Chapter 23 Photodynamic Therapy for Palliation of Infiltrative Endoluminal Obstruction at Left Secondary Carina and Left Lower Lobe Bronchus
Case Description
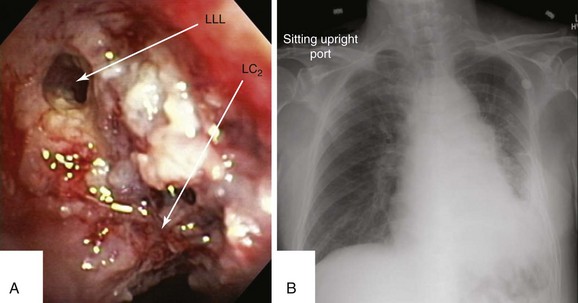
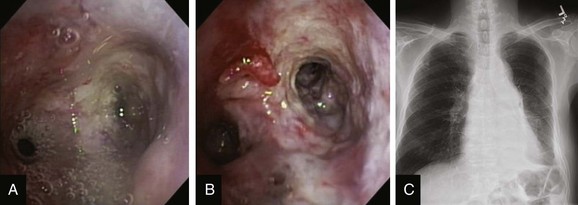
This patient was a 75-year-old man with stage IIIA squamous cell carcinoma who presented with worsening dyspnea, hemoptysis, and wheezing. A bronchoscopy performed by his pulmonologist showed near complete left lower lobe bronchial obstruction. He had undergone three cycles of chemotherapy, which were poorly tolerated. He refused further chemotherapy but agreed to receive external beam radiation therapy (EBRT), which unfortunately was complicated by radiation pneumonitis. Repeat bronchoscopy several weeks later showed recurrence of the tumor, prompting high-dose brachytherapy (dose and fractions unknown) at an outside facility. Follow-up bronchoscopy showed diffuse infiltrative tumor at the left secondary carina (LC2) nearly completely occluding the left lower lobe (LLL) bronchus. The segments in the left upper lobe (LUL) were infiltrated but patent, and the LLL bronchus was circumferentially narrowed by inflamed, necrotic, and friable mucosa, which was likely responsible for his hemoptysis and wheezing (Figure 23-1). His limited pulmonary function test (PFT) performed several months before showed FVC (forced vital capacity) of 2.26 L (69% of predicted), FEV1 (forced expiratory volume in one second) of 1.73 L (74% of predicted), and DLCO (diffusing capacity of the lungs for carbon monoxide) 68% of predicted. He was on no medications. On examination, the patient was cachectic, weighed 118 lbs, and had localized wheezes on the left side. After discussing various treatment alternatives, the patient decided to proceed with photodynamic therapy (PDT) to attempt restoration of LLL bronchial patency (see Figure 23-1).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had lobar obstruction due to endobronchial involvement from squamous cell carcinoma. Central airway obstruction from locally advanced lung cancer is seen in approximately 30% of patients previously treated with radiotherapy or chemoradiotherapy. This patient’s prior systemic chemotherapy and external beam radiation therapy (EBRT) did not preclude the use of photodynamic therapy (PDT) because PDT causes no direct injury to lung parenchyma. In one series, 85% of patients treated with PDT had received prior chemoradiotherapy.1 This patient’s previous brachytherapy did not preclude PDT either, and evidence indicates that the two modalities can be safely administered together or sequentially.2,3
Although bronchoscopy is essential in planning PDT, the response to PDT does not seem to correlate with the degree of airway obstruction or with tumor pathology. Preoperatively, the patient’s chest radiograph showed left lower lobe consolidation (see Figure 23-1); however, during initial evaluation of these patients, a computed tomography (CT) of the chest is probably preferable because CT may provide information regarding the extent of peribronchial involvement and airway distortion, which can be underestimated by bronchoscopy alone. Ventilation-perfusion studies may show absence or reduction of regional perfusion out of proportion to ventilation in the involved lung zone—a finding usually associated with extensive peribronchial involvement and poor outcome.4 Identification of significant peribronchial disease on CT has therapeutic implications because this is not treated using debulking techniques, and even if the airways are reopened, gas exchange may not improve if a reduction in local perfusion is due to tumor infiltration of the pulmonary vasculature.
Comorbidities
When considering PDT, physicians should be aware that patients with hepatic or renal impairment may need greater preventive measures for photosensitivity* because the commonly used photosensitizer, photofrin, is retained in the skin and in the reticuloendothelial system as well. In addition, interaction between photofrin† and other drugs is possible, and concomitant use of other photosensitizing agents such as tetracyclines, sulfonamides, phenothiazines, sulfonylurea hypoglycemic agents, thiazide diuretics, griseofulvin, and fluoroquinolones can increase the risk of photosensitivity reactions. Our patient had no evidence of hepatic or renal dysfunction and was on no medications.
Patient Preferences and Expectations
During the interview process, the patient wondered whether another treatment would be necessary, and if this could be safely repeated. We explained that during the cleanup bronchoscopy, performed a day or two after PDT, we could use the laser light to re-illuminate the lesion if persistent obstructing disease was present. In addition, two administrations of photofrin‡ within 30 to 45 days apart is possible if subsequent follow-up bronchoscopies continue to reveal symptomatic bronchial obstruction.
Procedural Strategies
Indications
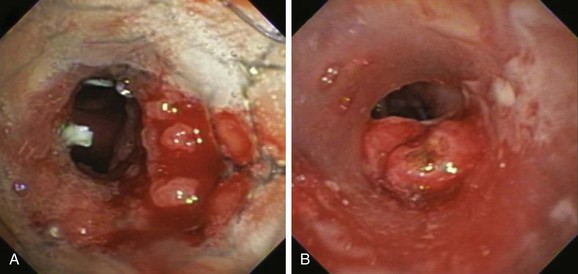
Described indications for PDT in the treatment of central airway obstruction include palliation of advanced malignant airway obstruction (especially chemoradiation-resistant lesions), tumor ingrowth through stents (Figure 23-2), and, rarely, benign disorders such as granulation tissue growth or papillomas.5,6 Accepted selection criteria for patients with locally advanced lung cancer consist of the following: (1) patients have inoperable or unresectable tumor with existing or impending related symptoms; (2) patients still have good performance status (Karnofsky Performance Status [KPS] index >50% or World Health Organization [WHO] scale ≤3); and (3) patients have no extrathoracic metastasis.7
PDT should be applied only to patients with intraluminal airway obstruction. Among these, the best response involves patients with mucosal involvement. Results from studies show that the mean duration of complete response was 22 weeks if more than 50% of the obstruction was due to mucosal disease, compared with only 7 weeks if the tumor was submucosal or extraluminal.4 In addition to the type of obstruction, the morphology (shape) of the treatment area leads to variations in response rates. In this regard, debulking and stent insertion add uniformity to the field of treatment by providing a more consistent shape and diameter of the obstructed airway. Although this could amplify PDT effect, airway stents may attenuate it by reducing light penetration.
Expected Results
In a review of 12 articles that describe a total of more than 600 patients with advanced lung cancer,8 bronchoscopic PDT was considered safe and effective for malignant endobronchial obstruction, providing symptomatic relief in all patients and a survival benefit for those without metastatic disease and with good performance status. Palliation of symptoms occurs in 74% to 100% of patients.5 Regardless of the pathology of the malignant obstruction (primary lung cancer, metastases), several factors influence the response to PDT. These include different optical properties of tissues that will influence the absorption of light and the depth of penetration (pigmentation, bleeding, and necrosis), extent of the lesion (superficial vs. “bulky” obstruction), and location of the lesion (large or small airways). Effects are less predictable with tumors located at acute angles, such as those noted in our case.
The basic principle of PDT is that the injected chemical (i.e., photofrin) accumulates more in rapidly growing tissues than in normal tissues. These molecules absorb light and produce cytotoxic oxygen species upon illumination with light of appropriate wavelength (600 to 800 nm).* This photochemical reaction leads to formation of reactive oxygen species, direct cell injury, and induction of apoptosis and vascular effects such as congestion, red cell extravasation within the tumor, and ischemic necrosis through neovascular shutdown mediated in part by thromboxane A2 release.9,10 The variability in clinical response noted in studies occurs when the interactions are inconsistent.
Results from randomized controlled studies* of patients with advanced-stage disease presented in abstract form show no survival advantage for PDT compared with neodymium-doped yttrium aluminum garnet (Nd:YAG) laser therapy, or PDT combined with radiotherapy compared with radiotherapy alone, but an advantage for PDT with radiotherapy was noted with respect to symptom control.11,12 One study randomized 211 patients to PDT or Nd:YAG laser therapy and found that the response was significantly greater 1 month after treatment with PDT than after treatment with Nd:YAG laser (55% vs. 30%).11 In another trial, the bronchial lumen was completely opened with no evidence of gross tumor in 14 of 20 patients receiving PDT plus radiotherapy compared with 2 of 21 patients receiving radiotherapy alone.12 Three of 20 patients experienced massive and fatal hemoptysis at 67, 187, and 567 days after treatment with PDT and radiotherapy,12 potentially suggesting that the addition of radiation after PDT may increase the risk for bronchoarterial fistula formation.
Noncontrolled studies have reported post-PDT reductions in dyspnea, hemoptysis, cough, or bronchial obstruction and atelectasis.13–16 One of these studies included 68 men and 32 women (mean age, 62.5 years) with advanced inoperable bronchogenic carcinoma and endobronchial obstruction; 43 patients had a functional WHO class of 2 or less, and 54 had a WHO class of greater than 2. Patients were followed and were re-treated on an as-needed basis every 6 to 8 weeks for 1 year, and then every 3 to 6 months until death; average PDT treatments per person numbered 1.47. Mean and median survival times for WHO of 2 or less were 17.8 and 14 months, respectively; for WHO of greater than 2, they were 6.9 and 4 months, respectively, potentially suggesting that PDT treatments should be offered to patients who are still functional with a reasonable performance status.16 When compared with Nd:YAG laser, PDT may offer a more durable response, and more patients may be symptom-free at 1 month in the PDT group.5
At least one study has shown that PDT may be better than Nd:YAG laser for symptom palliation in patients with central airway obstruction caused by non–small cell lung carcinoma (NSCLC).17 However, the two groups were unbalanced in terms of staging or severity of illness. This study included 31 patients (mean age, 65 years; KPS >40), and 57% of the PDT group had advanced NSCLC (IIIA, IV) versus 88% in the Nd:YAG laser group. Given these caveats, the study showed no difference between groups at 1 week in terms of symptoms or degree of obstruction, but the median time to treatment failure was 50 days for PDT versus 38 days for the Nd:YAG group (P = .03). Overall survival in this study favored the PDT group (265 vs. 95 days; P = .007), but again, this might be explained by the more advanced stage of disease in the Nd:YAG group.17
Expected complications after PDT include photosensitivity* (≈21%), which can be prolonged. Patients must avoid sun exposure for approximately 4 to 6 weeks after injection. Within days after treatment, however, airway obstruction may result from necrotic tissue, so a cleanup bronchoscopy† within 24 to 72 hours post intervention may be necessary when large obstructing lesions are treated. Other potential complications include dyspnea 30%, hemoptysis 16%, fever 16%, coughing 15%, pneumonia 12%, and bronchitis 10%.
Team Experience
Decisions about PDT probably should result from deliberations of a multidisciplinary lung cancer conference or clinic, including surgery, radiation oncology, pulmonary, and oncology subspecialties. An institution that offers PDT should have a readily available bronchoscopy team because PDT may require salvage bronchoscopies performed within 48 hours post illumination for tumor slough removal.‡ The bronchoscopy team should also be readily available during the treatment period because the risk of fatal hemoptysis is increased, especially in patients who have undergone multiple endobronchial procedures, previous radiation therapy, or metal stent placement.
The most common adverse effect related to PDT is skin photosensitivity, with an incidence between 5% and 28%. Lesser rates were reported from centers that had a dedicated member of the team assigned to provide counseling and information to patients.8 Great value is derived from having a dedicated PDT nurse to offer patient education and to explain the importance of adhering to protection from sunlight strategies.*
Therapeutic Alternatives
For patients with malignant obstruction, apart from traditional surgical resection, radiotherapy, and chemotherapy, several bronchoscopic options are available. Nd:YAG laser, brachytherapy, and cryotherapy have been used alone or in combination for symptom palliation. Rigid bronchoscopy with laser photocoagulation and coring out of the tumor is especially useful for tracheal or mainstem bronchial disease and when hemoptysis is present. In our patient, the lobar obstruction caused by mucosal disease was not appropriate for laser treatment because of a substantial infiltrative rather than endoluminal exophytic component. In addition, although stent insertion is an alternative in patients with malignant obstruction, our patient had a distal lobar location of disease (LLL) that was not ideal for stent placement because the LLL bronchus is both short and narrow. Cryotherapy usually preserves airway cartilage and was an alternative for this patient18; however, it was not ideal because of the absence of substantial exophytic disease. Further high-dose brachytherapy was not warranted because it had already been attempted without success.
Cost-Effectiveness
Since 1988, PDT has been approved by the U.S. Food and Drug Administration for palliative treatment of central airway lesions due to NSCLC.19 In 2004, the National Institute for Clinical Excellence stated, “Current evidence on safety and efficacy of PDT for advanced bronchial carcinoma is adequate to support its use to treat patients with inoperable NSCLC.”20
Techniques and Results
Anesthesia and Perioperative Care
Studies report flexible bronchoscopic PDT with patients under moderate sedation. Some studies have used general anesthesia and rigid bronchoscopy or endotracheal tube for ventilation, through which operators place the flexible instrument for tumor location and illumination. Use of general anesthetic and the rigid bronchoscope in patients with bronchial obstruction undergoing PDT allows good access for debridement, thereby achieving a thorough cleaning of the bronchial tree and ample room to maneuver in case of complications.8 In the opinion of many PDT experts, combined rigid and flexible instruments under general anesthesia are best for patients with obstructive tumors of the trachea and mainstem bronchi, but for other cases, the method depends on the experience of the operator and a patient’s comorbidities and personal choice.7 We decided to perform this particular procedure on our patient while under general anesthesia to allow better control of the diffuser in the distal left main and lower lobe bronchi and to have a secure airway in case the patient’s hemoptysis worsened during the procedure.
Technique and Instrumentation
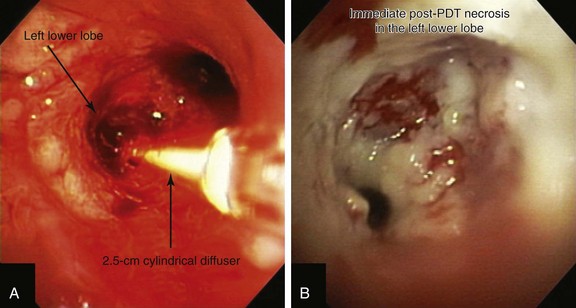
Light is delivered to the tumor by cylindrical fiberoptic diffusers passed through the working channel of the bronchoscope (Figure 23-3). The fiber may be positioned alongside the tumor when it is relatively flat, or it can be implanted within the tumor when it is bulky and exophytic. Cylindrical diffusers are commonly used for central airway obstruction and are available in several lengths; the choice of diffuser tip length* depends on the length of the tumor to be treated. Diffuser length should be properly sized to avoid exposure of nonmalignant tissue to light and to prevent overlapping of previously treated malignant tissue. Therefore, before light administration, the length of the tumor is assessed with bronchoscopy, and a suitable light fiber length is selected to transilluminate the tumor. Within lung and tumor tissue, 630 nm light will penetrate to a depth of 5 to 10 mm from the point of origin, depending on the power density and the length of the fiber.21 Argon-pumped dye laser and diode-based systems have been used as a light source. We used a compact diode-based laser (Diomed Ltd., Andover, Mass) that was available at our institution. This is a nonthermal laser, so no risks of airway fire are present. Photoactivation of photofrin is controlled by the total light dose delivered. In the treatment of endobronchial cancer, a light dose of 200 J/cm of diffuser length should be delivered. The total power output at the fiber tip is set to deliver the appropriate light dose using exposure times of 8 minutes and 20 seconds.21
A step-by-step approach to bronchoscopic PDT proceeds as follows: First, the treatment plan is developed by performing diagnostic flexible bronchoscopy, during which time the length of the tumor is assessed and the diffuser length is selected (see Figure 23-1). Then, in the presensitization phase, photofrin is injected; after a latent period of 2 to 3 days (which permits optimal differential concentration between tumor and healthy surrounding tissue), some investigators measure drug levels by point spectroscopy; others proceed straight to the illumination (irradiative) phase* using laser light of appropriate wavelength (630 nm) (see Figure 23-3). Light doses of 200 J/cm of diffuser length are used in endobronchial cancer for both palliation of obstructing cancer and treatment of superficial lesions.22 One to three days later, debridement is usually necessary when obstructing lesions are treated (Figure 23-4); before a second laser light treatment is provided, any residual tumor should be debrided, which may cause bleeding if performed vigorously. In fact, debridement of necrotic tissue should be discontinued if the volume of bleeding increases; this may indicate that debridement has gone beyond the zone of the PDT effect. Re-illumination can then be performed if residual obstruction remains, usually within 96 to 120 hours after the initial injection, but no further injection of photofrin should be given for such re-treatment with laser light. In fact, most patients receive two treatments during their hospitalization, and many return several weeks later for repeated treatments.23
Anatomic Dangers and Other Risks
PDT can completely destroy malignant tissues that have been adequately dosed with the photosensitizer and exciting light. This may result in necrosis that may not be apparent during the irradiative phase of treatment, when the full cross-sectional extent of the tumor may not be obvious to the bronchoscopist (see video on ExpertConsult.com) (Video V.23.1![]() ). In addition, further necrosis of the tumor develops hours to days after treatment. Thus when performed in hollow organs such as the airway or the esophagus, PDT can lead to fistula formation. Increased airway inflammation increases the effects of PDT and may lead to damage of adjacent nonmalignant but inflamed mucosa. Tissue sloughing can cause severe lobar and segmental airway obstruction and respiratory failure. Salvage bronchoscopy may be necessary as soon as 6 hours after treatment, and when main central airway treatment is performed, postprocedure intensive care unit (ICU) admission is probably warranted. Having ready access to flexible bronchoscopy, as well as a good grasping forceps or cryotherapy unit available, can be lifesaving. Local edema and formation of mucus from adjacent normal tissues may result in transient bronchitis, which may exacerbate coexisting chronic obstructive pulmonary disease (COPD) or asthma.
). In addition, further necrosis of the tumor develops hours to days after treatment. Thus when performed in hollow organs such as the airway or the esophagus, PDT can lead to fistula formation. Increased airway inflammation increases the effects of PDT and may lead to damage of adjacent nonmalignant but inflamed mucosa. Tissue sloughing can cause severe lobar and segmental airway obstruction and respiratory failure. Salvage bronchoscopy may be necessary as soon as 6 hours after treatment, and when main central airway treatment is performed, postprocedure intensive care unit (ICU) admission is probably warranted. Having ready access to flexible bronchoscopy, as well as a good grasping forceps or cryotherapy unit available, can be lifesaving. Local edema and formation of mucus from adjacent normal tissues may result in transient bronchitis, which may exacerbate coexisting chronic obstructive pulmonary disease (COPD) or asthma.
Results and Procedure-Related Complications
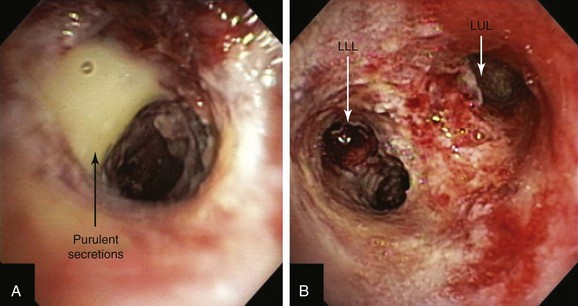
After administration of general anesthesia with propofol, our patient was intubated with a No. 8.0 endotracheal tube. Flexible bronchoscopy showed again circumferential neoplastic infiltration in the distal left mainstem bronchus (LMB) with near total occlusion of the left lower lobe (LLL) and involvement of the left secondary carina (LC2) and the left upper lobe (LUL) (see Figure 23-1). The 2.5 cm cylindrical diffuser was inserted, and a total of 300 joules (480 seconds) of light energy was applied using a 630 nm diode laser to treat the distal LMB and the LLL bronchus (see Figure 23-2). At the end of the procedure, the patient was extubated without difficulty. He was treated as an inpatient given the fact that he was very frail and lived 150 miles away from the hospital. The next day, he underwent a flexible bronchoscopy under moderate sedation, at which time the LLL bronchus was found to be occluded by necrotic debris. Pus was noticed in the distal segmental airways (see Figure 23-4).* Abundant saline washes were performed to rid the area of the obstructing material. Once this had been performed, we noted that patency to the LLL segmental bronchi had been restored (see Figure 23-4). Two days later, rigid bronchoscopy was performed because additional necrotic material had to be removed from the LLL bronchus (Figure 23-5). Patency to the left lower lobe was thus established (see video on ExpertConsult.com) (Video V.23.2![]() ). A small piece of cartilage that had likely been destroyed by tumor was noted, although this might have resulted from PDT-induced bronchial wall and mucosal necrosis (see video on ExpertConsult.com) (Video V.23.3
). A small piece of cartilage that had likely been destroyed by tumor was noted, although this might have resulted from PDT-induced bronchial wall and mucosal necrosis (see video on ExpertConsult.com) (Video V.23.3![]() ).
).
When light of two to three times the recommended dose (aka light overdose) has been administered to patients with superficial endobronchial tumors, increased symptoms and damage to normal tissue can be expected. Occasionally local edema and mucous formation from surrounding normal tissues may result in transient bronchitic symptoms, including cough and dyspnea. Necrotic tumor slough and secretions from bulky tumors may potentially cause respiratory distress, atelectasis, or pneumonia. Although rapid tumor vascular stasis is observed, cytotoxicity and cellular death of tumor cells are delayed in such a way that necrotic debris eventually forms at the site of treatment over the next 48 hours. This debris is tenacious and can be difficult or impossible to expectorate (see Figure 23-5), prompting the need for repeated bronchoscopic intervention.
Long-Term Management
Outcome Assessment
A clinic appointment a week later after bronchoscopy improved aeration in the LLL on chest radiograph, improved symptoms, and resolved airway obstruction (see Figure 23-5). Four weeks after the intervention, during a sunny day, the patient went outside of his house and developed sunburn with significant erythema, pain, and itching around the eyes. He went to the local emergency department, where he was diagnosed with PDT-induced sunburn and was given local anesthetic to be applied to the affected area for pain. When we discussed this issue with him, he told to us that a home health nurse had instructed him that he could leave the house after 4 weeks post intervention. He had neglected to call our nursing team to inform us of this recommendation.
Follow-up Tests and Procedures
When compared with electrocautery, more airway scarring and more subepithelial fibrosis may be seen after treatment with PDT or Nd:YAG laser. Prominent scar tissue formation and significant stenosis were found in four of six (67%) patients treated with PDT in one series—a finding that contrasts with the assumption that PDT is highly selective for tumor tissue. In the treatment of early lung cancer, normal airway mucosa is exposed more than in patients with advanced disease; however, the fact that normal mucosa can be affected, resulting in scar tissue formation, is important in the choice of diffuser length.24 A follow-up bronchoscopy is almost always warranted, not only to explore the need for additional bronchoscopic interventions, but also to rule out potential strictures in the treated area. If subsequent radiotherapy is planned, it is advisable to allow 2 to 4 weeks between PDT and radiation to ensure that the inflammatory response* produced by the PDT has subsided. Patients should be carefully evaluated for tumor erosion into a major blood vessel. If on follow-up bronchoscopy, evidence of tumor recurrence and obstruction is apparent, patients may receive a second course of PDT a minimum of 30 days after the initial therapy.† Our patient was not a candidate for further radiotherapy but could have undergone PDT again.
The interaction between PDT and radiation, chemotherapy, and stent insertion is important when multimodality treatments are planned for these patients with advanced-stage lung cancer. Certain chemotherapy drugs such as Adriamycin and mitomycin can enhance the PDT effect.25 Ionizing radiation has a synergistic effect and has been given before, after,26 or during the PDT treatment period.27 PDT in lung cancer is compatible when used in conjunction, or in sequence, with other standard systemic or endobronchial treatment methods.28 PDT can be combined with brachytherapy concomitantly or sequentially, as was done in our case.3 Also, PDT can be applied in sequence with Nd:YAG laser; in one series, Nd:YAG laser was used to debulk endobronchial obstructing tumors. The addition of PDT by its specific action resulted in long periods of local remission and provided an apparent survival benefit.28 When our patient was seen during follow-up, he informed us that he did not wish to undergo further bronchoscopic treatment. We chose to respect his decision, and we did not inquire as to his reasons.
Referrals
After PDT, the patient was referred back to his oncologist for consideration for further systemic treatment. In patients with advanced lung cancer, local control and distant control are not independent factors, and quality of life may not improve if only local or distal control has been achieved.29 Because medical treatments had been exhausted, we thought that palliative care consultation was appropriate. This recommendation was accepted by the patient and his family.
Quality Improvement
We assumed responsibility for not clarifying discharge instructions regarding post-PDT care and for not communicating this to the patient’s home health nurse, who had been arranged by his previous caregivers. Sunburn due to cutaneous hypersensitivity may be completely prevented with careful instruction and patient compliance with sunlight avoidance.5 Indeed, after 4 weeks, our patient should have tested his skin for photosensitivity. We apologized for our lack of satisfactory communication and shared our misgivings with the patient and with his family. Patients and surrogate decision makers need to know and fully understand the status of their health so they can make fully informed decisions regarding their care. Health care providers must not hesitate to advocate for patients in this regard. Often, problems of communication and other follow-up care issues can be resolved if an interventional team has the appropriate personnel and other resources necessary to provide telephone, computer-based, and in-person follow-up. This is not always the case, particularly in institutions that have not yet perceived the importance of an interventional service, or in institutions where administrators do not recognize the spectrum of disease and the substantial comorbidities often present in patients with central airway obstruction.
If a patient has been injured during medical treatment, full participation in subsequent informed consent processes is possible only if the details that led to an injury are fully understood.30 Because many patients with central airway obstruction may require repeated bronchoscopic or other treatments, withholding information relevant to the patient’s health care could be interpreted as dishonest and could severely undermine a physician’s relationship with the patient,30 drastically altering the patient’s ability to make an informed and correct decision regarding further intervention.
Discussion Points
1. List three indications for PDT in this patient.
2. List three potential contraindications to PDT.
3. Describe the instructions to be provided to a patient who has undergone PDT.
4. Describe the clinical indications and the physics of brachytherapy as compared with PDT.
Expert Commentary
Two non-PDT features of this case deserve review: the choice to treat the cancer systemically with chemotherapy then locally with radiation before offering direct treatment for the partial airway obstruction, and the diagnostic options used to assess the functional parameters of the left lower lobe airway obstruction. Marasso et al. reviewed 98 patients with advanced lung cancer and an obstructive airway component. Fifty patients were treated with chemotherapy and radiation first, followed by direct local airway manipulation; 48 patients had local airway treatment first followed by chemotherapy and radiation. The mean survival rates in the two groups were 5 and 14 months, respectively.39 He argued that optimizing airway function improved performance status, lessened post obstructive airway pathophysiology, and subsequently allowed better tolerance of chemotherapy and radiation. In my opinion, the discussion of airway intervention prior to chemotherapy/radiation is one that every multidisciplinary thoracic oncology team should have before providing any treatment for advanced lung cancer. The authors made an excellent point about CT scanning and how it provides key in-depth information about the left lower lobe consolidation not provided by the chest x-ray. In a similar manner, selective bronchography of the left lower lobe bronchial tree would add functional information that CT scan does not. Selective bronchography has been shown to be superior to the 4 week rule* or CT scans of the chest in predicting the reversibility of lobar or lung atelectasis caused by neoplastic bronchial obstruction.40 Selective bronchography provides functional information that still life imaging cannot address, and the procedure itself is low-cost, safe, and technically simple.
Because PDT involves the unique interaction of a device (laser) and a drug (photosensitizer) to create a clinical response, PDT users must intimately understand the photodynamic reaction and must perceive where individual cases can potentially result in unexpected responses. Figure 23-6 is a depiction of the PDT reaction within the airway and the key factors effecting responses. It will be useful to review this case point by point from Figure 23-6 to appreciate how variations can be minimized and the clinical response can be more predictable.
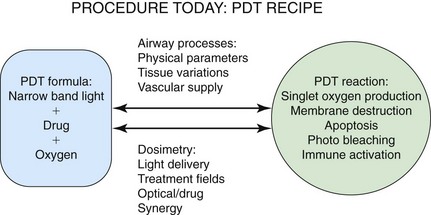
Figure 23-6 Photodynamic therapy (PDT) reaction within the airway and key factors effecting responses.
The authors commented that the degree of obstruction does not correlate with the PDT response; however, the degree of obstruction does dramatically affect dosimetry considerations, which in turn directly determine the PDT response. The first PDT dosimetry question a clinician should answer is whether the obstruction should be treated with interstitial or superficial catheter placement. Interstitial catheter placement requires a separate physics calculation that is independent of the airway’s area because the impaled tumor receives 100% of the delivered light energy. Superficial catheter placement light delivery is affected by shadowing, luminal area, percentage of tumor occupancy, and amount/type of secretions.41 Weighing the pros and cons of catheter placement to optimize the predictability of response is, in my opinion, underutilized.
This case presents several challenges related to the patient’s airway physical parameters: significant luminal diameter change over a very short length of airway, bloody secretions, irregular tumor edges with shadowing, and distal airway location affected by respiration movements. Tumor shape in this case would not allow an interstitial catheter placement, so the author’s discussion of stent placement before treatment with PDT is very relevant. An uncovered stent can resolve much of airway/tumor irregularity; bleeding would be better tamponaded, thus reducing secretions and causing the treatment area to become easier to define.42 Use of a 1 centimeter long catheter in this case would have lessened luminal diameter variation; the 2.5 cm catheter used appears to be treating totally different airway calculated areas with the same light/energy delivery at its proximal versus distal ends. Finally, strongly filtered fiberoptic bronchoscopy will allow real-time observation of catheter movement that digital scopes often cannot follow when the digital camera is super saturated with light. Tissue variations were well addressed by the authors, but strict airway toilet before light delivery must be emphasized.
Vascular supply in this case may have been affected by chemotherapy, radiation therapy (external beam or HDR), and stenting. Unfortunately a strong theoretical argument can be made for an augmented or reduced vascular supply. In my opinion, confirming significant drug uptake at the treatment site would answer the practical question of vascular supply and drug and oxygen delivery. This could have been accomplished by employing point spectrometric measurements just before the time of treatment. Additionally, taking measurements before and after treatment allows confirmation that an adequate photodynamic reaction has taken place by demonstrating drug consumption.43
Dosimetry cannot be calculated by a one size fits all approach. Dosimetry recommendations quoted by the authors were generated by trials for FDA approval of photofrin and reflect the understanding of the field 15 to 20 years ago. Preset diode laser options also encourage a nonadaptable approach to airway dosimetry. Programs that are employing PDT as a frontline oncologic treatment are universally customizing dosimetry to the individual’s clinical presentation. Customization takes into account light delivery variations, treatment field plasticity, tissue interference with light absorption, drug uptake variations, and synergistic effects of patient medications and previous oncologic treatments.43
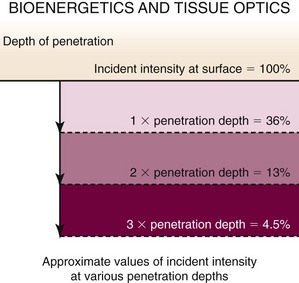
Figure 23-7 presents unpublished data from Dr. Tom Mang’s laboratory at the University of Buffalo and depicts the effects of tissue on light delivery. For airway tissue, one penetration depth is approximately 8 mm, and the assumption that a significant clinical PDT response can be seen to a depth of 8 mm comes from basic science information such as this. However, in our program, we have seen a significant tumoricidal response with 90% attenuation of light intensity and apoptosis within cells with 95% light intensity attenuation. This suggests that tumor cell death can occur out to 2.4 cm from the light source. Pre-PDT external beam radiation to the treatment field would have dramatically lowered this patient’s cellular sensitivity to singlet oxygen damage and would have changed significantly the predicted response depth. Drug uptake, oxygen delivery, HDR, current medications, stent placement, and previous chemotherapy also affect PDT response. This case presents so many variables that a truly predictable PDT response in my opinion was not possible. A positive outcome does not change how unpredictable PDT really was in this setting; however, the balancing factors included upfront full disclosure of risk benefits and limited available treatment options.
* The 4 week rule refers to the duration of collapse that is sometimes used by bronchoscopists to predict a response to bronchoscopic intervention in the setting of malignant central airway obstruction; identifying patients who meet the 4 week criteria can be unreliable because in clinical practice, definitive symptoms or imaging studies that can pinpoint duration of collapse are seldom found. In addition, the 4 week rule has not been studied in randomized clinical trials and is most often recommended on the basis of expert opinion rather than evidence-based data.
1. Moghissi K, Bond MG, Sambrook RJ, et al. Treatment of endotracheal or endobronchial obstruction by non-small cell lung cancer: lack of patients in an MRC randomized trial leaves key questions unanswered. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol). 1999;11:179-183.
2. Weinberg BD, Allison RR, Sibata C, et al. Results of combined photodynamic therapy (PDT) and high dose rate brachytherapy (HDR) in treatment of obstructive endobronchial non-small cell lung cancer (NSCLC). Photodiagn Photodyn Ther. 2010;7:50-58.
3. Freitag L, Ernst A, Thomas M, et al. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax. 2004;59:790-793.
4. Lam S, Müller NL, Miller RR, et al. Predicting the response of obstructive endobronchial tumors to photodynamic therapy. Cancer. 1986;58:2298-2306.
5. Loewen GM, Pandey R, Bellnier D, et al. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38:364-370.
6. Shikowitz MJ, Abramson AL, Steinberg BM, et al. Clinical trial of photodynamic therapy with meso-tetra (hydroxyphenyl) chlorin for respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2005;131:99-105.
7. Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J. 2006;28:200-218.
8. Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer. Eur Respir J. 2003;22:535-541.
9. Edell ES, Cortese DA. Photodynamic therapy: its use in the management of bronchogenic carcinoma. Clin Chest Med. 1995;16:455-463.
10. Maziak DE, Markman BR, MacKay JA, et al. Cancer Care Ontario Practice Guidelines Initiative Lung Cancer Disease Site Group. Photodynamic therapy in nonsmall cell lung cancer: a systematic review. Ann Thorac Surg. 2004;77:1484-1491.
11. Wieman TJ, Diaz-Jimenez JP, Moghissi K, et al. Photodynamic therapy (PDT) with photofrin is effective in the palliation of obstructive endobronchial lung cancer: results of two randomized trials [abstract]. Proc Am Soc Clin Oncol. 1998;17:464a.
12. Lam S, Grafton C, Coy P, et al. Combined photodynamic therapy (PDT) using photofrin and radiotherapy (XRT) versus radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. SPIE. 1991;1616:20-28.
13. Vincent RG, Dougherty TJ, Rao U, et al. Photoradiation therapy in advanced carcinoma of the trachea and bronchus. Chest. 1984;85:29-33.
14. Hugh-Jones P, Gardner WN. Laser photodynamic therapy for inoperable bronchogenic squamous carcinoma. Q J Med. 1987;243:565-581.
15. LoCicero J, Metzdorff M, Almgren C. Photodynamic therapy in the palliation of late stage obstructing non-small cell lung cancer. Chest. 1990;98:97-100.
16. Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg. 1999;15:1-6.
17. Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, et al. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J. 1999;14:800-805.
18. Homasson JP. Bronchoscopic cryotherapy. J Bronchol. 1995;2:145-153.
19. Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21:1404-1411.
20. National Institute for Clinical Excellence. Photodynamic therapy for advanced bronchial carcinoma. http://guidance.nice.org.uk/nicemedia/live/11101/31032/31032.pdf. Accessed May 6, 2011
21. Dougherty TJ, Marcus SL. Photodynamic therapy. Eur J Cancer. 1992;28:1734-1742.
22. Mang TS. Dosimetric concepts for PDT. Photodiagn Photodyn Ther. 2008;5:217-223.
23. Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg. 2010;89:1744-1749.
24. van Boxem AJ, Westerga J, Venmans BJ, et al. Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer. 2001;31:31-36.
25. Baas P, van Geel IP, Oppelaar H, et al. Enhancement of photodynamic therapy by mitomycin C: a preclinical and clinical study. Br J Cancer. 1996;73:945-951.
26. Schaffer M, Schaffer PM, Corti L, et al. Photofrin as a specific radiosensitizing agent for tumors: studies in comparison to other porphyrins, in an experimental in vivo model. J Photochem Photobiol B. 2002;66:157-164.
27. Madsen SJ, Sun CH, Tromberg BJ, et al. Effects of combined photodynamic therapy and ionizing radiation on human glioma spheroids. Photochem Photobiol. 2002;76:411-416.
28. Moghissi K, Dixon K, Hudson E, et al. Endoscopic laser therapy in malignant tracheo-bronchial obstruction using sequential Nd: YAG laser and photodynamic therapy. Thorax. 1997;52:281-283.
29. Sutedja TG. Photodynamic therapy in advanced tracheobronchial cancers. Diagn Ther Endosc. 1999;5:245-251.
30. Loren DJ, Gallagher T. Disclosure of harmful medical errors: the verdict. In: Colt HG, Quadrelli S, Friedman L. The Picture of Health: Medical Ethics and the Movies. New York: Oxford University Press, 2011.
31. Pinnacle Biologics. PDT with photofrin. www.photofrin.com. Accessed May 6, 2011
32. Ung YC, Yu E, Falkson C, et alLung cancer disease site group of Cancer Care Ontario’s Program in Evidence-Based Care. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
33. Bedwinek J, Petty A, Bruton C, et al. The use of high dose rate endobronchical brachytherapy to palliate symptomatic endobronchical recurrence of previously irradiated bronchogenic carcinoma. Int J Radiat Oncol Biol Phys. 1991;22:23-30.
34. Carvalho Hde A, Gonçalves SL, Pedreira WJr, et al. Irradiated volume and the risk of fatal hemoptysis in patients submitted to high dose-rate endobronchial brachytherapy. Lung Cancer. 2007;55:319-327.
35. Nag S, Dally M, de la Torre M, et al. Recommendations for implementation of high dose rate 192Ir brachytherapy in developing countries by the Advisory Group of International Atomic Energy Agency. Radiother Oncol. 2002;64:297-308.
36. Nag S, Abitbol AA, Anderson LL, et al. Consensus guidelines for high dose rate remote brachytherapy in cervical, endometrial, and endobronchial tumors. Clinical Research Committee, American Endocurietherapy Society. Int J Radiat Oncol Biol Phys. 1993;27:1241-1244.
37. Nag S, Kelly JF, Horton JL, et al. Brachytherapy for carcinoma of the lung. Oncology. 2001;15:371-381.
38. Huber RM, Fischer R, Hautmann H, et al. Palliative endobronchial brachytherapy for central lung tumors: a prospective, randomized comparison of two fractionation schedules. Chest. 1995;107:463-470.
39. Marasso A, Bernardi V, Gai R, et al. Radiofrequency resection of bronchial tumours in combination with cryotherapy: evaluation of a new technique. Thorax. 1998;53:106-109.
40. Downie GH, Childs JH, Landucci DL, et al. The use of selective bronchography in predicting reversal of neoplastic obstructive atelectasis. Chest. 2002;123:828-834.
41. Downie GH, Cuenca RE, Allison RR, et al. A comparison of interstitial and superficial light delivery for photodynamic therapy of intraluminal neoplasms. J Bronchol. 2002;9:193-196.
42. Allison R, Sibata C, Sarma K, et al. High-dose-rate brachytherapy in combination with stenting offers a rapid and statistically significant improvement in quality of life for patients with endobronchial recurrence. Cancer J. 2004;10:368-373.
43. Downie GH. Clinical considerations for airway photodynamic therapy. In: Report of the 13th World Congress for Bronchology (WCB) and 13th World Congress for Bronchoesophagology (WCBE). Medimond International Proceedings 2005;1-9.
* Patients should be informed that the period requiring precautionary measures for photosensitivity may sometimes be longer than 90 days.
† Photofrin is, at present, the most frequently used photosensitizer. Other variants of purified hematoporphyrin derivatives are marketed (e.g., Photosan in Germany).
‡ The usual dose is 2 mg/kg as slow intravenous injection over 3 to 5 minutes.
* The absorption spectrum in the 600 to 800 nm range facilitates depth of penetration, which is 3 to 5 mm.
* Neither paper reported whether assessment of response was blinded, and the response was not clearly defined.
* Mild to moderate erythema, but also includes swelling, itching, and burning sensation; occasionally induces pseudoporphyria state.
† This may not be necessary when PDT is used to treat early lung cancer.
‡ Appropriate equipment for slough removal should be available and may include large-channel bronchoscopes and cryotherapy probes.
* All patients who receive photofrin will be photosensitive and must observe precautions to avoid exposure of skin and eyes to direct sunlight or to bright indoor light (from examination lamps, including dental lamps, operating room lamps, and unshaded light bulbs at close proximity) for at least 30 days, but some may remain photosensitive for up to 90 days or more. However, exposure of the skin to ambient indoor light is beneficial because the remaining drug will be inactivated gradually and safely through a photobleaching reaction. Before any area of skin is exposed to direct sunlight or bright indoor light, the patient should test it for residual photosensitivity. A small area of skin should be exposed to sunlight for 10 minutes. If no photosensitivity reaction (erythema, edema, blistering) occurs within 24 hours, the patient can gradually resume normal outdoor activities. If some photosensitivity reaction occurs with the limited skin test, the patient should continue precautions for another 2 weeks before retesting.
* Fibers for the treatment of endobronchial cancer are generally 1, 2, and 2.5 cm tip lengths and in general are adequate for tumor necrosis within the central airways.
* It is important to realize that an appropriate wavelength of laser light is one that interacts with the particular photosensitizer used for photosensitization of the target. It is also relevant that the duration of the latent period is dependent on the chemical structure of the photosensitizing agent and its handling by tumor and normal cells.
* Prevention of infection is relevant for patients with significant lobar or segmental tumor obstruction. In such patients, post-PDT necrosis creates a culture medium for microorganisms. Chest physiotherapy, deep breathing exercises, incentive spirometry, and antibiotics may be needed.
* The inflammatory response from PDT will depend on tumor size and the extent of surrounding normal tissue that receives light.
† In general, up to three courses of PDT (each separated by a minimum of 30 days) can be given.
* This technique is usually performed in the following steps: the tumor location and length are identified on bronchoscopy and chest x-ray; one or more catheters are implanted next to the lesion via the working channel of the bronchoscope. The bronchoscope is then removed and the catheter(s) are fixed to the nose. Orthogonal films are taken after a phantom is positioned in the catheter(s). The target volume is then drawn on the films with a 1 to 2 cm safety margin with respect to the bronchoscopy image. The dose is usually prescribed to be delivered at 1 cm from an iridium-192 source.
* Because the surface area of a sphere (4πr2) is proportional to the square of the radius, as the emitted radiation gets farther from the source, it must spread out over an area that is proportional to the square of the distance from the source. Therefore, radiation passing through any unit area is inversely proportional to the square of the distance from the source.