers to participation in these activities remain a complex issue, as illustrated in Figure 18.1 previously.
Therapists should also consider the use of other exercise classes such as tai chi, yoga or ballroom dancing. In older adults with chronic conditions tai chi appears to be safe and effective in promoting balance control, flexibility and cardiovascular fitness, alongside the psychosocial benefits (Wang et al., 2004). Tai chi has been trialled with people with Parkinson’s disease, but currently there is a lack of robust literature in this area (Lee et al., 2008). More recently yoga has been proposed as one of a range of mind–body therapies that could be of benefit to people with neurological conditions (Wahbeh et al., 2008). Researchers are also turning to innovative options such as dance, e.g. tango or American ballroom, which is showing promise in improving measures of walking, balance and quality of life measures in people with Parkinson’s disease (Hackney & Earhart, 2009).
Virtual reality training
Advances in virtual reality technology mean that devices using computer and gaming technology, such as the Nintendo Wii ®, are now found in many people’s homes. The potential of these types of adjuncts to maximize task-orientated practice and increase energy expenditure are beginning to be explored. The use of games using the Nintendo Wii ®, for example, has shown to increase energy expenditure in a group of asymptomatic participants (Graves et al., 2007; Lanningham-Forster et al., 2009). Research in individuals with neurological disorders, such as cerebral palsy, is beginning to emerge (Deutsch et al., 2008).
Treadmill training
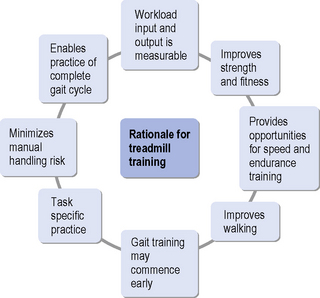
Shepherd and Carr (1999) argued that there are three reasons why treadmill training can support gait re-education:
Recent work has also suggested that treadmill training may also have positive effects on upper limb function, cognitive function and social participation (Ploughman et al., 2008; Smith & Thompson, 2008). It is important to note that these findings are currently based on limited research work. The effects of treadmill training beyond walking may therefore be co-incidental.
The original work on the effects of treadmill training involved animal studies (Barbeau et al., 1999). Animals with transected spinal cords were able to generate activity in lower limb muscles and produce a stepping motion. Research carried out on humans with incomplete spinal cord injury also showed it was possible to elicit similar activity when the individual was suspended in a harness and stepping was facilitated on a treadmill (Dobkin, 1999). This served to support the theory that humans may possess specific neural circuitry in the lumbar spinal cord that may have the ability for motor learning. The mechanism for motor learning appears as a consequence of repeated sensory inputs into lumbosacral motoneurones and interneurones, which leads to long-term potentiation. Repeated practice of the gait cycle, involving alternating loading and unloading of the lower-limbs and hip extension, seems to be the main sensory drive to promote the motor activity (Hesse, 1999).
Task-specific training on a treadmill is likely to induce expansion of subcortical and cortical locomotion areas in individuals following stroke and spinal cord injury (Dobkin, 1999; Hesse, 1999). It is also important to note, however, that whilst treadmill training has the potential to improve gait function in a number of neurological conditions, this approach may not necessarily be superior to other methods of gait training.Dickstein (2008) compared treatment outcomes of various therapeutic interventions on gait speed following stroke. The comparison indicated that gait speed can be improved through a variety of interventions but treadmill training was not found to be superior in this patient group. The authors concluded that ‘low-tech’ techniques can be as effective in gait training following stroke as more expensive treadmill and robotic approaches.
Readers are also reminded that treadmill walking can differ from overground walking and this can have profound effects on gait rehabilitation. Treadmill walking can result in an increase in cadence and a shortening of step length compared to overground walking. Wearing a harness dampens vertical spinal acceleration and reduces trunk rotation. Applying a body-weight support reduces the need to oppose gravity and this again reduces not only vertical, but also anterior-posterior acceleration (Aaslund & Moe-Nilssen, 2008). The altered visual experience of walking across a moving ground may also be difficult to cope with for some individuals.
Gait rehabilitation using treadmill training with partial body-weight support offers the possibility for active and task-specific gait training following stroke, even with individuals with low levels of functional ability (Hesse et al., 1999).Hesse et al. (1995a, 1995b) investigated the use of treadmill training and partial body-weight support with individuals following chronic stroke. These early studies showed some encouraging results, with changes in gait parameters such as stride length, cadence and velocity following the intervention, even if treadmill training may not be superior to other low tech approaches. Figure 18.2 shows a man with incomplete spinal cord injury at C5–C6 using a treadmill with harness body-weight support.

Figure 18.2 A man with incomplete spinal cord injury at C5–C6 using a treadmill with harness body weight support.
Smith et al. (1998) found normalization of reflexive activity in some individuals by the end of their programme, implying that reductions in spasticity could also accompany the strength gains seen in treadmill training. This indicates that there is no evidence to support the fear that treadmill training increases mass synergistic activity in the weaker side. Forrester et al. (2006) investigated the effects of treadmill training on central motor excitability. They showed, through transcranial magnetic stimulation, that treadmill training has the potential to alter the responsiveness of the lower-extremity central motor pathways.
As well as people with stroke, treadmill training has also been used by people with spinal cord injury (Behrman & Harkema, 2000; Dobkin, 2004), cerebral palsy (Schindl et al., 2000), MS (Giesser et al., 2007) and Parkinson’s disease (Fisher et al., 2008). Despite methodological limitations these studies suggest that treadmill training can be an effective tool for improving gait function in serveral neurological pathologies.Behrman and Harkema (2000) described the range of sensory cues needed to induce a reasonably normal reciprocal gait pattern when using treadmill training following spinal cord injury. Listed below, they could just as easily be adopted as aims for any treadmill training programme attempting to normalize gait:
Body-weight support and mechanized gait training
The optimum level of body-weight support seems to be that which allows maximum loading of the limb during the stance phase, whilst providing support for those individuals who are unable to maintain an upright posture independently. There is also agreement that as soon as an individual is progressing towards being able to balance independently whilst taking a step, the inclusion of practice of overground walking should be given more priority (Behrman & Harkema, 2000).
Hesse et al. (1997) suggested using a limit of 30% body-weight support; above this level heel strike and limb loading are compromised. Body-weight support has been shown to reduce double support time, and therefore may offer more scope to compensate for balance deficits, whilst maintaining an upright posture and producing a stepping motion (Barbeau et al., 1999).
Understandable concerns about the effort required by therapists during the treatment has led researchers to look at developing other assistive devices, such as the mechanized gait trainer developed by Hesse et al. (1999, 2000) and, more recently, robotic devices to add to the treadmill set-up. Aoyagi et al. (2007) designed their robotic system with the aim of working alongside the therapists who would be able to facilitate certain elements of the gait cycle. The robotic device would then remove the need for several therapists to be involved in coordinating several tasks at the same time. This particular system reported good technical qualities, but the complexity of instruments of this nature, in terms of attachments and adjustments, may limit their use in clinical practice. Lo & Triche (2008) investigated the use of body-weight supported treadmill training with and without robotic assistance in two groups of people with MS. Gait measurements improved significantly in both groups but the differences between groups were not significant, suggesting that the robotic device may not add anything to the outcome of the intervention, but that it may ease the work input by therapy staff.
Regnaux et al. (2008) suggested that robotic devices impose additional mechanical constraints on the gait cycle and the impacts of these on gait training are not yet fully understood.
It may be fair to conclude that new technology is being developed with the aim of easing the manual handling load of gait re-education, but at present there is no one system which seems to be able to address all necessary rehabilitation and therapy needs. Ultimately, it is unlikely that treadmill training will replace overground walking practice within neurorehabilitation. However, it clearly provides an addition to the repertoire of task-specific training programmes that have gained credibility. Figure 18.3 summarizes the rationale for using treadmill training in neurorehabilitation.
Resisted strength training in neurological rehabilitation
Interventions to improve muscle strength in neurological rehabilitation
There are a number of treatments available to therapists to improve muscle strength (Ada et al., 2006; Bohannon, 2007a; Dobkin, 2004), these include:
Meta-analysis of results from trials of these types of interventions in stroke has shown potential for improvements both at the impairment and activity levels (Ada et al., 2006).
Investigations into muscle weakness following stroke have concluded that there is reduced muscle strength in the limbs contralateral to the side of the brain lesion (Andrews & Bohannon, 2000; Bohannon, 1997b; Bohannon & Walsh, 1992; Sunnerhagen et al., 1999;). It must also be remembered that the limbs ipsilateral to the brain lesion are not ‘normal’, but also show signs of muscular weakness (Andrews & Bohannon, 2000; Harris et al., 2001). The trunk muscles, both contralateral and ipsilateral to the brain lesion, are weakened with all trunk movements being affected by the weakness (Bohannon, 1995; Bohannon et al., 1995; Tanaka et al., 1997). Lower limb paresis or weakness following a stroke is seen in about 4–15% of all strokes (Jungehulsing et al., 2008; Wandel et al., 2000). In those able to perform voluntary movements, lower limb strength may be reduced by 45% in the paretic side at the time they are discharged from rehabilitation (Andrews & Bohannon, 2000).
A minimum level of muscle strength is required to carry out physical activities and weakness can lead to substantial activity limitations. Brill et al. (2000) found evidence for this in the healthy population and studies of muscle strength of individuals with neurological conditions have reached similar conclusions (Bohannon, 2007b). Lower limb weakness has been correlated with a reduction in walking speed and endurance (Nakamura, 1988), a need for assistance with walking (Bohannon, 1988) and for assistance with transfers (Bohannon, 1989). Bohannon (2007a, 2007b) proposes that there are thresholds of force generation required to perform certain activities: for example, the achievement of sit to stand requires a minimum knee extensor force. The upper limb muscle weakness found in stroke contributes to reduced dexterity, hand grip and hand function (Canning et al., 2004; Ng & Shepherd, 2000). Contrary to common beliefs, flexor muscles in the upper limb are weaker than the extensor muscles (Andrews & Bohannon, 2000).
Two main causes of weakness are present following stroke; firstly, the ability to activate the muscle is compromised by poor descending motor control and, secondly, muscle weakness is related to disuse muscle atrophy after a period of decreased activity (Newsam & Baker, 2004). Central causes of weakness relate not only to the lack of descending drive, but also to changes in intrinsic motor neuron properties; these lead to lower motor unit firing rates, poor rate modulation and delayed recruitment (Ada et al., 2003a; Zijdewind & Thomas, 2003). Whilst stroke has been used as an example above, muscle weakness has also been demonstrated in other neurological pathologies such as: cerebral palsy (Maruishi et al., 2001), Parkinson’s disease (Allen et al., 2009), spinal cord injury (Arnold et al., 2006) and multiple sclerosis (Freeman et al., 2008).
Understanding the causes of muscle weakness, how they change with time and are affected by interventions may allow clinicians to target treatment interventions appropriately. Interventions that, for example, help to predominately maintain or improve the peripheral component may not result in any large strength-related changes in function, as these would depend on the ability of the central nervous system to adequately activate the motor neuron pool (Landau & Sahrmann, 2002).
Strength measurements are not frequently used in stroke rehabilitation. However, Bohannon (1997a) and Bohannon and Walsh (1992) have established that, despite their limitations, strength measurements can give useful information and can be reliable, provided that standardized procedures are followed.
Resisted strength training
The use of resisted strengthening exercises in neurological rehabilitation has now been broadly accepted as best clinical practice and should be implemented with the aim to maintain or increase muscle strength and function. The National Clinical Guidelines for Stroke (Royal College of Physicians, 2004) clearly recommend strengthening programmes in targeted muscles.
Evidence from other neurogical conditions also points toward muscle weakness as an impairment which could be improved with resisted strengthening exercises. Individuals with cerebral palsy (Scianni et al., 2009), Parkinson’s disease (Scandalis et al., 2001) and MS (de Boldt & McCubbin, 2002) have the potential to gain muscle strength and increase function.
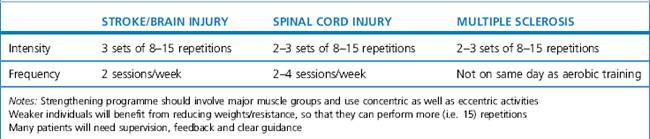
Clear evidence-based guidance for the prescription of resisted strength training programmes have yet to be developed for the majority of neurological conditions. The following guidance is based on the ACSM (2003) and offers broad help for those who prescribe and monitor strengthening exercises to patients with neurological conditions (see Table 18.5). It is important to note that this guidance is broad and requires careful assessment and potential modification with individual patients.
Progressive resisted exercise
Evidence is growing to support the use of progressive resisted exercise, i.e. progressively increasing the resistance during a strength training programme to improve muscle-generating force in neurological conditions such as cerebral palsy (Dodd et al., 2003) and stroke (Morris et al., 2004), with promising trends evident for improvements in activity over and above those seen with resisted strength training alone. An increase in transient muscle soreness has been reported, but this is not unexpected with resisted exercise (Taylor et al., 2005).
Morris et al. (2004) suggest that there are three important factors to a progressive resisted exercise protocol:
Taylor et al. (2005) stress the importance of allowing sufficient rest between each set of exercises. Body positioning during training may also be an important factor, such as training in functional positions or during functional tasks (Ada et al., 2004; Morris et al., 2004; Wolf et al., 2006, 2008). In less severely affected patients, training effects in such positions can be greater than those seen with resistance strength training (Winstein et al., 2004). Therefore, it is important to perform the training wherever possible in a functional position, for example, quadriceps-strengthening exercises during a sit to stand task.
Adjuncts to strength training
Biofeedback using electromyographic (EMG) signals from muscles is commonly used in musculoskeletal and women’s health by physiotherapists; the potential for this modality to aid in improving muscle strength in neurological rehabilitation is increasingly being proposed (Carr & Shepherd, 2006; Glinsky et al., 2007). However, evidence from robust clinical trials is still lacking (Scianni et al., 2009; Woodford & Price, 2007). A number of systematic reviews of controlled trials of electrical stimulation in a range of neurological conditions have highlighted its potential effects on strength (Ada & Foongchomcheay, 2002; Glanz et al., 1996; Glinsky et al., 2007; Kerr et al., 2004; Pomeroy et al., 2006; Singer, 1987). Although the majority of research is devoted to studies in the upper limb, there are promising studies in the lower limb where the majority of work has targeted the dorsiflexors to improve a known consequence of stroke: dropped foot (Burridge et al., 1997; Macdonnell et al., 1994; Merletti et al., 1978). The uptake of electrical stimulation in clinical practice remains low, which may relate to the lack of robust evidence and the lack of clinical guidelines for its use.
Robotic therapies are currently mostly confined to laboratory research. However, they are showing potential both for recovery of upper limb function following stroke (Prange et al., 2006) and lower limb function following stroke and spinal cord injury (see treadmill training above). Krebs et al. (2009) propose that the ability for robotic therapies to combine repetitive, intense, task-specific practice may be key to the neurological recovery seen with these modalities. Merholz et al. (2008) in their systematic review reported that improvements are more likely to be seen in muscle strength and arm motor function than in activities of daily living. Whether these therapies can be incorporated into day-to-day clinical practice remains untested.
The potential to combine principles from interventions to target muscle weakness warrants further investigation. For example, Newsam and Baker (2004) found that the combination of electrical stimulation and progressive resisted exercise showed strength improvements over and above that from progressive resisted exercise alone.
Constraint induced movement therapy
Programmes that incorporate an intensive therapy programme described as forced-use or constraint induced movement therapy (CIMT) have shown some of the most promising evidence thus far that motor recovery can be facilitated over and above the natural period of recovery after a stroke (Liepert et al., 2000). Indeed, this form of task-oriented training (Wolf et al., 2006, 2008) can result in functional improvements that, in less severely affected patients, can be greater than that seen with strength training (Winstein et al., 2004). Following any severe and sudden neurological insult there may be a period of shock or diaschesis; the threshold necessary for motor output then becomes elevated. It has been suggested that if successful motor output is not achieved, a conditioned suppression of activity may be the result (Sunderland & Tuke, 2005). Failure or difficulty in the attempt to move may then further suppress limb use, and this has been termed ‘learned non use’ (Taub et al., 1998).
After brain damage, such as that resulting from stroke, a complex pattern of neurological reorganization occurs (see Ch. 2). There have been reports of a reduction in motor cortex excitability, in the cortical representation of the paralysed muscles and an alteration in cortical activation (Liepert et al., 2001; Ward & Cohen, 2004). Non-invasive techniques for studying the human brain, such as transcranial magnetic stimulation and functional magnetic resonance imaging, are demonstrating the potential for CIMT to induce functionally relevant cortical reorganization (Levy et al., 2001; Sawaki et al., 2008).
Programmes using CIMT or modified CIMT focus attention towards the weaker limb and use repeated and extensive practice termed ‘shaping’ for up to 6 hours per day. The stronger arm is constrained in a sling and/or padded mitt. The motivation for motor output changes and subsequent accomplishment of a task then serves as a positive reinforcement for future attempts. Modified constraint or mCIMT, a variation of CIMT, is used to describe shorter periods of restraint over a longer intervention period and has also shown potential for improvements in motor control (Page et al., 2001).
Research in CIMT has demonstrated significant changes in measures of upper limb function in chronic (Page et al., 2004; Taub et al., 1993; Van der Lee et al., 1999; Wittenberg et al., 2003), subacute (Alberts et al., 2004; Page et al., 2002; Wolf et al., 2006) and acute stroke (Dromerick et al., 2000; Page et al., 2005). This body of evidence has resulted in CIMT being incorporated into the National Clinical Guidelines for Stroke (Royal College of Physicians, 2004). This recommends the use of CIMT with patients who have at least 10 degrees of active wrist and finger extension; who are more than 1 year post stroke; and are able to walk independently without an aid.
Some researchers and clinicians have questioned the suitability of all patients for CIMT. Inclusion criteria used in a number of studies were stringent and excluded a large proportion of stroke patients with significant impairments. Taub et al. (1998) discussed more relaxed criteria and more recently studies in patients outside these inclusion criteria have shown improvements in motor control (Bonifer et al., 2005; Page & Levin, 2007; Wu et al., 2007).
Tuke (2008), in a narrative review of CIMT, discussed the important elements for a CIMT programme:
Therapists and patients have raised some concerns about the practicalities of administering such an intensive programme, questioning patients’ ability to engage actively during the proposed hours of practice (Page et al., 2002). These concerns are being addressed by studies such as The EXCITE trial of CIMT, which showed significant improvements in motor function after a 2-week programme (Wolf et al., 2006) with functional improvements persisting at 1 and 2 years’ post-intervention (Wolf et al., 2008). Concerns over safety for patients with balance deficits are also being addressed by the use of padded mitts and excluded activities such as tasks involving balance (Dromerick et al., 2000; Sterr & Freivogel, 2003).Taub & Uswatte (2006) summarized that some of the questions remaining to be answered include:
Aaslund M.K., Moe-Nilssen R. Treadmill walking with body weight support: Effect of treadmill, harness and body weight support systems. Gait Posture. 2008;28:303-308.
Ada L., Foongchomcheay. Efficacy of electrical stimulation in preventing or reducing subluxation of the shoulder following stroke: a meta-analysis. Aust. J. Physiother.. 2002;48:257-267.
Ada L., Canning C., Shau-Ling L. Stroke patients have selective muscle weakness in shortened range. Brain. 2003;126(3):724-731.
Ada L., Dean C.M., Hall J.M., Bampton J., Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo controlled randomized trial. Arch. Phys. Med. Rehabil.. 2003;84:1486-1491.
Ada L., Dorsch S., Canning C. Strengthening interventions improve strength and improve activity after stroke: a systematic review. Aust. J. Physiother.. 2004;52:241-248.
Ada L., Dorsch S., Canning C.G. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust. J. Physiother.. 2006;52:241-248.
Ainsworth B.E. How do I measure physical activity in my patients? Questionnaires and objective methods. Br. J. Sports Med.. 2009;43(1):6-9.
Alberts J.L., Butler A.J., Wolf S.L. The effects of constraint induced therapy on precision grip: a preliminary study. Neurorehabil. Neural Repair. 2004;18:250-258.
Allen N.E., Canning C.G., Sherrington C., Fung V.S. Bradykinesia, muscle weakness and reduced muscle power in parkinson’s. Mov. Disord. 2009;24(5):1344-1351.
American College of Sports Medicine. Exercise Management for Persons with Chronic Diseases and Disability, vol. 2. Champaign: Human Kinetics. 2003.
American College of Sports Medicine. 2006 ACSM’s Guidelines for Exercise Testing and Prescription, seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
Andrews A.W., Bohannon R.W. Distribution of muscle strength impairments following stroke. Clin. Rehabil.. 2000;14:79-87.
Ang E.T., Wong P.T.H., Moochhalla S., et al. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118:335-345.
Aoyagi D., Ichinose W.D., Harkema S.J., et al. A robot and control algorithm that can synchronously assist in naturalistic motion during body-weight-supported gait training following neurologic injury. IEEE Trans. Neural Syst. Rehabil. Eng.. 2007;15:387-399.
Arnett S.W., Laity J.H., Agrawal S.K., Cress M.E. Aerobic reserve and physical functional performance in older adults. Age Ageing. 2008;37:384-389.
Arnold P.M., Filardi T.Z., Strang R.D., McMahon J.K. Early neurologic assessment of the patient with spinal cord injury. Top. Spinal Cord Inj. Rehabil.. 2006;12(1):38-48.
Barbeau H., Fung J., Visintin M. New approach to retrain gait in stroke and spinal cord injured subjects. Neurorehabil. Neural Repair. 1999;13:177-178.
Behrman A., Harkema S. Locomotion training after human spinal cord injury: a series of case studies. Phys. Ther.. 2000;80:688-699.
Blair S.N. Physical inactivity: a mayor public health problem. Br. Nutri. Found. Nutr. Bull.. 2007;32:113-117.
Bohannon R. Determinants of transfer capacity in patients with hemiparesis. Physiother. Can.. 1988;40:236-239.
Bohannon R. Selected determinants of ambulatory capacity in patients with hemiplegia. Clin. Rehabil.. 1989;3:47-53.
Bohannon R.W. Recovery and correlates of trunk muscle strength after stroke. Int. J. Rehabil. Res.. 1995;18:162-167.
Bohannon R.W. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20–79 years. Arch. Phys. Med. Rehabil.. 1997;78:26-32.
Bohannon R.W. Measurement and nature of muscle strength in patients with stroke. J. Neurol. Rehabil.. 1997;11:115-125.
Bohannon R. Muscle strength and muscle training after stroke. J. Rehabil. Med.. 2007;39:14-20.
Bohannon R. Knee extension strength and body weight determine sit to stand independence after stroke. Physiother. Theory. Pract.. 2007;23(5):291-297.
Bohannon R.W., Cassidy D., Walsh S. Trunk muscle strength is impaired multidirectionally after stroke. Clin. Rehabil.. 1995;9:47-51.
Bohannon R., Walsh S. Nature, reliability and predictive value of muscle performance measures in patients with hemiparesis following stroke. Arch. Phys. Med. Rehabil.. 1992;73:721-725.
Bonifer N.M., Andersen K.M., Arcieniegas D.B. Constraint induced movement therapy after stroke: efficacy for patients with minimal upper extremity motor ability. Arch. Phys. Med. Rehabil.. 2005;86:1867-1873.
Borg G. Borg’s Perceived Exertion and Pain Scales. Illinois: Human Kinetics, 1998.
Bowling A. Measuring Disease, second ed. Buckingham: Open University Press, 2001.
Bouchard C., Blair S.N., Haskell W.L. Physical Activity and Health. Champaign: Human Kinetics, 2007.
Brill P.A., Macera C.A., Davis D.R., et al. Muscular strength and physical function. Med. Sci. Sports Exerc.. 2000;32:412-416.
Burridge J., Taylor P., Hagan S., Wood D., Swain I. The effects of common peroneal nerve stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin. Rehabil.. 1997;11:201-210.
Canning C., Ada L., Adams R., O’Dwyer N. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin. Rehabil.. 2004;18:300-307.
Carr J.H., Shepherd R.B. The changing face of neurological rehabilitation. Revista Brasileira de Fisioterapia. 2006;10(2):147-156.
Chu K.S., Eng J.J., Dawson A.S., Harris J.E., Ozkaplan A., Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke. Arch. Phys. Med. Rehabil.. 2004;85:870-874.
Cluve A. Exercise and glycemic control in diabetes. Benefits, challenges, and adjustments to pharmacotherapy. Phys. Ther.. 2008;88:1297-1321.
Collen F.M., Wade D.T., Robb G.F., et al. The Rivermead Mobility Index: A further development of the Rivermead Motor Assessment. Int. Disabil. Stud.. 1991;10:61-63.
Craig C.L., Marshall A.L., Sjostrom M., et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc.. 2003;35:1381-1395.
Danielsson A., Willén C., Sunnerhagen K.S. Measurement of energy cost by the physiological cost index in walking after stroke. Arch. Phys. Med. Rehabil.. 2007;88:1298-1303.
de Bolt L., McCubbin J. The effects of home based resistance exercises on balance, power and mobility in adults with multiple sclerosis. Arch. Phys. Med. Rehabil.. 2004;85:290-297.
de Bruin E.D., Hartmann A., Uebelhart D., et al. Wearable systems for monitoring mobility-related activities in older people: a systematic review. Clin. Rehabil.. 2008;22:878-895.
Deutsch J.E., Borbely M., Filler J., Huhn K., Guarrera-Bowlby P. Use of a low cost, commercially available gaming console (Wii) for rehabilitation of and adolescent with cerebral palsy. Phys. Ther.. 2008;88(10):1196-1207.
Dickstein R. Rehabilitation of gait speed after stroke: A critical review of intervention approaches. Neurorehabil. Neural Repair. 2008;22:649-660.
Dishman R.K., Berthoud H.R., Booth F.W., et al. Neurobiology of Exercise. Obesity. 2006;13:345-356.
Ditor D.S., Latimer A.E., Ginis M.K., Arbour K.P., McCartney N., Hicks A.L. Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord. 2003;41:446-450.
Dobkin B.H. An overview of treadmill locomotor training with partial body weight support: a neurologically sound approach whose time has come for randomized clinical trials. Neurorehabil. Neural Repair. 1999;13:157-165.
Dobkin D. Strategies for stroke rehabilitation. Lancet Neurol.. 2004;3:528-536.
Dodd K.J., Taylor N.F., Graham H.K. A randomized clinical trial of strength training in young people with cerebral palsy. Dev. Med. Child Neurol.. 2003;45:652-657.
Dromerick A., Edwards D., Hahm M. Does the application of constraint induced movement therapy during acute rehabilitation reduce arm impairment after stroke? Stroke. 2000;31:2984-2988.
Elsworth C., Dawes H., Sackley C., et al. A study of perceved facilitators to physical activity in neurological conditions. Int. J. Ther. Rehabil.. 2009;16:17-23.
Finch E., Brooks D., Stratford P.W., et al. Physical Rehabilitation Outcome Measures, vol. 2. Toronto: Lippincott. 2002.
Finkelstein J., Lapshin O., Castro H., et al. Home-based physical telerehabilitation in patients with multiple sclerosis: A pilot study. J. Rehabil. Res. Dev.. 2008;45:1361-1374.
Fisher B.E., Wu A.D., Salem G.J., et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early parkinsons disease. Arch. Phys. Med. Rehabil.. 2008;89:1221-1229.
Forrester L.W., Hanley D.F., Macko R.F. Effects of Treadmill Exercise on Transcranial Magnetic Stimulation-Induced Excitability to Quadriceps After Stroke. Arch. Phys. Med. Rehabil.. 2006;87:229-234.
Freeman J.A., Porter B., Thompson A.J. Neurorehabilitation in multiple sclerosis. Top. Spinal Cord Inj. Rehabil.. 2008;14(2):63-75.
Giesser B., Beres-Jones J., Budovitch A., et al. Locomotor training using body weight support on a treadmill improves mobility in persons with multiple sclerosis: a pilot study. Mult. Scler.. 2007;13:224-231.
Glanz M., Klawansky S., Stason W., Berkey C., Chalmers T. Functional electrostimulation in poststroke rehabilitation: a meta analysis of the randomized controlled trials. Arch. Phys. Med. Rehabil.. 1996;77:549-553.
Glinsky J., Harvey L., Van Es P. Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: a systematic review. Physiother. Res. Int.. 2007;12(3):175-194.
Gordon N.F., Gulanick M., Costa F., et al. Physical activity and exercise recommendations for stroke survivors: An American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing, the Council on Nutrition, Physical Activity and Metabolism and the Stroke Council. Circulation. 2004;109:2031-2041.
Graves L., Stratton G., Ridgers N.D., Cable N.T. Comparison of energy expenditure in adolescents when playing new generation and sedentary computer games: cross sectional study. BMJ. 2007;335(7633):1282-1284.
Hackney M.E., Earhart G.M. Effects of dance on movement control in Parkinsons disease: a comparison of argentine tango and American ballroom. J. Rehabil. Med.. 2009;41(6):475-481.
Hale L.A., Pal J., Becker I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch. Phys. Med. Rehabil.. 2008;89:1765-1771.
Harris M.L., Polkey M.I., Bath P.M.W., et al. Quadriceps muscle weakness following acute hemiplegic stroke. Clin. Rehabil.. 2001;15:274-281.
Hartley S. Developing a self-management and exercise model for people with multiple sclerosis. Int. J. Ther. Rehabil.. 2009;16:34-42.
Hesse S., Bertelt C., Jahnke M., et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26:976-981.
Hesse S., Malezic M., Schaffrin A., et al. Restoration of gait by combined treadmill training and multichannel electrical stimulation in non-ambulatory hemiparetic patients. Scand. J. Rehabil. Med.. 1995;27:199-204.
Hesse S., Helm B., Krajnick J. Treadmill training with partial body weight support: influence of body weight release on the gait of hemiparetic patients. J. Neurol. Rehabil.. 1997;11:15-20.
Hesse S., Uhlenbrock D., Sarkodie-Gyan T. Gait pattern of severely disabled hemiparetic subjects on a new controlled gait trainer as compared to assisted treadmill walking with partial body weight support. Clin. Rehabil.. 1999;13:401-410.
Hesse S., Uhlenbrock D., Werner C., et al. A mechanized gait trainer for restoring gait in nonambulatory subjects. Arch. Phys. Med. Rehabil.. 2000;81:1158-1161.
Hicks A.L., Martin K.A., Ditor D.S., et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well being. Spinal Cord. 2003;41:34-43.
Janssen T.W.J., Hopman M.T.E. Spinal Cord Injury. In Skinner J.S., editor: Exercise Testing and Exercise Prescription for Special Cases, third ed, Philadelphia: Lippincott Williams & Wilkins, 2005.
Jungehulsing G., Muller-Nordhorn J., Nolte C., et al. Prevalence of stroke and stroke symptoms: a population-based survey of 28,090 participants. Neuroepidemiology. 2008;30(1):51-57.
Kang M., Zhu W., Ragan B.G., Frogley M. Exercise barrier severity and perseverance of active youth with physical disabilities. Rehabil. Psychol.. 2007;52:170-179.
Keith R.A., Granger C.V., Hamilton B.B., et al. The Functional Independence Measure: A new tool for rehabilitation. In: Eisenberg M.G., Grzesiak R.C., editors. Advances in Clinical Rehabilitation. Berlin: Springer, 1987.
Kerr C., McDowell B., McDonough S. Electrical stimulation in cerebral palsy: a review of effects on strength and motor function. Dev. Med. Child Neurol.. 2004;46:205-213.
Kileff J., Ashburn A. A pilot study of the effect of aerobic exercise on people with moderate disability multiple sclerosis. Clin. Rehabil.. 2005;19:165-169.
Krebs H.I., Volpe B., Hogan N. A working model of stroke recovery from rehabilitation roboticspractitioners. J. Neuroeng. Rehabil.. 2009;25(6):6.
Labronici R.H., Cunha M.C., Oliviera A., Gabbai A.A. Sport as integration of the physically handicapped in our society. Arq. Neuropsiquiatr.. 2000;50(4):1092-1099.
Landau W.M., Sahrmann S. Preservation of directly stimulated muscle strength in hemiplegia due to stroke. Arch. Neurol.. 2002;59(9):1453-1457.
Lanningham-Foster L., Foster R.C., McCrady S.K., Jensen T.B., Mitre N., Levine J.A. Activity promoting video games and increased energy expenditure. J. Pediatr.. 2009;154(6):819-823.
Lee M.S., Lam P., Ernst E. Effectiveness of tai chi for parkinsons disease : a critical review. Parkinsonism Relat. Disord.. 2008;14:589-594.
Levy C.F., Nichols D.S., Schmalbrock P.M., Keller P., Chakeres D. Functional MRI evidence of cortical reorganization in upper-limb stroke hemiplegia treated with constraint induced movement therapy. Am. J. Phys. Med. Rehabil.. 2001;80(1):4-12.
Liepert J., Bauder H., Miltner W.H., Taub E., Weiler C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210-1216.
Liepert J., Uhde I., Graf S., Leidner O., Weiller C. Motor cortex plasticity during forced use therapy in stroke patients: a preliminary study. J. Neurol.. 2001;248(4):315-321.
Lo A.C., Triche E.W. Improving gait in Multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil. Neural Repair. 2008;22:661-671.
Macdonnell R., Triggs W.W., Leikauskas J., Bourque M., Robb K., Day B. Functional electrical stimulation to the affected lower limb and recovery after cerebral infarction. J. Stroke Cerebrovas. Funct.. 1994;4:155-160.
Macko R.F., Ivey F.M., Forrester L.W. Task-orientated aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top. Stroke Rehabil.. 2005;12(1):45-57.
Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md. State. Med. J., February. 1965: 61-65.
Marcus B.H., Dubbert P.M., Forsyth L.H., et al. Physical activity behaviour change: issues in adoption and maintenance. Health Psychol.. 2000;19:32-41.
Maruishi M., Mano Y., Sasaki T., et al. Cerebral palsy in adults: independent effects of muscle strength and muscle tone. Arch. Phys. Med. Rehabil.. 2001;82:637-641.
McArdle W.D., Katch F.I., Katch V.L. Exercise Physiology, sixth ed. Baltimore: Lippincott Williams &Wilkins, 2006.
Merholz J., Platz T., Kugler J., Pohl M. Electromechanical and robot assisted arm training for improving arm function and activities of daily living after stroke. Cochrane Database Syst. Rev.. (4):2008. CDE006876
Merletti R., Selasci F., Latella D., et al. A control trial of muscle force recovery in hemiparetic patients during treatment with functional electrical stimulation. Scand. J. Rehabil. Med.. 1978;10:147-154.
Morris S., Dodd K., Morris M. Outcomes of progressive resistance training following stroke: a systematic review. Clin. Rehabil.. 2004;18:27-38.
Mostert S., Kesselring J. Effects of a short-term exercise training programme on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult. Scler.. 2002;8:161-168.
Mudge S., Stott S. Outcome measures to assess walking ability following stroke: a systematic review of the literature. Physiotherapy. 2007;93:198-200.
Nakamura R., Handa T., Watanabe S., Morohashi I. Walking cycle after stroke. J. Exp. Med.. 1988;154:241-244.
Newsam C., Baker L. Effect of an electric stimulation facilitation program on quadriceps motor unit recruitment after stroke. Arch. Phys. Med. Rehabil.. 2004;85:2040-2045.
Ng S.S.M., Shepherd R.B. Weakness in patients with stroke: implications for strength training in neurorehabilitation. Phys. Ther. Rev.. 2000;5:227-238.
Nudo R.J., Milliken G.W. Reorganization of movement representation in primary motor cortex following focal ischaemia infarcts in adult squirrel monkeys. J. Neurophysiol.. 1996;75:2144-2149.
Oman R.F., King A.C. The effect of life events and exercise program format on the adoption and maintenance of exercise behaviour. Health Psychol.. 2000;19:605-612.
Page S.J., Levine P. Modified constraint induced therapy in patients with chronic stroke exhibiting minimal movement ability in the affected arm. Phys. Ther.. 2007;87:872-878.
Page S.J., Levine P., Leonard A.C. Modified constraint-induced therapy in acute stroke: a randomised controlled pilot study. Neurorehabil. Neural Repair. 2005;19:27-32.
Page S., Levine P., Sisto S., Bond Q., Johnston M.V. Stroke patients and therapist opinion of constraint induced movement therapy. Clin. Rehabil.. 2002;16:55-60.
Page S.J., Sisto S.A., Johnston M.V., Levine P., Hughes M. Modified constraint induced therapy: a randomized, feasibility and efficacy study. J. Rehabil. Res. Dev.. 2001;38:583-590.
Page S.J., Sisto S., Levine P., McGrath R.E. Efficacy of modified constraint induced movement therapy in chronic stroke. Arch. Phys. Med. Rehabil.. 2004;85:14-18.
Pang M.Y., Eng J.J., Dawson A.S., Gylfadottir S. The use of exercise training in improving aerobic capacity in individuals with stroke: a meta analysis. Clin. Rehabil.. 2006;20:97.
Pariser G., Madras D., Weiss E. Outcomes of an aquatic exercise program including aerobic capacity, lactate threshold and fatigue in two individuals with multiple sclerosis. J. Neurol. Phys. Ther.. 2006;30(2):82-90.
Ploughman M., McCarthy J., Bossé M., et al. Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Arch. Phys. Med. Rehabil.. 2008;89:2041-2047.
Pomeroy V., King L., Pollock A., Baily-Hallam A., Langhorne P. Electrostimulation for promotingrecovery of movement or functional ability after stroke (review). Cochrane Database Syst. Rev.. (2):2006. CD003241. DOI: 10.1002/14651858.CD003241.pub2
Prange G.B., Jannink M.J., Groothuis-Oudshoorn C.G., Hermens H.J., Ijzerman M.J. Systematic review of the effect of robot aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev.. 2006;43(2):171-184.
Regnaux J.P., Saremi K., Marehbian J., et al. An accelerometry-based comparison of 2 robotic assistive devices for treadmill training of gait. Neurorehabil. Neural Repair. 2008;22:348-354.
Rietberg M.B., Brooks D., Uitdehaag B.M.J., et al. Exercise therapy for multiple sclerosis. Cochrane Database Syst. Rev.. 3, 2004. CD003980
Royal College of Physicians. National Clinical Guidelines for Stroke Intercollegiate Stroke Working Party. London: R. C. o. Physicians. 2004.http://www.rcplondon.ac.uk/pubs/books/stroke/stroke_guidelines_2ed.pdf.
Rogers A., Furler B.A., Brinks S., Darrah J. A systematic review of the effectiveness of aerobic exercise interventions for children with cerebral palsy: an AACPDM evidence report. Dev. Med. Child Neurol.. 2008;50:808-814.
Sawaki L., Butler A.J., Leng X., Wassenaar P.A., Mohammed Y.M., Blanton S., et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil. Neural Repair. 2008;22:505-513.
Scandalis T.A., Bosak A., Berliner J.C., et al. Resistance training and gait function in patients with Parkinson’s disease. Am. J. Phys. Med. Rehabil.. 2001;80:38-43.
Schindl M.R., Forstner C., Kern H., et al. Treadmill training with partial body weight support in nonambulatory patients with cerebral palsy. Arch. Phys. Med. Rehabil.. 2000;81:301-306.
Scianni A., Butler J.M., Ada L., Texeira-Salmel L.F. Muscle strengthening is not effective in children and adolescents with cerebral palsy: a systematic review. Aust. J. Physiother.. 2009;55(2):81-87.
Shepherd R.B., Carr J.H. Treadmill walking in neuro-rehabilitation. Neurorehabil. Neural Repair. 1999;13:171-173.
Singer B. Functional electrical stimulation of the extremities in the neurological patient: a review. Am. J. Physiother.. 1987;33(1):33-42.
Skidmore F.M., Mackman C.A., Pav B., et al. Daily ambulatory activity levels in idiopathic Parkinson disease. J. Rehabil. Res. Dev.. 2008;45:1343-1348.
Smith G.V., Macko R.F., Silver K.H.C., et al. Treadmill aerobic exercise improves quadriceps strength in patients with chronic hemiparesis following stroke: a preliminary report. J. Neurol. Rehabil.. 1998;12:111-117.
Smith A.D., Zigmond M.J. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp. Neurol.. 2003;184:31-39.
Smith P.S., Thompson M. Treadmill training post stroke: are there any secondary benefits? A pilot study. Clin. Rehabil.. 2008;22:997-1002.
Snook E.M., Motl R.W. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil. Neural Repair. 2009;23:108-116.
Sterr A., Freivogel S. Motor-Improvement following intensive training in low-functioning chronic hemiparesis. Neurology. 2003;61:842-884.
Stokes E.K. Outcome measurement. In: Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone Elsevier; 2009:191-201.
Sunderland A., Tuke A. Neuroplasticity, learning and recovery after stroke: a critical evaluation of constraint-induced therapy. Neuropsychol. Rehabil.. 2005;15(2):81-96.
Sunnerhagen K.S., Svantesson U., Lonn L., et al. Upper motor neurone lesion: their effects on muscle performance and appearance in stroke patients with minor motor impairment. Arch. Phys. Med. Rehabil.. 1999;80:155-161.
Sutoo D., Akiyama K. Regulation of brain function by exercise. Neurobiol. Dis.. 2003;13:1-14.
Tanaka S., Hachisuka K., Ogata H. Trunk rotatory muscle performance in post-stroke hemiplegic patients. Am. J. Phys. Med. Rehabil.. 1997;76:366-369.
Tang A., Sibley K.M., Thomas S.G., Bayley M.T., Richardson D., McIlroy W.E., et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters and functional capacity in subacute stroke. Neurorehabil. Neural Repair. 2009;23:398-406.
Tasiemski T., Bergstrom E., Savic G., Gardner B.P. Sports, recreation and employment following spinal cord injury: a pilot study. Spinal Cord. 2000;38(3):173-184.
Tasiemski T., Kennedy P., Gardner B.P., Taylor P. The association of sports and physical recreation with life satisfaction in a community sample of people with spinal cord injuries. NeuroRehabilitation. 2005;20:253-265.
Taub E.M., Novak T., Cook E., et al. Technique to improve chronic motor deficit after stroke. Arch. Phys. Med. Rehabil.. 1993;74:347-354.
Taub E., Crago J., Uswatte G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil. Psychol.. 1998;43:152-1570.
Taub E., Uswatte G. Constraint-induced movement therapy: answers and questions after two decades of research. NeuroRehabilitation. 2006;21(2):93-95.
Tuke A. Constraint-induced movement therapy: a narrative review. Physiotherapy. 2008;94:105-114.
Taylor N., Dodd K., Damiano D. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys. Ther.. 2005;85(11):1208-1223.
Van den Berg M.H., Ronday H.K., Peeters A.J., et al. Using internet technology to deliver a home-based physical activity intervention for patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum.. 2006;55:935-945.
Van der Lee J.W., Lankhorst G.F., Vogelaar T.W., Deville W.L., Bouter L.M. Forced use of the upper extremity in chronic stroke patients. Results from a single-blind randomised clinical trial. Stroke. 1999;30:2369-2375.
van der Ploeg H.P., Streppel K.R.M., van der Beek A.J., et al. Counselling increases physical activity behaviour nine weeks after rehabilitation. Br. J. Sports Med.. 2006;40:223-229.
van der Slot W.M.A., Roebroeck M.E., Landkroon A.P., et al. Everyday physical activity and community participation of adults with hemiplegic cerebral palsy. Disabil. Rehabil.. 2007;29:179-189.
Voorips L.E., Ravelli A.C.J., Dongelmans P.C.A., et al. A physical activity questionnaire for the elderly. Med. Sci. Sports Exerc.. 1991;23:974-979.
Wahbeh H., Siegward M.E., Oken B.S. Mind body interventions: applications in neurology. Neurology. 2008;70:2321-2328.
Wandel A., Jorgensen H., Nakayama H., Raaschou H., Olsen T. Prediction of walking function in stroke patients with initial lower extremity paralysis: the copenhagen stroke study. Arch. Phys. Med. Rehabil.. 2000;81:736-738.
Wang C., Cole J.P., Lau J. The effect of tai chi on health outcomes in patients with chronic conditions. Arch. Intern. Med.. 2004;164:493-501.
Ward N.S., Cohen L.G. Mechanisms underlying recovery of motor function after stroke. Arch. Neurol.. 2004;61(12):1844-1848.
Winstein C.J., Rose D.K., Tan S.M., Lewthwaite R., Chui H.C., Azen S.P. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch. Phys. Med. Rehabil.. 2004;85(4):620-628.
Wittenberg G.F., Chen R., Ishii K., Bushara K.O., Taub E., Gerber L.H. Constrain induced movement therapy in stroke: magnetic stimulation motor maps and cerebral activation. Neurorehabil. Neural Repair. 2003;17:48-57.
Wolf S.L., Winstein C.J., Miller J.P., et al. The EXCITE trial: retention of improved upper extremity function among stroke survivors receiving CI movement therapy. Lancet Neurol.. 2008;7(1):33-40.
Wolf S.L., Winstein C.J., Miller J.P., et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 2006;296(17):2094-2104.
Woodford H.J., Price C. Electromyographic feedback for the recovery of motor function after stroke. Stroke. 2007;38:1999-2000.
World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva: WHO, 2001.
Wu C.Y., Chen C.L., Tang S.F., Lin K.C., Huang Y.Y. Kinematic and clinical analyses of upper extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil.. 2007;88:964-970.
Yardley L., Donovan-Hall M. Predicting adherence to exercise-based therapy in rehabilitation. Rehabil. Psychol.. 2007;52:56-64.
Zijdewind I., Thomas C. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord Injury. J. Neurophysiol.. 2003;89:2065-2071.