CHAPTER 38 Petroclival Meningiomas
Middle Fossa Anterior Transpetrosal Approach
INTRODUCTION
Surgical removal of the pyramidal bone through the middle fossa has been used mainly for cerebellopontine angle tumors since 1970.1–6 Advantages of the approach were wider surgical exposure and minimal brain damage compared to the conventional subtemporal approach, because the tumor is accessed epidurally from the space of the resected pyramidal bone to spare brain retraction and sacrifice of the bridging veins. In 1977, the approach was applied to clival meningiomas.7 For petroclival meningiomas, a high tumor radicality was achieved because of removal of attached dura with the tumor. However, disadvantages were sacrificed hearing and risk of cerebrospinal fluid (CSF) rhinorrhea.
In 1985, limited bone resection only in the pyramidal apex, the anterior transpetrosal approach (APA), was introduced to preserve hearing in clipping of basilar trunk aneurysms8,9 and indicated for petroclival meningiomas in the 1990s.10,11 After 1999, other authors recommended this approach for petroclival meningiomas.12–14
CLASSIFICATION OF PETROCLIVAL MENINGIOMAS AND INDICATIONS FOR APA
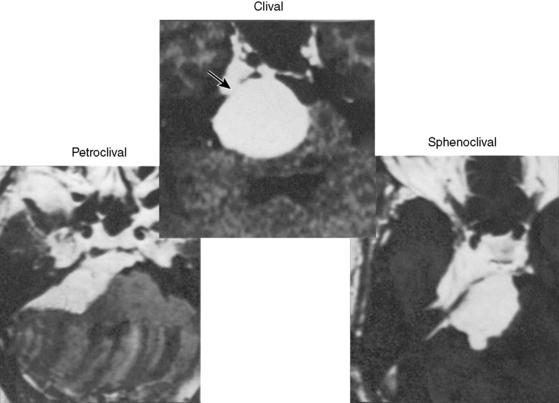
The petroclival meningiomas were classified into three types based on the extension of the tumor attachment (Fig. 38-1): (1) pure clival type, (2) sphenoclival type (middle fossa extension), and (3) petroclival type (extension along the posterior pyramid). The APA was indicated for the first and second types. The indication should not be selected based on the tumor size, but on the attachment size. The APA may absolutely be indicated for a tumor located in the midline clivus with extension into Meckel’s cave or in the middle fossa, and in a case with the tumor attachment on the upper half of the clivus and medial to the internal auditory meatus (IAM).
SURGICAL TECHNIQUE AND AVOIDING COMPLICATIONS
The anatomy and the surgical technique are described in refs. 15 to 17.
Exposure and Resection of the Pyramid
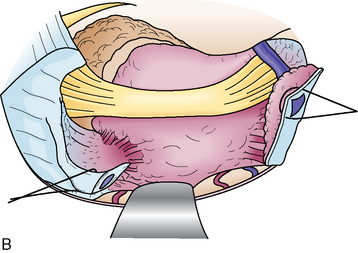
The dura mater is dissected and elevated from the temporal bone using a hooked retractor. The arcuate eminence is recognized medial to the EAM. The MMA is coagulated and cut at the foramen spinosum, and the periosteal dura, adhesive to the greater superficial petrosal nerve (GSPN), is cut to preserve the nerve. Tumor feeders commonly seen around GSPN or in the bone groove on the pyramid are coagulated. The interdural dissection is extended on the mandibular nerve to reduce tension of the dura, and the trigeminal impression is observed on the petrous apex. The axis of the microscope is shifted anteriorly to overlook the petrous apex. The IAM is located slightly anterior to the arcuate eminence, at a depth of 7 mm from the bone surface. The height of the arcuate eminence varies in each case, and must be checked by bone-targeted computed tomography (CT) before surgery. A line of maximal bone resection with preservation of the auditory structures is shown in Figure 38-2.
Dural Incision and Opening Meckel’s Cave
In large tumors, the posterior fossa is filled with the tumor mass. Tumor feeders from the tentorium can be coagulated to reflect the anterior leaflet of the tentorium. The trochlear nerve is commonly encased by the tumor at the point of dural entrance. The course of the trigeminal nerve varies according to the tumor origin and is classified into the following three types:18
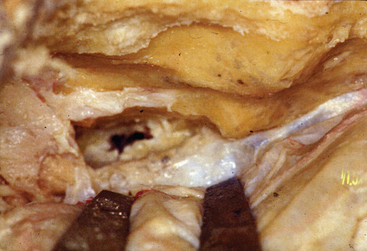
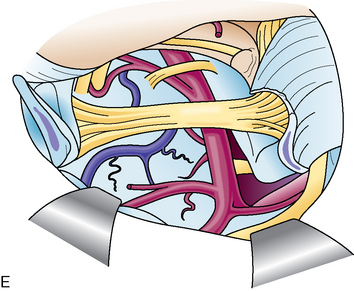
However, the trigeminal nerve is fixed at the entry portion of Meckel’s cave, and the dura on the cave is cut 1 cm anteriorly along the upper margin of the nerve. The dura on the cave, where two dural folds meet, is very tough against incision. A loose network of trigeminal nerve fibers (plexiform part) and the tumor are seen in the cave (Fig. 38-3). The tumor commonly locates medial to the nerve, and is removed by mobilizing the nerve fibers.
Tumor Removal Steps
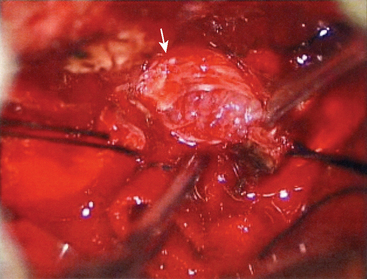
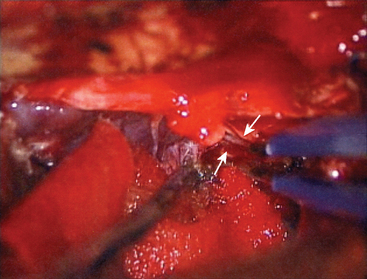
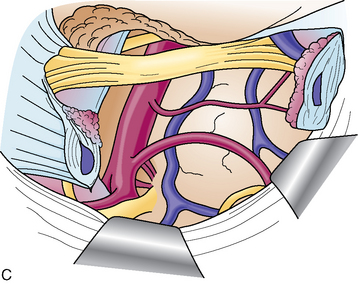
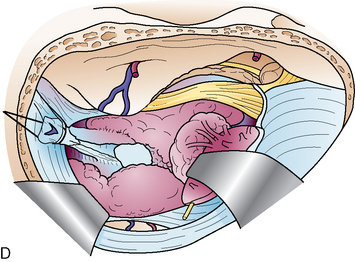
The first step is coagulation of the main tumor feeders commonly originating medial to Meckel’s cave (Fig. 38-4). This space is widened by mobilizing the tentorium superiorly and the trigeminal nerve inferiorly. In a tumor with a narrow attachment, note the abducens nerve commonly courses medial to the tumor. The second step is internal tumor decompression. After detachment of the feeders, bleeding may be minimal, and an ultrasonic aspirator is available. The tumor dome in the blind corner is gradually visualized by tumor retraction toward its base using Sugita’s hooked tumor retractor (Mizuho Ika Co., Tokyo, Japan). This technique is very useful to decrease the tension of arteries and cranial nerves, engulfed and overstretched by the tumor, and to obtain enough space for dissection. The third step is to release the arteries and cranial nerves carefully. The superior cerebellar artery (SCA) is sometimes encased in the tumor, and the tumor must be split sharply along the artery. The most important point is to spare injury to the basilar perforators, especially in a case with tumor invasion between the basilar trunk and brain stem. The anterior inferior cerebellar artery (AICA) commonly courses along the abducens nerve, on the lower margin of the tumor. The facial nerve is mostly deviated posteroinferiorly with a preserved arachnoid border (see Fig. 38-6C). The presence of the facial nerve can be confirmed neuroendoscopically. In the tentorial type, the facial nerve is protected by the elongated trigeminal nerve. The fourth step is to remove the tumor margin en bloc. Surgeons may be aware that the compressed brain stem is gradually returning to the normal position during surgery.
The presence of the VIIth nerve can be confirmed neuroendoscopically.
Closure and Postoperative Care
The following double layers are used for prevention of CSF leakage.
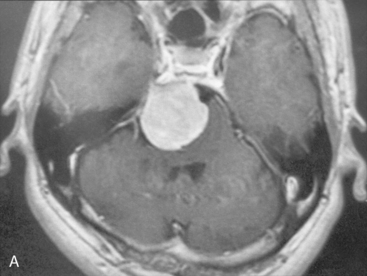
A 54-year-old man complained of mild ataxic gait. He had no cranial nerve deficit and his hearing was normal. MRI showed a 32-mm meningioma originating from the upper half of the clivus (Fig. 38-5A). Angiography showed a tumor feeder from the tentorial branch of the meningohypophyseal trunk. Via right APA, the tumor was observed medial to the trigeminal nerve, having its attachment on the clivus and the tentorium. The facial–auditory nerve complex might be deviated posteriorly and hidden by the elongated trigeminal nerve (Fig. 38-5B). By opening Meckel’s cave, the trigeminal nerve was mobilized inferiorly. The feeders were completely coagulated, and internal decompression became easier in the bloodless surgical field.
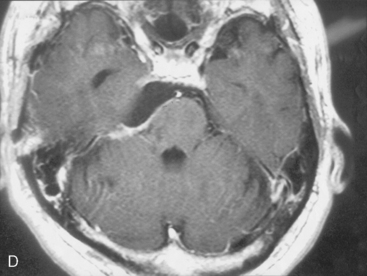
Medial structures such as the abducens nerve and basilar trunk could be separated from the tumor, by retraction of the tumor margin toward the surgeon. All of the tumor could be removed with preservation of cranial nerves and arteries (Fig. 38-5C). The patient’s postoperative course was uneventful, and mild facial hypesthesia was his only complaint. The tumor disappeared completely on the postoperative MRI, without retraction damage on the temporal lobe and cerebellum (Fig. 38-5D).
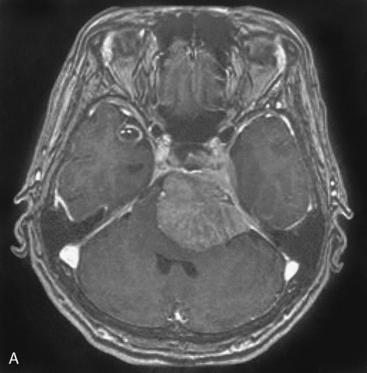
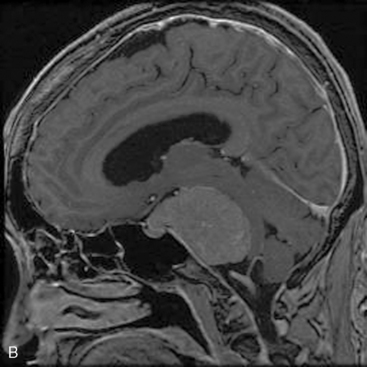
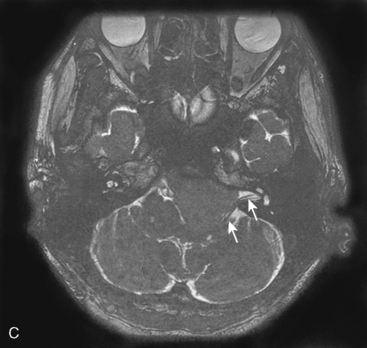
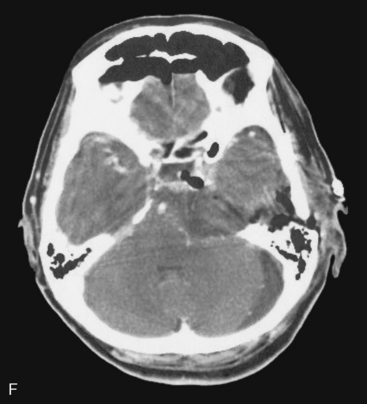
A 63-year-old woman complained of left facial hypesthesia. An MRI scan showed a 45-mm meningioma extending both in the middle and posterior fossae (Fig. 38-6A–C). Angiography demonstrated tumor feeders from the meningohypophyseal trunk (tentorial artery), and from branch of the middle meningeal artery. The anterior transpetrosal approach was performed. After removal of the middle fossa tumor, Meckel’s cave was opened and the tumor in the cave was removed (see Fig. 38-3). The tumor feeders were completely exposed and coagulated at surgery before tumor was decompressed (see Fig. 38-4). The tumor became softened by devascularization, and complete resection became easier (Fig. 38-6D, E). The postoperative course was uneventful, and the patient’s only complaint was mild diplopia at down gaze because of sacrifice of the trochlear nerve, encased by the tumor. Postoperative MRI showed no residual tumor (Fig. 38-6F).
SURGICAL RESULTS
Among 115 patients with petroclival meningiomas, 84 (73%) were operated via the APA. In eight patients showing middle fossa extension higher than the posterior clinoid process, the zygomatic arch was removed to decrease retraction damage to the temporal lobe. Forty-six percent of the cases showed a large sized tumor at or more than 40 mm in diameter (Table 38-1). The degree of tumor removal was confirmed by MRI 3 to 6 months after surgery. Gross total removal (GTR) was achieved in 57 (67.9%), subtotal removal (STR) in 22 (26.2%), and partial removal in 5 (5.9%) patients (Table 38-2).
| Small (<25 mm) | 24 (28.6%) |
| Medium (25–39 mm) | 21 (25.0%) |
| Large (>40 mm) | 39 (46.4%) |
| 84 (100.0%) |
TABLE 38-2 Degree of surgical removal
| Gross total removal | 57 (67.9%) |
| Subtotal removal | 22 (26.2%) |
| Partial removal | 5 (5.9%) |
The patient’s condition 1 year after surgery was independent (KPS ≥ 80) in 69 (82.1%) cases, temporarily dependent (KPS 60–70) in 7 (8.3%), and dependent (KPS ≤ 50) in 6 (7.1%) patients (Table 38-3). One patient died of surgical complications (cerebral venous thrombosis), and one committed suicide after being informed of tumor regrowth.
| Independent (KPS ≥ 80) | 69 (82.1%) |
| Temporary dependent (KPS 60–70) | 7 (8.3%) |
| Department (KPS ≤ 50) | 6 (7.1%) |
| Died | 2 (2.3%) |
| 84 (100%) |
ADVANTAGES AND DISADVANTAGES
[1] King T.T. Combined translabyrinthine-transtentorial approach to acoustic nerve tumors. Proc R Soc Med. 1970;63:780-782.
[2] House W.F. Surgery of acoustic tumors. Otolaryngol Clin North Am. 1973;6:245-266.
[3] Bochenek Z., Kukwa A. An extended approach through the middle cranial fossa to the internal auditory meatus and the cerebellopontine angle. Acta Otolaryngol. 1975;80:410-414.
[4] Kanzaki J., Kawase T., Sano H., et al. A modified extended middle cranial fossa approach for acoustic tumors. Arch Otorhinolaryngol. 1977;212:119-121.
[5] Hakuba A. Total removal of cerebellopontine angle tumors with a combined transpetrosal-transtentorial approach. No Shinkei Geka. 1978;6:347-354. [in Japanese]
[6] Shiobara R., Ohira T., Kanzaki J., Toya S. A modified extended middle cranial fossa approach for acoustic nerve tumors. J Neurosurg. 1989;68:358-365.
[7] Hakuba A., Nishimura S., Tanaka K., et al. Clivus meningioma: six cases of total removal. Neurol Med Chir. 1977;17:63-77. [in Japanese]
[8] Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach fo petroclival meningiomas. Neurosurgery. 1988;22:510-517.
[9] Kawase T., Toya S., Shiobara R., Mine S. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-867.
[10] Kawase T., Shiobara R., Toya S. Anterior transpetrosal-transtentorial approach for sphenopetro-clival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;2:869-876.
[11] Kawase T., Shiobara R., Toya S. Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas: selective pyramid resection and radicality. Acta Neurochir. 1994;129:113-120.
[12] Sekhar L.N., Schessek D.A., Bucur S.D., et al. Partial labyrinthectomy petrous apicectomy approach to neoplastic and vascular lesions of the petroclival area. Neurosurgery. 1999;44:537-550. discussion 550–2
[13] Goel A. Extended lateral subtemporal approach for petroclival meningiomas: report of experience with 24 cases. Br J Neurosurg. 1999;13:270-275.
[14] Seifert V., Raabe A., Zimmermann M. Conservative (labyrinth-preserving) transpetrosal approach to the clivus and petroclival region –indications, complications, results and lessons learned. Acta Neurochir (Wien). 2003;145:631-642. discussion 642
[15] Miller C., Loveren H.R., Keller J.T., et al. Transpetrosal approach; surgical anatomy and technique. Neurosurg. 1993;33:461-469.
[16] Kawase T. Technique of anterior transpetrosal approach. Op Tech. Neurosurg. 1999;2:10-17.
[17] Rhoton A.L. The posterior cranial fossa: Microsurgical anatomy & surgical approaches. Neurosurgery Suppl. 2000;47:235-240.
[18] Ichimura S., Kawase T., Onozuka S., et al. Variable origin of petroclival meningiomas; difference in symptoms and operative findings. Acta Neurochir (Wien). 2008;150:637-645.