CHAPTER 196 Pharyngitis and Adenotonsillar Disease
Infectious and inflammatory diseases involving the pharynx, tonsils, and adenoids account for a significant proportion of childhood illnesses and pediatric health care expenditures. They often result in two of the most common surgical procedures of childhood, tonsillectomy and adenoidectomy (Fig. 196-1). Clinical research has now helped illuminate this vast area of pediatric otolaryngology, including the effects of adenotonsillar hypertrophy on obstructive sleep apnea and the many possible sequelae of obstructive sleep apnea, the microbiologic flora of the tonsils and adenoids and their role in chronic adenotonsillar hypertrophy, the relationship between adenotonsillar hypertrophy and craniofacial growth, and new techniques for adenotonsillectomy with improved management of perioperative morbidity. This chapter reviews the current understanding of pharyngitis and adenotonsillar disease processes.
History
Celsus was the first to report removal of the tonsils.1 Describing his surgical technique, Celsus indicated that “the tonsils are loosened by scraping around them and then torn out.”1 Hemostasis was obtained using a vinegar mouthwash and painting the tonsillar fossa with a medication to reduce bleeding.2 Aëtius of Amida on the Tigris described a technique for tonsillectomy in the first half of the sixth century, in which a hook was used to snare the tonsil and a knife was used for amputation. He warned of the severe dangers of hemorrhage when excision was too deep.3 Subsequent surgical techniques were described by Paul of Aegina in 625, and Physick described a forceps to facilitate extirpation of the tonsil, which became the forerunner of the modern tonsil guillotine.1,4 Mackenzie improved on the Physick tonsillotome and popularized its use for surgery of the tonsils in the late nineteenth century.5
The adenoids were first described by the Danish physician Meyer. In his 1868 paper “Adenoid vegetations in the nasopharyngeal cavity,” Meyer described in detail his technique of posterior rhinoscopy to diagnose adenoid hypertrophy and recommended removal of adenoid tissue with the aid of a ring knife.6 In 1885, Gottstein described the first adenoid curette.6
Crowe and colleagues7 reviewed 1000 consecutive tonsillectomies performed between 1911 and 1917. In the study “Relation of tonsillar and nasopharyngeal infection to general systemic disorders,” they provided a detailed description of a meticulous surgical technique by sharp dissection and described using the Crowe-Davis mouth gag. The low complication rate they described compares favorably with rates in modern reports of tonsillectomy.
Anatomy
Palatine Tonsil
The palatine tonsil represents the largest accumulation of lymphoid tissue in Waldeyer’s ring and, in contrast to the lingual and pharyngeal tonsils, constitutes a compact body with a definite thin capsule on its deep surface.8 Tonsillar crypts, blind tubules from the epithelium on the surface of the tonsil that are lined with stratified squamous epithelium, extend deep into this tissue.
The tonsillar capsule is a specialized portion of the pharyngobasilar fascia that covers the surface of the tonsil and extends into it to form septa that conduct the nerves and vessels.8 The tonsil is not, therefore, easily separated from its capsule, but the capsule is united largely by loose connective tissue to the pharyngeal muscles. One can easily dissect the tonsil from its normal position by separating the capsule from the muscle through this loose connective tissue.
The tonsillar fossa is composed of three muscles: the palatoglossus muscle, which forms the anterior pillar; the palatopharyngeal muscle, which is the posterior pillar; and the superior constrictor muscle of the pharynx, which forms the larger part of the tonsillar bed.8 The muscular wall is thin, and immediately against it on the outer wall of the pharynx is the glossopharyngeal nerve. This nerve can be easily injured if the tonsillar bed is violated, and not uncommonly the nerve is temporarily affected by edema after tonsillectomy, which produces both a transitory loss of taste over the posterior third of the tongue and referred otalgia.
The arterial blood supply of the tonsil enters primarily at the lower pole, with branches also at the upper pole. There are typically three arteries at the lower pole: the tonsillar branch of the dorsal lingual artery anteriorly, the ascending palatine artery (a branch of the facial artery) posteriorly, and the tonsillar branch of the facial artery between them that enters the lower aspect of the tonsillar bed.8 At the upper pole of the tonsil, the ascending pharyngeal artery enters posteriorly, and the lesser palatine artery enters on the anterior surface. The tonsillar branch of the facial artery is the largest. Venous blood drains through a peritonsillar plexus about the capsule.8 The plexus drains into the lingual and pharyngeal veins, which in turn drain into the internal jugular vein.
The nerve supply of the tonsillar region is through the tonsillar branches of the glossopharyngeal nerve about the lower pole of the tonsil and through the descending branches of the lesser palatine nerves, which course through the pterygopalatine ganglion.8 The cause of referred otalgia with tonsillitis is through the tympanic branch of the glossopharyngeal nerve. Efferent lymphatic drainage courses through the upper deep cervical lymph nodes, especially the jugulodigastric or tonsillar node located behind the angle of the mandible.8
Adenoids
The adenoid or pharyngeal tonsils form the central part of the ring of lymphoid tissue surrounding the oropharyngeal isthmus. The adenoid is composed of lymphoid tissue, with its apex pointed toward the nasal septum and its base toward the roof and posterior wall of the nasopharynx. The adenoid is covered by a pseudostratified ciliated columnar epithelium that is plicated to form numerous surface folds. The adenoid develops as a midline structure by the fusion of two lateral primordia that become visible during early fetal life, are fully developed during the seventh month of gestation, and continue to grow until the fifth year of life, often causing some airway obstruction.6,9 Thereafter, the adenoid gradually atrophies, the nasopharynx grows, and the airway improves.10
The blood supply and drainage are from the ascending pharyngeal artery, the ascending palatine artery, the pharyngeal branch of the maxillary artery, the artery of the pterygoid canal, and contributing branches from the tonsillar branch of the facial artery.9 Venous drainage is to the pharyngeal plexus, which communicates with the pterygoid plexus and then drains into the internal jugular and facial veins. The nerve supply is from the pharyngeal plexus. The efferent lymphatic drainage of the adenoids is to the retropharyngeal and pharyngomaxillary space lymph nodes.9
Immunology of the Adenoids and Tonsils
The adenoids and tonsils are predominantly B-cell organs; B cells account for 50% to 65% of all adenoid and tonsillar lymphocytes.11 Approximately 40% of adenoid and tonsillar lymphocytes are T cells, and 3% are mature plasma cells. Conversely, 70% of the lymphocytes in peripheral blood are T cells.12 The immunoreactive lymphoid cells of the adenoids and tonsils are found in four distinct areas: the reticular cell epithelium, the extrafollicular area, the mantle zone of the lymphoid follicle, and the germinal center of the lymphoid follicle.11
Ample evidence shows that the adenoids and tonsils are involved in inducing secretory immunity and regulating secretory immunoglobulin production. They contain a system of channels covered by specialized endothelium that can mediate antigen uptake much like Peyer’s patches of epithelium in the bowel.13 Both the adenoids and tonsils are favorably located to mediate immunologic protection of the upper aerodigestive tract as they are exposed to airborne antigens. Both organs, specifically the tonsils, are particularly designed for direct transport of foreign material from the exterior to the lymphoid cells.11 This is in contrast to lymph nodes, which depend on antigenic delivery through afferent lymphatics. The tonsillar crypts are covered by stratified squamous epithelium. There are 10 to 30 of these crypts in the tonsils, and they are ideally suited to trapping foreign material and transporting it to the lymphoid follicles.11
The tonsils and adenoids rank among the secondary lymphatic organs. Intratonsillar defense mechanisms eliminate weak antigenic signals. Only when additional higher antigenic concentrations are presented does proliferation of antigen-sensitive B cells occur in the germinal centers.11 Low antigen doses effect the differentiation of lymphocytes to plasma cells, whereas high antigen doses produce B-cell proliferation. The generation of B cells in the germinal centers of the tonsils is considered by Siegel to be one of the most essential tonsillar functions.14
Immunoglobulins (Igs) produced by the adenoid include IgG, IgA, IgM, and IgD.11 IgG appears to pass into the nasopharyngeal lumen by passive diffusion.11 The tonsil produces antibodies locally as well as B cells, which migrate to other sites around the pharynx and periglandular lymphoid tissues to produce antibodies.
T-cell functions, such as interferon-γ production and, presumably, production of other important lymphokines, have been shown to be present in tonsils and adenoids.11 The role played by tonsillar and adenoid T cells in tumor response is still unknown.
The human tonsils are immunologically most active between ages 4 and 10 years. Involution of the tonsils begins after puberty, resulting in a decrease of the B-cell population and a relative increase in the ratio of T to B cells.11,15 Although the overall Ig-producing function is affected, considerable B-cell activity is still seen in clinically healthy tonsils even at age 80 years.16 The situation is different in disease-associated changes, such as when recurrent tonsillitis and adenoid hyperplasia are observed. Inflammation of the reticular crypt epithelium results in shedding of immunologically active cells and decreasing antigen transport function with subsequent replacement by stratified squamous epithelium.17 These changes lead to reduced activation of the local B-cell system, decreased antibody production, and an overall reduction in density of the B-cell and germinal centers in extrafollicular areas.17 In contrast to recurrent tonsillitis, the changes are less pronounced in adenoid hyperplasia, in which the immunoregulatory conditions necessary for maintenance of the B-cell population are well preserved. The reason is most likely that the reticular epithelium is less affected in inflammation of adenoids than of tonsils.
Reports conflict regarding the immunologic consequences of tonsillectomy and adenoidectomy, yet it is clear that no major immunologic deficiencies result from these procedures.16,18 Ogra19 showed a three- to fourfold drop in titers in children previously immunized with live poliovirus vaccine. Attempts to vaccinate seronegative children subjected to tonsillectomy and adenoidectomy have resulted in delayed and lowered nasopharyngeal secretory immune responses as measured by IgA antibodies to the poliovirus.19 Fortunately, poliovirus epidemics are no longer an annual threat. Serum IgA levels in patients who had undergone tonsillectomy were lower than in age-matched controls, but this immunologic change did not appear to be clinically significant. Some studies actually point to improved immunologic activity after tonsillectomy. One study showed better neutrophil chemotaxis after tonsillectomy, and another demonstrated increased IgG and IgM production, possibly as a result of unblocking of the suppression that the immune system was subject to before tonsillectomy.20,21 One large study, with a cohort of 1328 children, showed no higher incidence of atopic disease (asthma, allergic rhinitis, and eczema) in children who had adenotonsillectomy prior to age 8 than in those who had not undergone surgery.22
Bacteriology
Establishment of normal flora in the upper respiratory tract begins at birth. Actinomyces, Fusobacterium, and Nocardia are acquired by age 6 to 8 months.23 Subsequently, Bacteroides, Leptotrichia, Propionibacterium, and Candida are also established as part of the oral flora.24 Fusobacterium populations reach high numbers after dentition and reach maximal numbers at 1 year of age.23 The ratio of anaerobic to aerobic bacteria in saliva is approximately 10 : 1,24 because of variations in oxygen concentration throughout the oral cavity.
Healthy children up to 5 years of age can harbor known aerobic pathogens. Ingvarsson and colleagues24 reported that Streptococcus pneumoniae was recovered in 19% of healthy children, Haemophilus influenzae in 13%, group A Streptococcus in 5%, and Moraxella (Branhamella) catarrhalis in 36%. The frequency of pathogens decreases with age, possibly because of greater immunity. Changes in the pharyngeal bacteria flora noted during viral illnesses are thought to be a result of the increased adherence of Staphylococcus aureus as well as gram-negative enteric organisms.25 Oral pharyngeal colonization during illness-free periods was found to vary from 12% to 18% for gram-negative enteric organisms and from 5% to 14% for S. aureus.26 During an episode of viral upper respiratory tract infection, the colonization rates for gram-negative organisms and S. aureus increased to 60% and 43%, respectively.
Brodsky and Koch27 found substantive differences between the types and numbers of aerobic bacteria found in nondiseased and diseased adenoids. The core samples of normal adenoids showed that 75% of children who were relatively free of upper respiratory disease, otitis media, and symptoms of adenoid obstruction had either no bacterial growth on culture or bacteria that are considered part of the normal flora and not pathogenic. Core samples in adenoids of only 45% of children who had chronic adenoid infection and 39% who had obstructive adenoid hypertrophy had no bacteria growth or only normal flora; the bacteria found in these children were more likely to be β-lactamase producers.
Infections of Waldeyer’s Ring
Many organisms can induce inflammation of Waldeyer’s ring. These include aerobic as well as anaerobic bacteria, viruses, yeasts, and parasites. Some of the infectious organisms are part of the normal oral pharyngeal flora, and others are external pathogens. Because the oropharynx is colonized by many organisms, most infections of Waldeyer’s ring are polymicrobial. These organisms work synergistically and can be demonstrated in mixed aerobic and anaerobic infections.28
Another feature of mixed infections is the ability of organisms resistant to an antimicrobial agent to protect an organism susceptible to that agent by the production of an antibiotic-degrading enzyme that is secreted into the tissues.29 Because of the polymicrobial nature of most infections around Waldeyer’s ring, it is often difficult to interpret data derived from clinical samples obtained from mucosal surfaces and to differentiate between organisms that are colonized and those that are invaders.
Viruses
Herpangina caused by coxsackievirus is characterized by small vesicles with erythematous bases that become ulcers and are spread over the anterior pillar tonsils, palate, and posterior pharynx (Fig. 196-2). Herpes simplex virus commonly causes the well-known “cold sore.” This virus can also cause exudative or nonexudative pharyngitis, mainly in older children and young adults. In younger children, the herpesvirus may induce gingivostomatitis.
Management of viral infections is nonspecific and symptomatic. Antibiotics are helpful in cases of secondary bacterial infection.30
Epstein-Barr Virus
One particular type of viral infection that deserves special attention is the Epstein-Barr virus (EBV). EBV induces the mononucleosis syndrome, which consists of high fever; general malaise; large, swollen, dirty-gray tonsils (Fig. 196-3); sore throat; dysphagia; and odynophagia. Petechiae located at the junction of the hard and soft palate are highly suggestive of EBV infection, although not pathognomonic. Often patients with EBV infection have hepatosplenomegaly with resultant liver damage. The most common method of transmission is oral contact.
Diagnosis of EBV is confirmed by laboratory studies. A differential blood count showing 50% lymphocytosis with 10% atypical lymphocytes is characteristic of EBV infection. Serologic studies include monospot and other serum heterophil antibody titer measurements. Results of these tests may be negative initially, and repeat testing in 1 to 2 weeks is warranted if clinical suspicion of EBV infection is strong. The heterophil antibody titers are detected by the Paul-Bunnell-Davidsohn or ox-cell hemolysis test. If the heterophil antibody agglutination test result is negative, the disease may still be present. Only 60% of patients with infectious mononucleosis have a positive result within the first 2 weeks after onset of the illness; 90% have a positive result 1 month after onset.31 EBV-specific serologic assays have become the method of choice for confirmation of acute or convalescent EBV infection. Figure 196-4 shows the serologic response time. Management of this condition is symptomatic. Recovery may take weeks, and antibiotics are used to treat secondary bacterial infections. Ampicillin should be avoided because a rash may occur despite previous intake without similar reactions. Upper airway obstruction from severely enlarged tonsils can be life-threatening and should be managed promptly with the insertion of a nasopharyngeal airway and short-term high-dose steroid therapy. If the obstruction is severe and unrelieved by these measures, a tonsillectomy or tracheotomy may be necessary.
Neisseria
Pharyngitis as a result of Neisseria gonorrhoeae is common in homosexual men. It has been detected in 6% of adolescents with acute pharyngitis.32 Although the infection is often asymptomatic, it can result and persist after treatment. Acute exudative tonsillitis is a manifestation of gonococcal pharyngitis. The clinical syndrome may range from an asymptomatic to an exudative pharyngitis, but most cases tend to fall toward the exudative pharyngitis end of the spectrum. Nonetheless, disseminated gonococcemia can result even from mild or asymptomatic infection. Penicillin and tetracycline are the most effective therapeutic agents.
Vincent’s Angina
Vincent’s angina is secondary to Spirochaeta denticulata and Vincent’s fusiform bacillus Borrelia vincentii (Treponema vincentii). This condition arises slowly, manifesting both mild local and systemic symptoms. The infection arises most commonly in overcrowded conditions. Patients present with complaints of high fever, headache, sore throat, and physical findings of cervical lymphadenopathy and a membrane on the tonsil that, when removed, reveals an ulcer that remains confined to the surrounding tissue and usually heals in 7 to 10 days.33 Management consists of penicillin therapy. Local treatment in the form of oral hygiene is helpful. This condition may be confused with trench mouth, which is caused by the same organisms but in which oral cavity ulcers include the gums and oral mucous membranes.34
Corynebacterium diphtheriae
The incidence of Corynebacterium diphtheriae infection has declined markedly since the introduction of diphtheria vaccination. This organism causes an early exudative pharyngotonsillitis with a thick pharyngeal membrane. Infection can then spread to the throat, tonsils, palate, and larynx.35 C. diphtheriae organisms also produce a lethal exotoxin that can damage cells in distant organs. Today only 200 to 300 cases of diphtheria occur in the United States each year, usually—but not exclusively—in people who have not been immunized.36 The organism is a gram-positive pleomorphic aerobic bacillus that can be identified in a routine throat culture, particularly when the microbiologist is made aware of a clinical suspicion of this diagnosis. Only toxigenic strains infected with a bacteriophage can cause diphtheria.30 Laryngeal inflammation combined with an exudative, necrotic, gray pharyngeal membrane may result in airway obstruction. Removal of this membrane causes bleeding. Early diagnosis is critical, and the goal of therapy is neutralization of unbound toxin with antitoxin. Myocarditis and neurologic sequelae resembling features of Guillain-Barré syndrome or poliomyelitis may result.35,36 The organism is identified by fluorescent antibody studies. The presence of Klebs-Löffler bacillus in the membrane can be diagnosed with Gram staining.34 Because diphtheria is an emergency condition, antitoxin must be given in the first 48 hours of onset to be effective. Airway obstruction should be managed with tracheotomy. Penicillin in high doses should be administered.
Streptococcal Tonsillitis-Pharyngitis
Group A Streptococcus is the most common bacterial cause of acute pharyngitis.37 The public health importance of this infection lies not only in its frequency but also on the fact that it is a precursor of two serious sequelae, acute rheumatic fever and poststreptococcal glomerulonephritis. Although the incidence of rheumatic fever has decreased, all groups of β-hemolytic streptococci have been associated with rheumatic fever.23
Acute streptococcal tonsillitis is a disease of childhood, with a peak incidence at about 5 to 6 years of age, but can occur in children younger than 3 years and in adults older than 50 years.34 Outbreaks may arise in epidemic forms in institutional settings such as recruit camps and daycare facilities. Acute tonsillitis manifests as a dry throat, malaise, fever, fullness of the throat, odynophagia, dysphagia, otalgia, headache, limb and back pain, cervical adenopathy, and shivering. Examination reveals a dry tongue, erythematous, enlarged tonsils, and yellowish white spots on the tonsils. In severe cases, a tonsillar or pharyngeal membrane or purulent exudate may exist along with jugulodigastric lymph node enlargement.34
The diagnosis of acute tonsillitis is made mainly on clinical grounds. The clinical manifestations of streptococcal and nonstreptococcal pharyngitis overlap so broadly that it is often impossible to make the diagnosis with certainty. For this reason, most authorities recommend that the diagnosis of group A β-hemolytic streptococcal (GABHS) pharyngitis be verified or ruled out by microbiologic tests in patients who appear likely, on the basis of clinical and epidemiologic considerations, to have this illness.37,38 The time-honored method of diagnostic confirmation is the throat culture. This is a simple and extremely useful test but sampling must be skillfully performed by swabbing the posterior pharynx and tonsillar areas.37 The specimen must also be appropriately processed and read. Such selective use of throat cultures represents good medical practice.
One of the major problems in the use of throat cultures in everyday medical practice has been the delay in obtaining results. The delay can range from 18 to 48 hours, which holds up the start of appropriate management but does not increase the likelihood of development of rheumatic fever. Nevertheless, it can be difficult for physicians to persuade parents of the wisdom of withholding antibiotics until results are known, especially if their children are cranky and febrile. If group A streptococcal pharyngitis is treated early in the clinical course, the period of communicability is reduced.39 Management is thus initiated before culture results are available and unfortunately may not be terminated even when negative results are obtained. Development of rapid strep detection tests for the detection of the group A streptococcal antigen has therefore represented a useful advance.
Several rapid tests to detect group A streptococcal antigen in the pharynx have been developed. They employ either latex agglutination or enzyme-linked immunosorbent assay (ELISA) methods to extract the antigen from a swab. The streptococcal group A carbohydrate may be detected within a matter of minutes. The test kits are suitable for use in a physician’s office. Although the rapid detection tests are highly specific, they are unfortunately not as sensitive as routine throat culture.37,40,41 A negative rapid detection test result may prompt the practitioner to withhold antibiotic therapy while awaiting culture results. Most guidelines suggest that throat cultures should be performed when the body temperature is greater than 38.3° C or when the illness is characterized exclusively by a sore throat.42 The most accurate and cost-effective method to diagnose acute GABHS infection is the use of the rapid strep test. This is followed by standard throat culture in patients with a negative rapid strep test result and a strong suspicion of streptococcal tonsillitis.
Even optimally obtained and processed, throat cultures are not without flaws. Throat culture cannot reliably differentiate acute from chronic infection. The treatment of all patients who have positive culture results leads to over-management. There are occasional false-negative results (approximately 10% of cases), although one report has found that patients with false-negative results are most likely carriers who do not require treatment.37 Studies have reported that a single throat culture is 90% to 97% sensitive and 90% specific for GABHS growth.43 The carrier state can be elucidated by serologic testing. A true infection is demonstrated by a positive throat culture result and at least a two-dilutional rise in the antistreptolysin-O titer.44 A GABHS carrier without acute infection has a positive culture result with no change in dilution titer.45 Excluding the diagnosis of group A streptococcal pharyngitis is quite important because the majority of patients with acute pharyngitis do not have “strep” throat.
Therapy has usually been directed at the aerobic pathogens traditionally associated with tonsillitis (e.g., GABHS). Penicillin is still the agent of choice in most cases. However, anaerobic bacteria play a major role in the complications associated with tonsillitis, so they are probably also involved in recurrent tonsillitis. One study has documented the prevalence of Bacteroides cultured from chronically inflamed tonsils.46 Anaerobes also have been implicated in acute tonsillitis.23 Clinical failure of penicillin should lead to the suspicion of β-lactamase–producing organisms. The reason for the treatment failure could be that these organisms either act as pathogens themselves, so-called direct pathogens, or protect susceptible pathogens from the effects of β-lactamase antibiotics, making them so-called indirect pathogens. In such cases, the patient complains of sore throat that never resolves completely despite penicillin management. An alternative to the use of penicillin is to use a penicillin plus a β-lactamase inhibitor such as clavulanic acid (e.g., amoxicillin/clavulanic acid). Other alternatives are clindamycin and a combination of erythromycin and metronidazole. Institution of antibiotic therapy within 24 to 48 hours of symptom onset will result in decreased symptoms associated with sore throat, fever, and adenopathy 12 to 24 hours sooner than without antibiotic administration. The use of antibiotics also minimizes the chance of suppurative complications and diminishes the likelihood of acute rheumatic fever.47,48 Ten full days of therapy is necessary, as eloquently demonstrated by Schwartz and colleagues,49 who showed that children receiving 10 days of therapy have lower clinical and bacteriologic recurrence rates than children receiving only 7 days of therapy.
There appears to be no need for further management for asymptomatic carriers because the carrier state does not lead to suppurative or nonsuppurative complications nor is the patient likely to spread the disease to others.50 Although asymptomatic patients need neither culture nor management if results of follow-up culture are positive, certain high-risk situations should be approached differently. For example, if a family member had rheumatic fever or if a family has been experiencing recurrent streptococcal illness, another course of antibiotics would be recommended for the carrier. Patients who become symptomatic after an appropriate course of therapy may indeed represent true management failures for which a second course of therapy would also be justified.50
Tonsillar Concretions/Tonsilloliths
Tonsillar concretions or tonsilloliths (Fig. 196-5) arise from retained material and bacterial growth in the tonsillar or adenoid crypts and may exist in patients with or without a history of inflammatory disorders in either the tonsils or adenoids.51 The clinical presentation of fetor oris (halitosis) and sore throat as well as the presence of whitish, expressible, foul-tasting, and foul-smelling cheesy lumps from the tonsils characterizes the tonsillar concretions in many patients. Local management involves simple expression of the concretions by the patient, the use of pulsating jets of water to clean the pockets of debris mechanically, or application of topical silver nitrate to the tonsillar crypts in an effort to chemically cauterize and obliterate them. Persistent problems with pain, halitosis, foreign body sensation, or otalgia may require surgical removal of the tonsils as definitive therapy.
Complications of Tonsillitis
Nonsuppurative Complications
Scarlet fever is secondary to acute streptococcal tonsillitis or pharyngitis with production of endotoxins by the bacteria.33 Manifestations include an erythematous rash; severe lymphadenopathy with a sore throat; vomiting; headache; fever; erythematous tonsils and pharynx; tachycardia; and a yellow exudate over the tonsils, pharynx, and nasopharynx. The membrane that is present over the tonsils is usually more friable than that seen with diphtheria. A strawberry tongue with a rash and large glossal papillae is a good diagnostic sign. Diagnosis of scarlet fever is made by culture and positive result of the Dick test, which is an intradermal injection of dilute streptococcal toxin.34 Management of this condition involves intravenous administration of penicillin G. Otologic complications may include necrotizing otitis media with complete loss of the tympanic membrane and ossicles.
Fortunately, acute rheumatic fever is an uncommon illness in the United States today. The incidence of rheumatic fever following sporadic streptococcal infection is approximately 0.3%.50 A patient with rheumatic fever who does not comply with penicillin prophylaxis should have a tonsillectomy and adenoidectomy because patients who have undergone surgery have a lower infection rate with β-hemolytic streptococcus.52
Poststreptococcal glomerulonephritis may be seen after both pharyngeal and skin infections. The incidence is approximately 24% after exposure to nephrogenic strains, but these strains account for less than 1% of the total pharyngeal strains.50 Typically, an acute nephritic syndrome develops 1 to 2 weeks after a streptococcal infection. The infection is secondary to the presence of a common antigen of the glomerulus with the streptococcus. Penicillin management may not decrease the attack rate, and there is no evidence that antibiotic therapy affects the natural history of glomerulonephritis. A tonsillectomy may be necessary to eliminate the source of infection.
Suppurative Complications
Peritonsillar Infections
Peritonsillar abscess most commonly occurs in patients with recurrent tonsillitis or in those with chronic tonsillitis that has been inadequately treated. The spread of infection is from the superior pole of the tonsil with pus formation between the tonsil bed and the tonsillar capsule.33 This infection usually occurs unilaterally and the pain is quite severe, with referred otalgia to the ipsilateral ear a few days after the onset of tonsillitis. Drooling is caused by odynophagia and dysphagia. Trismus is frequently present as a result of irritation of the pterygoid musculature by the pus and inflammation. There is gross unilateral swelling of the palate and anterior pillar with displacement of the tonsil downward and medially with reflection of the uvula toward the opposite side. Cultures of the peritonsillar abscess usually show a polymicrobial infection, both aerobic and anaerobic.53
Cellulitis should be differentiated from abscess in the management of peritonsillar infections. Some abscesses may be clinically obvious, whereas others are less obvious. When there is extension of infection of the peritonsillar abscess, computed tomography (CT) with contrast enhancement may be indicated (Fig. 196-6).
The choice between needle aspiration and incision and drainage in the management of peritonsillar abscesses is controversial. Traditional management has consisted of incision and drainage, with tonsillectomy 4 to 12 weeks later. Some surgeons advocate immediate tonsillectomy or Quinsy tonsillectomy as definitive management to ensure complete drainage of the abscess and to alleviate the need for a second hospitalization for an interval tonsillectomy.54 Each therapeutic modality has advantages in certain situations. If incision and drainage or needle aspiration fails to drain an abscess adequately, a Quinsy tonsillectomy is indicated. In patients with a prior history of recurrent peritonsillar abscess or recurrent tonsillitis severe enough to warrant tonsillectomy, a Quinsy tonsillectomy should be considered. Quinsy tonsillectomy is particularly favored in children because they are likely to experience further episodes of tonsillitis, and needle aspiration or incision and drainage with a child under local anesthesia is often difficult or impossible.
Parapharyngeal Space Abscess
An abscess can develop in the parapharyngeal space if infection or pus drains from either the tonsils or from a peritonsillar abscess through the superior constrictor muscle.33 The abscess is located between the superior constrictor muscle and the deep cervical fascia and causes displacement of the tonsil on the lateral pharyngeal wall toward the midline. Involvement of the adjacent pterygoid and paraspinal muscle with the inflammatory process results in trismus and a stiff neck. The thickness of the overlying sternocleidomastoid muscle often prevents the detection of fluctuance by palpation.
Retropharyngeal Space Infections
The superior limit of the retropharyngeal space is the cranial base. Inferiorly, the retrovisceral space extends into the mediastinum to approximately the level of the tracheal bifurcation. The buccal pharyngeal fascia is adherent to the prevertebral fascia in the midline, so that infection in the retropharyngeal space is unilateral. Lateral neck radiography, CT, or ultrasonography may help ascertain whether there is cellulitis or a true abscess (Fig. 196-7). The source of the retropharyngeal abscess is a chain of lymph nodes present on either side of the midline in the retropharyngeal space. These lymph nodes receive drainage from the nose, paranasal sinuses, pharynx, and eustachian tube.
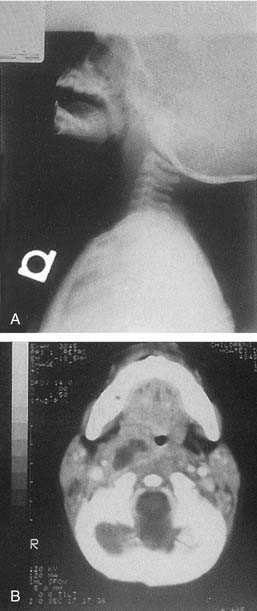
Figure 196-7. A, Lateral neck radiograph showing a retropharyngeal abscess. B, Computed tomography scan of the same patient.
A transoral approach is recommended for incision and drainage of retropharyngeal space abscesses. If the abscess extends inferiorly below the hyoid bone (shown on the CT scan), an external approach should be used. The patient must undergo oral intubation, which can be done safely by introduction of the tube on the side opposite the abscess to avoid aspiration of purulent material. The patient must be positioned in the head-down Trendelenburg position, and packing should be placed around the endotracheal tube inferiorly. Gram staining, culture, and antimicrobial sensitivity staining should be performed on the purulent material. A small vertical incision is made in the lateral aspect of the posterior pharyngeal wall at a point between the junction of the lateral one third and medial two thirds of the distance between the midline of the pharynx and the medial aspect of the retromolar trigone.55 The space is gently probed with a hemostat to break up the loculations and drain the abscess. A drain is not used because of the possibility of aspiration if swallowed postoperatively. If the abscess extends laterally to involve the parapharyngeal space, it should be drained through an external approach.
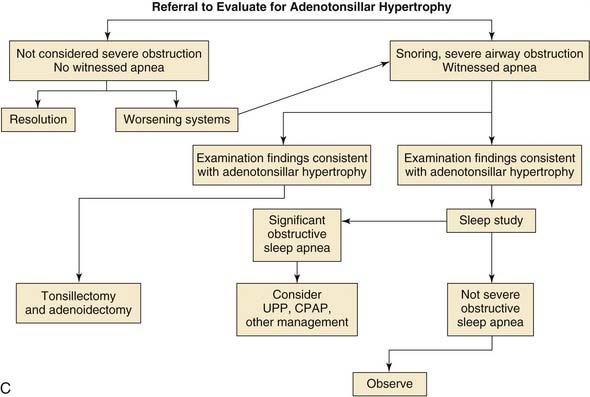
Chronic Adenotonsillar Hypertrophy
Etiology
Chronic adenotonsillar hypertrophy—manifesting as various degrees of airway obstruction in children—has become the most common indication for adenotonsillectomy in the United States.56 Typically, the tonsils and adenoids are very small at birth and progressively enlarge over the first four years of life as a result of increased immunologic activity (Figs. 196-8 and 196-9). Brodsky and colleagues27,57 reported that hypertrophied and chronically infected tonsils and adenoids had greater loads of pathogenic bacteria, especially β-lactamase producers, than nondiseased tonsils and adenoids.57,58 These studies were based on core samples that were believed to be more accurate than surface cultures of the tonsils and adenoids. It is possible that equilibrium exists between the normal flora of the adenotonsillar tissue and their local immunologic response and that this equilibrium can become disrupted with recurrent acute viral or bacterial infections or colonization with pathogenic bacteria, resulting in hypertrophied lymphoid tissue.27
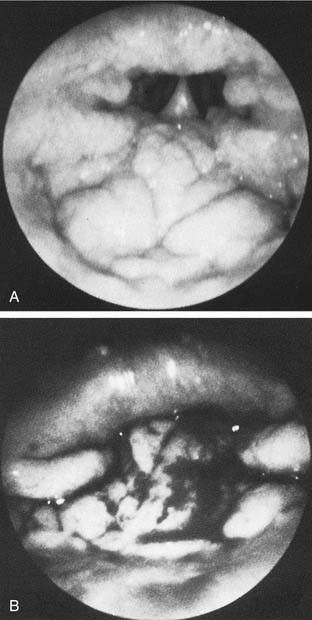
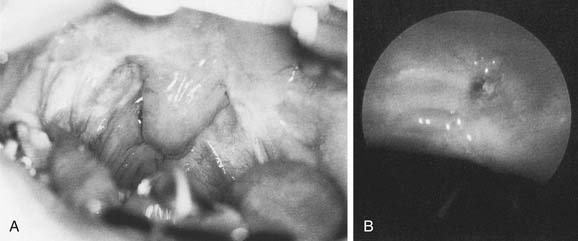
Figure 196-9. A, Endoscopic view of mild adenoid hypertrophy. B, Severe adenoid hypertrophy with total choanal obstruction.
In addition to chronic bacterial infection, exposure to second-hand smoke has been implicated as a cause of adenotonsillar hypertrophy in children.8
Airway Obstruction
Obstructive sleep apnea is the most common indication for tonsillectomy in the pediatric population. Pediatric obstructive sleep apnea, confirmed by polysomnography, is reported to have an incidence of 1% to 3%.59–61 The obstructive apnea is almost always associated with hypertrophy of the tonsils and adenoids. The tonsil and adenoid tissue, when large, fills the area of the oropharynx and nasopharynx, obstructing airflow. This obstruction is worse when the patient is supine and asleep owing to the effects of gravity and the relaxation of surrounding nasopharyngeal and oropharyngeal soft tissue. The obstruction can result in a mildly compromised airway, which leads to snoring, and most children with airway obstruction related to adenotonsillar hypertrophy have a history of significant snoring at night.28,31,59,62,63 The obstruction may lead to intermittent complete airway obstruction with subsequent apnea and oxygen desaturation. The apnea typically is short and usually associated with a brief arousal wherein the patient repositions himself or herself and opens the airway. However, if the apnea is prolonged, oxygen desaturation can occur. Such desaturation episodes put stress on the cardiovascular system.
Before the syndrome of obstructive sleep apnea was widely recognized, children sometimes presented with pulmonary hypertension and cor pulmonale, failure to thrive, and developmental delay.64 Such severe consequences rarely occur now, but the sleep disturbance manifests as multiple clinical symptoms that are commonly seen. Some of the more common symptoms that occur during the sleep of affected children are loud “heroic” breathing, diaphoresis, apnea, gasping, mouth-breathing, restless sleep, enuresis, drooling, night terrors, and sleepwalking. During the day affected children may suffer from daytime sleepiness, morning headache, dry mouth, halitosis, swallowing difficulty, behavioral difficulties and hyponasal speech, or, rarely, hypernasal speech (rhinolalia aperta) caused by enlarged tonsils impinging on normal palatal movement.63 Behavioral difficulties include hyperactivity, inattentiveness in the classroom, problems with academic performance, and rebellious or aggressive behavior. The aforementioned behavioral difficulties are also clinical manifestations of the most commonly diagnosed psychiatric diagnosis in children—attention deficit–hyperactivity disorder (ADHD).
Today obstructive sleep apnea and its consequences are widely recognized, and for that reason it is the primary indication for tonsillectomy in this country.65 There is no debate about the existence of the obstructive sleep apnea syndrome or that adenotonsillectomy is the treatment of choice. However, there is considerable debate about the methods of diagnosis and therefore considerable discussion about exactly how large a group of children is affected by the syndrome (see Chapter 183 for a full discussion).
Attention Deficit–Hyperactivity Disorder
Numerous articles in the literature have shown a significant relationship between sleep-disordered breathing and the symptoms of ADHD. One study investigated 996 children aged 4 to 5 years seen consecutively in a community health clinic. Of the 782 for whom the questionnaires were completed, 95 or 12% of the children were found to snore on most nights. A group of 66 children considered at high risk for sleep disturbance was selected from these 95 children and compared with a control group who had no symptoms of sleep disturbance. The study used a modified version of the Conner’s behavioral scale to assess symptoms of hyperactivity. These 66 children were studied with overnight oximetry and video monitoring. According to results of the overnight study, only 7 of the 66 children in the high-risk group had documented obstructive sleep apnea syndrome, but all the children in this high-risk group had significantly higher scores on parental and teacher reports of hyperactive behavior.60
Another study looked at the relationship between the symptoms of sleep-disordered breathing and problem behavior including hyperactivity and inattention. The study enrolled 3019 5-year-old children. The researchers used a questionnaire to evaluate for symptoms of sleep-disordered breathing as well as problems with behavior, and had a subset of 219 children’s families complete the Revised Conners’ Parent Rating Scale as a means of validating the results obtained with limited initial questioning. Symptoms of sleep-disordered breathing were present in 25% of the children. A strong association was found between parent-reported sleep problems and the parent-reported incidence of inattention, hyperactivity, and aggressiveness. Interestingly, a dose-response effect on problem behaviors was seen for both snoring frequency and snoring loudness. These results remained significant even when data were adjusted for sex, race, maternal education level, maternal marital status, household income, and respiratory history. Such adjustments had not been made in previous reports on the link between SDB and ADHD.66
A different study evaluated 2076 children and found 71 with behavioral or academic problems. These children had significantly more problems with snoring and difficulty breathing. This association was found to be strongest with children who had academic problems or in children whose specific behavioral problem was consistent with ADHD.67
Other studies have examined this issue from the opposite direction, evaluating a group of children diagnosed with neuropsychological problems and searching for signs and symptoms of SDB. These studies show that children with neuropsychological problems have a higher percentage of sleep problems than normal controls. Marcotte and associates looked at 200 children referred for psychiatric evaluation for possible ADHD; 79 children were diagnosed with ADHD, learning disability, or combined ADHD–learning disability. Through parent questionnaires, the investigators found a high incidence of reported sleep problems at night in comparison with the incidence in a control group of children. They did not find any difference in the reported length of sleep. Thus, the effect seems to depend on quality, not quantity, of sleep.68
Another study surveyed a group of parents at a child psychiatry unit and a group of patients at a general pediatric clinic. Children at the psychiatry clinic with the diagnosis of ADHD had a 33% incidence of habitual snoring, compared with 11% of the other children at the psychiatry clinic and 9% in those at the general pediatric clinic. Higher snoring scores derived from the validated pediatric sleep questionnaires were associated with higher levels of inattention and hyperactivity, again pointing to an apparent dose-response relationship.69
The pathophysiology of ADHD is unknown, but there are suggestions that an abnormal sleep pattern may be one causal factor. It has long been known that stimulants improve behavior in patients with ADHD. Stimulants are believed to work because ADHD is a disorder caused by hypovigilance rather than hypervigilance.70 If hypovigilance is indeed the mechanism, it makes sense that a child with decreased or poor sleep secondary to obstructive sleep apnea syndrome would be much more likely to suffer from symptoms of ADHD. Consequently, if the sleep obstruction was removed, it should follow that the quality of sleep would improve and therefore symptoms of ADHD would diminish.
One small article in the European literature did show that symptoms of ADHD diminished after adenotonsillectomy.71 At this point, however, no large prospective studies have looked carefully at this issue. This is an area of active research in the literature and one that receives a lot of attention, but still more work must be done to better clarify the cause-and-effect relationships.
Neurocognitive Development
Multiple studies have indicated that children with a childhood history of snoring and SDB may have alteration in normal neurocognitive development. One study evaluated 297 first-grade children whose school performance was in the lowest 10th percentile in their class ranking. With both a parental questionnaire and overnight pulse oximetry monitoring and transcutaneous monitoring of partial pressure of carbon dioxide, the group was screened for signs and symptoms of a sleep disorder. The researchers found that 54 (18.1%) of these children had sleep-associated gas exchange abnormalities. Parents of these children were all offered surgical treatment, and 24 underwent tonsillectomy and adenoidectomy; parents of the remaining 30 refused surgical treatment. The group undergoing surgical treatment had a significant improvement in school scores the following year, whereas, the group refused treatment and a control group had no change in year-to year scores.72
In another study, the author sent questionnaires to the parents of seventh and eighth graders whose test scores placed them in either the top 25% or bottom 25% of the class. These students were matched for age, gender, race, address, and schools. The parents were sent questions related to their child’s sleeping habits from age 2 to 6 years, in which the primary indicators of sleep disturbance were snoring frequency and loudness. Only those with reported loud frequent snoring during childhood years were included in the group with suspected sleep disorder. The few children with current snoring were excluded. The investigators ultimately had 800 students in each group and found that frequent and loud snoring was reported in 12.9% of the low-performing students, but in 5.1% of the high-performing children.73 To the investigators, these results indicated that a significant sleep disturbance during the early important years of neurocognitive development may create deficits that cannot be overcome later in life, after the sleep disturbance has resolved.
One study compared the results of neurocognitive testing between 16 snoring children and 13 normal controls aged 5 to 10 years. Of the snoring children, 13 completed polysomnograms and none had clinically defined obstructive sleep apnea. The researchers found that the snoring children had lower attention, memory, and intelligence scores. They concluded that the impact of clinically mild SDB may be greater than suspected and may significantly affect future development of the child.74
Another study looked at a large community sample of 5-year-old children. The investigators screened the children for SDB and found that those with symptoms of SDB had significantly lower scores on a wide range of neurocognitive studies. These studies included assessments of executive function, memory, and general intellectual ability. All the test results were adjusted for any potentially confounding sociodemographic or health issues.66 This study found that the differences in cognitive function were present even if the children with polysomnography-confirmed obstructive sleep apnea were excluded. Thus, even in children with what has been referred to as “primary snoring” were shown in this study to have measurable neurocognitive deficits.
The mechanism underlying possible cognitive deficits remains unclear. Three major processes occur during the sleep of children with upper airway obstruction—episodic hypoxia, repeated arousal leading to sleep deprivation or sleep fragmentation, and periodic or continuous alveolar hypoventilation combined with intermittent hypercapnia. Which of these sequelae or in what proportion they may be responsible for cognitive impairment is not known.73 Central nervous system development is an ongoing process from infancy to adolescence, and developing neural elements are most likely more susceptible to injury. Neurocognitive deficits in children affected with SDB may be more prominent in the area of the prefrontal cortex because these areas do not finish development until adolescence.75
The National Institutes of Health–sponsored 2003 National Sleep Disorders Research Plan states, “In recent years it has become apparent that SDB and snoring are not as innocuous as previously thought.” The report continues that failure to diagnose and treat such disorders in a timely manner may lead to long-lasting residual consequences. “However, the point of transition between what constitutes pathology and what is normal has yet to be defined.”76 In an editorial, one researcher active in this area discussed the accumulating evidence of a causal relationship between pediatric SDB and neurocognitive deficits. He concluded his thoughts, “The remaining but complex challenge will be to define at what age, and by what means, intervention will be required to prevent long-term neurocognitive dysfunction.”77
An article by Garetz78 offers a good review of the literature surrounding the issues of behavior and cognition associated with SDB. She discusses the need for further research while pointing out that none of the studies in this area has been randomized, and few studies have addressed well how ethnicity and obesity may play a role in these areas. Clearly, many questions are left unanswered at this point. First, we do not know what the dose-response relationship is between SDB and neurocognitive impairment. How long should a child be allowed to snore before treatment is recommended? We know snoring resolves spontaneously in many children, so how long do we allow a child’s snoring to persist before we have concern that cognitive function will be affected? Is the tolerable length of time related to the age of the child—is a child more sensitive to the effects of sleep disturbance at 3 years or 7 years, and thus should we wait less time in a 3- year-old with significant SDB? These are all questions that further research must address.
Enuresis
Enuresis is another indicator of severe underlying airway obstruction in children. Weider and associates79 described enuresis related to chronic adenotonsillar hypertrophy and significant airway obstruction that was relieved by adenotonsillectomy. In this study, all patients with secondary enuresis (developed later during childhood) showed response to adenotonsillectomy, whereas 24 patients with primary enuresis (congenitally present) showed no response to surgery, possibly as a result of other neurologic factors. A proposed cause of enuresis is poor nocturnal regulation of antidiuretic hormone release that is related to disorders of rapid eye movement (REM) sleep.
Growth Problems
Children with chronic adenotonsillar hypertrophy and airway obstruction may also present with failure to thrive.64,80 A review of the literature further confirmed this apparent relationship between SDB secondary to adenotonsillar hypertrophy and the risk of growth failure in children.81 This relationship may be related to abnormal regulation of growth hormone. During rapid eye movement sleep, growth hormone may be severely disrupted in children with obstructive sleep apnea.80 Several studies have confirmed a postoperative increase in circulating insulin-like growth factor and its binding proteins in children with obstructive sleep apnea secondary to adenotonsillar hypertrophy.82,83
Cardiopulmonary Complication
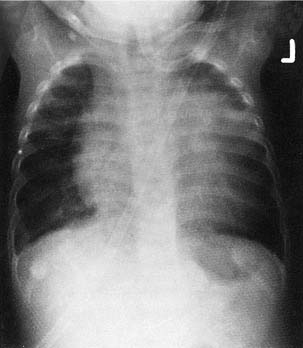
Severe cases of sleep obstruction may result in cor pulmonale, pulmonary vascular hypertension, and alveolar hypoventilation, all of which may be reversed by adenotonsillectomy (Fig. 196-10).19,84,85 The etiology of cor pulmonale is related to chronic upper airway obstruction, which leads to pulmonary ventilation-perfusion abnormalities and chronic alveolar hypoventilation. The result is chronic hypercapnia and hypoxia with respiratory acidemia, pulmonary artery vasoconstriction, and right ventricular dilation. Eventual cardiac failure may occur.31,86,87 Relief of upper airway obstruction by adenotonsillectomy will eventually reverse this condition. However, increased partial pressure of carbon dioxide (PCO2) values may persist after relief of the obstruction, occasionally requiring prolonged endotracheal intubation and mechanical ventilation until PCO2 values return to normal.
Craniofacial Growth and Adenotonsillar Hypertrophy
Chronic mouth-breathing secondary to adenotonsillar hypertrophy and upper airway obstruction has been shown to affect craniofacial growth patterns in children. As early as 1872, Tomes88 reported that children who were chronic mouth-breathers secondary to adenoid hypertrophy displayed evidence of malocclusion and maxillofacial growth abnormalities. Mouth-breathing leads to downward and backward displacement of the mandible and tongue and potential postural changes of the head and neck that may secondarily affect dental occlusion and jaw growth.89 Numerous animal and human studies have demonstrated the effect of chronic nasal obstruction on maxillofacial growth patterns. Harvold90 showed that chronic nasal obstruction may lead to narrow maxillary dental arches in rhesus monkeys. Linder-Aronson and colleagues91 demonstrated the classic stigmata of adenoid facies in children with chronic nasopharyngeal obstruction from adenoid hypertrophy. These consisted of longer total anterior face height with a tendency toward a retrognathic mandible in comparison with controls.
Adenotonsillectomy has been shown to reverse some of these findings.91,92 Other investigators have confirmed the relationship between chronic nasal obstruction, adenotonsillar hypertrophy, and increased facial height.93,94 A later study showed a significant correlation between adverse cephalometric data and adenotonsillar hypertrophy in a group of children 4 to 12 years old.95 Although there is significant support in the literature for the effect of upper airway obstruction and adenotonsillar hypertrophy on craniofacial growth, other studies have been less conclusive about this relationship.94,96,97 Maxillofacial growth disturbances in children who are mouth-breathers may also be multifactorial in etiology.98 As a result of this apparent relationship among upper airway obstruction, adenotonsillar hypertrophy, and craniofacial growth disturbance, an otolaryngologic evaluation may be warranted in children undergoing orthodontic procedures for malocclusion who show evidence of adenotonsillar hypertrophy and mouth-breathing.
Diagnostic Assessment of the Tonsils and Adenoids
The physical examination of patients with suspected adenotonsillar disease should include a thorough head and neck examination to rule out manifestations of chronic adenotonsillar hypertrophy (i.e., the craniofacial stigmata of chronic nasal obstruction, which includes open-mouth breathing, an elongated face, dark circles under the eyes, and evidence of dental malocclusion). Other possible causes of nasal obstruction and these findings (e.g., turbinate hypertrophy secondary to allergic rhinitis) should also be evaluated. It is important to listen to the patient’s speech to assess the presence of hyponasality. Having the child repeat words such as “Mickey Mouse,” which emphasize nasal emission, and “baseball,” which does not, better illuminates the presence of hyponasality.4
Brodsky and coworkers57 described an assessment scale for tonsillar hypertrophy. In this scale, 0 indicates that the tonsils do not impinge on the airway; 1+ indicates less than 25% airway obstruction; 2+ indicates 25% to 50% airway obstruction; 3+ indicates 50% to 75% airway obstruction; and 4+ indicates more than 75% airway obstruction. It is also important to assess the palate on oral examination. Patients with evidence of overt or submucous cleft palate are at increased risk for the development of velopharyngeal insufficiency (VPI) after adenoidectomy. Submucous cleft palate may manifest only as a bifid uvula. However, notching of the posterior hard palate may be palpable, and an obvious translucent line through the mid–soft palate may be evident (Fig. 196-11). Although diagnostic assessment of the tonsils is apparent with oral examination, evaluation of adenoid tissue is much more difficult because it is not easily accessible on physical examination.
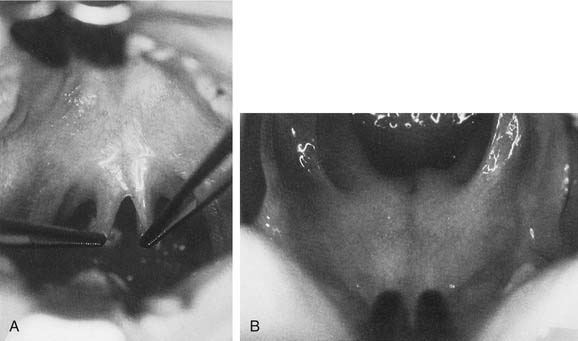
Figure 196-11. A, Bifid uvula in a patient with a submucous cleft palate. B, Submucous cleft palate.
Lateral neck radiography may be helpful in assessing adenoid hypertrophy (Fig. 196-12). Fujioka and associates99 determined that the adenoid-nasopharyngeal ratio, measured by lateral neck radiography, closely correlated with clinical symptomatology related to adenoid hypertrophy. This conclusion has been confirmed by other reports.100 However, physiologic variations during nasal and oral breathing may affect these nasopharyngeal dimensions as measured by lateral neck radiography.101 Furthermore, during an upper respiratory or sinonasal infection, significant nasopharyngeal secretions and purulence can obscure the nasopharynx and give the false impression of adenoid obstruction.
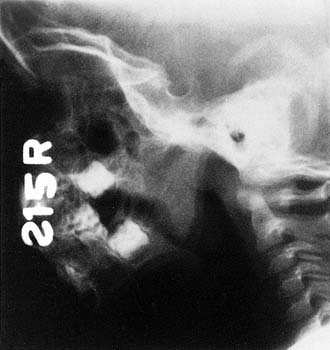
Figure 196-12. Lateral neck radiograph demonstrating significant adenoid hypertrophy and nasopharyngeal obstruction.
Flexible endoscopic nasopharyngoscopy may also be valuable in the assessment of adenotonsillar disease. With appropriate topical anesthesia, pediatric endoscopes, and reassurance from the physician, children generally tolerate this procedure well. The presence of adenoid tissue obstructing the posterior nasal choana will be apparent with this technique. The degree of hypopharyngeal extension of tonsillar hypertrophy may also be apparent. Sometimes tonsils that do not appear significantly large on oral examination can be seen to impinge upon and actually push back the epiglottis when viewed from above with the flexible laryngoscope. VPI secondary to tonsillar hypertrophy limiting palatal movement will be apparent on nasopharyngoscopy. The presence of adenoiditis may also be diagnosed from the presence of purulent secretions covering the adenoid pad. Wormald and Prescott102 and Wang and others103 demonstrated the greater efficacy of flexible nasopharyngoscopy in comparison with lateral neck radiography and clinical symptomatology in the assessment of adenoid hypertrophy in children. Rhinomanometry has also been demonstrated to correlate with the presence of nasal obstruction secondary to adenoid hypertrophy; however, this test is not well tolerated by children, is difficult and time-consuming to administer, and is probably not of clinical benefit in its current form.104
The role of polysomnographic testing in children has been previously reported.64,80,105 Because of the high cost of polysomnography, it is important for the otolaryngologist to be selective in referring patients for this diagnostic modality. Furthermore there are few dedicated pediatric sleep laboratories and the wait for a study can often be very long. Patients with obvious symptoms related to adenotonsillar hypertrophy confirmed by physical examination most likely do not require polysomnographic testing. Patients with significant symptomatology—suggesting sleep apnea or a significant sleep disturbance without evidence of significant adenotonsillar hypertrophy on examination—should undergo polysomnographic testing to determine the severity of the sleep disturbance. Children with significant comorbidities that may increase surgical risk are also candidates for a polysomnogram to help in the preoperative risk-benefit assessment of surgical intervention. A polysomnogram may also help in the evaluation of the neurologically compromised child, in order to differentiate between obstructive and central apnea, because surgery is not likely to improve the latter. Screening chest radiography and electrocardiography are also recommended in the preoperative assessment of severe cases.
Preoperative Assessment
Preoperative assessment in patients undergoing adenotonsillectomy is crucial and may reveal problems that could complicate either surgery or the patient’s postoperative course. It is crucial to detect the existence of any coagulation abnormalities. A family history of coagulation disorders or easy bruising may be a warning sign of an underlying bleeding disorder that warrants further hematologic evaluation. Routine evaluation of coagulation parameters before surgery in patients undergoing adenotonsillectomy is controversial. Manning and colleagues,106 examining the records of 994 of 1050 consecutive patients undergoing tonsillectomy, adenoidectomy, or adenotonsillectomy, determined that evidence of coagulation disorders in patients with no clinical history of or findings consistent with a hematologic disorder was extremely low, thereby not justifying routine preoperative coagulation studies. This conclusion was confirmed by Close and associates,100 who suggested that excessive bleeding associated with tonsillectomy was usually not the result of an identifiable coagulation disorder.
Conversely, Kang and coworkers107 studied the risk of postoperative hemorrhage in 1061 children undergoing adenotonsillectomy. In this study, 2.5% of the children had at least one abnormality on preoperative coagulation screening, which consisted of determinations of prothrombin time, partial thromboplastin time, bleeding time, and platelet count. These researchers suggested that, although hematologic disorders were diagnosed infrequently by preoperative coagulation screening, the coagulation profile may detect patients who are more likely to bleed postoperatively.107 This suggestion is consistent with the findings of Bolger and colleagues,108 who demonstrated abnormal initial coagulation screening results in 11.5% of patients undergoing adenotonsillectomy. It is apparent that patients who have an obvious family or clinical history of excessive bleeding or an underlying hematologic disorder require close monitoring of coagulation profiles and consultation with a hematologist. Use of preoperative coagulation screening should be left to the discretion of the surgeon until its role is better clarified by further study.
Patients with other medical conditions may require further testing or preoperative consultation. Patients with a history of significant bronchospasm may require pulmonary medicine evaluation and management in the perioperative period. Black patients should be screened for sickle cell disease preoperatively, and girls of reproductive age should undergo a preoperative serum β-human chorionic gonadotropin test to rule out pregnancy. Velocardiofacial syndrome may require preoperative angiography to determine the presence of abnormally medially displaced carotid arteries, which may be at risk during tonsillectomy.109
Adenotonsillectomy
The technique of adenotonsillectomy has evolved significantly over the past 2000 years, and various techniques are used today for extirpation of the tonsils and adenoids. Multiple tonsillectomy techniques exist, including the sharp dissection initially described by Crowe and associates7; various electrocauterization techniques; lasers (including potassium-titanyl–phosphate [KTP]110 and CO252); the tonsil guillotine; as well as newer technologies and techniques such as Coblation (ArthroCare Corporation, Austin, TX), the Harmonic Scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH), and the Microdebrider (Medtronic Xomed, Inc., Jacksonville, FL). The number of adenotonsillectomies performed in the United States appears to have peaked in the 1940s and 1950s.56 In 1959, 1.4 million tonsillectomies were performed. This number decreased to 500,000 in 1979 and fell even further to 340,000 in 1985.50,56 In the past 30 years there has been a significant decrease in the number of adenotonsillectomies performed in the United States, as well as a change in the preoperative indications for surgery. Whereas chronic infection was the primary surgical indication for adenotonsillectomy in the 1950s and 1960s, airway obstruction and obstructive sleep apnea have now become the most common preoperative indications for surgery.56 Apparent drops in the number of adenotonsillectomies performed in the United States reflect a higher degree of selectivity by otolaryngologists and referring primary care physicians.
Indications
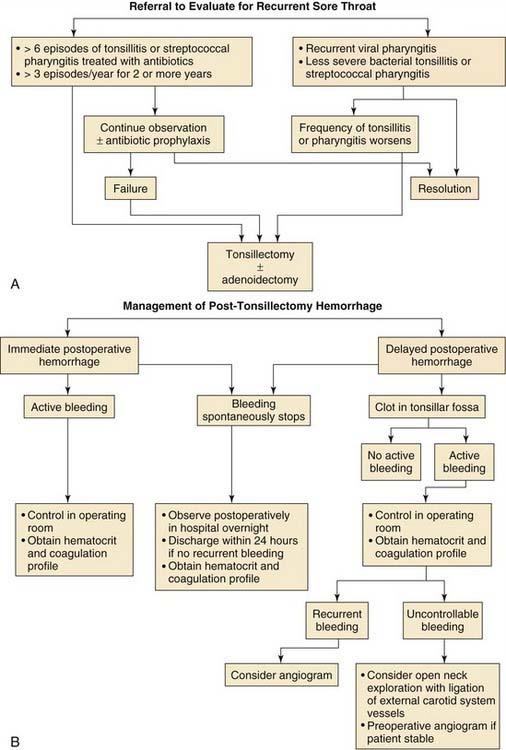
The current indications for tonsillectomy and adenoidectomy are shown in Boxes 196-1 and 196-2. Generally, the indications for adenotonsillectomy can be related primarily to chronic upper airway obstruction in conjunction with adenotonsillar hypertrophy, which manifests as snoring, obstructive sleep apnea, or chronic infectious conditions such as chronic recurrent tonsillitis.
Box 196-1 Surgical Indications for Tonsillectomy
Infection
Upper Airway Obstruction
Patients with airway obstruction related to adenotonsillar hypertrophy typically present with excessive snoring and sometimes with witnessed brief apneic events. The severity of the condition can often be elicited with a history supplied by the parents. However, there may often be no indications of airway obstruction other than loud snoring. It has been demonstrated that patients who snore loudly at night may manifest significant sleep apnea on polysomnographic testing when no significant parental history of witnessed apnea has been noted.111 Patients who have obvious adenotonsillar hypertrophy on physical examination, a significant history of loud snoring with the associated symptoms of restless, disturbed sleep, or daytime somnolence do not necessarily need preoperative polysomnographic testing. If the parent is unclear about the severity of airway obstruction or if the physical findings and history are not consistent, preoperative polysomnographic testing is warranted to demonstrate the severity of airway obstruction.
Chronic Infections
Patients with chronic recurrent tonsillitis or chronic tonsillitis may benefit from tonsillectomy and possibly adenoidectomy. In 1995, the American Academy of Otolaryngology–Head and Neck Surgery Clinical Indicators Compendium stated that patients who have three or more infections of the tonsils and/or adenoids per year despite adequate medical therapy are candidates for tonsillectomy and adenoidectomy.112 Other indications are chronic tonsillitis unresponsive to medical therapy that results in a persistent foul taste or halitosis and recurrent tonsillitis associated with the streptococcal state that has not responded to β-lactamase–resistant antimicrobial therapy.112 The efficacy of elective tonsillectomy for recurrent sore throats has been demonstrated by numerous investigators.113,114 The efficacy of elective tonsillectomy has also been shown in children with recurrent throat infections, including GABHS.114
In a study by Paradise and associates,114 187 children participated in a randomized clinical trial. Inclusion criteria for the study were seven or more sore throat episodes in the preceding year that were treated with antibiotics; five or more sore throat episodes in the 2 preceding years; or three or more episodes in each of the 3 preceding years. Elective tonsillectomy or adenoidectomy resulted in better status than that in a control group of children who were managed nonsurgically. Factors that should be taken into account in the consideration of surgery for recurrent throat infections in children are (1) the severity of each episode, (2) how well infections have responded to medical therapy, and (3) quality of life issues (e.g., number of school days missed).
Tonsillectomy is the management of choice for peritonsillar abscess in children who do not tolerate needle aspiration. Peritonsillar abscesses that have been successfully drained by needle aspiration are not necessarily absolute indications for tonsillectomy. However, tonsillectomy is definitely indicated in cases of recurrent peritonsillar abscess.54,115–117
Adenoidectomy
Indications
Adenoid hypertrophy or chronic adenoiditis may cause significant problems requiring adenoidectomy in situations in which the tonsils themselves are not diseased and are not contributing to symptomatology. Patients with chronic adenoid hypertrophy causing craniofacial morphology problems, excessive snoring, or, possibly, quality of life issues (e.g., poor olfaction) are candidates for adenoidectomy.118 Adenoid hypertrophy may be confirmed by flexible nasopharyngoscopy or lateral cervical radiography.
Patients with a history of chronic recurrent sinusitis may also benefit from adenoidectomy. Patients with chronic sinusitis and significant adenoid hypertrophy may initially benefit from adenoidectomy rather than undergoing more extensive sinus surgery.119 In addition, patients with chronic purulent rhinitis secondary to chronic adenoiditis may also have response to adenoidectomy if their rhinitis has not responded well to appropriate medical therapy. Evidence now points to a possible reason for the efficacy of adenoidectomy in these situations. Coticchia and colleagues120 showed that biofilms were prominent in the adenoids of children with chronic sinusitis. The group compared pediatric adenoids removed for chronic sinusitis with those removed for obstructive sleep apnea. In the sinusitis-related adenoids, 94.9% of the mucosal surface was covered with dense mature biofilms, compared with just 1.9% of the surface in the hypertrophy-only adenoids. This study suggests that the biofilms may be a natural reservoir for resistant bacteria and that their removal during adenoidectomy may be the reason for the observed benefit of the procedure.
Patients with hyponasal speech (rhinolalia clausa) are also candidates for adenoidectomy. Hypernasal speech (rhinolalia aperta) is a contraindication to adenoidectomy. However, in unusual cases, excessive tonsillar hypertrophy may impede palatal movement, which may improve after tonsillectomy.121
Although surgical intervention should be considered in cases of severe nasal obstruction related to adenoid hypertrophy, there is evidence that alternative medical therapy exists to manage adenoid hypertrophy. Demain and Goetz122 demonstrated that aqueous nasal beclomethasone therapy led to significant improvement of nasal obstruction secondary to adenoid hypertrophy, which was confirmed by pre- and post-management flexible nasopharyngoscopy. In addition, patients with underlying inhalant allergies may benefit from antihistamine therapy and, possibly, from allergic immunologic desensitization therapy.
Adenoidectomy and Otitis Media
The role of the adenoids in the etiology of otitis media has been controversial. However, several investigations have demonstrated that appropriate management of the adenoids plays an important role in the diagnosis and management of chronic otitis media in children. Numerous reports have demonstrated that adenoidectomy alone or in conjunction with myringotomy tube placement may reduce the incidence of future episodes of otitis media and possibly reduce the necessity for future ventilation tubes.123,124 Gates and colleagues123 randomly assigned 578 children between the ages of 4 and 8 years to four management groups; group 1 underwent bilateral myringotomy and no ventilation tube placement; group 2 underwent placement of ventilation tubes; group 3 underwent adenoidectomy alone; and group 4 underwent adenoidectomy and placement of ventilation tubes. They demonstrated with statistical significance that patients who underwent adenoidectomy in conjunction with bilateral myringotomy had significantly lower postmanagement morbidity, as measured by hearing loss secondary to middle-ear effusion and the number of subsequent placements of ventilation tubes. Paradise and associates124 randomly studied 213 children who had previously received ventilation tubes that had since extruded. These patients were randomized into two groups, those undergoing adenoidectomy and those who did not (control group). The adenoidectomy group had statistically significant less otitis media than control subjects. Another, large study further demonstrated the effectiveness of adenoidectomy in this regard. Kadhim and associates,125 evaluating data on 50,000 children younger than 10 years who had undergone myringotomy tube placement, found that adenoidectomy at the time of tube placement decreased the odds of needing subsequent tube placement regardless of whether or not the child was having adenotonsillar disease. The actual mechanism by which adenoidectomy affects the course of otitis media in children is unclear. Surgical extirpation of the adenoids may remove a nasopharyngeal nidus of contaminated tissue that secondarily acts as a source of infection in the middle ear, or adenoidectomy may simply remove an anatomic obstruction of the eustachian tube. The actual size of the adenoid pad has not necessarily been implicated in the etiology of chronic otitis media with effusion. Many studies have been unable to determine the relationship between adenoid size and chronic otitis media with effusion.126–129
On the basis of current evidence, adenoidectomy should be considered in children undergoing primary ventilation tube placement who have symptomatology suggestive of chronic nasal obstruction or adenoid hypertrophy that is confirmed by nasopharyngoscopy or nasopharyngeal radiography. Patients who require subsequent sets of ventilation tubes also may be candidates for adenoidectomy, regardless of adenoid size or symptomatology. If surgery is elected, proper technique is important, as Bluestone115 and McKee130 reported that surgical manipulation of the eustachian tube during adenoidectomy may predispose children to eustachian tube malfunction and the subsequent development of otitis media with effusion.
Adenotonsillectomy: Surgical Technique
Many surgical techniques have been described for extirpation of the tonsils and adenoids. Crowe and associates7 described the first meticulous surgical dissection technique with use of sharp instrumentation. More recently, however, dissection with electrocautery has become the most popular and common technique. Other current techniques are the Coblator (ArthroCare Corporation), the Microdebrider, the ultrasonic Harmonic Scalpel, bipolar electrocautery, and, less frequently, CO2 or KTP laser tonsillectomy.62,110,131–135 A 2007 survey of pediatric otolaryngologists showed monopolar cautery to be by far the most common method of tonsillectomy, favored by 53% of the respondents. Coblation at 16% was the second most common method, cold dissection combined with electrocautery was third at 10%, followed by bipolar electrocautery at 6%.136 Evidence also suggests that sharp dissection may lead to slightly less postoperative pain. However, there may be less intraoperative blood loss with electrocautery techniques.132,137,138 Guillotine tonsillectomy techniques are still used and have been shown to have a low complication rate.140–141 In China, the technique of guillotine tonsillectomy in children without anesthesia has been described.142
Of the new technologies, Coblation tonsillectomy has received possibly the most attention and acceptance. Coblation technology utilizes a system of radiofrequency bipolar electrical current that passes through a medium of normal saline, which results in the production of a plasma field of sodium ions. These energized ions are able to break down intercellular bonds and effectively vaporize tissue at a temperature of only 60° C. This vaporization theoretically results in effective dissection with less postoperative pain from thermal injury.143,144 The technique can be utilized for complete tonsillectomy or for intracapsular tonsillectomy, otherwise known as tonsillotomy, in which the tonsil is debulked, leaving a small amount of lymphoid tissue to cover the inferior constrictor muscle. Multiple newer studies suggest decreased pain and recovery time with Coblation than with electrocautery and the Harmonic Scalpel.145–147 These studies report no higher incidence of postoperative hemorrhage with this technique. Some researchers, however, have found a higher bleeding rate with the Coblator, and this issue remains a concern for some writers.148–150
The Harmonic Scalpel utilizes ultrasonic technology to cut and coagulate tissues, resulting in minimal tissue damage from thermal trauma. The device converts electrical energy from a generator into mechanical vibration through a transducer that consists of piezoelectric ceramics, generating a back-and-forth vibration of the blade at a frequency of approximately 55.5 kHz. The device can cut tissue on low- and high-power settings and can also coagulate bleeding tissue. Initially, the Harmonic Scalpel found its use in laparoscopic surgery in the field of pediatric and general surgery. However, its use in tonsillectomy has been reported to have encouraging results.103,151 Wiatrak and Willging151 reported on a prospective study consisting of 117 patients undergoing either traditional electrocautery technique or Harmonic Scalpel surgery. They found a trend toward decreased postoperative pain over the first 3 postoperative days and a significant increase in the ability to sleep comfortably through the first three postoperative nights in children undergoing Harmonic Scalpel surgery. The duration of the procedure was longer for the Harmonic Scalpel than for electrocautery. The complication rates were equivalent in the two groups. At this time the Harmonic Scalpel cannot be utilized for adenoidectomy, so other techniques must be used for that operation. A concern about its use is higher costs incurred for each blade utilized for tonsillectomy. Other studies have shown the Harmonic Scalpel to be an effective instrument for tonsillectomy but have not found it to have a significant advantage over electrocautery.152
Subtotal tonsillectomy has become a more popular technique over the last several years. The concept of partial intracapsular tonsillectomy or tonsillotomy is not new to otolaryngology.153 This procedure can be performed using any of the described instruments but is now most commonly performed with powered instrumentation.154,155 Ideally in this technique, the only tissue manipulated and dissected is the tonsil itself. No mucosal cuts are made, the peritonsillar capsule is not dissected, and there should not be any direct cauterization of the peritonsillar fascia and underlying pharyngeal musculature. All of the preceding features are thought to make the postoperative pain and discomfort much less with this technique. With use of a microsurgical debrider, the hypertrophied tonsils are sequentially shaved behind the levels of the anterior and posterior tonsillar pillars. Exposure is obtained by retraction of the anterior tonsillar pillar with a Herd retractor. Hemostasis is obtained with suction cautery. Multiple studies point to a reduced need for pain medicine and more rapid return to normal activity and normal diet with subtotal microsurgical debrider tonsillectomy.156–158 The incidence of postoperative bleeding is not increased with this technique and may actually be slightly less than with traditional electrocautery.159 However, at least one study did not show a significant difference in return to normal activity and pain resolution between these two methods.160 The indications for this technique are controversial, with some advocating this technique as appropriate for all indications in all ages and others stating that it should be performed only in children without a history of tonsillitis, owing to the concern about persistent tonsillitis in the residual tonsil tissue. Cost is a factor in the use of disposable microsurgical debrider blades for partial intracapsular tonsillectomy. At this point there is little evidence that the residual tissue is associated with recurrent tonsillitis as the child ages, but more long-term data are needed for confirmation. The most significant concern with this technique is the possibility of regrowth and the subsequent recurrence of obstructive symptoms. In one series of 278 patients, the incidence of regrowth was 3.2%.161 This event is rare but does occur, and the patient’s parents must be made aware of this possibility during the informed consent discussion for this procedure.
The microsurgical debrider can be effective for performing adenoidectomy. Koltai and others have reported on and shown the safety and efficacy of this technique.155,162–165 Koltai, Chan, and Younes166 report that the utilization of power-assisted adenoidectomy allows more precise, rapid, and safe removal of adenoid tissue. This technique is especially useful in removing adenoid tissue that extends through the choanae and into the nasal cavity. However, the cost of microsurgical debrider blades continues to be an issue related to its use. Stanislaw and coworkers,167 in a prospective evaluation of power-assisted adenoidectomy or curette adenoidectomy in 90 children, found that power-assisted adenoidectomy was 20% faster, involved 27% less blood loss, and provided a more complete resection and better control of depth of reception. It also had higher surgeon satisfaction.
Technique Description
Electrocautery tonsillectomy using a meticulous dissection technique and low electrocautery wattage results in very minimal intraoperative blood loss and acceptable postoperative discomfort. This technique is described in detail. Instrumentation is shown in Figure 196-13.
Patients should not eat solid foods for 8 hours before surgery. If the patient is closely supervised, he or she may drink clear liquids until 3 hours before surgery. Preoperative sedation with midazolam hydrochloride (0.5 to 1 mg/kg) approximately 30 minutes before surgery may be necessary for patients with excessive preoperative anxiety. Anesthesia is typically induced by mask ventilation, with subsequent intravenous access after the anesthetic has taken effect. Patients usually receive intravenous antibiotics such as ampicillin (20 mg/kg; maximum dose, 1 g). Telian and colleagues168 demonstrated that intravenous antibiotics followed by a 1-week course of postoperative oral antibiotics significantly decreased morbidity after tonsillectomy.
Patients who have a significant history of obstructive sleep apnea or are younger than 3 years receive intraoperative corticosteroids, most commonly dexamethasone (0.5 mg/kg). Catlin and Grimes169 reported that one dose of intraoperative intravenous corticosteroids improved recovery time in children following tonsillectomy. However, Ohlms and associates170 and Volk and coworkers171 demonstrated no significant effects on recovery time of a single dose of intravenous steroids. We use corticosteroids to potentially reduce airway edema in patients who are more susceptible to airway complications after tonsillectomy. Although we do not routinely use peritonsillar infiltration with local anesthetic agents, numerous reports have described short-term benefit of this procedure with regard to postoperative pain in the immediate recovery period after tonsillectomy.172–174 Peritonsillar infiltration with local anesthesia may also decrease intraoperative blood loss in patients undergoing sharp-dissection tonsillectomy.172
After induction of general anesthesia and placement of an endotracheal tube, the patient is placed in Rose’s position (Fig. 196-14A) and a Crowe-Davis mouth gag is placed for exposure of the oropharynx. A red rubber catheter is placed through the right nares, brought back out through the oral cavity, and clamped with a Kelly clamp to retract the soft palate for better visualization of the nasopharynx. At this point the hard and soft palate are examined by visual inspection and palpation for evidence of a submucous cleft palate. Adenoidectomy should not be performed in the presence of an occult submucous cleft palate unless absolutely necessary. Conservative adenoidectomy should be considered in these situations. The Microdebrider is especially useful in this rare situation.
Adenoidectomy in the United States is still most commonly performed with adenoid curettes, which have either straight or curved handles.175 The LaForce adenotome also has been described as an instrument for adenoid extirpation.176 The nasopharynx is inspected with a nasopharyngeal mirror to assess the size of the adenoid pad and guide placement of the curette. The curette is placed high in the nasopharynx so it abuts the posterior aspect of the nasal septum. The adenoid pad is then carefully curetted, with care taken not to penetrate deeply into the prevertebral region. Care must also be taken during use of the curette not to extend too far laterally and thus potentially risking damage to the torus tubarius and eustachian tube orifice. Small curettes may be used to remove residual adenoid tissue in the posterior choanal region. This step entails removal of the superior portion of the adenoid pad and preservation of the inferior aspect with cautery or curettes. Packs are placed into the nasopharynx to obtain hemostasis.
After tonsillectomy has been performed, the nasopharynx is reexamined, and hemostasis is obtained with suction electrocautery. It is important to remember that the mouth-gag tongue blade places pressure on the base of tongue and should be released intermittently to allow blood circulation in the tongue and possibly aid in reducing postoperative pain. A throat pack may be used to prevent blood and secretions from entering the esophagus. Swallowing of blood and secretions may lead to postoperative nausea and vomiting. In addition, blood oozing around the endotracheal tube may form a laryngeal clot, which may lead to postextubation airway obstruction. Children undergoing adenotonsillectomy typically have an uncuffed endotracheal tube, which may allow leakage of air around the tube with positive-pressure ventilation. Fires and endotracheal tube explosions have been reported during tonsillectomy, presumably related to oxygen leaking around uncuffed endotracheal tubes.177
After removal of the tonsillar fossa packs, residual bleeding may be controlled with electrocautery on a low-wattage setting. Significant bleeding vessels may have to be clamped and ligated. Great care should be taken in the use of suture ligatures in the tonsillar fossa. The external maxillary and lingual arteries may inadvertently be ligated in the region of the inferior tonsillar pole, thus leading to sloughing and severe hemorrhage. In addition, the internal carotid artery, which is in close proximity to the tonsillar fossa, may be damaged inadvertently, resulting in complications such as pseudoaneurysm formation and excessive hemorrhage.52 After hemostasis has been obtained in the oropharynx and nasopharynx, the oral and nasal cavities are thoroughly irrigated. The hypopharynx is suctioned to evacuate all secretions and potential blood clots, and the stomach is also suctioned at this point. The patient is then awakened and extubated.
In general, accepted practice is to admit for overnight admission patients who have a significant history of obstructive sleep apnea, those younger than 3 years, and those with other medical problems or extenuating circumstances. If they are taking oral fluids adequately, all patients undergoing outpatient surgery are discharged after an observation period of approximately 2 to 4 hours. Intravenous fluids are administered according to deficit replacement and maintenance until oral intake resumes satisfactorily; lactated Ringer’s solution or 0.5 normal saline is used. Acetaminophen with or without codeine is given for analgesia. A 1-week course of oral antibiotics is also prescribed. The patient’s diet advances from clear liquids on the day of surgery to a soft diet the following day and then solid foods subsequently as tolerated. Soft foods are generally well tolerated. However, patients with unrestricted diets have not been shown to have significant complication rates postoperatively.6,58,178 Patients or their parents are instructed to return immediately to the emergency room if there is any evidence of bright red bleeding from the nose or oral cavity.
Inpatient versus Outpatient Procedures
It has been clearly demonstrated in the literature that adenotonsillectomy in appropriately selected children may be performed on an outpatient basis.14,25,105,179,180 It is important that patients are observed postoperatively and that they obtain significant postoperative intravenous rehydration. Postoperative observation times discussed in the literature vary from 136 minutes to 6 to 8 hours.133,181 Although short-stay outpatient tonsillectomy has been demonstrated as a safe and cost-effective procedure, it is important preoperatively to identify outpatients who are at risk for postoperative complications and who may benefit from longer observation periods following surgery.
Numerous writers believe that children younger than 3 years should be admitted for observation after surgery because they are at increased risk for postoperative complications, most of which are airway-related.94,180,182–185 The ultimate decision for postoperative admission lies with the surgeon. Other patients who may warrant admission after adenotonsillectomy include those with (1) a history of an abnormal bleeding disorder or abnormal preoperative coagulation profile, (2) evidence of severe obstructive sleep apnea, (3) underlying craniofacial abnormalities such as Down syndrome, and (4) systemic disorders such as cardiopulmonary disease or asthma. Postoperative admission also should be considered for patients undergoing tonsillectomy for peritonsillar abscess, for patients who live a long distance from the hospital (>60 miles or 1 hour driving time), or for patients whose home situation is not consistent with close postoperative observation.116,117,186
Complications
Serious postoperative complications secondary to surgery of the tonsils and adenoids are primarily related to pain, hemorrhage, airway obstruction, postoperative pulmonary edema, VPI, nasopharyngeal stenosis, and death.187 Meticulous attention to surgical technique and technical advances in modern anesthesiology have significantly reduced the number of complications related to adenotonsillectomy. Paradise reported an overall complication rate of 14%; all of these complications were self-limiting.124 Richmond described a series of 794 patients undergoing adenotonsillectomy, of whom 4.2% had variable degrees of postoperative bleeding and 1.3% had self-limiting postoperative airway obstruction. In 9409 pediatric patients undergoing adenotonsillectomy, Crysdale and Russel188 reported an overall hemorrhage rate of 2.15%, with only 0.06% of patients requiring a second general anesthetic for hemostasis. For reasons other than the hemorrhage (including fever, airway distress, pneumonia, inadequate oral intake, vomiting, and pain), 0.6% of the patients required delayed hospitalization. The incidence of serious surgical complications has been reported to be approximately 15 cases per 1000.189 Pulmonary abscess, although dreaded in the past, is now extremely rare. However, this complication may still be encountered, as may other rare complications, including Horner’s syndrome, optic neuritis, meningitis, brain abscess, paralysis of the glossopharyngeal and recurrent laryngeal nerves, salivary fistulas from the submandibular gland to the tonsillar fossa, and mediastinal emphysema.187,190,191
Postoperative Hemorrhage
Postoperative hemorrhage is the most common serious complication of adenotonsillectomy. Reported postoperative hemorrhage rates range from 0.5% to 10%, although variable rates have been reported with different surgical techniques.188,192–195 Meticulous attention to surgical technique and intraoperative hemostasis, regardless of the technique used, will lead to acceptable postoperative hemorrhage rates. Detection of underlying coagulation disorders by history and physical findings or through preoperative screening in appropriately selected patients will help avert significant intraoperative blood loss. Appropriate preoperative and intraoperative measures are taken in conjunction with a hematology consult. Certain analgesics (e.g., ketorolac tromethamine) have been associated with increased hemorrhage rates when given in the perioperative period and should be avoided in patients undergoing adenotonsillectomy.63,196
The extensive blood supply of the tonsillar fossa is composed of a complex network of anastomosing arteries primarily arising from the ipsilateral external carotid artery system. However, there are contributions from the internal carotid and vertebral arteries, and contralateral vessels circulate through the circle of Willis.197 Therefore, extensive intraoperative or postoperative hemorrhage may not always cease after ligation of the external carotid artery. In rare, severe cases, ligation of other branches (e.g., the maxillary, facial, lingual, or superior thyroid artery) may be necessary.192
The arterial blood supply of the nasopharynx and adenoids arises from both the internal and external carotid arterial system. However, bleeding in this region can be controlled most commonly with compression and electrocautery. In severe cases, application of caustic agents (e.g., tannic acid, bismuth subgallate) or hemostatic agents (e.g., Avitene) may be beneficial. Aberrant arterial vessels in the oropharynx and nasopharynx may be damaged intraoperatively (Fig. 196-15). McKenzie and Wolfe97 reported an aberrant carotid artery arising in Rosenmüller’s fossa that was injured during adenoidectomy, necessitating subsequent ligation of the common carotid artery with resultant transient hemiplegia.
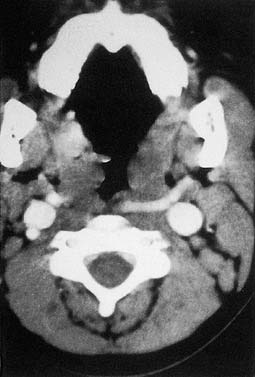
Figure 196-15. Computed tomography scan with contrast enhancement demonstrating an aberrant artery in the left retropharyngeal region.
Bleeding can be intraoperative, immediately postoperative (within 24 hours), or delayed (after 24 hours). Intraoperative hemorrhage may be related to an underlying coagulopathy or possibly to major arterial damage in severe cases. Steps to control major intraoperative hemorrhage include use of suction cautery or ligation or, possibly, placement of a pack in the tonsillar fossa and over-suturing of the tonsillar pillars to provide constant compression of the fossa. Suture ligatures may damage underlying arterial vessels, thereby worsening the bleeding or possibly leading to delayed postoperative hemorrhage and should be placed with extreme care if they are used.87,198 In severe cases, ligation of larger arteries through an open-neck exploration may be necessary. However, this should be an extremely uncommon step. Persistent hemorrhage from the nasopharynx after adenoidectomy is often related to residual remnants of adenoid tissue that should be completely removed to best obtain hemostasis.
Delayed postoperative bleeding, although typically not as dangerous as intraoperative or immediately postoperative bleeding, may be fatal if not managed appropriately. Patients or parents should be well informed before discharge about the possibility of delayed postoperative hemorrhage and should be told to return for immediate evaluation if there is any evidence of bright red bleeding from the nose or mouth. The most common time for delayed postoperative bleeding is between the fifth and seventh postoperative days. When hemorrhage occurs, patients should be returned to the operating room for immediate hemostasis if active bleeding is confirmed on physical examination. If no active bleeding is apparent and a blood clot is evident in the tonsillar fossa, it should not be disturbed. However, if the surgeon cannot determine whether active bleeding is taking place, the clot should be suctioned to allow better examination. Blood clots often hide bleeding vessels and may prevent appropriate coagulation as a result of fibrinolysis.199 Patients with delayed hemorrhage who are not actively bleeding and have a blood clot present in the tonsillar fossa should at least be admitted for overnight observation. A coagulation profile and hematocrit measurement should be obtained.
Airway Obstruction and Pulmonary Edema
Postoperative airway obstruction may occur after adenotonsillectomy, especially in children younger than 3 years.58,182 Edema of the tongue, nasopharynx, and palate may necessitate temporary replacement of a nasal trumpet and also may require intravenous corticosteroid therapy. Dislodging of pharyngeal clots may obstruct the larynx postoperatively, resulting in death.200 Therefore, all clots should be evacuated from the pharynx before termination of the procedure.
Velopharyngeal Insufficiency
Velopharyngeal insufficiency (VPI), or hypernasality, is a relatively unusual complication primarily related to adenoidectomy. In patients with excessively large tonsils and preoperative VPI, the velopharyngeal insufficiency may actually improve after the obstructive tonsils have been removed. Patients with a history of cleft palate or evidence of an occult submucous cleft palate on physical examination (see Figure 196-11) should not undergo adenoidectomy unless it is absolutely necessary. In these situations conservative or partial adenoidectomy should be performed. The actual incidence of VPI after adenoidectomy in otherwise healthy children has been estimated to be between 1 in 750 and 1 in 1459.189,201 It is important to determine preoperatively whether there is a family history of VPI or whether the patient had a prior history of hypernasal speech or nasal regurgitation as an infant. These signs may herald the development of postoperative VPI. If an underlying speech abnormality exists preoperatively, speech therapy evaluation should be performed. It also is important to determine whether reported hyponasal speech is truly related to a nasal obstruction or is actually related to hypernasal speech, which can be misinterpreted by parents.
Nasopharyngeal Stenosis
Nasopharyngeal stenosis is an extremely difficult management problem that requires surgical intervention for satisfactory resolution. This situation may arise from excessive cauterization with extensive mucosal destruction involving the nasopharynx, lateral nasopharyngeal wall, and superior tonsillar pillar along with excessive resection of posterior tonsillar pillar tissue. Other predisposing factors are performance of surgery during an acute episode of pharyngitis or purulent sinusitis, revision adenoid surgery with removal of lateral pharyngeal bands, and keloid formation.202 Figi203 described 37 cases of nasopharyngeal stenosis, 22 of which were secondary to adenotonsillectomy.109 Guggenheim204 reported 18 cases of nasopharyngeal stenosis secondary to adenoidectomy caused by stripping of the lymphoid tissue from the torus tubarius, Rosenmüller’s fossa, and the lateral nasopharynx.205
Numerous techniques have been described for surgical repair using a combination of skin flaps, local mucosal flaps, stents, skin grafts, and scar incisions.206 Cotton207 described a technique using incision of the stenosis with rotation of a laterally and inferiorly based pharyngeal flap to reline the nasopharynx. Occasionally, second-stage repair is necessary. Physical findings in patients with nasopharyngeal stenosis typically consist of scarring of the uvula down to the posterior pharyngeal wall with possibly a small opening visible on mirror examination or flexible nasopharyngoscopy (Fig. 196-16). The majority of the scarring is based laterally in the region of the posterior tonsillar pillars. This results in partial or complete obstruction of the nasopharyngeal airway and can be extremely debilitating.
Cervical Spine Complications
A rare complication of adenoidectomy or tonsillectomy is atlantoaxial subluxation (Grisel’s syndrome). Although up to 10% of patients may report neck pain after adenoidectomy, patients with Grisel’s syndrome have decalcification of the anterior arch of the atlas and laxity of the anterior transverse ligament between the atlas and axis.63,208 This leads to complaints of stiff neck with spasm of the sternocleidomastoid or deep cervical muscle. The patient holds the head to one side with slight rotation toward the opposite side. Radiographic evaluation in suspected cases should include anteroposterior and lateral cervical spine radiography in addition to flexion-extension radiographs, which may detect mild subluxation. In severe cases, CT and possibly magnetic resonance imaging may demonstrate evidence of decalcification, which may be associated with cervical spine osteomyelitis. Most cases of atlantoaxial subluxation are related to infection or trauma and only rarely are secondary to adenotonsillectomy.209 Patients with Down syndrome are more prone to traumatic atlantoaxial subluxation following adenotonsillectomy. Great care should be taken in cervical spine manipulation during surgery in patients with Down syndrome.104
Management of atlantoaxial subluxation after adenoidectomy or adenotonsillectomy should be individualized according to the duration and the symptoms present. Intravenous antimicrobial therapy and possibly cervical traction may be necessary. In the presence of cervical osteomyelitis, long-term (4-8 weeks) intravenous antimicrobial therapy, coupled with cervical stabilization, will be necessary.209 Retropharyngeal abscess after adenoidectomy has also been reported.190
Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance and behavior in 4-5 year olds. Arch Dis Child. 1993;68:360-366.
American Academy of Otolaryngology–Head and Neck Surgery. 1995 Clinical Indicators Compendium. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery; 1995.
American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866-878.
Bluestone CD. Current indications for tonsillectomy and adenoidectomy. Ann Otol Rhinol Laryngol Suppl. 1992;155:58.
Bluestone CD, Wittel RA, Paradise JL, et al. Eustachian tube function as related to adenoidectomy for otitis media. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:1325-1339.
Coticchia J, Zuliani G, Coleman C, et al. Biofilm surface area in the pediatric nasopharynx: chronic rhinosinusitis vs obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2007;133:110-114.
Derkay CS, Darrow DH, Welch C, et al. Post-tonsillectomy morbidity and quality of life in pediatric patients with obstructive tonsils and adenoid: Microdebrider vs electrocautery. Otolaryngol Head Neck Surg. 2006;143:114-120.
Garetz SL. Behavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep-disordered breathing: summary of the literature. Otolaryngol-Head Neck Surg. 2008;138(Suppl):S19-S26.
Gates GA, Avery CA, Cooper JCJr, et al. Chronic secretory otitis media: effects of surgical management. Ann Otol Rhinol Laryngol Suppl. 1989;138:2-32.
Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope. 1988;98:58.
Gates GA, Avery CA, Prihoda TJ, et al. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med. 1987;317:1444-1451.
Goldstein NA, Fatima M, Campbell TF, et al. Child behaviour and quality of life before and after tonsillectomy and adenoidectomy. Arch Otolaryngol Head Neck Surg. 2002;128:770-775.
Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458-464.
Gozal D. Sleep disordered breathing and school performance in children. Pediatrics. 1998;102:616-620.
Gozal D, Pope DWJr. Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107:1394-1399.
Javed F, Sadri M, Uddin J, et al. A completed audit cycle on post-tonsillectomy haemorrhage rate: Coblation versus standard tonsillectomy. Acta Otolaryngol. 2007;127(Mar):300-304.
Kay DJ, Mehta V, Goldsmith AJ. Perioperative adenotonsillectomy management in children: current practices. Laryngoscope. 2003;113:592-597.
Koltai PJ, Chan J, Younes A. Power-assisted adenoidectomy: total and partial resection. Laryngoscope. 2002;112:29.
Koltai PJ, Solares CA, Mascha EJ, et al. Intracapsular partial tonsillectomy for tonsillar hypertrophy in children. Laryngoscope. 2002;112(Suppl 100):17-19.
Littlefield PD, Hall DJ, Holtel MR. Radiofrequency excision versus monopolar electrosurgical excision for tonsillectomy. Otolaryngol Head Neck Surg. 2005;133:51-54.
Paradise JL, Bluestone CD, Bachman RZ, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children: results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310:674-683.
Paradise JL, Bluestone CD, Rogers KD, et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement: results of parallel randomized and nonrandomized trials. JAMA. 1990;263:2066-2073.
Telian SA. Sore throat and antibiotics. Otolaryngol Clin North Am. 1986;19:103.
1. Curtin JM. The history of tonsil and adenoid surgery. Otol Clin North Am. 1987;20:415.
2. Catlin FI. Pulmonary complications of tonsillectomy as originally described by Samuel J. Crowe, M.D. Laryngoscope. 1981;91:52.
3. Weir N. Otolaryngology: an Illustrated History. London: Butterworth & Co; 1990.
4. Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551.
5. Mackenzie M. The pharynx. In: Diseases of the Pharynx. New York: William Wood and Co.; 1880.
6. Thornval A. Wilhelm Meyer and the adenoids. Arch Otolaryngol Head Neck Surg. 1969;90:383.
7. Crowe SJ, Watkins SS, Rothholz AS. Relation of tonsillar and naso-pharyngeal infections to general systemic disorders. Bull Johns Hopkins Hosp. 1917;28:1.
8. Hollinshead WH. The pharynx and larynx. In: Hollinshead WH, editor. Anatomy for Surgeons: The Head and Neck. Philadelphia: JB Lippincott, 1982.
9. Goeringer GC, Vidic B. The embryogenesis and anatomy of Waldeyer’s ring. Otolaryngol Clin North Am. 1987;20:207.
10. Jeans WD, Fernando DC, Maw AR, et al. A longitudinal study of the growth of the nasopharynx and its contents in normal children. Br J Radiol. 1981;54:117.
11. Richtsmeier WJ, Shikhari AM. The physiology and immunology of the pharyngeal lymphoid tissue. Otolaryngol Clin North Am. 1987;20:219-228.
12. Hanson LA. Comparative immunological studies of the immune globulins of human milk and of blood serum. Int Arch Allergy Appl Immunol. 1961;18:241.
13. Howie AJ. Scanning and transmission electron microscopy on the epithelium of human palatine tonsils. J Pathol. 1980;130:191.
14. Siegel G. The influence of tonsillectomy on cell mediated immune response. Arch Otolaryngol Head Neck Surg. 1984;239:205.
15. Richtsmeier WJ. Human interferon production in tonsil and adenoid tissue cultures. Am J Otolaryngol. 1983;4:325.
16. Brandtzaeg P, Surjan LJr, Berdal P. Immunoglobulin systems of human tonsils I: control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentration. Clin Exp Immunol. 1978;31:367-381.
17. Surjan L, Brantzaeg P, Berdal P. Immunoglobulin system of human tonsils II: patients with chronic tonsillitis or tonsillar hyperplasia: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol. 1978;31:382.
18. Siegel G. Theoretical and clinical aspects of the tonsillar function. Int J Pediatr Otorhinolaryngol. 1983;6:61.
19. Ogra PL. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody responses to poliovirus. N Engl J Med. 1971;284:59.
20. Sainz M, Gutierrez F, Moreno PM, et al. Changes in immunologic response in tonsillectomized children I: Immunosuppression in recurrent tonsillitis. Clin Otolaryngol Allied Sci. 1992;17:376-379.
21. Sennaroglu L, Onerci M, Hascelik G. The effect of tonsillectomy and adenoidectomy on neutrophil chemotaxis. Laryngoscope. 1993;103:1349-1351.
22. van Hattum ES, Balemans WAF, Rovers MM, et al. Adenoidectomy and/or tonsillectomy in childhood is not associated with atopic disease later in life. Clin Exp Allergy. 2006;369:40-43.
23. Brook I. The clinical microbiology of Waldeyer’s ring. Otolaryngol Clin North Am. 1987;20:259.
24. Ingvarsson L, Lundgren K, Irving J. The bacterial flora in the nasopharynx in healthy children. Acta Otolaryngol Suppl (Stockh). 1982;386:94.
25. Selinger DS, Reed WP, McLaren LC. Model for studying bacterial adherence to epithelial cells infected with viruses. Infect Immun. 1981;32:941.
26. Ramirez-Ronda CH, Fuxench-Lopea Z, Nevarez M. Increased pharyngeal bacterial colonization during viral illness. Arch Intern Med. 1981;141:1599.
27. Brodsky L, Koch RJ. Bacteriology and immunology of normal and diseased adenoids in children. Arch Otolaryngol Head Neck Surg. 1993;119:821.
28. Brook I, Walker RI. Pathogenicity of anaerobic gram-positive cocci. Infect Immun. 1984;45:320.
29. Brook I, Yocum P. In vitro protection of group A beta hemolytic streptococci from penicillin and cephalothin by Bacteroides fragilis. Chemotherapy. 1983;29:18.
30. Telian SA. Sore throat and antibiotics. Otolaryngol Clin North Am. 1986;19:103.
31. Petersdorf RG, editor. Harrison’s Principles of Internal Medicine, 10th ed, New York: McGraw-Hill, 1983.
32. Wiesner PJ. Gonococcal pharyngeal tonsillar infections. Clin Obstet Gynecol. 1981;18:121.
33. Zucconi M, Strambi LF, Pestalozza G, et al. Habitual snoring and obstructive sleep apnea syndrome in children: effects of early tonsil surgery. Int J Pediatr Otorhinolaryngol. 1993;26:235-243.
34. Zalzal GH, Cotton RT. Pharyngitis and adenotonsillar disease. In: Cummings C, et al, editors. Otolaryngology–Head and Neck Surgery. St. Louis: Mosby, 1986.
35. Dobie RA, Robey DN. Clinical features of diphtheria in the respiratory tract. JAMA. 1979;242:2197.
36. Teele DW. Inflammatory diseases of the mouth and pharynx. In: Bluestone CD, Stool SE, editors. Pediatric Otolaryngology. Philadelphia: WB Saunders, 1983.
37. Bisno AL. Acute pharyngitis: etiology and diagnosis. Pediatrics. 1996;97:949.
38. Committee on Infectious Diseases of the American Academy of Pediatrics. 1994 Red Book: Report of the Committee on Infectious Diseases, 23rd ed. Elk Grove Village, IL: The American Academy of Pediatrics; 1994.
39. Randolph MF, Gerber MA, DeMeo KK, et al. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J Pediatr. 1985;106:870-875.
40. Facklam RR. Specificity study of kits for detection of group A streptococci directly from throat swabs. J Clin Microbiol. 1987;25:504.
41. Gerber MA. Comparison of throat cultures and rapid strep tests for diagnosis of streptococcal pharyngitis. Pediatr Infect Dis J. 1989;8:820.
42. Honikman LH, Massel BF. Guidelines for the selective use of throat cultures in the diagnosis of streptococcal respiratory infections. Pediatrics. 1971;48:573.
43. Schulman ST. Streptococcal pharyngitis: diagnostic considerations. Pediatr Infect Dis J. 1994;13:567.
44. Shapiro NL, Cunningham MJ. Streptococcal pharyngitis in children. Curr Opin Otolaryngol Head Neck Surg. 1995;3:369.
45. Amir J, Shechter Y, Eilam N, et al. Group A beta-hemolytic streptococcal pharyngitis in children younger than 9 years. Isr J Med Sci. 1994;30:619-622.
46. Brook I, Yocum P. Bacteriology of chronic tonsillitis in young adults. Arch Otolaryngol Head Neck Surg. 1984;110:803.
47. Gerber MA, Markowitz M. Management of streptococcal pharyngitis reconsidered. Pediatr Infect Dis J. 1985;4:518.
48. Krober MS, Bass JW, Michels GN. Streptococcal pharyngitis: placebo-controlled double-blind evaluation of clinical response to penicillin therapy. JAMA. 1985;253:1271.
49. Schwartz RH, Weintzen RW, Pedreira F. Penicillin V therapy for group A streptococcal pharyngitis. JAMA. 1981;246:1790.
50. Palumbo FM. Pediatric considerations of infections and inflammations of Waldeyer’s ring. Otolaryngol Clin North Am. 1987;20:311.
51. Pruet CW, Duplan DA. Tonsil concretions and tonsilloliths. Otolaryngol Clin North Am. 1987;20:305.
52. Martinez SA, Akin DP. Laser tonsillectomy and adenoidectomy. Otolaryngol Clin North Am. 1987;20:371.
53. Sugita R, Kawamura S, Icikawa G, et al. Microorganisms isolated from peritonsillar abscess and indicated chemotherapy. Arch Otolaryngol Head Neck Surg. 1982;108:655-658.
54. Christensen PH, Schonsted-Madsen U. Unilateral immediate tonsillectomy as the treatment of peritonsillar abscesses: results with special attention to pharyngitis. J Laryngol Otol. 1983;97:1105.
55. Yellon RF, Bluestone CD. Head and neck space infections in children. In Bluestone CD, Stool SE, Kenna MA, editors: Pediatric Otolaryngology, 3rd ed, Philadelphia: WB Saunders, 1996.
56. Rosenfeld RM, Green RP. Tonsillectomy and adenoidectomy: changing trends. Ann Otol Rhinol Laryngol. 1990;99:187.
57. Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13:149.
58. Brodsky L, Radomski K, Gendler J. The effect of post-operative instructions on recovery after tonsillectomy and adenoidectomy. Int J Pediatr Otorhinolaryngol. 1993;25:133.
59. Gisalson T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. Chest. 1995;107:963-966.
60. Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance and behavior in 4-5 year olds. Arch Dis Child. 1993;68:360-366.
61. Redline S, Tishler PV, Schluchter M, et al. Risk factors for sleep disordered breathing n children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527-1532.
62. MacGregor FB, Albert DM, Bhattacharyya AK. Post-operative morbidity following pediatric tonsillectomy: a comparison of bipolar diathermy dissection and blunt dissection. Int J Pediatr Otorhinolaryngol. 1995;31:1.
63. Rusy LM, Houck CS, Sullivan LJ. A double-blind evaluation of ketorolac tromethamine versus acetaminophen in pediatric tonsillectomy: analgesia and bleeding. Anesth Analg. 1995;80:226-229.
64. Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31.
65. Frank Y, Kravath RE, Pollak CP. Obstructive sleep apnea and its therapy: clinical and polysomnographic manifestations. Pediatrics. 1983;71:737.
66. Gottlieb DJ, Vezina RM, Chase C, et al. Symptoms of sleep disordered breathing in 5-year old children are associated with sleepiness and problem behaviors. Pediatrics. 2003;112:870-877.
67. Weissbluth M, Davis AT, Poncher J, et al. Signs of airway obstruction during sleep and behavioral, developmental and academic problems. J Dev Behav Pediatr. 1983;4:119-121.
68. Marcotte AC, Thatcher PV, Butters M, et al. Parental report of sleep problems in children with attentional and learning disorders. Dev Behav Pediatr. 1998;19:178-186.
69. Chervin RD, Dillon JE, Bassetti C, et al. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185-1192.
70. Weinburg WA, Harper CR. Vigilance and its disorders. Neurol Clin. 1993;11(Feb):59-78.
71. Ali NJ, Pitson D, Straddling JR. Sleep-disordered breathing: effects of adenotonsillectomy on behavior and psychological functioning. Eur J Pediatr. 1996;155:56-62.
72. Gozal D. Sleep disordered breathing and school performance in children. Pediatrics. 1998;102:616-620.
73. Gozal D, Pope DWJr. Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107:1394-1399.
74. Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458-464.
75. Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1-16.
76. National Institutes of HealthNational Heart, Lung, and Blood Institute. 2003 National Sleep Disorders Research Plan. Washington, DC: U.S. Department of Health and Human Services; 2003. NIH Publication No. 03-5209. Available at http://www.nhlbi.nih.gov/health/prof/sleep/res_plan/sleep-rplan.pdf
77. Hunt CE. Neurocognitive outcomes in sleep-disordered breathing. J Pediatr. 2004;145:430-432.
78. Garetz SL. Behavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep-disordered breathing: summary of the literature. Otolaryngol Head Neck Surg. 2008;138(Suppl):S19-S26.
79. Weider DJ, Sateia MJ, West RP. Nocturnal enuresis in children with upper airway obstruction. Otolaryngol Head Neck Surg. 1991;105:427.
80. Leach J, Olson J, Hermann J, et al. Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:741-744.
81. Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediatr Otorhinolaryngol. 2006;70:769-778.
82. Aydogan M, Toprak D, Hatun S, et al. The effect of recurrent tonsillitis and adenotonsillectomy on growth in childhood. Int J Pediatr Otorhinolaryngol. 2007;71:1737-1742.
83. Nieminen P, Lopponen T, Tolonen U, et al. Growth and biochemical marker of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109:e55.
84. Brown OE, Manning SC, Ridenour B. Cor pulmonale secondary to tonsillar and adenoidal hypertrophy: management considerations. Int J Pediatr Otorhinolaryngol. 1988;6:131-139.
85. Mullens PD, Nagaraj HS, McMurray GT. Upper airway obstruction resulting in cor pulmonale. J Ky Med Assoc. 1978;76:223.
86. Luke MJ, Mehrizi A, Folger GMJr, et al. Chronic nasopharyngeal obstruction as a cause of cardiomegaly, cor pulmonale, and pulmonary edema. Pediatrics. 1966;37:762-768.
87. Myer CMIII. Tonsillectomy. Patient of the Month Program. 1988;18:11.
88. Tomes C. On the developmental origin of the V-shaped contracted maxilla. Monthly Rev Dent Surg. 1872;1:2.
89. Oulis CJ, Vadiakas GP, Ekonomides J, et al. The effect of hypertrophic adenoids and tonsils on the development of posterior crossbite and oral habits. J Clin Pediatr Dent. 1994;18:197-201.
90. Harvold EP. Neuromuscular and morphological adaptations in experimentally induced oral respiration. In: McNamara JMJ, editor. Naso-Respiratory Function and Craniofacial Growth. Ann Arbor, MI: Center for Human Growth and Development, 1979. Craniofacial Growth Series, Monograph 9
91. Linder-Aronson S, Woodside DG, Lundstrom A. Mandibular growth direction following adenoidectomy. Am J Orthod Dentofacial Orthop. 1986;89:273.
92. Gryczynska D, Powajbo K, Zakrzewska A. The influence of tonsillectomy on obstructive sleep apnea children with malocclusion. Int J Pediatr Otorhinolaryngol (Suppl). 1995;32:S225.
93. Dunn GF, Green LJ, Cunat JJ. Relationships between variation of mandibular morphology and variation of nasopharyngeal airway size in monozygote twins. Angle Orthod. 1973;43:129-135.
94. Wiatrak BJ, Myer CMIII, Andrews TM. Complications of adenotonsillectomy in children under 3 years of age. Am J Otolaryngol. 1991;12:170.
95. Ozdemir H, Altin R, Sogut A, et al. Craniofacial differences according to AHI scores of children with obstructive sleep apnoea syndrome: cephalometric study in 39 patients. Pediatr Radiol. 2004;34:393-399.
96. Hartsook JT. Mouthbreathing as a primary etiologic factor in the production of malocclusion. J Dent Child. 1946;13:91.
97. McKenzie W, Wolfe CI. Carotid abnormalities and adenoid surgery. J Laryngol Otol. 1959;73:596.
98. Watson WG. Open bite: a multifactorial event. Am J Orthod. 1981;80:443.
99. Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoid size in children: adenoid-nasopharyngeal ratio. Am J Radiol. 1979;133:401.
100. Close HL, Kryzer TC, Nowlin JH, et al. Hemostatic assessment of patients before tonsillectomy: a prospective study. Otolaryngol Head Neck Surg. 1994;111:733-738.
101. Britton PD. Effect of respiration on nasopharyngeal radiographs when assessing adenoidal enlargement. J Laryngol Otol. 1989;103:71.
102. Wormald PJ, Prescott CAJ. Adenoids: comparison of radiological assessment methods with clinical and endoscopic findings. J Laryngol Otol. 1992;106:342.
103. Wang DY, Clement P, Kaufman L, et al. Fiberoptic examination of the nasal cavity and nasopharynx in children. Acta Otorhinolaryngol Belg. 1991;45:323-329.
104. Parker AJ, Maw AR, Powell JE. Rhinomanometry in the selection for adenoidectomy and its relation to preoperative radiology. Int J Pediatr Otorhinolaryngol. 1989;17:155.
105. Richardson MA, Seid AB, Cotton RT, et al. Evaluation of tonsils and adenoids in sleep apnea syndrome. Laryngoscope. 1980;90:1106-1110.
106. Manning SC, Beste D, McBride T, et al. An assessment of preoperative coagulation screening for tonsillectomy and adenoidectomy. Int J Pediatr Otorhinolaryngol. 1987;13:237-244.
107. Kang J, Brodsky L, Danziger I, et al. Coagulation profile as a predictor for post-tonsillectomy and adenoidectomy (T+A) hemorrhage. Int J Pediatr Otorhinolaryngol. 1994;28:157-165.
108. Bolger WE, Parsons DS, Potempa L. Preoperative hemostatic assessment of the adenotonsillectomy patient. Otolaryngol Head Neck Surg. 1990;103:396.
109. Finkelstein Y, Zohar Y, Nachmani A, et al. The otolaryngologist and the patient with velocardiofacial syndrome. Arch Otolaryngol Head Neck Surg. 1993;119:563-569.
110. Strunk CL, Nichols ML. A comparison of the KTP/532-laser tonsillectomy vs. traditional dissection/snare tonsillectomy. Otolaryngol Head Neck Surg. 1990;103:966.
111. Carroll JL, McColley SA, Marcus CL, et al. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610-618.
112. American Academy of Otolaryngology–Head and Neck Surgery. 1995 Clinical Indicators Compendium. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery; 1995.
113. Mawson SR, Adlington P, Evans M. A controlled study evaluation of adenotonsillectomy in children. J Laryngol Otol. 1967;81:777.
114. Paradise JL, Bluestone CD, Bachman RZ, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children: results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310:674-683.
115. Bluestone CD, Wittel RA, Paradise JL, Felder H. Eustachian tube function as related to adenoidectomy for otitis media. Trans Am Acad Ophthalmol Otolaryngol. 1972;176:1325.
116. Herzon FS. Peritonsillar abscess: incidence, current management practices and a proposal for treatment guidelines. Laryngoscope (Suppl). 1995;74:1.
117. Lockhart R, Parker GS, Tami TA. Role of Quinsy tonsillectomy in the management of peritonsillar abscess. Ann Otol Rhinol Laryngol. 1991;100:569.
118. Ghorganian SN, Paradise JL, Doty RL. Odor perception in children in relation to nasal obstruction. Pediatrics. 1983;72:510.
119. Rosenfeld RM. Pilot study of outcomes in pediatric rhinosinusitis. Arch Otolaryngol Head Neck Surg. 1995;121:729.
120. Coticchia J, Zuliani G, Coleman C, et al. Biofilm surface area in the pediatric nasopharynx: chronic rhinosinusitis vs obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2007;133:110-114.
121. MacKenzie-Stepner K, Witzel MA, Stringer DA, et al. Velopharyngeal insufficiency due to hypertrophic tonsils: a report of 2 cases. Int J Pediatr Otorhinolaryngol. 1987;14:57-63.
122. Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995;95:355.
123. Gates GA, Avery CA, Prihoda TJ, et al. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med. 1987;317:1444-1451.
124. Paradise JL, Bluestone CD, Rogers KD, et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement: results of parallel randomized and nonrandomized trials. JAMA. 1990;263:2066-2073.
125. Kadhim AL, Spilsbury K, Semmens JB, et al. Adenoidectomy for middle ear effusion; a study of 50,000 children over 24 years. Laryngoscope. 2007;117:427-433.
126. Gates GA, Avery CA, Cooper JCJr, et al. Chronic secretory otitis media: effects of surgical management. Ann Otol Rhinol Laryngol Suppl. 1989;138:2-32.
127. Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope. 1988;98:58.
128. Mandel EM, Bluestone CD, Takahashi H, et al. Effect of adenoidectomy on eustachian tube function: preliminary results of a randomized clinical trial. Adv Otorhinolaryngol. 1992;47:227.
129. Roydhouse N. Adenoidectomy for otitis media with mucoid effusion. Ann Otol Rhinol Laryngol. 1980;89:312.
130. McKee WJE. A controlled study of the effects of tonsillectomy and adenoidectomy in children. Br J Prev Soc Med. 1963;17:49.
131. Andrea M. Microsurgical bipolar cautery tonsillectomy. Laryngoscope. 1993;103:1177.
132. Linden BE, Gross CW, Long TE, et al. Morbidity in pediatric tonsillectomy. Laryngoscope. 1990;100:120-124.
133. Nishimura T, Yagisawa M, Suzuki A, et al. Laser tonsillectomy. Acta Otolaryngol Suppl (Stockh). 1988;454:313-315.
134. Pang YT. Pediatric tonsillectomy: bipolar electrodissection and dissection/snare compared. J Laryngol Otol. 1995;109:733.
135. Weimert TA, Babyak JW, Richter HJ. Electrodissection tonsillectomy. Arch Otolaryngol Head Neck Surg. 1990;116:186.
136. Walner DL, Parker N, Miller RP. Past and present instrument use in pediatric adenotonsillectomy. Otolaryngol Head Neck Surg. 2007;137:49-53.
137. Leach JL, Manning S, Schaefer SD. Comparison of two methods of tonsillectomy. Laryngoscope. 1993;103:619.
138. Salam MA, Cable HR. Post-tonsillectomy pain with diathermy and ligation techniques: a prospective randomized study in children and adults. Clin Otolaryngol. 1992;17:517.
139. McGuire NG. A method of guillotine tonsillectomy with an historical review. J Laryngol Otol. 1967;81:187.
140. Unlu Y, Tekalan SA, Cemiloğlu R, et al. Guillotine and dissection tonsillectomy in children. J Laryngol Otol. 1992;106:817.
141. Wake M, Glossop P. Guillotine and dissection tonsillectomy compared. J Laryngol Otol. 1989;403:588.
142. Yuan HC, Tang SJ, Cheng RQ. Clinical observation on guillotine tonsillectomy without anesthesia. Acta Otolaryngol (Stockh). 1988;454:273.
143. Temple RH, Timms MS. Paediatric Coblation tonsillectomy. Int J Pediatr Otorhinolaryngol. 2001;61:195-198.
144. Timms MS, Temple RH. Coblation tonsillectomy: a double blind randomized controlled study. J Laryngol Otol. 2002;116:450-452.
145. Parsons SP, Cordes SR, Comer B. Comparison of posttonsillectomy pain using the ultrasonic scalpel, coblator, and electrocautery. Otolaryngol Head Neck Surg. 2006;134:106-113.
146. Glade RS, Pearson SE, Zalzal GH, et al. Coblation adenotonsillectomy: an improvement over electrocautery technique. Otolaryngol Head Neck Surg. 2006;134:852-855.
147. Littlefield PD, Hall DJ, Holtel MR. Radiofrequency excision versus monopolar electrosurgical excision for tonsillectomy. Otolaryngol Head Neck Surg. 2005;133:51-54.
148. Javed F, Sadri M, Uddin J, et al. A completed audit cycle on post-tonsillectomy haemorrhage rate: Coblation versus standard tonsillectomy. Acta Otolaryngol. 2007;127:300-304.
149. Windfuhr JP, Deck JC, Remmert S. Hemorrhage following Coblation tonsillectomy. Am Otol Rhinol Laryngol. 2005;114:749-756.
150. Noon AP, Hargreaves S. Increased post-operative haemorrhage seen in adult Coblation tonsillectomy. J Laryngol Otol. 2003;117:704-706.
151. Wiatrak BJ, Willging JP. Harmonic scalpel tonsillectomy in children: a randomized, prospective study. Otolaryngol Head Neck Surg. 2003;128:318.
152. Collison PJ, Weiner R. Harmonic scalpel versus conventional tonsillectomy: a double-blind clinical trial. Ear Nose Throat J. 2004;82:707-710.
153. Hultcrantz E, Linder A, Markstrom A. Tonsillectomy or tonsillotomy? A randomized study comparing postoperative pain and long-term effects. Int J Pediatr Otorhinolaryngol. 1999;51:171.
154. Koltai PJ. The safety and efficacy of intracapsular (partial) tonsillectomy (IP) for pediatric obstructive sleep disordered breathing (OSDB). Paper presented at annual meeting of American Society of Pediatric Otolaryngology, Nashville, TN, May 4-5, 2003; in press.
155. Koltai PJ, Solares CA, Mascha EJ, et al. Intracapsular partial tonsillectomy for tonsillar hypertrophy in children. Laryngoscope. 2002;112(Suppl 100):17-19.
156. Mixson CM, Weinberger PM, Austin MB. Comparison of Microdebrider subcapsular tonsillectomy to harmonic scalpel and electrocautery total tonsillectomy. Am J Otolaryngol. 2007;38:13-17.
157. Derkay CS, Darrow DH, Welch C, et al. Post-tonsillectomy morbidity and quality of life in pediatric patients with obstructive tonsils and adenoid: Microdebrider vs electrocautery. Otolaryngol Head Neck Surg. 2006;143:114-120.
158. Lister MT, Cunningham MJ, Benjamin B, et al. Microdebrider tonsillotomy vs electrosurgical tonsillectomy: a randomized, double-blind paired control study of postoperative pain. Arch Otolaryngol Head Neck Surg. 2006;132:599-604.
159. Schmidt R, Herzog A, Cook S, et al. Complications of tonsillectomy: a comparison of techniques. Arch Otolaryngol Head Neck Surg. 2007;133:925-928.
160. Sobol SE, Wetmore RF, Marsh RR, et al. Postoperative recovery after Microdebrider intracapsular or monopolar electrocautery tonsillectomy. Arch Otolaryngol Head Neck Surg. 2006;132:270-274.
161. Sorin A, Bent JP, April MM, et al. Complications of Microdebrider-assisted powered intracapsular tonsillectomy and adenoidectomy. Laryngoscope. 2004;114:297-304.
162. Koltai PJ. Powered instrumentation in pediatric otolaryngology. Adv Otolaryngol Head Neck Surg. 2000;14:25.
163. Koltai PJ, Chan J, Younes A. Power-assisted adenoidectomy: total and partial resection. Laryngoscope. 2002;112:29.
164. Murray N, Fitzpatrick P, Guarisco LJ. Powered partial adenoidectomy. Arch Otolaryngol Head Neck Surg. 2002;128:792-796.
165. Rodriguez K, Murray N, Guarisco JL. Power-assisted partial adenoidectomy. Laryngoscope. 2002;112(Suppl 100):26-28.
166. Koltai PJ, Kalathia AS, Stanislaw P, et al. Power-assisted adenoidectomy. Arch Otolaryngol Head Neck Surg. 1997;123:685.
167. Stanislaw PJr, Koltai PJ, Feustel PJ. Comparison of power-assisted adenoidectomy vs. adenoid curette adenoidectomy. Arch Otolaryngol Head Neck Surg. 2000;126:845.
168. Telian SA, Handler SD, Fleisher GR, et al. The effect of antibiotic therapy on recovery after tonsillectomy in children: a controlled study. Arch Otolaryngol Head Neck Surg. 1986;112:610-615.
169. Catlin FI, Grimes WJ. The effect of steroid therapy on recovery from tonsillectomy in children. Arch Otolaryngol Head Neck Surg. 1991;117:649.
170. Ohlms LA, Wilder RT, Weston B. Use of intraoperative corticosteroids in pediatric tonsillectomy. Arch Otolaryngol Head Neck Surg. 1995;121:737.
171. Volk MS, Martin P, Brodsky L, et al. The effects of preoperative steroids in tonsillectomy patients. Otolaryngol Head Neck Surg. 1993;109:726-730.
172. Broadman LM, Patel RI, Feldman BA, et al. The effects of peritonsillar infiltration on the reduction of intraoperative blood loss and post-tonsillectomy pain in children. Laryngoscope. 1989;99:578-581.
173. Jebeles JA, Reilly JS, Gutierrez JF, et al. Tonsillectomy and adenoidectomy pain reduction by local bupivacaine infiltration in children. Int J Pediatr Otorhinolaryngol. 1993;25:149-154.
174. Stuart JC, MacGregor FB, Cairns CS, et al. Peritonsillar infiltration with bupivacaine for paediatric tonsillectomy. Anaesth Intensive Care. 1994;22:679-682.
175. Kay DJ, Mehta V, Goldsmith AJ. Perioperative adenotonsillectomy management in children: current practices. Laryngoscope. 2003;113:592-597.
176. Kornblut AD. A traditional approach to surgery of the tonsils and adenoids. Otol Clin North Am. 1987;20:349.
177. Keller C, Elliott W, Hubbell RN. Endotracheal tube safety during electrodissection tonsillectomy. Arch Otolaryngol Head Neck Surg. 1992;118:643.
178. Hall MD, Brodsky L. The effect of post-operative diet on recovery in the first twelve hours after tonsillectomy and adenoidectomy. Int J Pediatr Otorhinolaryngol. 1995;31:215.
179. Gabalski EC, Mattucci KF, Setzen M, et al. Ambulatory tonsillectomy and adenoidectomy. Laryngoscope. 1996;106:77-80.
180. Helmus C. Tonsillectomy and adenoidectomy in the one- and two-year old child. Laryngoscope. 1979;89:1764.
181. Helmus C, Grin M, Westfall R. Same-day-stay adenotonsillectomy. Laryngoscope. 1990;100:593.
182. Berkowitz RG, Zalzal GH. Tonsillectomy in children under 3 years of age. Arch Otolaryngol Head Neck Surg. 1990;116:685.
183. Gerber ME, O’Connor DM, Adler E, et al. Selected risk factors in pediatric tonsillectomy. Arch Otolaryngol Head Neck Surg. 1996;122:811-814.
184. Rothschild MA, Catalano P, Biller HF. Ambulatory pediatric tonsillectomy and the identification of high-risk subgroups. Otolaryngol Head Neck Surg. 1994;110:203.
185. Tom LW, DeDio RM, Cohen DE, et al. Is outpatient tonsillectomy appropriate for young children? Laryngoscope. 1992;102:277-280.
186. Weinberg E, Brodsky L, Stanievich J, et al. Needle aspiration of peritonsillar abscess in children, review. Arch Otolaryngol Head Neck Surg. 1993;119:169-172.
187. Pratt LW, Hornberger HR, Moore VJ. Mediastinal emphysema complicating tonsillectomy and adenoidectomy. Ann Otol Rhinol Laryngol. 1962;71:158.
188. Crysdale WS, Russel D. Complications of tonsillectomy and adenoidectomy in 9409 children observed overnight. Can Med Assoc J. 1986;135:1139.
189. Gibb AG. Unusual complications of tonsil and adenoid removal. J Laryngol Otol. 1969;83:1159.
190. Pratt LW. Tonsillectomy and adenoidectomy: mortality and morbidity. Trans Am Acad Ophthalmol Otolaryngol. 1970;74:1146.
191. Tolczynski B. Tonsillectomy, its hazards and their prevention. Eye Ear Nose Throat Mon. 1969;48:378-385.
192. Kornmesser HJ. Haemorrhage and haemostasis in face, visceral cranium, neck and middle ear region (author’s transl) [in German]. Arch Otorhinolaryngol Head Neck Surg. 1978;219:209.
193. Maniglia AJ, Kushner H, Cozzi L. Adenotonsillectomy: a safe outpatient procedure. Arch Otolaryngol Head Neck Surg. 1989;115:92.
194. Roy A, De la Rosa C, Vecchio YA. Bleeding following tonsillectomy. Arch Otolaryngol Head Neck Surg. 1976;102:9.
195. Szeremeta W, Novelly NJ, Benninger M. Postoperative bleeding in tonsillectomy patients. Ear Nose Throat J. 1996;75:373.
196. Gallagher JE, Blauth J, Fornadley JA. Perioperative ketorolac tromethamine and postoperative hemorrhage in cases of tonsillectomy and adenoidectomy. Laryngoscope. 1995;105:606.
197. Sholehvar J, Hunsicker RC, Stool SE. Arteriography in posttonsillectomy hemorrhage. Arch Otolaryngol Head Neck Surg. 1972;95:581.
198. Gardner JF. Sutures and disasters in tonsillectomy. Arch Otolaryngol Head Neck Surg. 1968;88:551.
199. Rasmussen N. Complications of tonsillectomy and adenoidectomy. Otolaryngol Clin North Am. 1987;20:383.
200. Cummings GO. Morbidities and mortalities following 20,000 tonsillectomies and adenoidectomies. Laryngoscope. 1954;64:647.
201. Witzel MA, Rich RH, Margar-Bacal F, et al. Velopharyngeal insufficiency after adenoidectomy: an 8-year review. Int J Pediatr Otorhinolaryngol. 1986;11:15-20.
202. Lehman WB, Pope TH, Hudson WR. Nasopharyngeal stenosis. Laryngoscope. 1968;78:371.
203. Figi FA. Cicatricial stenosis of the nasopharynx: correction by means of skin graft. Plast Reconstr Surg. 1947;2:97.
204. Guggenheim P. Cicatricial stenosis of the nasopharyngeal isthmus: result of lateral adenoidectomy. Arch Otolaryngol Head Neck Surg. 1963;77:13.
205. Guida RA, Mattucci KF. Tonsillectomy and adenoidectomy: an inpatient or outpatient procedure? Laryngoscope. 1990;100:491.
206. D’Amelio R, Palmisano L, LeMoli S, et al. Serum and salivary IgA levels in normal subjects: comparison between tonsillectomized and non-tonsillectomized subjects. Int Arch Allergy Appl Immunol. 1982;68:256-259.
207. Cotton RT. Nasopharyngeal stenosis. Arch Otolaryngol Head Neck Surg. 1985;3:146.
208. Derkay CS, Kenna MA, Pang D. Refractory torticollis: an uncommon complication of adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 1987;14:87.
209. Baker LL, Bower CM, Glasier CM. Atlantoaxial subluxation and cervical osteomyelitis: two unusual complications of adenoidectomy. Ann Otol Rhinol Laryngol. 1996;105:295.