CHAPTER 29 Pharmacogenomics
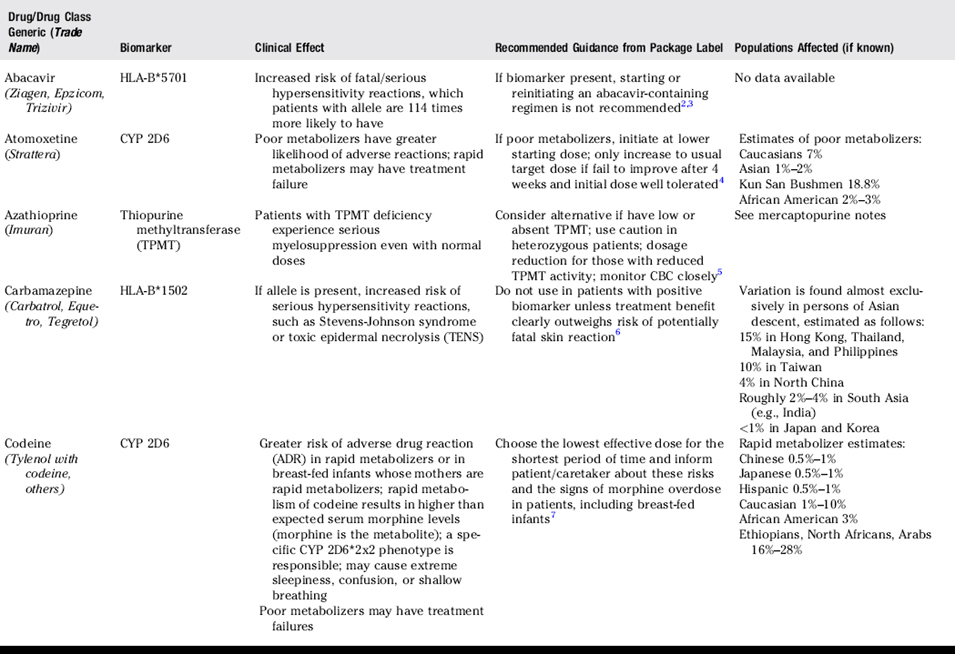
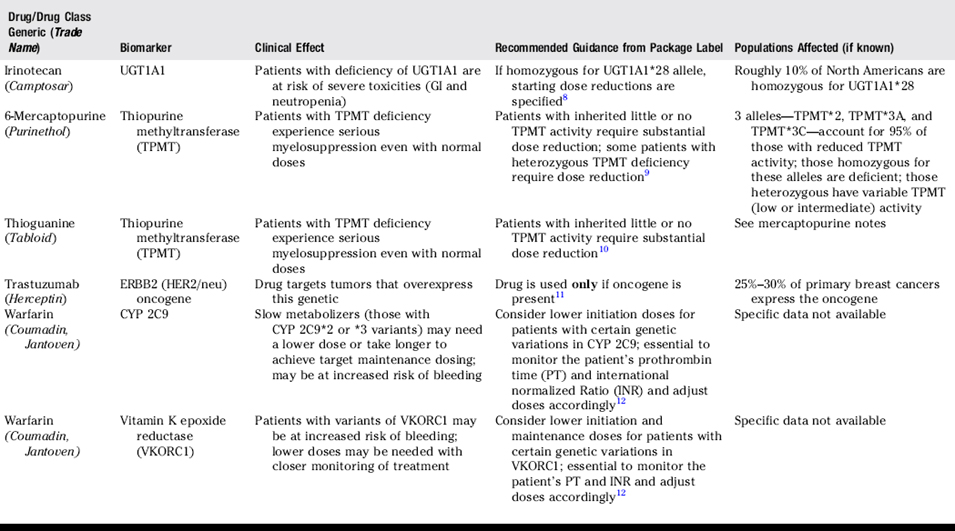
The field of pharmacogenomics is in its infancy. However, important and tangible recommendations for personalized prescribing are beginning to emerge. Pharmacogenomic variables are now identified for a few commonly prescribed drugs. Recommendations regarding laboratory testing and prescriber actions are now incorporated into the official prescribing literature for these drugs (Table 29-1). For other drugs, recommendations are not yet defined (Table 29-2). Pharmacogenomics is a rapidly changing discipline, and many factors, including health care costs, patient preferences, and the overall health of the patient will ultimately influence the individualization of any therapy.1
| Drug/Drug Class | Biomarker | Potential Clinical Effect |
|---|---|---|
* Defined action may be available in future, currently prescriber actions not well defined.
Pharmacists and other health care providers are encouraged to familiarize themselves with current guidance and rapid changes occurring in the field of pharmacogenomics. Current guidance has resulted in strong recommendations for screening before initiation of drug therapy or for the adjustment of drug dosages during therapy for selected drugs. Continuing education programs are available through organizations such as the American Medical Association and Food and Drug Administration.13
1. U.S. Department of Health and Human Services. Report of the Secretary’s Advisory Committee on Genetics, Health, and Society. Realizing the potential of pharmacogenomics: opportunities and challenges. May 2008 http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_PGx_report.pdf Accessed March 2009
2. Phillips E.J. Genetic screening to prevent abacavir hypersensitivity reaction: are we there yet? Clin Infect Dis. 2006;43:103-105.
3. Ziagen, Epzicom. Trizivir package inserts: Research Triangle Park. NC: GlaxoSmithKline, 2009.
4. Strattera package insert, 2008 Indianapolis:Eli Lilly and Company
5. Imuran package insert, 2008 San Diego:Prometheus Laboratories
6. Tegretol package insert, 2008 East Hanover:Novartis
7. Tylenol with codeine package insert, 2007 Raritan:Ortho-McNeil Pharmaceuticals
8. Camptosar package insert, 2008 New York:Pfizer
9. Purinethol package insert, 2007 Sellersville:Gate Pharmaceuticals
10. Tabloid package insert, 2004 Research Triangle Park:GlaxoSmithKline
11. Herceptin package insert, 2008 San Francisco:Genentech, Inc
12. Coumadin package insert, 2007 Princeton:Bristol-Myers Squibb Co
13. The American Medical Association and the Food and Drug Administration (FDA). Pharmacogenomics and Personalized Medicine. June 2007 Web-based training ama.learn.com/login Accessed March 2009
REVIEW QUESTIONS
(Answers and Rationales on page 384.)