Chapter 22 PET/CT and SPECT/CT Hybrid Imaging
RATIONALE FOR INTEGRATING NUCLEAR IMAGING AND CT
Myocardial Perfusion Imaging (See Chapters 14–16)
Myocardial perfusion imaging is a robust approach to diagnosing obstructive CAD, quantifying the magnitude of myocardium at risk, assessing the extent of tissue viability, and guiding therapeutic management (i.e., selection of patients for revascularization). The extensive published literature on single-photon emission computed tomography (SPECT) suggests that its average sensitivity for detecting greater than 50% angiographic stenosis is 87% (range, 71% to 97%), whereas the average specificity is 73% (range, 36% to 100%).1 With the use of attenuation-correction methods, the specificity improves, especially among patients undergoing exercise stress testing.1 With PET perfusion imaging, the reported average sensitivity for detecting greater than 50% angiographic stenosis is 91% (range, 83% to 100%), whereas the average specificity is 89% (range, 73% to 100%).2
Despite its widespread use and acceptance, a recognized limitation of this approach is that it often uncovers only coronary territories supplied by the most severe stenosis, and consequently it is relatively insensitive to accurately delineate the extent of obstructive angiographic CAD, especially in the setting of multivessel CAD. For example, in a recent study of 101 patients with significant angiographic left main coronary stenosis, Berman et al. reported that by perfusion assessment alone, high-risk disease with moderate to severe perfusion defects (involving > 10% myocardium at stress) was identified in only 59% by quantitative analysis.3 Conversely, absence of significant perfusion defect (>5% myocardium) was seen in 15% of patients. Similar results have been reported with PET imaging.4
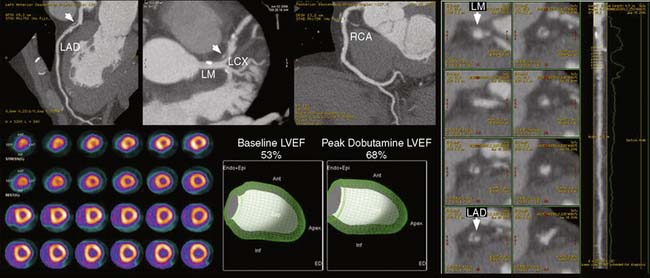
Recent evidence suggests that two quantitative approaches may be able to help mitigate this limitation, at least in part. One of them relates to PET’s unique ability to assess left ventricular function at rest and during peak stress (as opposed to post stress with SPECT).4 The data suggest that in normal subjects, left ventricular ejection fraction (LVEF) increases during peak vasodilator stress.4 In patients with obstructive CAD, however, the delta change in LVEF (from baseline to peak stress) is inversely related to the extent of obstructive angiographic CAD. Indeed, patients with multivessel or left main disease show a frank drop in LVEF during peak stress even in the absence of apparent perfusion defects. In contrast, those without significant CAD or with one-vessel disease show a normal increase in LVEF. Consequently, the diagnostic sensitivity of gated PET for correctly ascertaining the presence of multivessel disease increases from 50% to 79%.4
The second approach is based on the ability of PET to enable absolute measurements of myocardial blood flow (in mL/min/g) and coronary vasodilator reserve. In patients with so-called balanced ischemia or diffuse CAD, measurements of coronary vasodilator reserve would uncover areas of myocardium at risk that would generally be missed by performing only relative assessments of myocardial perfusion.5 It is important to point out, however, that neither of these approaches has been tested in prospective clinical trials.
Another limitation of the myocardial perfusion imaging approach is that it fails to describe the presence and extent of subclinical atherosclerosis.6,7 This is not unexpected, since the myocardial perfusion imaging method is designed and targeted on the identification of flow-limiting stenoses. This is potentially important, especially in patient subgroups with intermediate-high clinical risk in whom there may be extensive subclinical CAD, and may explain at least in part the limitations of perfusion imaging alone to identify low-risk patients among those with high clinical risk (e.g., diabetes, end-stage renal disease).8
Cardiac Computed Tomography (See Chapter 21)
Using state-of-the-art technology in carefully selected patients, it is possible to obtain high-quality images of the coronary arteries. The available evidence suggests that on a per-patient basis, the average weighted sensitivity for detecting at least one coronary artery with greater than 50% stenosis is 94% (range, 75% to 100%), whereas the average specificity is 77% (range, 49% to 100%).9 The corresponding average positive predictive value (PPV) and negative predictive value (NPV) are 84% (range, 50% to 100%) and 87% (range, 35% to 100%), respectively, and the overall diagnostic accuracy is 89% (range, 68% to 100%).
Two multicenter, single-vendor trials evaluating the diagnostic accuracy of CTA-64 have been completed and recently published.10,11 The results of these two studies confirm the robustness of CTA-64 for complete visualization of the coronary tree. The Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial enrolled 230 patients with a disease prevalence of 25%. On a patient-based model, the sensitivity, specificity, PPV and NPV to detect =50% or =70% stenosis were 95%, 83%, 64%, and 99%, respectively, and 94%, 83%, 48%, 99%, respectively. The study reported no differences in sensitivity and specificity for nonobese compared with obese subjects, whereas calcium scores ≥400 reduced specificity significantly. The Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography (CorE 64) trial11 enrolled 291 patients with a disease prevalence of 56% and provides additional evidence that is somewhat discordant to the ACCURACY trial and to initial results from single-center studies. The CorE 64 study excluded patients with calcium scores over 600. On a per-patient basis, the sensitivity for detecting at least one coronary artery with ≥50% stenosis was 85%, considerably lower than in single-center studies and in the ACCURACY trial using similar technology, whereas the specificity was 90%, higher than previously reported. The corresponding average PPV and NPV were 91% and 83%, respectively, surprisingly different than most previous studies. On a per-vessel basis, the reported sensitivity for detecting coronary arteries with =50% stenosis was 75%, whereas the specificity was 93%. The corresponding PPV and NPV were 82% and 89%, respectively.
Except for the ACCURACY study, these reported accuracies of CTA to date should be interpreted in light of the relatively narrow range of CAD likelihood in patients examined (i.e., high or intermediate-high), as evidenced by the high prevalence of obstructive CAD in these series (56% to 62%).9,11 Further, results are generally limited to relatively large vessel sizes (≥1.5 mm), excluding the results of smaller or uninterpretable vessels (generally distal vessels and side branches), the inclusion of which lowers sensitivity. An ongoing problem with CT is that high-density objects such as calcified coronary plaques and stent struts limit its ability to accurately delineate the degree of coronary luminal narrowing.12,13 Of note, the CorE 64 trial excluded patients with high calcium scores (>600).11 From a clinical perspective, a normal CTA is helpful inasmuch as it effectively excludes the presence of obstructive CAD and the need for further testing, defines a low clinical risk, and makes management decisions straightforward. Because of its limited accuracy to define stenosis severity and predict flow-limiting disease,14,15 however, abnormal CTA results are more problematic to interpret and to use as the basis for defining the potential need of invasive coronary angiography and myocardial revascularization.
CLINICAL APPLICATIONS OF DUAL-MODALITY IMAGING
Attenuation Correction (See Chapters 6 and 7)
One of the most basic uses of CT in dual-modality imaging scanners (e.g., PET/CT and SPECT/CT) is for patient positioning and correction of the in homogeneity of the scintigraphic data caused by overlapping soft tissue (so-called attenuation correction). Unlike CT for calcium scoring or coronary angiography, acquisition parameters for CT-based transmission imaging vary with the configuration of the CT scanner (e.g., 8-, 16-, 64-multidetector CT) and clinical protocol, and several approaches have been proposed.16,17 However, the general scan settings for CT transmission imaging adhere to the following principles: (1) a slow gantry rotation speed (e.g., 1 sec/revolution), combined with a relatively high pitch (e.g., 0.5 to 0.6:1), (2) nongated scan, (3) a high tube potential (e.g., 140 kVp) and a low tube current (~10 to 20 mA), and (4) a CT acquisition obtained during tidal expiration breath hold or shallow breathing.
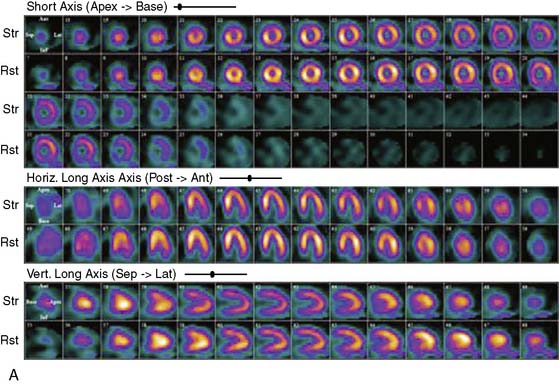
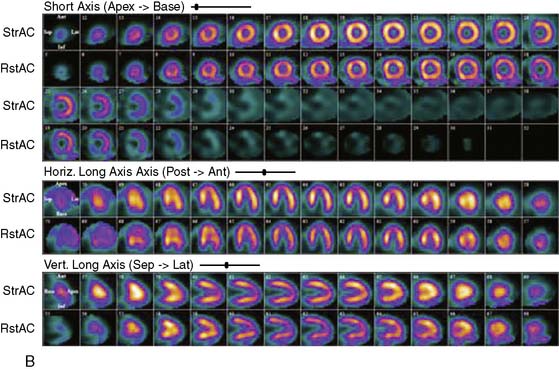
Proper attenuation correction for SPECT increases diagnostic certainty and reduces the number of equivocal studies (Fig. 22-1).18,19 However, CT-based attenuation correction also poses significant challenges for cardiac imaging.20 Attenuation correction errors leading to artifacts have been reported in 30% to 60% of cases with both SPECT/CT and PET/CT (Fig. 22-2).20,21 These artifacts are usually related to misalignments between the emission and CT transmission data sets caused by patient, cardiac, and/or respiratory motion,20,22 leading to regional defects and frequent in homogeneity in quantitative tracer distribution.23 Proper quality control and availability of registration software are crucial before interpretation of SPECT/CT and PET/CT images.
Localization of Targeted Molecular Imaging Agents
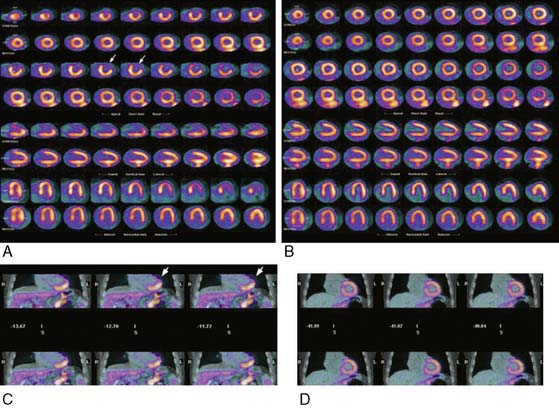
An emerging advantage of dual-modality imaging relates to the ability of CT to provide an anatomic roadmap that is critical for localization of targeted imaging agents. This has proven useful in both clinical and research applications in oncology imaging. For cardiovascular imaging, dual-modality approaches may be especially useful for imaging of atherosclerosis, where the CT can help delineate plaques and the localization of the targeted imaging probe in relation to those plaques (Fig. 22-3).24 The proposed integration between PET and MRI may expand the possibilities for characterization of both myocardial tissue and vasculature.25
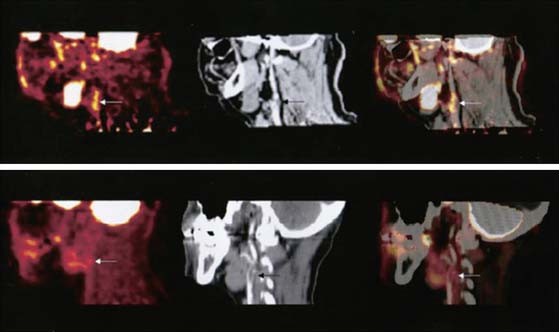
Figure 22-3 The upper row (left to right) shows PET, contrast CT, and coregistered PET/CT images in the sagittal plane, from a 63-year-old man who had experienced two episodes of left-sided hemiparesis. Angiography demonstrated stenosis of the proximal right internal carotid artery; this was confirmed on the CT image (black arrow). The white arrows show 18FDG uptake at the level of the plaque in the carotid artery. As expected, there was high 18FDG uptake in the brain, jaw muscles, and facial soft tissues. The lower row (left to right) demonstrates a low level of 18FDG uptake in an asymptomatic carotid stenosis. The black arrow highlights the stenosis on the CT angiogram, and the white arrows demonstrate minimal 18FDG accumulation at this site on the 18FDG PET and coregistered PET/CT images.
(Reproduced with permission from Rudd JH, Warburton EA, Fryer TD, et al: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography, Circulation 105:2708–2711, 2002.)
Integrating Calcium Scoring with Myocardial Perfusion Imaging
Voluminous plaques are more prone to calcification, and stenotic lesions frequently contain large amounts of calcium.26 There is growing, consistent evidence that coronary artery calcium (CAC) scores are generally predictive of a higher likelihood of ischemia (reflecting obstructive CAD) on myocardial perfusion imaging, and the available data support the concept of a threshold phenomenon governing this relationship.27–29 Indeed, the frequency of myocardial ischemia increases significantly with increasing CAC scores, especially among patients with CAC ≥400 (Fig. 22-4).27–29 A recent study reported that a CAC score ≥709 increased the sensitivity of SPECT MPI for detecting patients with obstructive CAD despite normal regional perfusion,30 probably reflecting the ability of the CAC score to uncover the presence of extensive atherosclerosis in selected patients with balanced ischemia. Given the fact that CAC scores are not specific markers of obstructive CAD,31 however, one should be cautious in considering integrating this information into management decisions regarding coronary angiography, especially in patients with normal perfusion imaging. Conversely, CAC scores less than 400, especially in symptomatic patients with intermediate likelihood of CAD, may be less effective in excluding CAD, especially in young subjects and women.32 In a recent study of symptomatic patients with intermediate likelihood of CAD, the absence of CAC only afforded an NPV of 84% to exclude ischemia.29
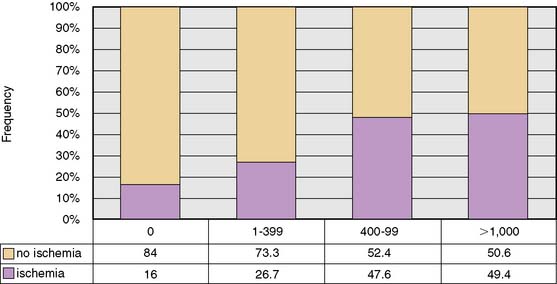
Figure 22-4 Frequency of inducible ischemia by myocardial perfusion imaging in patients with Agatston CAC score ≥400.
(Reproduced from Schenker MP, Dorbala S, Hong EC, et al: Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease, Circulation 117:1693–1700, 2008.)
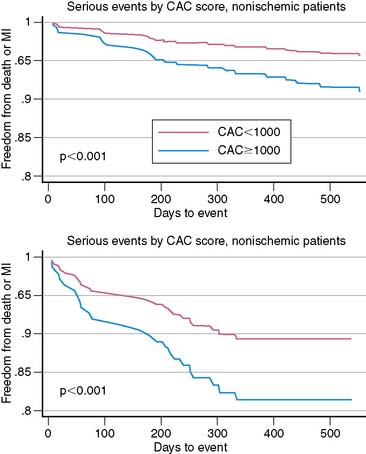
The potential to acquire and quantify rest and stress myocardial perfusion and non-contrast CT scan for CAC scoring from a single dual-modality study may offer a unique opportunity to expand the prognostic value of stress nuclear imaging. The rationale for this integrated approach is predicated on the fact that the perfusion imaging approach is designed to uncover only obstructive atherosclerosis and is thus insensitive for detecting subclinical disease. The CAC score, reflecting the anatomic extent of atherosclerosis,33 may offer an opportunity to improve the conventional models for risk assessment using nuclear imaging alone (especially in patients with normal perfusion), a finding that may serve as a more rational basis for personalizing the intensity and goals of medical therapy in a more cost-effective manner. For example, recent data suggest that quantification of CAC scores at the time of stress nuclear imaging using a dual-modality approach can enhance risk predictions in patients with suspected CAD.29 In a consecutive series of 621 patients undergoing stress PET imaging and CAC scoring in the same clinical setting, risk-adjusted analysis demonstrated that for any degree of perfusion abnormality, there was a stepwise increase in adverse events (death and myocardial infarction) with increasing CAC scores. This finding was observed in patients with and without evidence of ischemia on PET MPI. Indeed, the annualized event rate in patients with normal PET MPI and no CAC was substantially lower than among those with normal PET MPI and a CAC ≥1000 (Fig. 22-5). Likewise, the annualized event rate in patients with ischemia on PET MPI and no CAC was lower than among those with ischemia and a CAC ≥1000. Although CT coronary angiography as an adjunct to perfusion imaging could expand the opportunities to identify patients with noncalcified plaques at greater risk of adverse cardiovascular events, it is unclear how much added prognostic information there is in the contrast CT scan over the simple CAC scan.34
Integrating CT Coronary Angiography and Myocardial Perfusion Imaging for Diagnosis and Management of CAD
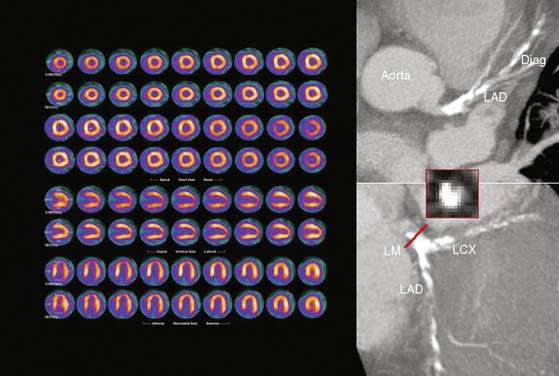
As discussed, CTA provides excellent diagnostic sensitivity for stenoses in the proximal and mid segments (>1.5 mm in diameter) of the main coronary arteries. Although the refinements implemented in the latest generation of CT technology have substantially reduced the number of nonevaluable coronary segments, the spatial resolution is still relatively limited compared to invasive angiography, and the sensitivity of this approach is reduced substantially in more distal coronary segments and side branches.9 This limitation is unlikely to change, because a significant improvement in spatial resolution for CT will have to be coupled with a substantial increase in radiation dose in order to maintain noise constant. However, this limitation of CT can be offset by the MPI information that is generally unaffected by the location of coronary stenoses. First clinical results appear encouraging, and they support the notion that dual-modality imaging may offer superior diagnostic information with regard to identification of the culprit vessel.35–37 For example, Rispler et al. reported a significant improvement in specificity (63% to 95%) and PPV (31% to 77%) without a change in sensitivity or NPV for detection of obstructive CAD as defined by quantitative coronary angiography in a cohort of 56 patients with known or suspected CAD undergoing hybrid SPECT/CTA imaging.37 On the other hand, CTA improves the detection of multivessel CAD, which as discussed earlier is one of the main pitfalls of stress perfusion scintigraphy (Fig. 22-6). As noted, the incremental value seems most pronounced for coronary stenoses in distal segments and diagonal branches, and in vessels with extensive coronary lesions or heavy calcifications on CTA.
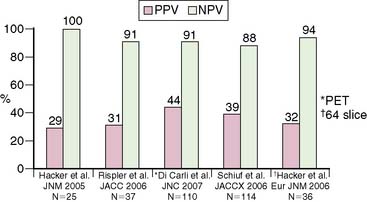
Nonetheless, one of the most compelling arguments supporting a clinical role of dual-modality imaging is its potential ability for optimizing management decisions. The importance of stress perfusion imaging in the integrated strategy is the ability of noninvasive estimates of jeopardized myocardium to identify which patients may benefit from revascularization—that is, differentiating high-risk patients with extensive scar versus those with extensive ischemia (Fig. 22-7). The advantages of this approach are clear: avoidance of unnecessary catheterizations that expose patients to risk and the potential for associated cost savings.38 CTA is an excellent method to exclude CAD, but its ability to accurately assess the degree of luminal narrowing as a surrogate for physiologic significance is only modest. Recent data from multiple laboratories using either sequential (CTA followed by SPECT)6,39–41 or hybrid imaging (SPECT/CT or PET/CT)7,19,42,43 suggest that the positive predictive value of CTA for identifying coronary stenoses producing objective evidence of stress-induced ischemia is suboptimal (Fig. 22-8).
The value of ischemia information for optimizing clinical decision making has been demonstrated by multiple studies. The nonrandomized Coronary Artery Surgery Study (CASS) registry reported that surgical revascularization in patients with CAD improved survival only among those with three-vessel disease with severe ischemia on exercise stress testing, while medical therapy was a superior initial therapy in patients without this finding.44 Nonrandomized observational data using risk-adjustment techniques and propensity scores have also demonstrated the ability of stress perfusion imaging to identify which patients may accrue a survival benefit from revascularization.45 The benefit of an ischemia-guided approach to management is further supported by invasive estimates of flow-limiting stenosis (e.g., fractional flow reserve [FFR]).46 In the setting of an FFR above 0.75, revascularization can be safely deferred without increased patient risk, despite the presence of what visually appears to be a significant stenosis.46 Indeed, cardiac event rates are extremely low in these patients, even lower than predicted if treated with PCI.47 This differential risk appears to be sustainable in the long term.48
Further support came in a recent report from a randomized clinical trial evaluating the efficacy of revascularization decisions using an angiographically guided versus a functionally guided (as assessed by FFR) PCI in patients with multivessel CAD.49 In this study, routine use of an FFR-guided approach significantly reduced the rate of the composite endpoint of death, nonfatal myocardial infarction, and repeat revascularization by 28% at 1 year compared to the angiographically guided strategy. In addition, both groups have high and comparable rates of angina-free patients at 1 year.49 Furthermore, in patients with visually defined left main coronary disease, an FFR above 0.75 was associated with excellent 3-year survival and freedom from major adverse cardiovascular events.47 Conversely, event rates are increased when lesions with FFR less than 0.75 are not revascularized.50
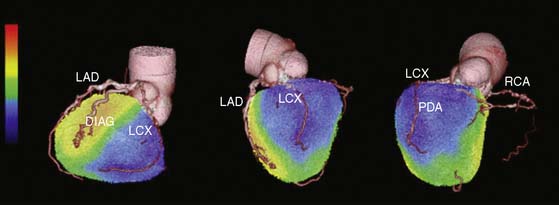
Together, these data suggest that by identifying which patients have sufficient ischemia to merit revascularization, stress perfusion imaging may play a significant role in the selection of patients for catheterization within a strategy based on identification of patient benefit. From the previous discussion, this physiologic data would have greater clinical impact than visually defined coronary anatomy for revascularization decision making. In patients with multivessel CAD, the value of dual-modality imaging would allow better localization of the culprit stenosis and may offer a more targeted approach to revascularization (Fig. 22-9).
CHALLENGES, POTENTIAL OPPORTUNITIES, AND UNRESOLVED ISSUES FOR HYBRID IMAGING
Single-Setting Dual-Modality Versus Sequential Imaging
Second, we need to develop the evidence that would support which patients benefit from dual-modality testing from both diagnostic and prognostic perspectives. As discussed, there is some evidence that assessment CAC scores in combination with stress nuclear imaging may offer an opportunity to improve risk assessment, especially in symptomatic patients without prior CAD.29 However, evaluation of CAC can be achieved without the need of advanced multidetector CT technology. For patients with equivocal stress nuclear test results, CT coronary angiography can be performed as a sequential test in a different scanner. Conversely, stress nuclear imaging is often used as a follow-up of patients with abnormal CTA studies to define hemodynamic significance of coronary stenosis. In such cases of sequential testing, the two data sets can be registered and fused off-line for interpretation and reporting.40,51,52 Thus, the specific impact of hybrid imaging on treatment strategy and subsequently on outcome remains to be determined in prospective and long-term studies.
Radiation Dosimetry
The potential diagnostic appeal of a dual-modality approach must also be weighed against the challenges that it poses with regard to the added radiation dose to patients. This is a key issue as conventional spiral CTA protocols using retrospective ECG-gating have been shown to be associated with high total radiation doses between 9.4 and 21.4 mSv.53,54 Hybrid cardiac imaging with CTA and SPECT MPI has been reported to expose patients to excessively high radiation doses up to 41.5 mSv,37 which has prevented acceptance and the widespread use of this technology. However, this is changing rapidly. New low-dose CTA acquisition protocols with prospective electrocardiogram (ECG) triggering have recently been introduced and shown to offer a tremendous reduction of radiation dose to an average of 2.1 mSv55–57 at maintained accuracy,56 making this technique most suitable for hybrid imaging. Hybrid imaging with prospectively triggered CTA in conjunction with a stress-only SPECT MPI protocol may offer a significant reduction in dose.56
1. Klocke F.J., Baird M.G., Lorell B.H., Bateman T.M., Messer J.V., Berman D.S., O’Gara P.T., Carabello B.A., Russell R.O.Jr, Cerqueira M.D., St John Sutton M.G., DeMaria A.N., Udelson J.E., Kennedy J.W., Verani M.S., Williams K.A., Antman E.M., Smith S.C.Jr, Alpert J.S., Gregoratos G., Anderson J.L., Hiratzka L.F., Faxon D.P., Hunt S.A., Fuster V., Jacobs A.K., Gibbons R.J., Russell R.O. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42(7):1318-1333.
2. Di Carli M.F., Dorbala S., Meserve J., El Fakhri G., Sitek A., Moore S.C. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48(5):783-793.
3. Berman D.S., Kang X., Slomka P.J., Gerlach J., de Yang L., Hayes S.W., Friedman J.D., Thomson L.E., Germano G. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14(4):521-528.
4. Dorbala S., Vangala D., Sampson U., Limaye A., Kwong R., Di Carli M.F. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48(3):349-358.
5. Parkash R., deKemp R.A., Ruddy Td T, Kitsikis A., Hart R., Beauschene L., Williams K., Davies R.A., Labinaz M., Beanlands R.S. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11(4):440-449.
6. Schuijf J.D., Wijns W., Jukema J.W., Atsma D.E., de Roos A., Lamb H.J., Stokkel M.P., Dibbets-Schneider P., Decramer I., De Bondt P., van der Wall E.E., Vanhoenacker P.K., Bax J.J. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48(12):2508-2514.
7. Di Carli M.F., Dorbala S., Curillova Z., Kwong R.J., Goldhaber S.Z., Rybicki F.J., Hachamovitch R. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007;14(6):799-809.
8. Shaw L.J., Iskandrian A.E. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11(2):171-185.
9. Di Carli M.F., Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115(11):1464-1480.
10. Budoff M.J., Dowe D., Jollis J.G., Gitter M., Sutherland J., Halamert E., Scherer M., Bellinger R., Martin A., Benton R., Delago A., Min J.K. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-1732.
11. Miller J.M., Rochitte C.E., Dewey M., Arbab-Zadeh A., Niinuma H., Gottlieb I., Paul N., Clouse M.E., Shapiro E.P., Hoe J., Lardo A.C., Bush D.E., de Roos A., Cox C., Brinker J., Lima J.A. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336.
12. Hoffmann U., Moselewski F., Cury R.C., Ferencik M., Jang I.K., Diaz L.J., Abbara S., Brady T.J., Achenbach S. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient-versus segment-based analysis. Circulation. 2004;110(17):2638-2643.
13. Mollet N.R., Cademartiri F., Krestin G.P., McFadden E.P., Arampatzis C.A., Serruys P.W., de Feyter P.J. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol. 2005;45(1):128-132.
14. Leber A.W., Becker A., Knez A., von Ziegler F., Sirol M., Nikolaou K., Ohnesorge B., Fayad Z.A., Becker C.R., Reiser M., Steinbeck G., Boekstegers P. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47(3):672-677.
15. Raff G.L., Gallagher M.J., O’Neill W.W., Goldstein J.A. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46(3):552-557.
16. Gould K.L., Pan T., Loghin C., Johnson N.P., Sdringola S. Reducing radiation dose in rest-stress cardiac PET/CT by single poststress cine CT for attenuation correction: quantitative validation. J Nucl Med. 2008;49(5):738-745.
17. Lautamaki R., Brown T.L., Merrill J., Bengel F.M. CT-based attenuation correction in (82)Rb-myocardial perfusion PET-CT: incidence of misalignment and effect on regional tracer distribution. Eur J Nucl Med Mol Imaging. 2008;35(2):305-310.
18. Masood Y., Liu Y.H., Depuey G., Taillefer R., Araujo L.I., Allen S., Delbeke D., Anstett F., Peretz A., Zito M.J., Tsatkin V., Wackers F.J. Clinical validation of SPECT attenuation correction using x-ray computed tomography-derived attenuation maps: multicenter clinical trial with angiographic correlation. J Nucl Cardiol. 2005;12(6):676-686.
19. Malkerneker D., Brenner R., Martin W.H., Sampson U.K., Feurer I.D., Kronenberg M.W., Delbeke D. CT-based attenuation correction versus prone imaging to decrease equivocal interpretations of rest/stress Tc-99m tetrofosmin SPECT MPI. J Nucl Cardiol. 2007;14(3):314-323.
20. Gould K.L., Pan T., Loghin C., Johnson N.P., Guha A., Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48(7):1112-1121.
21. Goetze S., Wahl R.L. Prevalence of misregistration between SPECT and CT for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol. 2007;14(2):200-206.
22. McQuaid S.J., Hutton B.F. Sources of attenuation-correction artefacts in cardiac PET/CT and SPECT/CT. Eur J Nucl Med Mol Imaging. 2008;35(6):1117-1123.
23. Slomka P.J., Le Meunier L., Hayes S.W., Acampa W., Oba M., Haemer G.G., Berman D.S., Germano G. Comparison of myocardial perfusion 82Rb PET performed with CT- and transmission CT-based attenuation correction. J Nucl Med. 2008;49(12):1992-1998.
24. Rudd J.H., Warburton E.A., Fryer T.D., Jones H.A., Clark J.C., Antoun N., Johnstrom P., Davenport A.P., Kirkpatrick P.J., Arch B.N., Pickard J.D., Weissberg P.L. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708-2711.
25. Judenhofer M.S., Wehrl H.F., Newport D.F., Catana C., Siegel S.B., Becker M., Thielscher A., Kneilling M., Lichy M.P., Eichner M., Klingel K., Reischl G., Widmaier S., Rocken M., Nutt R.E., Machulla H.J., Uludag K., Cherry S.R., Claussen C.D., Pichler B.J. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459-465.
26. Wexler L., Brundage B., Crouse J., Detrano R., Fuster V., Maddahi J., Rumberger J., Stanford W., White R., Taubert K. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94(5):1175-1192.
27. He Z.X., Hedrick T.D., Pratt C.M., Verani M.S., Aquino V., Roberts R., Mahmarian J.J. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244-251.
28. Berman D.S., Wong N.D., Gransar H., Miranda-Peats R., Dahlbeck J., Arad Y., Hayes S.W., Friedman J.D., Kang X., Polk D., Hachamovitch R., Rozanski A. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923-930.
29. Schenker M.P., Dorbala S., Hong E.C., Rybicki F.J., Hachamovitch R., Kwong R.Y., Di Carli M.F. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117(13):1693-1700.
30. Schepis T., Gaemperli O., Koepfli P., Namdar M., Valenta I., Scheffel H., Leschka S., Husmann L., Eberli F.R., Luscher T.F., Alkadhi H., Kaufmann P.A. Added value of coronary artery calcium score as an adjunct to gated SPECT for the evaluation of coronary artery disease in an intermediate-risk population. J Nucl Med. 2007;48(9):1424-1430.
31. Nallamothu B.K., Saint S., Bielak L.F., Sonnad S.S., Peyser P.A., Rubenfire M., Fendrick A.M. Electron-beam computed tomography in the diagnosis of coronary artery disease: a meta-analysis. Arch Intern Med. 2001;161(6):833-838.
32. Knez A., Becker A., Leber A., White C., Becker C.R., Reiser M.F., Steinbeck G., Boekstegers P. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol. 2004;93(9):1150-1152.
33. Sangiorgi G., Rumberger J.A., Severson A., Edwards W.D., Gregoire J., Fitzpatrick L.A., Schwartz R.S. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126-133.
34. Mahmarian J.J. Computed tomography coronary angiography as an anatomic basis for risk stratification: deja vu or something new? J Am Coll Cardiol. 2007;50(12):1171-1173.
35. Gaemperli O., Schepis T., Koepfli P., Valenta I., Soyka J., Leschka S., Desbiolles L., Husmann L., Alkadhi H., Kaufmann P.A. Accuracy of 64-slice CT angiography for the detection of functionally relevant coronary stenoses as assessed with myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34(8):1162-1171.
36. Namdar M., Hany T.F., Koepfli P., Siegrist P.T., Burger C., Wyss C.A., Luscher T.F., von Schulthess G.K., Kaufmann P.A. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med. 2005;46(6):930-935.
37. Rispler S., Keidar Z., Ghersin E., Roguin A., Soil A., Dragu R., Litmanovich D., Frenkel A., Aronson D., Engel A., Beyar R., Israel O. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49(10):1059-1067.
38. Shaw L.J., Hachamovitch R., Berman D.S., Marwick T.H., Lauer M.S., Heller G.V., Iskandrian A.E., Kesler K.L., Travin M.I., Lewin H.C., Hendel R.C., Borges-Neto S., Miller D.D. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. Economics of Noninvasive Diagnosis (END) Multicenter Study Group. J Am Coll Cardiol. 1999;33(3):661-669.
39. Hacker M., Jakobs T., Matthiesen F., Vollmar C., Nikolaou K., Becker C., Knez A., Pfluger T., Reiser M., Hahn K., Tiling R. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med. 2005;46(8):1294-1300.
40. Gaemperli O., Schepis T., Kalff V., Namdar M., Valenta I., Stefani L., Desbiolles L., Leschka S., Husmann L., Alkadhi H., Kaufmann P.A. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging. 2007;34(7):1097-1106.
41. Gaemperli O., Schepis T., Valenta I., Husmann L., Scheffel H., Duerst V., Eberli F.R., Luscher T.F., Alkadhi H., Kaufmann P.A. Cardiac image fusion from stand-alone SPECT and CT: clinical experience. J Nucl Med. 2007;48(5):696-703.
42. Rispler S., Roguin A., Keidar Z., Ghersin E., Aronson D., Dragu R., Engel A., Israel O., Beyar R. Integrated SPECT/CT for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2006;47:115A.
43. Hacker M., Jakobs T., Hack N., Nikolaou K., Becker C., von Ziegler F., Knez A., Konig A., Klauss V., Tiling R. Combined use of 64-slice computed tomography angiography and gated myocardial perfusion SPECT for the detection of functionally relevant coronary artery stenoses. First results in a clinical setting concerning patients with stable angina. Nuklearmedizin. 2007;46(1):29-35.
44. Weiner D.A., Ryan T.J., McCabe C.H., Chaitman B.R., Sheffield L.T., Fisher L.D., Tristani F. The role of exercise testing in identifying patients with improved survival after coronary artery bypass surgery. J Am Coll Cardiol. 1986;8(4):741-748.
45. Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900-2907.
46. Kern M.J., Lerman A., Bech J.W., De Bruyne B., Eeckhout E., Fearon W.F., Higano S.T., Lim M.J., Meuwissen M., Piek J.J., Pijls N.H.J., Siebes M., Spaan J.A.E. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321-1341.
47. Bech G.J., De Bruyne B., Pijls N.H., de Muinck E.D., Hoorntje J.C.A., Escaned J., Stella P.R., Boersma E., Bartunek J., Koolen J.J., Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928-2934.
48. Pijls N.H., van Schaardenburgh P., Manahoran G., Boersma E., Bech J.W., van’t Veer M., Bar F., Hoorntjie J., Koolen J., Wijns W., De Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. 2007;49:2105-2111.
49. Tonino P.A., De Bruyne B., Pijls N.H., Siebert U., Ikeno F., van’t Veer M., Klauss V., Manoharan G., Engstrom T., Oldroyd K.G., Ver Lee P.N., MacCarthy P.A., Fearon W.F. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224.
50. Chamuleau S.A.J., Meuwissen M., Koch K.T., van Eck-Smit B.L.F., Tio R.A., Tijssen J.G.P., Piek J.J. Usefulness of fractional flow reserve for risk stratification of patients with multivessel coronary artery disease and an intermediate stenosis. Am J Cardiol. 2002;89:377-380.
51. Santana C.A., Garcia E.V., Faber T.L., Sirineni G.K., Esteves F.P., Sanyal R., Halkar R., Ornelas M., Verdes L., Lerakis S., Ramos J.J., Aguade-Bruix S., Cuellar H., Candell-Riera J., Raggi P. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. J Nucl Cardiol. 2009.
52. Slomka P.J. Software approach to merging molecular with anatomic information. J Nucl Med. 2004;45(Suppl 1):36S-45S.
53. Mollet N.R., Cademartiri F., de Feyter P.J. Non-invasive multislice CT coronary imaging. Heart. 2005;91(3):401-407.
54. Hausleiter J., Meyer T., Hadamitzky M., Huber E., Zankl M., Martinoff S., Kastrati A., Schomig A. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113(10):1305-1310.
55. Husmann L., Wiegand M., Valenta I., Gaemperli O., Schepis T., Siegrist P.T., Namdar M., Wyss C.A., Alkadhi H., Kaufmann P.A. Diagnostic accuracy of myocardial perfusion imaging with single photon emission computed tomography and positron emission tomography: a comparison with coronary angiography. Int J Cardiovasc Imaging. 2008;24(5):511-518.
56. Herzog B.A., Husmann L., Landmesser U., Kaufmann P.A. Low-dose computed tomography coronary angiography and myocardial perfusion imaging: cardiac hybrid imaging below 3 mSv. Eur Heart J. 2008.
57. Javadi M., Mahesh M., McBride G., Voicu C., Epley W., Merrill J., Bengel F.M. Lowering radiation dose for integrated assessment of coronary morphology and physiology: first experience with step-and-shoot CT angiography in a rubidium-82 PET-CT protocol. J Nucl Cardiol. 2008;15(6):783-790.