Chapter 21 Phenols
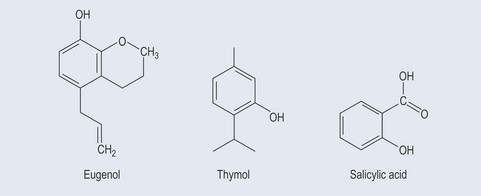
Phenols constitute probably the largest group of plant secondary metabolites, varying in size from a simple structure with an aromatic ring to complex ones such as lignins. Although many of the essential oils are terpenes, some are phenolic compounds, for example thymol from Thymus spp. (thyme) (Figure 21.1). Many simple phenols are responsible for taste, for example eugenol in cloves. They are called the phenylpropanoids because they originate from phenylalanine (see Figure 11.1, p. 82) and they have a six-carbon (C6) and three-carbon (C3) structure (Figure 21.2). They comprise several groups:
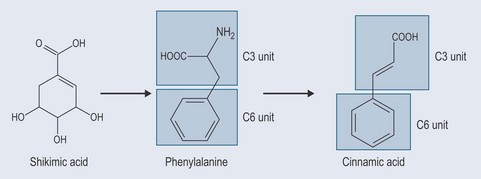
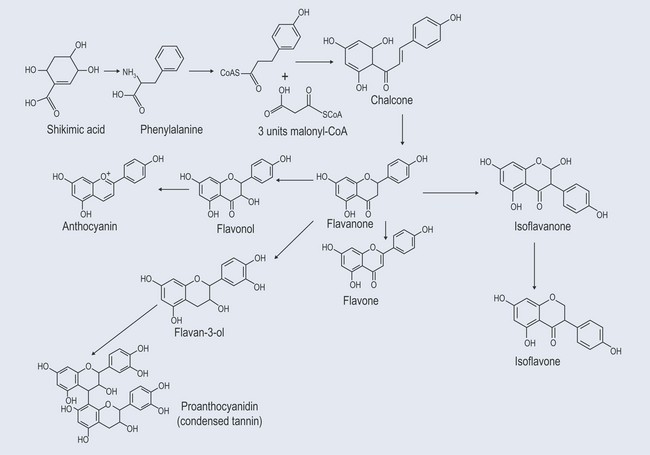
Figure 21.2 Showing the development of simple phenylpropenes from shikimic acid and demonstrating the C3 and C6 units.
Many of these are attached to sugars that have to be cut or cleaved before activity (see Chapter 24 ‘Glycosides’, p. 181).
Shikimic Acid
The starting point for the production of phenylpropanoids is shikimic acid (Figure 21.2). Shikimic acid (or shikimate) is the precursor for:
Abridged Phenylpropanoids
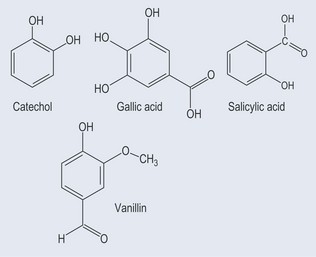
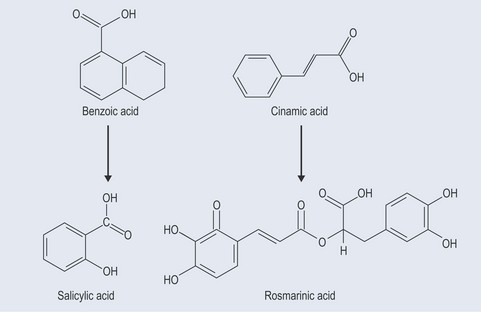
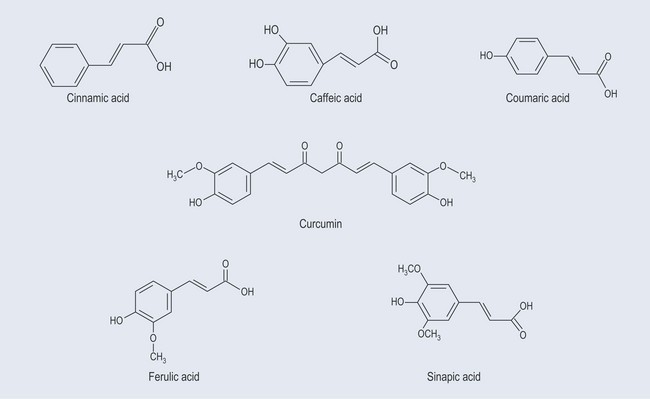
These possess no side chain or a side chain with one carbon atom (Figure 21.3).
Simple Phenols
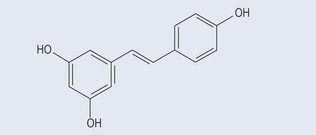
Stilbenes
These are found in the heartwood of plants. They are thought to be responsible for ‘French paradox’ whereby deaths from heart disease in France are lower than in other countries. Resveratrol (Figure 21.6), a component of red wine, acts not only as an antioxidant and an anti-inflammatory but also as an antitumour agent. It is found in blueberries, bilberries, cranberries, and in the skins and seeds of grapes.
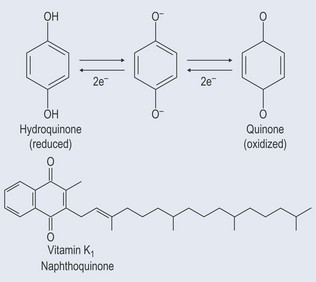
Quinones
Derived from the oxidation of phenols, quinones are closely involved in photosynthesis because they have the ability to gain and lose electrons, which enables the conversion of light energy in a form of energy a plant can use (see Chapter 2 ‘Atoms’, p. 10). They are able to carry electrons between the components that are involved in light reactions (Figure 21.7). Quinones are very lipid soluble.
Naphthoquinones
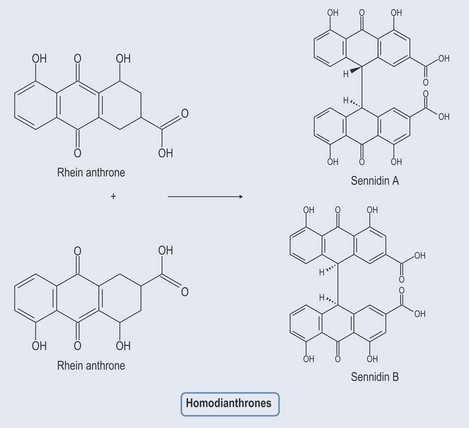
Anthraquinones
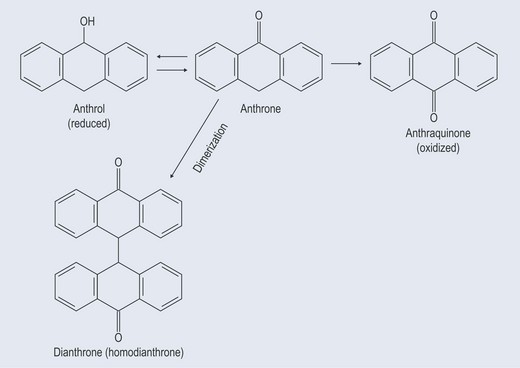
Figure 21.8 Diagram showing the relationship between anthraquinones, anthrones, dianthols and dianthrones.
• Action of Anthraquinones
• Relationship Between Anthraquinone Derivatives
Hypericin is two of the same anthrone joined together to form a homoanthrone.
• Pharmacokinetics of Anthraquinone Action
The glycosides of anthraquinones and dianthrones are polar (see Chapter 3 ‘Bonds’, p. 15) and therefore not easily absorbed across the phospholipid membranes of the cells of the gut wall. Once the bacteria have cleaved the sugar off, the compound becomes non-polar and is absorbed across the non-polar cell membrane of the gut wall cells.
The action of an anthrone (Figures 21.8 and 21.9) is excessively vigorous, which is why certain herbs, such as Frangula (buckthorn bark), have to be stored before use. The storage time allows the anthrones to become oxidized so that they form the less reactive anthraquinone glycosides.
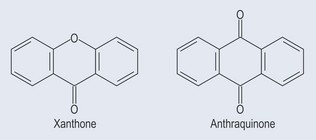
Xanthones
Contribute to the yellow colour of the gentian root. Xanthones (Figure 21.10) are mainly found in:
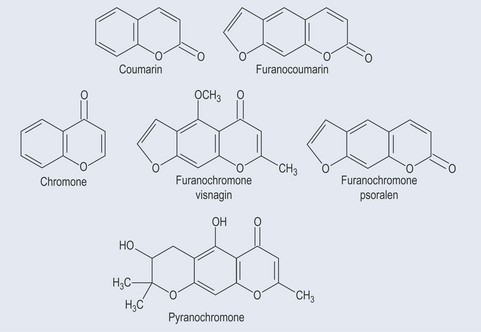
Coumarins
Coumarins are commonly found in:
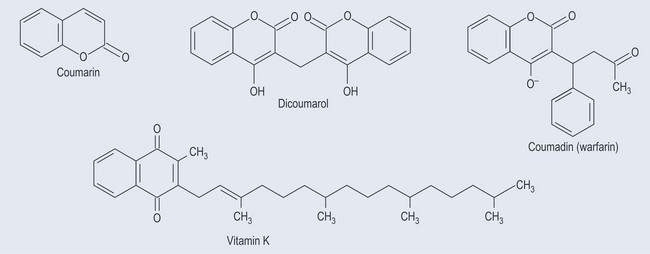
Anticoagulant Actions
Coumarin itself is not an anticoagulant. However, it can be converted into the dicoumarol (usually when the plant is damaged), which is an anticoagulant (Figure 21.12). Dicoumarol interferes with the activation by vitamin K of clotting, as it is similar in structure to vitamin K, and competes with the enzyme involved in vitamin K production (see Figure 28.1, p. 212). Dicoumarol is similar in structure to vitamin K, so is able to inhibit vitamin K production.
Psoralens (Furanocoumarins)
Psoralens are coumarins that possess a furan ring (see Figure 21.11, and compare with the structure of furanose sugar in Figure 9.4, p. 65), which is why they are also called furanocoumarins. This extra ring on the coumarin structure means that they absorb light readily, so consideration of the exposure a patient who is given herbs containing furanocoumarins will be getting to sunlight is important.
Flavonoids
Formation of Flavonoids
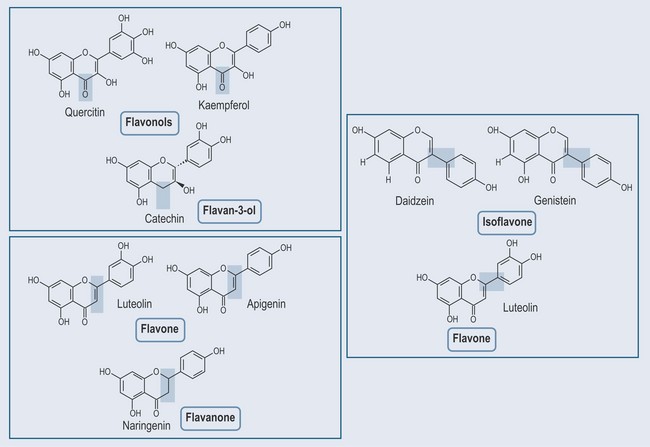
The formation of flavonoids is illustrated in Figure 21.13. The structures of different flavonoids are shown in Figure 21.14.
Colours Associated with Flavonoids
Colour is dependent on the placement of the hydroxyl groups around the basic skeleton:
• Chalcones
• Isoflavones
Differ from flavones by the position of the phenyl group (see Figure 21.14). Found in:
Properties of Soy Isoflavones
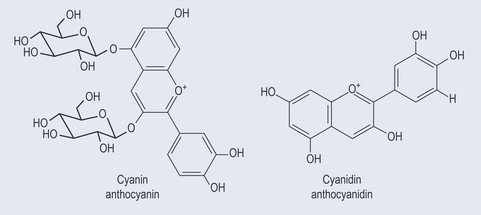
• Anthocyanidins (Figure 21.15)
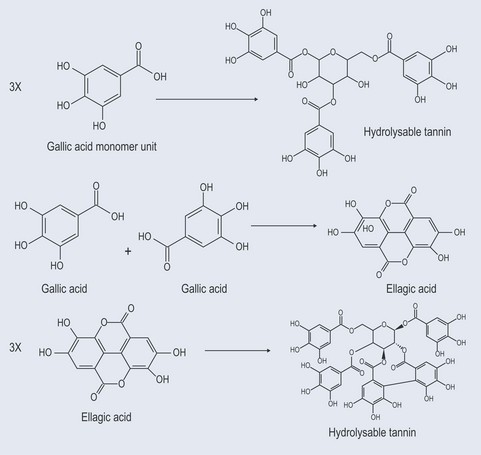
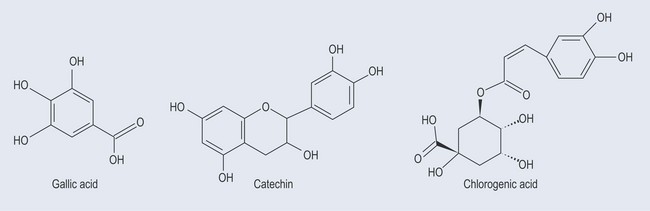
Tannins
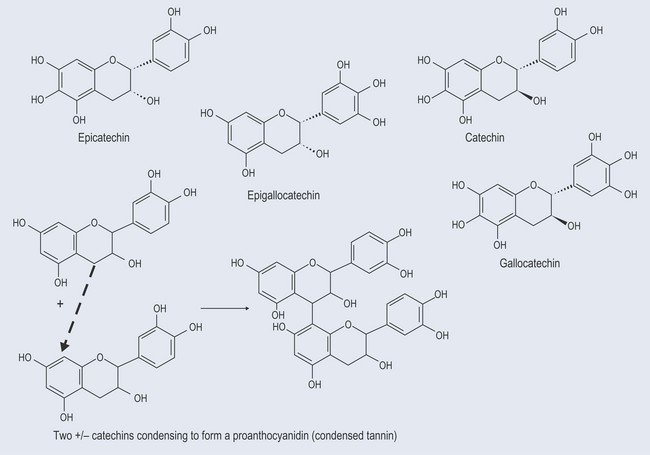
Proanthrocyanidins (Condensed Tannins)
The Importance of Tannin Interactions
Tannins provide astringent and haemostatic properties to a compound. They interact with:
• Reaction with Carbohydrates
• Reaction with Proteins
How Binding Takes Place: Hydrophobic and Hydrogen Bonding
Precipitation is very important and care should be taken when mixing herbs containing tannins; a highly tanninized herb might bind useful constituents. Herbalists can usually see this straight away when they mix tinctures, as they become cloudy. One of the reason that strong black tea is supposed to be good for diarrhoea is that it binds with the proteins in the gut wall and acts as a barrier to prevent water loss.
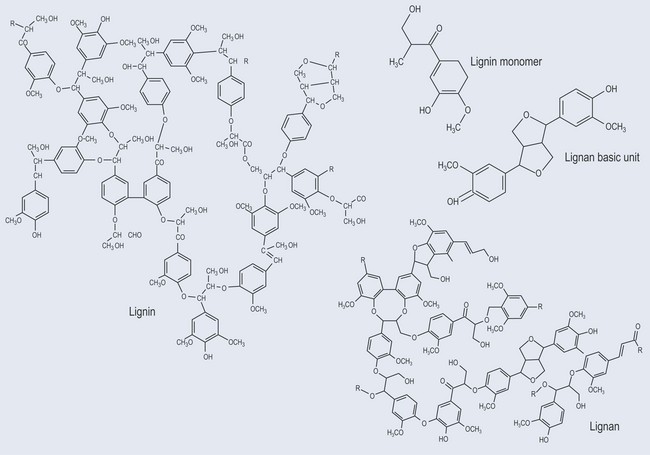
Lignin
Lignans and Lignins
Lignans are made up of two units of a phenylpropene derivative joined by C3 side chains or between the aromatic ring and C3 ring (Figure 21.19). They are found in:
Plant lignans are found in foods such as whole grains, legumes, seeds, fruits and vegetables. The best source of plant lignans are flax seeds. Once in the gut, bacteria change the precursors into mammalian lignans (enterolactone and enterodiol). These lignans appear to have a weak oestrogenic activity, although the effect does not occur by direct stimulation of the oestrogen receptors.