Chapter 189 Pertussis (Bordetella pertussis and Bordetella parapertussis)
Treatment
Goals of therapy are to limit the number of paroxysms, to observe the severity of the cough, to provide assistance when necessary, and to maximize nutrition, rest, and recovery without sequelae (Table 189-1). Infants <3 mo of age with suspected pertussis are always admitted to hospital, as are those between 3 and 6 mo of age unless witnessed paroxysms are not severe, and patients of any age if significant complications occur. Prematurely born young infants and children with underlying cardiac, pulmonary, muscular, or neurologic disorders have a high risk for severe, potentially fatal disease.
Table 189-1 CAVEATS IN ASSESSMENT AND CARE OF INFANTS WITH PERTUSSIS
Antibiotics
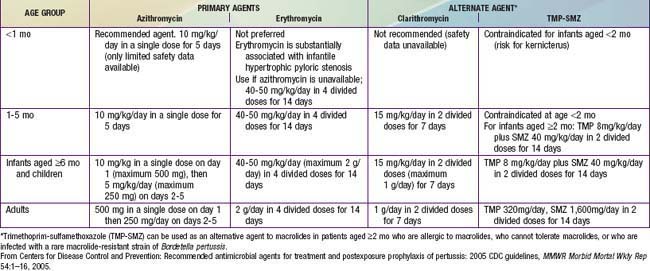
An antimicrobial agent is always given when pertussis is suspected or confirmed, primarily to limit the spread of infection and secondarily for possible clinical benefit. Macrolides are preferred agents and are similar to one another in terms of in vitro activity (Table 189-2). Resistance has been reported rarely. A 7- to 10-fold relative risk for infantile hypertrophic pyloric stenosis (IHPS) has been reported in neonates treated with orally administered erythromycin. Azithromycin is the preferred agent for most patients and particularly in neonates, although cases of IHPS have followed its use. All infants <1 mo of age treated with any macrolide should be monitored for symptoms of pyloric stenosis. Benefits of post-exposure prophylaxis for infants far outweigh risk of IHPS.
Care of Household and Other Close Contacts
A macrolide agent should be given promptly to all household contacts and other close contacts, such as those in daycare, regardless of age, history of immunization, and symptoms (see Table 189-2). The same age-related drugs and doses used for prophylaxis are used for treatment. Visitation and movement of coughing family members in the hospital must be assiduously controlled until erythromycin has been taken for 5 days. In close contacts <7 yr of age who have received fewer than 4 doses of pertussis-containing vaccines, vaccination should be initiated or continued to complete the recommended series. Children <7 yr of age who received a 3rd dose >6 mo before exposure or a 4th dose ≥3 yr before exposure should receive a booster dose. Individuals ≥9 yr of age should be given a Tdap (adolescent/adult formulation of tetanus and diphtheria toxoids and acellular pertussis) booster if they have not previously received Tdap. Unmasked health care personnel (HCP) exposed to untreated cases should be evaluated for post-exposure prophylaxis and follow-up. Coughing HCP with or without known exposure to pertussis should be promptly evaluated for pertussis.
Prevention
Universal immunization of children with pertussis vaccine, beginning in infancy with periodic reinforcing doses through adolescence and adulthood, is central to the control of pertussis (Chapter 165). There is no serologic correlate of protection from B. pertussis.
American Academy of Pediatrics, Committee on Infectious Diseases. Prevention of pertussis among adolescents: recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. Pediatrics. 2006;117:965-978.
Castagnini LA, Munoz FM. Clinical characteristics and outcomes of neonatal pertussis: a comparative study. J Pediatr. 2010;156:498-500.
Centers for Disease Control and Prevention. Local health department costs associated with response to a school-based pertussis outbreak—Omaha, Nebraska, Sept-Nov, 2008. MMWR. 2011;60(1):5-8.
Centers for Disease Control and Prevention. Use of mass Tdap vaccination to control an outbreak of pertussis in a high school—Cook County, Illinois, September 2006—January 2007. MMWR Morbid Mortal Wkly Rep. 2008;57:796-799.
Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Morbid Mortal Wkly Rep. 2005;54:1-16.
Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep. 2006;55(RR-3):1-34.
Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among pregnant and postpartum women and their infants: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep. 2008;57:1-51.
Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304(8):890-896.
Dylag AM, Shah SI. Administration of tetanus, diphtheria, and acellular pertussis vaccine to parents of high-risk infants in the neonatal intensive care unit. Pediatrics. 2008;122:e550-e555.
Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics. 2009;123:1446-1451.
Greenberg DP, Doemland M, Bettinger JA, et al. Epidemiology of pertussis and Haemophilus influenzae type b disease in Canada with exclusive use of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus—Haemophilus influenzae type b pediatric combination vaccine and an adolescent-adult tetanus-diphtheria-acellular pertussis vaccine: implications for disease prevention in the United States. Pediatr Infect Dis J. 2009;28:521-528.
Halasa NB, Barr FE, Johnson JE, et al. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics. 2003;112:1274-1278.
Halperin SA. Recommendation for an adolescent dose of tetanus and diphtheria toxoids and acellular pertussis vaccine: reassurance for the future. J Pediatrics. 2006;149:589-591.
Halperin SA. The control of pertussis—2007 and beyond. N Engl J Med. 2007;356:110-113.
McIntyre P, Wood N. Pertussis in early infancy: disease burden and preventive strategies. Curr Opin Infect Dis. 2009;22:215-223.
McColloster P, Vallbona C. Graphic-output temperature data loggers for monitoring vaccine refrigeration: implications for pertussis. Am J Pub Health. 2011;101(1):46-47.
Mooi FR, van Loo IHM, van Gent M, et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg Infect Dis. 2009;15:1206-1212.